Abstract
Purpose
The aim of this study was to conduct a prospective pilot study comparing the diagnostic performance of MRI alone and 18F-FDG simultaneous PET/MRI using a diuresis protocol in bladder cancer patients.
Methods
Twenty-two bladder cancer patients underwent 18F-FDG PET/MRI, using intravenous furosemide and oral hydration for bladder clearance. A radiologist scored probability of tumor in 3 locations (urinary bladder, pelvic lymph nodes, nonnodal pelvis) using 1- to 3-point scale (1 = negative, 2 = equivocal, 3 = definite tumor). A nuclear medicine physician reviewed fused PET/MRI images, after which scores were reassigned based on combined findings. Follow-up pathologic and imaging data served as reference. Performances of MRI alone and PET/MRI were compared.
Results
Of these patients, 82%, 38%, and 18% were positive for bladder, pelvic nodal, and nonnodal pelvic tumor, respectively. At a score of 3, PET/MRI exhibited greater accuracy for detection of bladder tumor (86% vs 77%), metastatic pelvic lymph nodes (95% vs 76%), and nonnodal pelvic malignancy (100% vs 91%). In the bladder, PET changed the level of suspicion in 36% of patients (50% increased suspicion, 50% decreased suspicion), with 75% of these changes deemed correct based on reference standard. For pelvic lymph nodes, PET changed suspicion in 52% (36% increase, 64% decrease), with 95% of changes deemed correct. For nonnodal pelvis, PET changed suspicion in 9% (100% increase), with 100% deemed correct.
Conclusions
Additional PET information helped to appropriately determine level of suspicion in multiple anatomic sites for otherwise equivocal findings on MRI alone. Although requiring larger studies, findings suggest a possible role for simultaneous PET/MRI to assist bladder cancer management.
Keywords: bladder cancer, cystoscopy, FDG, lymph nodes, PET/MRI
Bladder cancer management is complex, in part due to limitations of available imaging modalities, such that there is an unmet medical need for novel imaging strategies that improve the accuracy of bladder cancer evaluation. A broad range of potential applications for novel imaging strategies includes initial detection and diagnosis; local, regional, and distant staging; monitoring for neo-adjuvant therapy response; and detection of disease recurrence after treatment.1–4 Such applications of novel imaging may have important implications in initial diagnosis, prognosis, and treatment selection in bladder cancer patients. Standard imaging in bladder cancer includes conventional CT and MRI, which are both prone to challenges. For example, the series of interventions that bladder cancer patients undergo, including cystoscopy, biopsy, transurethral resection, as well as both intravesical and systemic therapy, may lead to nonspecific mural thickening and irregularity that can mimic or obscure bladder tumors.5–7 Conventional imaging is also suboptimal in the accurate detection of pelvic lymph node metastases.6,7
18F-FDG PET/CT has been explored as an alternative approach for bladder cancer evaluation,8,9 although it has not been broadly adopted.10 A primary limitation of PET is that accumulation of excreted FDG in the bladder lumen greatly limits the detection of focal tumors.10 However, recent investigations have addressed this issue using diuresis protocols. For instance, protocols have entailed administrating oral hydration and an intravenous diuretic during the period between FDG administration and PET acquisition, with additional instructions for the patient to forcibly void prior to onset of imaging.10–13 This approach has been shown to facilitate visualization of abnormal increased FDG activity within mural bladder lesions10–13 (in addition to reducing radiation exposure to pelvic organs). However, even when using a diuresis protocol, the bladder can potentially undergo substantial changes in volume and contour between the separate PET and CT components of a single PET/CTexamination because of continual urinary excretion and subsequent changes in bladder distension, thereby hindering the coregistration of these 2 image sets.14,15 Moreover, MRI, rather than CT, may be specifically desired given the documented role of MRI in local T staging and the differentiation of recurrent tumor from posttreatment changes of the bladder wall.2,4,16 However, obtaining separate PET/CTand MRI examinations entails greater patient inconvenience, does not overcome coregistration limitations, and may not be practical in some contexts.
Simultaneous PET/MRI systems have increased in clinical availability in recent years and may help address these issues.17,18 PET/MRI combines the unique benefits of both modalities (high-contrast resolution and multiparametric nature of MRI; metabolic information of PET) with the patient convenience and practicality of a single imaging session. In addition, unlike PET/CT, modern PET/MRI systems perform spatially and temporally simultaneous acquisitions, thereby improving the alignment of normal and pathologic structures between the 2 modalities.14,19 Indeed, a recent investigation demonstrated improved coregistration of the bladder wall, FDG-avid bladder lesions, and FDG-avid pelvic lymph nodes on PET and MRI acquisitions when performed in a simultaneous, rather than a sequential, fashion.15
These considerations raise the possibility that PET/MRI may offer a novel approach for the imaging assessment of bladder cancer patients that addresses known challenges of CT, PET/CT, and MRI alone for this purpose. Indeed, while requiring validation in clinical studies, PET/MRI could potentially facilitate numerous aspects of bladder cancer management. Therefore, our aim in this investigation was to conduct a prospective pilot study comparing the diagnostic performance of MRI alone and 18F-FDG simultaneous PET/MRI using a diuresis protocol in bladder cancer patients.
METHODS
Patients
Our institutional review board approved this HIPAA-compliant prospective study. The pilot trial was approved for a total of 30 PET/MRI examinations. Written informed consent was obtained from all subjects. Patients with a known history of bladder cancer who were scheduled to undergo clinically indicated MRI urography were recruited to have their examination performed as a simultaneous PET/MRI. A total of 24 patients were recruited to undergo a total of 30 PET/MRI examinations (one PET/MRI examination in 18 patients, 2 PET/MRI examinations in 6 patients). One patient was initially enrolled, but unable to complete the study because of inability to fit within the PET/MRI scanner. An additional patient underwent PET/MRI to evaluate a locally recurrent mass following radical cystectomy and was also excluded from the present data analysis. In patients who underwent 2 PET/MRI examinations, only the first examination was evaluated in the current study. This process left a final included cohort of 22 patients (mean age, 66.5 ± 9.4 years; 19 men, 3 women) undergoing a total of 22 PET/MRI examinations (16 examinations performed for initial staging, 6 performed following prior therapy). The inclusion of patients both before and after therapy provided a combination of patients both with and without viable bladder tumor within the study cohort. Six of these patients were included in an earlier study comparing coregistration accuracy between simultaneously and sequentially acquired PET and MRI image sets.15
PET/MRI Protocol
Patients underwent imaging using a single whole-body simultaneous PET/MRI system (Siemens Biograph mMR, Siemens Medical Solutions, Erlangen, Germany). The system consists of a PET detector designed using MR-compatible avalanche photodiodes, housed within a 3-T MRI system. Initially, 10.0 mCi of FDG was injected, after which the patient waited approximately 1 hour before undergoing imaging. Diuresis was achieved via 0.5 mg/kg of intravenous furosemide, oral hydration via 1 to 1.5 L of water, and instructions to forcibly void before imaging.15 The furosemide and hydration were administered 45 minutes after FDG administration in the first 14 patients and 15 minutes after FDG administration in the remaining 8 patients.
PET/MRI examinations comprised conventional T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and diffusion-weighted imaging (DWI) of the abdomen and pelvis. T2-weighted imaging included high-resolution axial and sagittal acquisitions of the urinary bladder. Diffusion-weighted imaging was performed using b values of 0, 400, and 800 s/mm2, with inline reconstruction of the apparent diffusion coefficient map using a monoexponential fit. In addition, dynamic contrast-enhanced MRI of the bladder was performed using intravenous injection of 0.1 mmol/kg of Gadobutral (Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) followed by a 20-mL saline flush, both administered at a rate of 3 mL/se. Dynamic contrast-enhanced was obtained using a continuously acquired radial golden-angle sequence with joint parallel imaging and compressed sensing reconstruction (spatial resolution 1 × 1 × 3 mm, reconstructed temporal resolution of 2.3 seconds).20 After completion of the bladder dynamic contrast-enhanced sequence, conventional volumetric breath-hold fat-suppressed gradient-echo T1WI was performed of the abdomen and pelvis, followed by coronal T1-weighted MR urography of the upper and lower tracts in the excretory phase.
18F-FDG PET was performed simultaneously as the high-resolution axial T2WI of the bladder, thus occurring before any postcontrast MRI sequences. PET was acquired in sinogram mode for 5 minutes (transaxial FOV, 600 mm; transaxial matrix, 172 × 172; axial FOV, 258 mm).15 An iterative 3D algorithm was used for PET reconstruction, using 3 iterations and 21 subsets.15 Attenuation correction was performed via a breath-hold dual-echo Dixon-based sequence.21 Quantitative T2 mapping and diffusion kurtosis imaging of bladder masses were also performed for investigational purposes, although these were not assessed as part of the present analysis.
Image Analysis
A radiologist (A.B.R., with 7 years of experience in body MRI at the start of this study) performed an initial prospective assessment of each examination at the time of clinical interpretation. As part of this evaluation, the radiologist scored the probability of tumor in each of 3 anatomic locations (urinary bladder, pelvic lymph nodes, nonnodal pelvis) using a 1- to 3-point scale (1 = no findings suspicious for tumor, 2 = findings equivocal for tumor, 3 = findings definitely representing tumor). The nonnodal pelvis included other organs, peritoneal implants, and bones. These assessments were based on a composite evaluation of all available MRI sequence (precontrast and postcontrast T1WI, T2WI, DWI). The upper abdomen was not evaluated using this scoring scheme, given that simultaneous PET was performed only on the pelvis.
Subsequently, simultaneous PET/MRI of the pelvis was viewed in conjunction with 1 of 2 nuclear medicine physicians (K.P.F. and F.P., with 11 and 24 years of experience, respectively). The MIM fusion viewer (version 5.4; MIM Software, Cleveland, Ohio) was used to achieve image fusion. The nuclear medicine physician evaluated images for the presence of visually increased FDG activity, assessing for both increased activity corresponding with abnormalities identified on MRI as well as potential additional sites of increased activity without an MRI correlate. Based on this further evaluation, an additional set of scores was assigned for the probability of tumor in each of the 3 locations using a 1- to 3-point scale, based on the following scheme: an MRI score of 1 was raised to a score of 2 in the presence of increased FDG activity and remained a score of 1 otherwise; an MRI score of 2 was decreased to a score of 1 in the absence of corresponding increased FDG activity and raised to a score of 3 in the presence of corresponding increased FDG activity; an MRI score of 3 remained a score of 3, regardless of the presence or absence of corresponding increased FDG activity. A score of 3 on MRI alone was not decreased in the absence of corresponding increased FDG activity, given that a score of 3 indicated a finding that was definitive for tumor. PET imaging was not used to assist local T staging of bladder cancer, given insufficient spatial resolution of PET, compared with that of MRI, for this purpose.
Reference Standard
Following completion of imaging and interpretation of all of the PET/MRI examinations, the previously noted radiologist and a medical oncologist (A.V.B., with 5 years of experience in the specialized management of bladder cancer) jointly reviewed the electronic medical record for all patients. All available follow-up clinical, cystoscopic, laboratory, pathologic, and imaging data were evaluated in order to determine a reference standard for the presence or absence of tumor in each of 3 evaluated anatomic locations. For assessment of the bladder, histological results from bladder biopsy performed at the time of cystoscopy served as reference, with carcinoma in situ classified as reference standard negative. The bladder was also classified as negative for tumor if cystoscopy was normal and no biopsies were therefore taken. For assessment of pelvic lymph nodes, pathologic results from biopsy or lymph node dissection served as reference. If no pathologic reference standard was available, then either progressive nodal enlargement on serial examinations or decrease in size following systemic therapy was considered indicator of nodal metastasis; stability of nodes on serial imaging or an absence of visualized nodes on both the MRI and PET, as well as on follow-up imaging, was considered indicator of an absence of nodal metastases. Anatomic sites in a given patient were excluded from analysis if the site could not be reliably classified in terms of the presence or absence of tumor based on these reference criteria.
Statistical Analysis
The sensitivity and specificity of MRI alone and of PET/MRI were computed for each of the 3 anatomic sites, when considering both thresholds of 2 or 3 on the previously noted 1- to 3-point scale to be positive for tumor. In addition, the fraction of examinations in which the additional PET information raised or lowered the level of suspicion for tumor for each anatomic site was computed. For such examinations, the fraction of case in which the change in suspicion was ultimately deemed to be correct based on the reference standard was computed. Statistical analysis was performed using Excel for Macintosh (version 12.1.10; Microsoft, Redmond, Wash).
RESULTS
In terms of the presence of bladder tumor, 82% (18/22) of patients were considered reference standard positive (8 by radical cystectomy, 10 by cystoscopy and biopsy), and 18% (4/22) were considered reference standard negative (2 with negative pathology at cystoscopic biopsy; 2 with normal cystoscopy). The presence of metastatic pelvic lymph nodes could not be evaluated in 1 patient based on the applied reference standard. In terms of the presence of metastatic pelvic lymph nodes among the remaining patients, 38% (8/21) were considered reference standard positive (2 positive at radical cystectomy with pelvic lymph node dissection, 3 with progressive nodal growth on serial imaging, 2 with decrease in size following initiation of systemic therapy, 1 with decrease in size following systematic therapy with increase in size on subsequent follow-up imaging); 57% (12/21) were considered reference standard negative (5 negative at pelvic lymph node dissection during radical cystectomy, 1 negative at biopsy, 2 with small nodes stable on follow-up imaging, 4 with an absence of nodes on MRI and PET, as well as any available follow-up imaging); and 1 patient could not be classified for this end point. In terms of nonnodal pelvic tumor, 18% (4/22) were considered reference standard positive (1 with biopsy-proven peritoneal implants, 1 with peritoneal implants showing progressive increase in size on follow-up imaging, 1 with biopsy-proven osseous metastasis, and 1 with both osseous metastasis showing progressive increase in size on follow-up imaging and biopsy-proven extensive prostatic involvement by tumor), and 82% (18/22) were considered reference standard negative (all with an absence of any suspicious nonnodal pelvic findings on MRI and PET and no evidence of metastasis on any imaging or pathologic follow-up evaluation).
Table 1 shows the diagnostic performance of MRI alone and of simultaneous PET/MRI for detection of tumor in various anatomic sites. At a threshold score 2 or greater on the 1- to 3-point scale used for interpretation, PET/MRI resulted in higher accuracy compared with MRI alone for detection of bladder tumor (86% vs 77%) and of metastatic pelvic lymph nodes (95% vs 71%). At a threshold of 3 on the 1- to 3-point scale, PET/MRI resulted in higher accuracy for detection of bladder tumor (86% vs 77%), metastatic pelvic lymph nodes (95% vs 76%), and nonnodal pelvic malignancy (100% vs 91%).
TABLE 1.
Diagnostic Performance of MRI Alone and of Simultaneous PET/MRI for Detection of Tumor in Various Anatomic Sites
| Threshold | Outcome | Session | Bladder | Pelvic Lymph Nodes | Nonnodal Pelvis |
|---|---|---|---|---|---|
| ≥2 | Sensitivity | MRI | 94% (17/18) | 100% (8/8) | 100% (4/4) |
| PET/MRI | 89% (16/18) | 88% (7/8) | 100% (4/4) | ||
| Specificity | MRI | 0% (0/4) | 54% (7/13) | 100% (18/18) | |
| PET/MRI | 75%% (3/4) | 100% (13/13) | 100% (18/18) | ||
| Accuracy | MRI | 77% (17/22) | 71% (15/21) | 100% (22/22) | |
| PET/MRI | 86% (19/22) | 95% (20/21) | 100% (22/22) | ||
| 3 | Sensitivity | MRI | 72% (13/18) | 38% (3/8) | 50% (2/4) |
| PET/MRI | 89% (16/18) | 88% (7/8) | 100% (4/4) | ||
| Specificity | MRI | 100% (4/4) | 100% (13/13) | 100% (18/18) | |
| PET/MRI | 75% (3/4) | 100% (13/13) | 100% (18/18) | ||
| Accuracy | MRI | 77% (17/22) | 76% (16/21) | 91% (20/22) | |
| PET/MRI | 86% (19/22) | 95% (20/21) | 100% (22/22) |
Table 2 shows the frequency of examinations in which the additional PET information changed the level of suspicion for tumor in the various anatomic sites, as well as the fraction of such cases in which the change was considered correct based on the available reference standard. For assessment of the bladder, additional PET information changed the level of suspicion in 36% (8/22) of patients (50% an increase in suspicion, 50% a decrease of suspicion), with 75% of these changes considered correct based on the reference standard. For assessment of pelvic lymph nodes, additional PET information resulted in a change in suspicion in 52% (11/21) of patients (36% an increase, 64% a decrease), with 95% of these changes considered correct. For assessment of the nonnodal pelvis, additional PET information resulted in a change in suspicion in 9% (2/22) of patients (100% an increase in suspicion), with 100% of these changes considered correct.
TABLE 2.
Frequency of Examinations in Which Additional PET Information Changed the Level of Suspicion for Tumor and the Fraction of Such Cases in Which the Change Was Considered Correct Based on the Reference Standard
| Impact of PET/MRI Relative to MRI Alone | Bladder | Pelvic Lymph Nodes | Nonnodal Pelvis | |
|---|---|---|---|---|
| Any change in suspicion | % Of all patients | 36% (8/22) | 52% (11/21) | 9% (2/22) |
| % Correct | 75% (6/8) | 91% (10/11) | 100% (2/2) | |
| Increase in suspicion | % Of patients with change in suspicion | 50% (4/8) | 36% (4/11) | 100% (2/2) |
| % Correct | 75% (3/4) | 100% (4/4) | 100% (2/2) | |
| Decrease in suspicion | % Of patients with change in suspicion | 50% (4/8) | 64% (7/11) | 0% (0/2) |
| % Correct | 75% (3/4) | 86% (6/7) | N/A | |
Figures 1 to 7 show examples of the role of additional PET information in adjusting the level of suspicion for tumor in the various anatomic locations.
FIGURE 1.
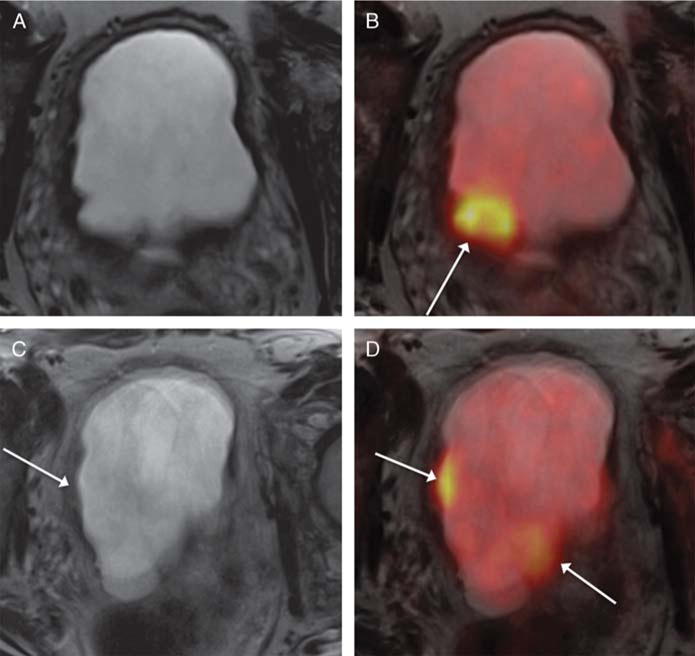
A 68-year-old man with muscle-invasive high-grade bladder cancer on prior biopsy, undergoing simultaneous 18F-FDG PET/MRI. A and B, Axial T2-weighted images show regions of mild nonspecific mural thickening (eg, arrow, B) that was considered equivocal for presence of tumor. C and D, Fused PET/MR images show multiple focal regions of increased metabolic activity (arrows, C and D), which increased confidence for the presence of bladder tumor. Subsequent radical cystectomy demonstrated multifocal tumor.
FIGURE 7.
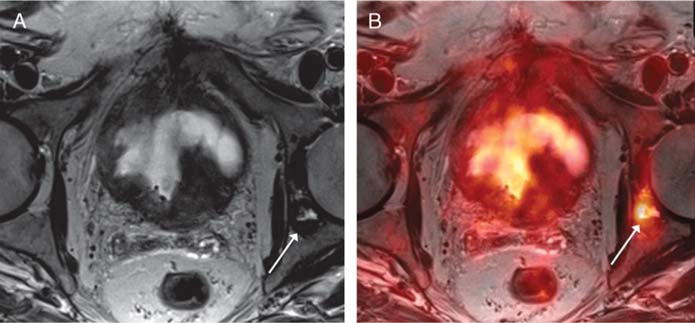
An 82-year-old man with prior biopsy showing high-grade non–muscle-invasive bladder cancer, undergoing simultaneous 18F-FDG PET/MRI. A, Axial T2-weighted image shows a left acetabular lesion (arrow) that was considered possibly degenerative, given its proximity to the hip joint, and equivocal for osseous metastasis. B, Fused PET/MR image shows corresponding marked increased metabolic activity (arrow), raising suspicion that the lesion represents an osseous metastases. Subsequent bone biopsy demonstrated metastatic urothelial carcinoma.
DISCUSSION
In this prospective pilot study, we performed 18F-FDG PET/MRI in patients scheduled to undergo clinically indicated MR urography for bladder cancer evaluation. The simultaneous PET information helped refine determinations of the level of suspicion for tumor in all evaluated anatomic sites within the pelvis, which could have important implications for improving clinical staging in bladder cancer. In particular, the presence or absence of corresponding abnormal FDG activity was helpful for raising or lowering the level of suspicion for tumor when findings from MRI alone were equivocal, as well as for introducing suspicion for tumor in some cases in which no abnormality was detected using MRI alone. For instance, MRI alone commonly demonstrated nonspecific thickening of the bladder wall, possibly representing sequela of prior interventions, or nonspecific small pelvic lymph nodes. In such cases, PET information helped to more confidently stratify the likelihood that such findings represented bladder tumor or metastatic nodes, with the modified levels of suspicion for tumor based on PET data being correct in the large majority of instances (75%–100%, depending on the given analysis). The largest impact of the additional PET information was in the assessment of pelvic lymph nodes (ie, an alternation in the level of suspicion for nodal metastases in approximately half of patients, largely because of a decrease in the level of suspicion for nonspecific pelvic lymph nodes detected on MRI that exhibited no PET correlate), although PET was also occasionally helpful for raising the level of suspicion in the nonnodal pelvis. These findings support a potential role for simultaneous PET/MRI to improve noninvasive imaging assessment in bladder cancer patients and thereby influence treatment decision making. The improved accuracy for detection of pelvic lymph node metastases is of particular importance, given that this assessment is suboptimal using current imaging, yet has a large role in management. For instance, the presence of a true-positive lymph node greatly impacts overall prognosis, the role of cystectomy, and planning (eg, the number of cycles) of chemotherapy.
Past studies have shown diagnostic value of 18F-FDG PET/CT in bladder cancer patients when performed using a diuresis protocol.11,12 However, MRI may specifically be desired in bladder cancer patients given MRI’s roles in T staging and assessment for local recurrence after treatment. In addition, the sequential nature of the acquisitions in PET/CT may contribute to greater misregistration of the bladder between the 2 modalities as a result of active bladder filling occurring between the 2 acquisitions.15 One exploratory study in oncology patients showed that PET/MRI contributed to clinical management more often than did PET/CT and that PET/MRI provided information affecting patient care that was unavailable from PET/CT.22 However, in that study, only 3 of 134 patients had bladder cancer, and results were not provided separately for these patients.22 Given the encouraging findings from our pilot study, additional dedicated investigations applying PET/MRI for bladder cancer assessment are warranted.
While requiring validation in larger studies, our preliminary observations suggest that PET/MRI may have a role in the routine clinical evaluation of bladder cancer patients. PET/MRI provides a comprehensive examination, integrating the high-contrast resolution and multiparametric assessment of MRI with the metabolic information of PET, in the convenience of a single imaging session. In our study, we have demonstrated the ability to perform this examination in a practical fashion within the context of standard patient care. PET/MRI could be applied in an array of clinical scenarios that require optimal characterization of the primary tumor, as well as more accurate detection of local and distant metastases, whether for initial treatment selection, monitoring of treatment response, or surveillance for recurrent tumor. In addition, future investigations could incorporate quantitative metrics from the 2 modalities, including apparent diffusion coefficient and SUV, to further improve prognostic assessment.
The primary limitation of this study is its small sample size, consistent with our intent to conduct a pilot study of a previously unexplored clinical application of PET/MRI. In addition, given that the data for this study were derived from prospective clinical interpretations provided by the interpreting radiologist and nuclear medicine physician, interreader variability was not evaluated. Also, we explored the utility of only a single PET/MRI system, although other clinical PET/MRI systems are commercially available. Finally, we evaluated only the diagnostic accuracy of PET/MRI based on the available reference standard. The impact of PET/MRI on other downstream clinical outcomes, such as patient survival, was not assessed.
In conclusion, we conducted a prospective pilot study of 18F-FDG simultaneous PET/MRI using a diuresis protocol in bladder cancer patients. The additional information from PET was useful for refining the level of suspicion for tumor involving the bladder, pelvic lymph nodes, and nonnodal pelvis, in comparison with MRI findings alone. In particular, the presence or absence of corresponding increased FDG activity in findings that were equivocal on MRI alone usually resulted in an appropriate increase or decrease, respectively, in the level of suspicion for tumor in such regions, with the largest impact of PET information in the assessment of pelvic lymph nodes. If validated in larger studies, the findings indicate a clinically practical role for simultaneous PET/ MRI to assist the management of bladder cancer patients.
FIGURE 2.
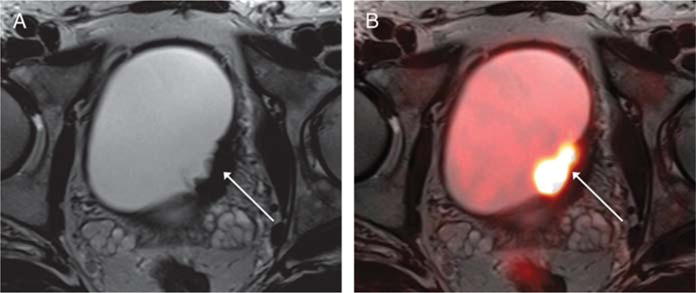
A 52-year-old man with muscle-invasive high-grade bladder cancer on prior biopsy, undergoing simultaneous 18F-FDG PET/MRI. A, Axial T2-weighted image shows irregular sessile wall thickening of the left lateral bladder wall (arrow) that was considered equivocal for presence of tumor. B, Fused PET/MR image shows corresponding increased metabolic activity in the region of wall thickening (arrow), which increased confidence for the presence of bladder tumor. Subsequent radical cystectomy demonstrated tumor in this region.
FIGURE 3.
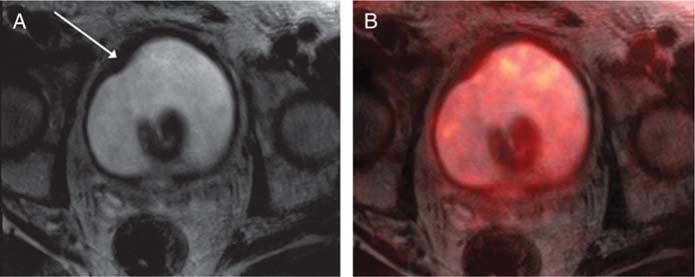
A 72-year-old man with prior intravesical Bacillus Calmette-Guerin therapy for high-grade non–muscle-invasive bladder cancer, undergoing simultaneous 18F-FDG PET/MRI. A, Axial T2-weighted image shows mild sessile thickening of the right anterolateral bladder wall (arrow) that was considered equivocal for presence of tumor. B, Fused PET/MR image shows no corresponding increased metabolic activity in the region of thickening, which decreased the level of suspicion for tumor in this region. Subsequent cystoscopy was normal, without evidence of tumor.
FIGURE 4.
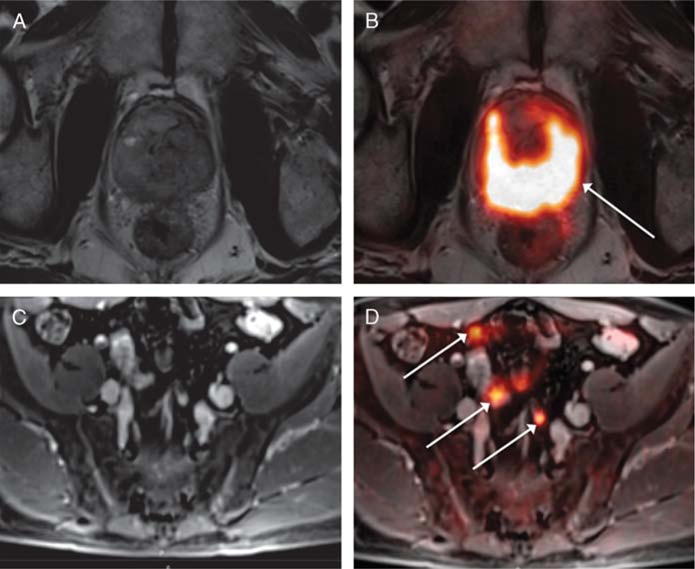
A 62-year-old man with prior biopsy showing high-grade non–muscle-invasive bladder cancer, undergoing simultaneous 18F-FDG PET/MRI. A, Axial T2-weighted image shows diffuse heterogeneity of the prostate without discrete lesion. B, Fused PET/MR image shows diffuse marked increased metabolic activity throughout the prostate (arrow), which raised the level of suspicion for prostatic involvement by tumor. Subsequent prostate biopsy demonstrated extensive prostatic invasion by urothelial carcinoma. C, On postcontrast axial T1-weighted image of the pelvis, potential pelvic lymph nodes are difficult to differentiate from surrounding vessels and bowel loops. D, Fused PET/MR image shows marked increased activity within numerous pelvic lymph nodes (arrows), which raised suspicion for nodal metastases. The nodes decreased in size following treatment with systemic chemotherapy.
FIGURE 5.
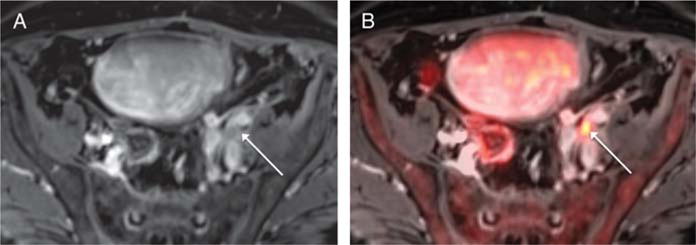
A 68-year-old man with prior biopsy showing high-grade non–muscle-invasive bladder cancer, undergoing simultaneous 18F-FDG PET/MRI. A, On postcontrast axial T1-weighted image of the pelvis, a mildly prominent left external iliac left node (arrow) is somewhat obscured by surrounding iliac vessels. B, Fused PET/MR image shows marked increased activity within this node (arrow), which raised suspicion for nodal metastasis. The node decreased in size following treatment with systemic chemotherapy.
FIGURE 6.
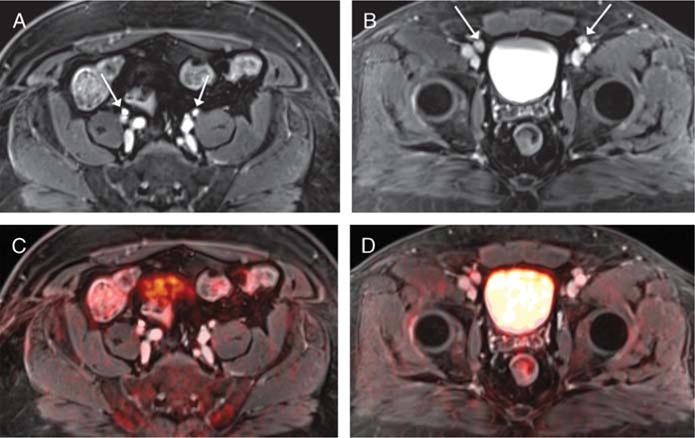
A 59-year-old man with prior transurethral resection and neoadjuvant chemotherapy for bladder cancer, undergoing simultaneous 18F-FDG PET/MRI. A and B, Postcontrast axial T1-weighted images of the pelvis show numerous mildly prominent proximal and distal iliac chain lymph nodes bilaterally (arrow) that were considered equivocal for nodal metastases. C and D, Fused PET/MR images show no corresponding increased metabolic activity, lowering the level of suspicion for nodal metastases. Nodal biopsy was benign, and the nodes remained stable in size on follow-up imaging.
Footnotes
The NYU School of Medicine Institutional Review Board approved this study.
Conflicts of interest and sources of funding: This study was supported by the Society of Abdominal Radiology Howard S. Stern Research Grant.
References
- 1.Hafeez S, Huddart R. Advances in bladder cancer imaging. BMC Med. 2013;11:104. doi: 10.1186/1741-7015-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DA, Durand M, Gumpeni N, et al. Role of magnetic resonance imaging in bladder cancer: current status and emerging techniques. BJU Int. 2012;110:1463–1470. doi: 10.1111/j.1464-410X.2012.11129.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ. Recent advances in treatment of advanced urothelial carcinoma. Curr Urol Rep. 2012;13:147–152. doi: 10.1007/s11934-012-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: role of MR imaging. Radiographics. 2012;32:371–387. doi: 10.1148/rg.322115125. [DOI] [PubMed] [Google Scholar]
- 5.Sadow CA, Silverman SG, O’Leary MP, et al. Bladder cancer detection with CTurography in an academic medical center. Radiology. 2008;249:195–202. doi: 10.1148/radiol.2491071860. [DOI] [PubMed] [Google Scholar]
- 6.Roy C, Bierry G, Matau A, et al. Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol. 2010;20:1803–1811. doi: 10.1007/s00330-010-1736-4. [DOI] [PubMed] [Google Scholar]
- 7.Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol. 2012;61:326–340. doi: 10.1016/j.eururo.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Ho L, Quan V, Henderson R, et al. High-grade urothelial carcinoma of the prostate on FDG PET-CT. Clin Nucl Med. 2007;32:746–747. doi: 10.1097/RLU.0b013e318123f83e. [DOI] [PubMed] [Google Scholar]
- 9.Kibel AS, Dehdashti F, Katz MD, et al. Prospective study of [18F] fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–4320. doi: 10.1200/JCO.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang N, Wang YL, Zeng L, et al. Feasible Method to enable clear visualization of suspected bladder cancer with 18F-FDG PET/CT. Clin Imaging. 2014;38:704–709. doi: 10.1016/j.clinimag.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Coquan E, Lasnon C, Joly F, et al. Diuretic F-FDG PET/CT for therapy monitoring in urothelial bladder cancer. Eur J Nucl Med Mol Imaging. 2014;41:1818–1819. doi: 10.1007/s00259-014-2800-0. [DOI] [PubMed] [Google Scholar]
- 12.Nayak B, Dogra PN, Naswa N, et al. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. 2013;40:386–393. doi: 10.1007/s00259-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 13.Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–19. doi: 10.4103/0971-3026.59746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brendle CB, Schmidt H, Fleischer S, et al. Simultaneously acquired MR/PET images compared with sequential MR/PET and PET/CT: alignment quality. Radiology. 2013;268:190–199. doi: 10.1148/radiol.13121838. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkrantz AB, Balar AV, Huang WC. Comparison of coregistration accuracy of pelvic structures between sequential and simultaneous imaging during hybrid PET/MRI in patients with bladder cancer. Clin Nucl Med. 2015;40:637–641. doi: 10.1097/RLU.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HJ, Pui MH, Guo Y, et al. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging. 2014;39:135–141. doi: 10.1007/s00261-013-0038-0. [DOI] [PubMed] [Google Scholar]
- 17.Fraum TJ, Fowler KJ, McConathy J, et al. PET/MRI for the body imager: abdominal and pelvic oncologic applications. Abdom Imaging. 2015;40:1387–1404. doi: 10.1007/s00261-015-0390-3. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkrantz AB, Friedman K, Chandarana H, et al. Current status of hybrid PET/MRI in oncologic imaging. Am J Roentgenol. 2016;206:162–172. doi: 10.2214/AJR.15.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakheja R, Demello L, Chandarana H, et al. Comparison of the accuracy of PET/CT and PET/MRI spatial registration of multiple metastatic lesions. Am J Roentgenol. 2013;201:1120–1123. doi: 10.2214/AJR.13.11305. [DOI] [PubMed] [Google Scholar]
- 20.Parikh N, Ream JM, Zhang HC, et al. Performance of simultaneous high temporal resolution quantitative perfusion imaging of bladder tumors and conventional multi-phase urography using a novel free-breathing continuously acquired radial compressed-sensing MRI sequence. Magn Reson Imaging. 2016;34:694–698. doi: 10.1016/j.mri.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eiber M, Martinez-Moller A, Souvatzoglou M, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38:1691–1701. doi: 10.1007/s00259-011-1842-9. [DOI] [PubMed] [Google Scholar]
- 22.Catalano OA, Rosen BR, Sahani DV, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269:857–869. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]


