ABSTRACT
The capsular polysaccharides in different serotypes of Klebsiella pneumoniae (KP) coded by the (CPS) gene cluster are characterized by a conserved and a hyper-variable region. We performed a virulence study by switching genes in the highly conserved region of the CPS cluster between strains. Six genes in the CPS conserved region in serotype K20, including galF, acidPPc, wzi, wza, wzb and wzc, were knocked out and replaced by the homologous genes from serotype K1. Compared to the parental K20 strain, the mutants showed a decline in lethality (LD50) in mice from 10-fold to > 105-fold and were categorized in terms of the effect on virulence as low (L) for galF and acidPPC, moderate (M) for wzi, and high (H) for wza, wzb and wzc. Although substituting the acidPPC gene from K1 for acidPPC in the K20 strain fully restored virulence, substitution with the wzi, wza, wzb or wzc homologs from K1 did not. The restoration with wzi from K1 led to a partial restoration of virulence, with the LD50 in mice changing from 104 to 103 CFU. For the wza, wzb and wzc genes, Complementation of K20 wza, wzb and wzc from K1 resulted in varied degrees of lethality in mice. Variable improvement in serum killing and phagocytosis was observed when the knockout mutants were compared with the gene-switched strains. In conclusion, homologous genes for capsule synthesis failed to exhibit the same functionality when switched between serotypes and virulence was decreased in different degree in according to the genes' homology.
KEYWORDS: capsule and virulence, Klebsiella pneumoniae, liver abscess
Introduction
Klebsiella pneumoniae is an opportunistic pathogen that causes both community and nosocomial infections.1 Recently, a new invasive syndrome has been defined in community-acquired liver abscesses with or without complications such as meningitis or endophthalmitis.2 This invasive syndrome has been mostly attributed to K. pneumoniae with the capsular serotypes K1 or K2. Bacterial cell surface polysaccharides, including both capsular- (K antigen) and lipo-polysaccharides, have been well documented as important virulent factors in the establishment of infection.3,4 Capsular polysaccharides (CPS) are a major barrier to macrophage or neutrophil phagocytosis. More than 77 serotypes of K. pneumoniae have been distinguished, and strains that produce CPS are generally more virulent than non-capsulated strains. Although serotypes K1 and K2 have been shown to be highly virulent in general, strains that are equivalently virulent have also been isolated from other serotypes. It has been speculated that each structural gene in the capsule synthesis gene cluster plays a different role in virulence. Previous studies have shown that the capsule synthesis gene cluster in the different serotypes has 2 distinct regions.5,6 One region consists of highly conserved genes (>50% nucleotide sequence similarity) while the other region is hypervariable. Each of these structural genes is serotype specific, and may or may not be replaceable with the homologous gene from another serotype.6 Although previous study has transferred plasmid carrying whole CPS genes cluster of serotype K2 into K21,7 homologous replacement of individual gene has not been studied.
In this study, we have collected a serotype K20 strain with a high lethality in mice with ≤102 colony forming units (cfu) suggesting the CPS genes were responsible for their hyper-virulence. We studied the contribution of genes in the highly conserved region of the CPS cluster to virulence. By using homologous gene exchange, we substituted homologous genes from a serotype K1 isolate into a serotype K20 isolate that had similar lethality in mice and assessed whether the genetic background of K20 would support CPS synthesis with homologous genes from K1. Virulence was also assessed in these gene-switched strains.
Methods and materials
Clinical isolation of serotypes K1 and K20 and multi-locus sequence typing (MLST)
Serotypes K1 (NVT-1001) and K20 (NVT-20312) were both isolated from liver abscess patients.8,9 The serotyping was performed as previously described.9 MLST was performed according to the Institute Pasteur scheme. Sequences of 7 housekeeping genes were obtained from isolates from liver abscess patients and carriers. Sequence information was compared with the information on the MLST web site (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html). Alleles and sequence types (STs) were assigned accordingly. Sequences of any alleles that were not in the database were submitted to the curator, and new allele numbers were obtained. Strains that had a difference in 2 or more alleles were considered to be unrelated.
Sequencing of the CPS gene cluster for serotype K1 and K20 isolates
Primers were designed based on the nucleotide sequence of the complete genome of K. pneumoniae serotype K1 NTUH-K2044 (GenBank accession number NC012731) and serotype K20 KP NTUH-KP13 (GenBank accession number AB289648) and used for PCR amplification of the corresponding K1 strain NVT1001 and K20 strain 312 sequences using Phusion Flash High-Fidelity PCR master mix (Finnzymes Oy, Espoo, Finland). The length of CPS gene cluster including the flanking region amplified using the primer sets was designed within total length CPS gene cluster which 20,611 bp for K1 and 19,503 bp for K20 (see Table S1 in the supplemental material). All sequence analyses and protein homology searches were conducted using the NCBI database (http://www.ncbi.nlm.nih.gov/).
Detection of virulence determinants for hypervirulent strain
Previously described virulence determinants including hypermucoviscous phenotype, aerobactin (iucA), yersiniabactin (irp2), salmochelin (iroB), enterobactin (entB), allantoin Metabolism (allS) and iron-uptake system (kfu) were detected according to previously published reports.2,10,11
Construction of in-frame deletion mutants in Serotype K20 and CPS gene exchange from serotype K1 to K20
Plasmid pUT-kmy consists of an R6K origin of replication, an mobRP4 origin of transfer, and a kanamycin resistance cassette,9 and was ligated with a sacB gene to generate plasmid pUT-KB to construct allelic exchange mutants. Plasmid pUT-KB is a suicide vector containing a counter-selection marker sacB that originates from Bacillus subtilis.12 When this gene is expressed on an integrated pUT-KB, it confers a sucrose-sensitive phenotype, which enables positive selection with sucrose to detect the loss of the vector. To make in-frame deletion mutants and exchange serotype K1 alleles into K20 homologous genes, an allelic exchange method was performed as previously described.13 Briefly, DNA fragments of the partial galF (UDP-Glucose pyrophosphorylase), acidPPc (acid phosphatase homolog), wzi (surface assembly), wza (putative capsule polysaccharide export protein), wzb (protein tyrosine phosphatase) or wzc (tyrosine-protein kinase) genes and the flanking regions were amplified from K20 using PCR with the primers sets listed in Table S1. The PCR fragments were generated and cloned into pUT-KB, resulting in the plasmids p-galF, p-acidPPc, p-wzi, p-wza, p-wzb and p-wzc. For homologous recombination, the plasmids p-galF, p-acidPPc, p-wzi, p-wza, p-wzb and p-wzc were transformed into E. coli S17-1 λpir using the heat shock method and mobilized into the K20 strain via conjugation. Single-crossover strains were selected from brilliant green containing inositol-nitrate-deoxycholate (BIND) plates supplemented with kanamycin (50 mg/ml), while the growth of non-K. pneumoniae strains was effectively suppressed on the BIND plates.9 A kanamycin-resistant transconjugant was selected, and the insertion of p-galF, p-acidPPc, p-wzi, p-wza, p-wzb or p-wzc was verified via PCR. After incubation in 20 ml BHI for 6 hours in the absence of kanamycin at 37°C, the fully grown cultures were spread onto LB plates supplemented with 10% sucrose. After a double crossover occurred, sucrose-resistant, kanamycin-sensitive colonies were selected, and in-frame deletions were obtained.
The procedures to obtain homologous recombinants for the allelic exchange of acidPPc, wzi, and wza from K1 were similar to the procedures used to obtain in-frame deletion mutants. An in-frame deletion, ΔacidPPc, Δwzi, or Δwza, was selected for homologous recombination. For the restoration with homologous acidPPc, wzi, or wza genes from K1 (NVT 1001), DNA fragments of the entire acidPPc, wzi, or wza genes with the flanking regions were amplified from NVT1001. The PCR fragments were then cloned into pUT-KB, resulting in plasmids containing pacidPPc, pwzi, or pwza. Homologous recombination was performed as described above for the in-frame deletions. K20 recombinants containing an entire acidPPc, wzi, or wza gene from K1 NVT1001 were confirmed via DNA sequencing.
Virulence assessment by neutrophil phagocytosis and the serum resistance assay
A neutrophil phagocytosis assay was performed as previously described.14. Serum bactericidal activity was measured using the method of Podschun et al.,15 with a slight modification. Isolation of neutrophils and serum from healthy volunteers was ethically approved by a human IRB committee (IRB-1-103-05-155). Briefly, a serum bactericidal assay was performed by incubating the bacteria in 75% normal human serum (NHS). The survival rate in 75% NHS was measured by viable counts after 30 min. of incubation. Each individual test was performed 3 times to calculate the standard deviation expressed with an error bar.
Serum agglutination test for K1 and K20
Parental isolates of K1 and K20 and mutants derived from them were serotyped using a capsule swelling reaction with antisera obtained from the Health Protection Agency and assessed by serum agglutination as previously described.16
Determination of K. pneumoniae dose causing 50% lethal dose (LD50) in mice
To determine the LD50 in mice, 10 mice were used as a sample population for each bacterial concentration. A 10-fold dilution series of colony forming unit (cfu) of K. pneumoniae was made and BALB/c mice were injected intraperitoneally with 0.1 ml of each dilution. The mice used in this study were approved by the animal use committee (approval NHRI-IACUC-103014-A). Symptoms and signs of infection were observed for 14 days. The survival of the inoculated mice was recorded, and the LD50 was calculated using SigmaPlot version 7.0 from SPSS Inc. (Chicago, IL).
Results
Genetic structure of the K1 and K20 capsular polysaccharide synthesis gene cluster
Comparison of the amino acid sequence of the K1 and K20 capsular polysaccharide synthesis genes indicated a conserved region with an amino acid sequence similarity from 99–54% (Fig. 1). The hyper-variable region of homologous genes had a nucleotides' sequence similarity with <32% between K1 and K20.
Figure 1.
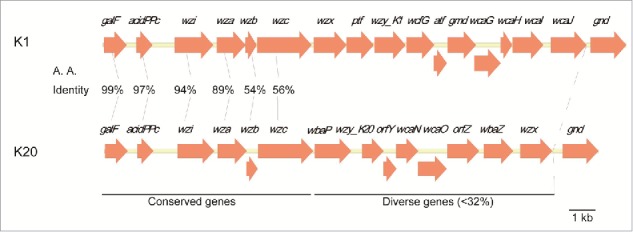
Capsular polysaccharide synthesis gene cluster in serotypes K1 and K20 of K. pneumoniae
Characteristics of K1 and K20 isolates used for knockout and switching of the capsular homologous genes
PCR analysis of the 7 housekeeping genes studied revealed that the K1 and K20 isolates belonged to MLST 23 and MLST 268 and had the gapA, infB, mdh, pgi, phoE, rpoB, and tonB alleles 2, 1, 1, 1, 9, 4, 12 and 2, 1, 2, 1, 7, 1, 81, respectively (Table 1). Both K1 and K20 carried chromosomal and plasmid regulators of the mucoid phenotype A (rmpA) genes including c-rmpA, p-rmpA and p-rmpA2 and had a similar lethality in mice by intra-peritoneal (IP) injection, with LD50 values of 102 CFU (Table 1). Detection of virulence determinants including hypermucoviscous phenotype, iucA, irp2, iroB, entB, allS and kfu showed that both serotype K1 and K20 contained all detected determinants except K20 isolate did not have hypermucoviscous phenotype and carry alls gene (Table 2).
Table 1.
Characteristics of the serotype K1 and K20 strains for capsule-related genes switched in this study.
| PCR |
Alleles |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | LD50 (CFU) | c-rmpA | p-rmpA | p-rmpA2 | MLST | gapA | infB | mdh | pgi | phoE | rpoB | tonB |
| K1 | 102 | + | + | + | ST23 | 2 | 1 | 1 | 1 | 9 | 4 | 12 |
| K20 | 102 | + | + | + | ST268 | 2 | 1 | 2 | 1 | 7 | 1 | 81 |
Note. CFU: colony form unit; c-rmpA: chromosome regulator of mucoid phenotype A; p-rmpA: plasmid rmpA; p-rmpA2: plasmid rmpA2; LD50: Lethal Dose 50%; MLST: Multi-locus sequence typing.
Table 2.
Virulence characteristics of the serotype K1 and K20 strains.
Virulence effects of knockout mutants of ΔgalF, ΔacidPPc, Δwzi, or Δwza, Δwzb or Δwzc
Compared to the parental K20 strain, gene knockout mutants showed a variable decline in lethality (LD50) in mice, from 10-fold to > 105-fold and the effect on virulence could be categorized into low (L), moderate (M), or high (H) (Table 3). The galF and acidPPc genes encoded polycistronic mRNAs driven by a P1 promoter, and a low effect of these genes on virulence was observed (Table 3). The remaining, wzi, wza, wzb or wzc gene was driven by a P2 promoter. The lost of these genes showed disruption of the capsule surface assembly. The mutant Δwzi, had a moderate effect with a reduced lethality of 100-fold (Table 3). The deletion of the Δwza, Δwzb or Δwzc genes that are involved in CPS polymerization caused a significant decrease in virulence (LD50 >107) indicating the importance of these genes in capsule synthesis (Table 3).
Table 3.
Virulence effect on mice lethality (LD50), resistance to serum complement killing and neutrophil phagocytosis in knockout mutants, switching conserved genes from serotype K1 into K20 and their complemented mutants: (1) ΔgalF, ΔacidPPc, K20Δ -acidPPc:(K1)acidPPc and restoration of ΔacidPPc::acidPPc in mutant acidPPc, with low effect (L) in LD50; (2) Δwzi, K20Δ wzi::(K1)wzi and restoration of wzi in mutant Δwzi, with moderate effect (M) in LD50; (3) Δwza, K20Δ wza::(K1)wza, Δwzb and Δwzb and restoration of K20Δ wza:: wza.
| Strain | LD50 (cfu) | Survival rate in 75% NHS (%) | Relative serum resistance* | Ingested bacterial (%) | Relative phagocytosis sensitivity# |
|---|---|---|---|---|---|
| K20 | 102 | 27.74 ± 6.29 | 1 | 46.78 ± 9.41 | 1 |
| Low effect on virulence (L) | |||||
| K20Δ galF | 103 | 4.33 ± 1.72 | 0.16 | 52.16±1.79 | 1.12 |
| K20Δ acidPPc | 103 | 8.15 ± 2.08 | 0.29 | 49.69 ± 1.81 | 1.06 |
| K20Δ -acidPPc:(K1)acidPPc | 102 | 32.16 ± 6.57 | 1.16 | 52.24 ± 4.70 | 1.12 |
| K20Δ acidPPc:: acidPPc | 102 | 24.46 ± 10.98 | 0.88 | 50.94 ± 4.26 | 1.09 |
| Moderate effect on virulence (M) | |||||
| K20Δ wzi | 104 | 1.50 ± 0.52 | 0.05 | 83.83 ± 6.91 | 1.79 |
| K20Δ wzi::(K1)wzi | 103 | 28.03 ± 4.55 | 1.01 | 29.12 ± 4.77 | 0.62 |
| K20Δ wzi::wzi | 102 | 23.03 ± 9.07 | 0.83 | 48.34 ± 4.59 | 1.03 |
| High effect on virulence (H) | |||||
| K20Δ wza | > 107 | 0 | 0.00 | 86.28 ± 3.50 | 1.84 |
| K20Δ wza::(K1)wza | > 106 | 0.03 ± 0.02 | 0.001 | 83.30 ± 6.55 | 1.78 |
| K20Δ wza:: wza | 102 | 23.40 ± 3.15 | 0.84 | 49.68 ± 2.05 | 1.06 |
| K20Δ wzb | > 107 | 0 | 0.00 | 91.22 ± 2.56 | 1.95 |
| K20Δ wzb:: (K1)wzb | 106 | 123.33 ± 16.80 | 4.45 | 70.27 ± 6.60 | 1.50 |
| K20Δ wzb:: wzb | 102 | 34.60 ± 3.04 | 1.24 | 47.49 ± 4.91 | 1.02 |
| K20Δ wzc | > 107 | 0 | 0.00 | 92.71 ± 1.94 | 1.98 |
| K20Δ wzc:: (K1)wzc | 104 | 3.63 ± 1.00 | 0.13 | 47.99 ± 3.23 | 1.03 |
| K20Δ wzc:: wzc | 102 | 31.46 ± 6.76 | 1.13 | 41.81 ± 6.92 | 0.89 |
Note.
Relative serum sensitivity, survival rate in 75% NHS of parental K20 isolate/ survival rate in 75% NHS of mutant. <1 referred to decease resistant to serum resistance and >1 referred to increase resistant to serum resistance.
Relative phagocytosis sensitivity, neutrophil phagocytosis rate measured after a 15-minute incubation with FITC-labeled bacteria of isogenic mutants compared with parental K20. Bold: significant change of serum resistance with reference to parental K20.
Resistance to serum complement killing and neutrophil phagocytosis in knockout mutants and the complemented mutants
Compared to the parental K20 strain, the mutant ΔgalF had no significant difference in survival in 75% normal human serum (NHS), and no change in serum resistance was observed. In contrast, the ΔacidPPc mutant exhibited a significant increase in survival in 75% NHS and became highly serum resistant, indicating the importance of acidPPc in serum complement killing (Table 3). Both the ΔgalF and ΔacidPPc mutants showed a slight increase of phagocytic uptake in neutrophils, but their susceptibility to phagocytosis was not significantly changed. In addition to the phagocytosis results, K20 antibody agglutination assays showed that the ΔgalF and ΔacidPPc mutants showed positive agglutination with anti-K20 antibody, indicating that the knockout of ΔgalF and ΔacidPPc did not greatly affect CPS synthesis (Figure S2). A complemented mutant of K20, ΔacidPPc::acidPPc, exhibited an identical LD50 and susceptibility of serum killing as the parental K20 strain.
The Δwzi mutant showed a significantly decreased survival rate in 75% NHS and a significantly increased phagocytic uptake in neutrophils, indicating a loss of virulence (Table 3). The complemented K20 mutant Δwzi::wzi showed restored phagocytic uptake in neutrophils comparable to the K20 parent. A K20 antibody agglutination assay showed that Δwzi mutants showed positive agglutination with anti-K20 antibody, indicating that the knockout of wzi did not prevent CPS synthesis (Figure S3).
Knockout mutants Δwza, Δwzb or Δwzc showed a significant reduction in 75% NHS and an increase in phagocytic uptake by neutrophils were observed for all 3 mutants. The complemented K20 mutant Δwza::wza showed a restored survival rate in 75% NHS and phagocytic uptake in neutrophils similar to the K20 parent (Table 3). Unlike ΔgalF, ΔacidPPc and Δwzi mutants, the Δwza, Δwzb or Δwzc mutants did not agglutinate with anti-K20 antibody, indicating that knock out of Δwza, Δwzb or Δwzc completely prevented or greatly reduced CPS synthesis (Figure S3). The complemented mutant K20Δwza::wza showed positive agglutination with K20 antibody.
The characteristics and effects on virulence of switching the genes acidPPc, wzi, wza, wzb or wzc from K1 into K20
Because the similarity of the galF amino acid sequence between K1 and K20 was 99%, switching K1-galF into K20-galF was not done. Only the acidPPc genes were exchanged to represent the effect of switching a K1 homologous gene into K20 in the low virulence effect group. The switching of K1-acidPPc into K20 restored virulence in terms of serum resistance, phagocytic resistance and the LD50 compared to the K20 parent (Table 3). Switching in the K20::K1-acidPPc mutant did not result in anti-K1 serum agglutination and agglutination with an anti-K20 serum test was unchanged (Table 3 and Figure S4).
The Δwzi knockout mutant showed a moderate impact on LD50 and had a 102-fold decreased in lethality compared to K20 parent strain. The substitution of K1-wzi into K20 caused a 10-fold decrease in LD50 compared to the K20 parent strain. On the other hand, a 10-fold increase LD50 of K1-wzi compared to a K20-Δwzi knockout mutant had been demonstrated. The results indicated a partial restoration of virulence after gene exchange. No change in serum resistance and agglutination with anti-K20 serum was observed after switching wzi from K1 into K20 (Table 3 and Figure S5) but an increased phagocytic resistance was observed in a K20-Δwzi.:K1-wzi complemented strain. The increased phagocytic resistance K20-Δwzi.:K1-wzi did not contribute to a higher LD50 compared to the K20 parent.
All three exchange mutants, K20::K1-wza,-wzb and -wzc, did not show restored virulence compared to the K20 parent, and no change was noted in the anti-K20 agglutination assay. Switching K20::K1-wza,-wzb did not restore phagocytic resistance, while switching K20::K1-wzc completely restored phagocytic resistance. Switching of ::K1-wza resulted in a similar LD50 and serum resistance in the K20-Δwza knockout mutant (Table 3). An increase in serum resistance and the LD50 was observed in the ::K1-wzb exchange mutant compared to the K20Δwzb knockout mutant (Table 3 and Figure. S6). Compared to the K20 parental strain, the LD50 of the K20Δwzb::K1-Δwzb exchange mutant was 104-fold less than the K20 parent. A significant increase in the serum resistance of the K20::K1-Δwzb exchange mutant was also observed compared to the K20 parent strain (Table 3). In the ::K1-Δwzc exchange mutant, the LD50 and serum resistance were partially restored. A 100-fold difference in the LD50 was found compared to the parent (Table 3). The exchange of conserved homologous genes from K1 to K20 showed that homologous genes for capsule synthesis from different serotypes of K. pneumoniae did not have the same functionality as the original genes, except for acidPPc, a gene that had >97% nucleotide similarity in the 2 strains. Even with a similarity as high as 94% (wzi), an incomplete restoration of virulence was observed among strains in which a homologous gene exchange occurred.
Discussion
Serotype-specific K. pneumoniae capsular polysaccharides are an important factor contributing to bacterial virulence.2,17,18 Specific capsular serotypes have been documented with a high frequency to show hyper-virulence in mouse and human infections.19 A cluster of genes are involved in capsule synthesis and driven by 2 promotors.6 Previous studies have shown that 6 genes, galF, acidPPc, wzi, wza, wzb and wzc, are conserved among different capsular serotypes.5,6 These conserved genes have also been proposed for the identification of capsular serotypes, including wzi and wzc.5,20 In this study, we have tried to elucidate the role of these conserved genes in virulence and whether they can function in a similar manner after exchanging a homologous gene from one serotype to another. We found these genes can be categorized into 3 types according to their different effects on the degree of virulence.
Among the 6 conserved genes studied, each individual gene made a different contribution to lethality in mice. The genes galF and acidPPC, which are driven by the P1 promotor, showed less influence on lethality. The wzi gene, which is responsible for surface assembly, showed a relatively moderate effect on lethality. The genes, Δwza, Δwzb or Δwzc, which are involved in CPS polymerization and CPS production,21 had a high effect on virulence. Loss of these genes results in the loss of CPS synthesis, conferring high susceptibility to neutrophilic phagocytosis. In general, knockout mutants of genes driven by the P2 promotor showed a loss of serum resistance and became more susceptible to neutrophilic phagocytosis (Table 3), but ΔgalF showed only greater susceptibility to serum killing but no effect on phagocytosis was evident, indicating normal CPS production.
Replacement with homologous genes wzi, wza, wzb or wzc from serotype K1 was unable to fully restore virulence, except for acidPPC. The restoration with acidPPC from K1 resulted in an equivalent virulence to that of the K20 parent for serum killing and phagocytosis, indicating that acidPPC from K1 can function as acidPPC in K20. In the wzi knockout mutant in K20, wzi from the serotype K1 isolate did not completely restore virulence in terms of lethality. For comparison between K20::K1-wzi and parental K20, K20::K1-wzi showed a significant improvement in phagocytosis but not in serum resistance indicating that K1-wzi gene can perform a partial function as original K20-wzi on capsular polysaccharides synthesis and complement synthesis (Table 3). The increase in resistance to phagocytosis did not contribute to virulence as determined by the lethality experiments in mice. Whether replacement of K20 by K1-wzi is not only affecting phagocytosis but other function contributing to virulence needs further study. For the wza, wzb and wzc genes, the substitution of wza, wzb and wzc from K1 into knockout mutants caused variable changes in lethality. The restoration with wza from K1 improved phagocytosis and serum resistance, and led to no change in lethality. Thus, the restoration with wza from K1 does not affect K20 capsular synthesis. The restoration with wzb resulted in a significant increase in serum resistance but no effect on phagocytosis, leading to a small change in the LD50 compared to a wzb knockout mutant. In contrast, the restoration of wzc from K1 significantly changed the phagocytosis rate compared to a wzc knockout mutant and led to a significant improvement in lethality. The wzc from K1 partially contributes to capsule synthesis in K20. This observation also indicates that the anti-phagocytic function of the capsule is more important than resistance to complement killing in terms of virulence.
Since the present study was focused only on the effect homologues genes switching, a serotype switching effect after the whole cluster of capsular polysaccharides genes' exchanging is of interest. Further study on serotype switching between serotypes is warrant in order to see the effect on virulence after serotype switched. In conclusion, the homologous genes for capsule synthesis in different serotype of K. pneumoniae are unable to function perfectly the same after switching. Overall, virulence declined even if the amino acid homology between the serotypes was very high.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST-104-2314-B-016-019-MY-3) and the National Health Research Institutes, (NHRI-IV-104-PP06), Taiwan.
References
- [1].Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998; 11:589-603; PMID:9767057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma L, Lu PL, Siu LK, Hsieh MH. Molecular typing and resistance mechanisms of imipenem-non-susceptible Klebsiella pneumoniae in Taiwan: results from the Taiwan Surveillance of Antibiotic Resistance (TSAR) study, 2002–2009. J Med Microbiol 2012; 62(Pt 1):101-7; PMID: 23002067; https://doi.org/ 10.1099/jmm.0.050492-0 [DOI] [PubMed] [Google Scholar]
- [3].Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, Alberti S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 2002; 70:2583-90; PMID:11953399; https://doi.org/ 10.1128/IAI.70.5.2583-2590.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yeh KM, Chiu SK, Lin CL, Huang LY, Tsai YK, Chang JC, Lin JC, Chang FY, Siu LK. Surface antigens contribute differently to the pathophysiological features in serotype K1 and K2 Klebsiella pneumoniae strains isolated from liver abscesses. Gut Pathog 2016; 8:4; PMID:26893615; https://doi.org/ 10.1186/s13099-016-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pan YJ, Lin TL, Chen YH, Hsu CR, Hsieh PF, Wu MC, Wang JT. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS One 2013; 8:e80670; PMID:24349011; https://doi.org/ 10.1371/journal.pone.0080670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shu HY, Fung CP, Liu YM, Wu KM, Chen YT, Li LH, Liu TT, Kirby R, Tsai SF. Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology 2009; 155:4170-83; PMID:19744990; https://doi.org/ 10.1099/mic.0.029017-0 [DOI] [PubMed] [Google Scholar]
- [7].Ofek I, Kabha K, Athamna A, Frankel G, Wozniak DJ, Hasty DL, Ohman DE. Genetic exchange of determinants for capsular polysaccharide biosynthesis between Klebsiella pneumoniae strains expressing serotypes K2 and K21a. Infect Immun 1993; 61:4208-16; PMID:8104896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, Chen TL, Chang FY, Koh TH. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol 2007; 45:466-71; PMID:17151209; https://doi.org/ 10.1128/JCM.01150-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yeh KM, Lin JC, Yin FY, Fung CP, Hung HC, Siu LK, Chang FY. Revisiting the importance of virulence determinant magA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: exact role in serotype K1 capsule formation. J Infect Dis 2010; 201:1259-67; PMID:19785524; https://doi.org/ 10.1086/606010 [DOI] [PubMed] [Google Scholar]
- [10].Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 2009; 4:e4982; PMID:19319196; https://doi.org/ 10.1371/journal.pone.0004982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 2014; 82:2356-67; PMID:24664504; https://doi.org/ 10.1128/IAI.01667-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reyrat JM, Pelicic V, Gicquel B, Rappuoli R. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect Immun 1998; 66:4011-7; PMID:9712740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsai YK, Fung CP, Lin JC, Chen JH, Chang FY, Chen TL, Siu LK. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother 2011; 55:1485-93; PMID:21282452; https://doi.org/ 10.1128/AAC.01275-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, Wang JJ, Huang LY, Siu LK. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect 2004; 6:1191-8; PMID:15488738; https://doi.org/ 10.1016/j.micinf.2004.06.003 [DOI] [PubMed] [Google Scholar]
- [15].Podschun R, Teske E, Ullmann U. Serum resistance properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentralbl Hyg Umweltmed 1991; 192:279-85; PMID:1777008 [PubMed] [Google Scholar]
- [16].Abate G, Koh TH, Gardner M, Siu LK. Clinical and bacteriological characteristics of Klebsiella pneumoniae causing liver abscess with less frequently observed multi-locus sequences type, ST163, from Singapore and Missouri, US. J Microbiol Immunol Infect 2012; 45:31-6; PMID:22138655; https://doi.org/ 10.1016/j.jmii.2011.09.002 [DOI] [PubMed] [Google Scholar]
- [17].Lin JC, Koh TH, Lee N, Fung CP, Chang FY, Tsai YK, Ip M, Siu LK. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog 2014; 6:21; PMID:24987462; https://doi.org/ 10.1186/1757-4749-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin WR, Lu PL, Siu LK, Chen TC, Lin CY, Hung CT, Chen YH. Rapid control of a hospital-wide outbreak caused by extensively drug-resistant OXA-72-producing Acinetobacter baumannii. Kaohsiung J Med Sci 2011; 27:207-14; PMID:21601165; https://doi.org/ 10.1016/j.kjms.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nassif X, Sansonetti PJ. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun 1986; 54:603-8; PMID:2946641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decre D. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 2013; 51:4073-8; PMID:24088853; https://doi.org/ 10.1128/JCM.01924-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ho JY, Lin TL, Li CY, Lee A, Cheng AN, Chen MC, Wu SH, Wang JT, Li TL, Tsai MD. Functions of some capsular polysaccharide biosynthetic genes in Klebsiella pneumoniae NTUH K-2044. PLoS One 2011; 6:e21664; PMID:21765903; https://doi.org/ 10.1371/journal.pone.0021664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007; 45:284-93; PMID:17599305; https://doi.org/ 10.1086/519262 [DOI] [PubMed] [Google Scholar]
- [23].Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 2005; 43:4178-82; PMID:16081970; https://doi.org/ 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


