Abstract
Objectives
Given the known risk factors for NEC, we hypothesized that metabolic dysfunction reflected in routinely collected newborn screening data would be associated with NEC in an at risk population.
Study Design
We conducted a retrospective cohort study using discharge records for all preterm neonatal intensive care unit admissions in California from 2005 to 2009. Infants with linked state newborn screening results were included. A model-development cohort of 94,110 preterm births from 2005 to 2008 was used to develop a risk-stratification model that was then applied to a validation cohort of 22,992 births from 2009.
Results
Fourteen acylcarnitines and acylcarnitine ratios were associated with increased risk of developing NEC. Each log unit increase in C5 and FC/(C16+18:1) was associated with a 78% and a 76% increased risk for developing NEC, respectively (OR 1.78, 95% CI 1.53 – 2.02, and OR 1.76, 95% CI 1.51 – 2.06). Six acylcarnitines, along with birth weight and total parenteral nutrition, were able to identify 89.8% of newborns with NEC in the model-development cohort (AUC=0.898, 95% confidence interval (CI) 0.889 – 0.907) and 90.8% of the newborns with NEC in the validation cohort (AUC=0.908, 95% CI 0.901 – 0.930).
Conclusions
These findings demonstrate that abnormal fatty acid metabolism is associated with prematurity and the development of NEC. Metabolic profiling through newborn screening may serve as an objective biologic surrogate of risk for the development of disease and thus facilitate disease prevention strategies.
Keywords: metabolism, prematurity, newborn screen, fatty acids
Introduction
Necrotizing enterocolitis (NEC) is a leading cause of morbidity and mortality among preterm infants. NEC is an acquired disease of the neonatal period marked by inflammation and necrosis of the gastrointestinal tract. The ambiguity of presenting symptoms of NEC and the low specificity of common diagnostic tests lead to delayed diagnosis and inability to initiate targeted therapies.(1)
The underlying pathophysiology of NEC is multifactorial involving a combination of developmental immaturity, variable feeding practices, and bacterial colonization of the gut.(2) Metabolism emerges at the intersection of these predisposing variables as an under-explored feature that likely impacts disease onset. Since no prior studies have conclusively identified high-risk infants based upon measurable predisposing biologic features, there has been little progression in the understanding of the inciting pathophysiologic basis for NEC beyond prematurity.(3-5)
Newborn screening (NBS) reports essential biomarkers that taken together are utilized to identify possible metabolic dysfunction associated with genetic disease. It is now well established that NBS metabolites including amino acids and acylcarnitines vary according to gestational age and birth-weight.(6-10) Gestational age and newborn weight as measures of developmental immaturity have long been used to aid in the determination of risk for acquired diseases of prematurity like NEC. As an alternative, NBS panels may be used to identify a predisposing metabolic phenotype that is associated with an acquired disease of prematurity such as NEC.
Abnormal fatty and organic acid metabolism of prematurity as indicated by acylcarnitine profiles may be implicated in the pathogenesis of NEC. Prematurity associated disturbances in nutrient metabolism, enteric dysmotility and gut colonization can result in excess fermentation and the accumulation of organic and short-chain fatty acids that have been shown to contribute to intestinal mucosal injury and necrosis in both human subjects and animal models that closely mimic human NEC.(11-15) We hypothesized that an association between newborn acylcarnitine profiles and the subsequent development of NEC could further refine age and weight associated risk in biologic terms.
Patients and Methods
Patient populations
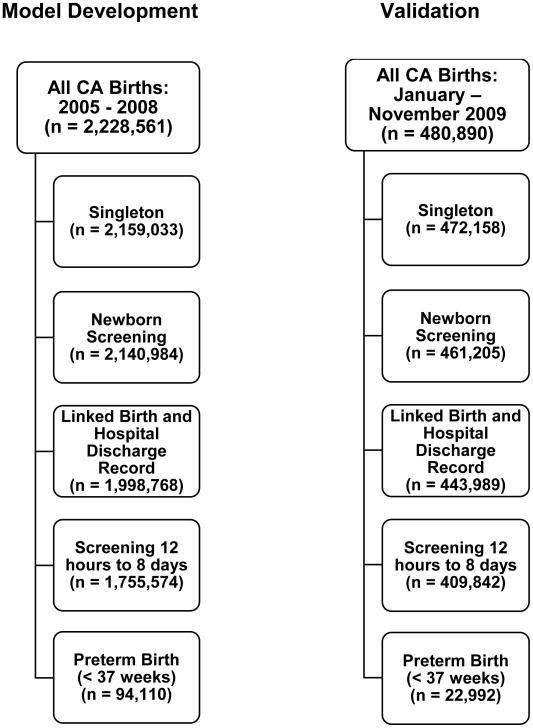
To explore the relationship between premature newborn metabolism and NEC, we used newborn screening results from more than 100,000 singleton preterm newborns born in California between 2005 and 2009. The model-development cohort consisted of singleton preterm (< 37 completed weeks gestation) newborns (n = 94,110). All subjects were born in California between 2005 and 2008, had routine newborn screening through the Genetic Disease Screening Program within the California Department of Public Health with a serum draw between 12 hours to 8 days of birth, and had linked birth certificate and hospital discharge records. The naïve validation cohort consisted of 22,992 preterm singletons with births between January 1 and November 30, 2009 who also had newborn screening based on serum collected between 12 hours and 8 days of birth and also had linked, birth, and hospital discharge records. Details regarding the populations from which the model-development and validation cohorts were drawn are included in Figure 1(online only).
Figure 1.
Model Development and Validation cohort exclusions.
Legend: Details regarding populations from which the model-development and validation cohorts were drawn.
Acylcarnitine measurements
We obtained acylcarnitine measurements, hours/days after birth at testing, race/ethnicity, and information about whether the infant had been on total parenteral nutrition between birth and the time of testing from the newborn screening records. Birth certificate and hospital discharge records were linked to newborn screening data through the California Office of Statewide Health Planning and Development from which we obtained information on total days gestation, birth weight, and diagnosis of NEC (by ICD-9 code 777.5). Details regarding the newborn screening program and testing of acylcarnitines have been described in detail elsewhere.(16, 17) In brief, all newborns included in the present study had acylcarnitines measured in dried blood specimens collected by heel-stick at birth hospitals between 12 hours and 8 days after birth. Following collection, specimens were sent to a state-approved laboratory for testing using a standardized tandem mass spectrometry assay (MS2 2000 system (PerkinElmer Life Sciences, Shelton, CT)). Specimens were tested using a NeoGram acylcarnitine derivatized reagent kit (PerkinElmer). For all samples, testing was based on the MS2 system operated in the positive ion mode (source voltage: 5500 V). Acylcarnitines were measured by precursor ion scanning using precursors of m/z 85, and quantitated by comparison to stable-isotope internal standards. All information on acylcarnitines measured as part of routine newborn screening was included in analyses. This included values for twenty acylcarnitines (C2, C3, C3DC, C4, C5, C5:1, C5DC, C6, C8, C8:1, C10, C10:1, C12, C14, C14:1, C16, C16:1, C18, C18:1, C18:1OH, and free carnitine (FC)) and two acylcarnitine ratios (FC/(C16+C18:1) and C3/C2).
Model Development and Validation cohort analyses
We performed two distinct phases of analysis. First, we evaluated whether there was an association between acylcarnitines and a subsequent diagnosis of NEC in the 2005 - 2008 model-development cohort. Second, we evaluated the performance of these acylcarnitines and acylcarnitine ratios in identifying preterm infants at risk for NEC in the 2005 to 2008 model-development cohort and in the 2009 validation cohort (wherein inclusion in the 2009 cohort was limited to those with a birth before December due to a change in lab assay in December, 2009).
Analysis of individual acylcarnitines
Crude association testing in the model-development cohort included comparing preterm newborns with and without NEC by characteristic and by the log of acylcarnitine level and ratio. The chi square test was used to compare groups on race/ethnicity, sex, total parenteral nutrition (yes or no), age in days at acylcarnitine testing, gestational age (gestational age <32, 32-36 wks) by birth weight grouping (< 1500, 1500-2499, ≥ 2500 grams). Race/ethnicity was derived from the birth certificate record where the reporting parent selected from a list of predefined categories. We used the two-tailed Wilcoxon Rank Sum Test for initial comparison of the distribution of acylcarnitine level and ratios between preterm infants with and without NEC. We then performed logistic regression to calculate odds ratios and 95% confidence intervals to identify the relationship between a natural log-unit increase in acylcarnitine levels or ratios and the risk of NEC wherein both crude- and characteristic- adjusted risks were evaluated.
Multivariate analysis of acylcarnitines
Final model development for combined characteristic and acylcarnitine effects utilized backward stepwise regression methods where p < .10 was used as the threshold for entering the model and p < .05 was used as the threshold for remaining. We evaluated performance of the final logistic model for NEC prediction in both the model-development and validation cohorts. Receiver operator characteristic curves and associated area under the curve statistics were evaluated overall, by day of testing, and by gestational age.
Statistical software and study approval
All analyses were performed using Statistical Analysis Software (SAS) version 9.3 (Cary, NC) based on data received by the Genetic Disease Screening Program as of December 31, 2013. This study was approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California by waiver of informed consent.
Results
Patient characteristics
Most newborns in the training cohort were Hispanic (50.92%) or non-Hispanic White (27.05%) and had newborn screening obtained between 12 hours and 2 days of life (69.18%). Approximately 1 in 127 preterm infants was ultimately diagnosed with NEC. Of those that developed NEC, the highest frequency was seen in newborns with births before 32 completed weeks of gestation with birthweight < 1500 grams (Table 1). Preterm newborns with NEC in the model-development and validation cohorts differed from those without NEC by race/ethnicity, use of total parenteral nutrition at the time of testing, day of life at testing, and by gestational age by birth weight grouping (Table 1).
Table 1.
Descriptive characteristics – Training and testing cohorts: Preterm births with and without necrotizing enterocolitis.
| Model Development | Validation | |||||||
|---|---|---|---|---|---|---|---|---|
| All n = |
No NEC n (%) |
NEC n (%) |
X2 | All n = |
No NEC n (%) |
NEC n (%) |
X2 | |
| Sample | 94,110 | 93,366 | 744 | 22,992 | 22,876 (99.50) | 116 (0.50) | ||
| Race/Ethnicity | ||||||||
| White | 25,460 (27.05) | 25,270 (27.07) | 190 (25.54) | 6,021 (26.19) | 5,992 (26.19) | 29 (25.00) | ||
| Hispanic | 47,923 (50.92) | 47,579 (50.96) | 344 (46.24) | 11,663 (50.73) | 11,608 (50.74) | 55 (47.41) | ||
| Asian | 8,509 (9.04) | 8,436 (9.04) | 73 (9.81) | 2,213 (9.63) | 2,203 (9.58) | 10 (8.62) | ||
| Black | 7,547 (8.02) | 7,448 (7.98) | 99 (13.31) | 1,883 (8.19) | 1,869 (8.17) | 14 (12.87) | ||
| Native American | 260 (0.28) | 257 (0.28) | 3 (0.40) | 53 (0.23) | 52 (9.63) | 1 (0.86) | ||
| “Other Race” | 3,116 (3.31) | 3,091 (3.31) | 25 (3.36) | 818 (3.56) | 815 (3.56) | 3 (2.59) | ||
| Unknown | 1,295 (1.38) | 1,285 (1.38) | 10 (1.34) | 30.95b | 341 (1.48) | 337 (1.47) | 4 (3.45) | 7.94 |
| Sexa | ||||||||
| Male | 42,678 (45.35) | 42,346 (45.35) | 332 (44.62) | 12,571 (54.68) | 12,506 (54.67) | 65 (56.03) | ||
| Female | 51,396 (54.61) | 50,984 (54.61) | 412 (55.38) | 0.45 | 10,412 (45.29) | 10,361 (45.29) | 51 (43.97) | 0.13 |
| Total Parenteral Nutrition | ||||||||
| Yes | 13,928 (14.80) | 13,506 (14.47) | 422 (56.72) | 5,800 (25.23) | 5,701 (24.92) | 99 (85.34) | ||
| No | 80,182 (85.20) | 79,860 (85.53) | 322 (43.28) | 1045.16b | 17,192 (74.77) | 17,175 (75.08) | 17 (14.66) | 223.39b |
| Hours/Days at Testing | ||||||||
| 12 hours – 2 days | 65,108 (69.18) | 64,786 (69.39) | 322 (43.28) | 16,158 (70.28) | 16,112 (70.43) | 46 (39.66) | ||
| 3 – 4 days | 19,310 (20.52) | 19,066 (20.42) | 244 (32.80) | 4,700 (20.44) | 4,660 (20.77) | 40 (34.48) | ||
| 5 – 6 days | 8,669 (9.21) | 8,517 (9.12) | 152 (20.43) | 1,961 (8.53) | 1,936 (8.46) | 25 (21.55) | ||
| 7 – 8 days | 1,023 (1.09) | 997 (1.07) | 26 (3.49) | 270.27b | 173 (0.75) | 168 (0.73) | 5 (4.31) | 69.60b |
| Gestational Age | ||||||||
| < 32 Weeks | 17,550 (18.65) | 16,982 (18.19) | 568 (76.34) | 4,269 (18.57) | 4,180 (18.27) | 89 (76.72) | 260.80 b | |
| 32 – 36 Weeks | 76,560 (81.35) | 76,384 (81.81) | 176 (23.66) | 1645.51b | 18,723 (81.43) | 18,696 (81.73) | 27 (23.28) | |
| Gestational Age by Birth Weight | ||||||||
| < 32 Weeks | ||||||||
| < 1500 grams | 7,828 (8.32) | 7,363 (7.89) | 465 (62.50) | 2,153 (9.36) | 2,072 (9.06) | 81 (69.83) | ||
| 1500 – 2499 grams | 4,285 (4.55) | 4,189 (4.49) | 96 (12.90) | 1,057 (4.69) | 1,049 (4.59) | 8 (6.90) | ||
| ≥ 2500 grams | 5,437 (5.78) | 5,430 (5.82) | 7 (0.94) | 1,059 (4.61) | 1,059 (4.63) | -- | ||
| 32 – 36 Weeks | ||||||||
| < 1500 grams | 1,817 (1.93) | 1,782 (1.91) | 35 (4.70) | 469 (2.04) | 464 (2.03) | 5 (4.31) | ||
| 1500 – 2499 grams | 25,290 (26.87) | 25,169 (26.96) | 121 (16.26) | 6,821 (29.67) | 6,803 (29.74) | 18 (15.52) | ||
| ≥ 2500 grams | 49,453 (52.55) | 49,433 (52.95) | 20 (2.69) | 3208.05b | 11,433 (49.73) | 11,429 (49.96) | 4 (3.45) | 522.91 |
36 preterm births in the “No NEC” grouping in the model development set and 9 preterm births in the “No NEC” grouping in the validation set had no sex designation.
p < .001
Analysis of individual acylcarnitines
The distribution of acylcarnitines and acylcarnitine ratios in preterm infants with and without NEC was different across all measures except for log C2, log C6 and log C18:1OH (Table 2, online). Fourteen of the 23 acylcarnitine measures were associated with per log unit increases in NEC risk after adjustment for race/ethnicity, use of total parenteral nutrition, days at test (by grouping), and gestational age by birth weight grouping (Table 3, online). Each log unit increase in C5 was associated with a 78% increased risk for NEC after adjustment (odds ratio 1.78, 95% confidence interval 1.53 – 2.02). Each log unit increase in FC/(C16+18:1) was associated with a 76% increase in risk for NEC after adjustment (odds ratio 1.76, 95% confidence interval 1.51 – 2.06) (Table 3, online).
Table 2 (online only).
Comparison of acylcarnitines measured in preterm births with and without necrotizing enterocolitis: Model Development Cohort.
| Acylcarnitine (nmol/mL) | No NEC Mean (SD) |
NEC Mean (SD) |
z = | P Value |
|---|---|---|---|---|
|
|
|
|
|
|
| log C2 | 3.27 (0.29) | 3.25 (0.33) | -1.57 | 0.12 |
| log C3 | 0.69 (0.43) | 0.77 (0.53) | 5.36 | <.001 |
| log C3DC | -2.56 (0.33) | -2.71 (0.33) | -12.10 | <.001 |
| log C4 | -1.29 (0.53) | -0.96 (0.52) | 17.98 | <.001 |
| log C5 | -1.80 (0.60) | -1.09 (0.58) | 29.35 | <.001 |
| log C5:1 | -3.30 (0.60) | -3.12 (0.57) | 7.63 | <.001 |
| log C5DC | -2.30 (0.38) | -2.38 (0.41) | -5.63 | <.001 |
| log C6 | -2.67 (0.59) | -2.70 (0.60) | -1.58 | 0.11 |
| log C8 | -2.63 (0.60) | -2.47 (0.64) | 6.66 | <.001 |
| log C8:1 | -2.19 (0.59) | -2.10 (0.73) | 3.27 | 0.001 |
| log C10 | -2.55 (0.59) | -2.82 (0.65) | -11.38 | <.001 |
| log C10:1 | -2.82 (0.59) | -2.68 (0.74) | 5.49 | <.001 |
| log C12 | -1.87 (0.55) | -2.35 (0.60) | -21.85 | <.001 |
| log C14 | -1.56 (0.46) | -1.86 (0.49) | -16.87 | <.001 |
| log C14:1 | -2.11 (0.55) | -2.37 (0.53) | -12.51 | <.001 |
| log C16 | 0.78 (0.44) | 0.33 (0.44) | -20.28 | <.001 |
| log C16:1 | -1.74 (0.54) | -2.11 (0.58) | -17.41 | <.001 |
| log C18 | -0.29 (0.36) | -0.35 (0.36) | -4.27 | <.001 |
| log C18:1 | 0.06 (0.34) | -0.08 (0.36) | -10.89 | <.001 |
| log C18:1OH | -3.54 (0.62) | -3.59 (0.60) | -1.80 | 0.07 |
| log FC | 3.77 (0.40) | 4.00 (0.46) | 14.08 | <.001 |
| log FC/(C16+C18:1)a | 2.58 (0.45) | 3.13 (0.46) | 29.82 | <.001 |
| log C3/C2a | -2.58 (0.38) | -2.48 (0.43) | 7.34 | <.001 |
Abbreviations: SD, standard deviation.
Ratio of acylcarnitine measurements in nmol/mL.
Table 3 (online only).
Association between per log unit increase in acylcarnitines/ratios and necrotizing enterocolitis.
| Adjusted ORa | 95% CI | P Value | |
|---|---|---|---|
|
|
|
|
|
| log C2 | 1.07 | 0.85 – 1.34 | 0.57 |
| log C3 | 1.21 | 1.03 – 1.41 | 0.02 |
| log C3DC | 0.72 | 0.58 – 0.91 | 0.01 |
| log C4 | 1.24 | 1.07 – 1.43 | 0.003 |
| log C5 | 1.78 | 1.53 – 2.02 | <.001 |
| log C5:1 | 1.22 | 1.06 – 1.40 | 0.01 |
| log C5DC | 0.81 | 0.72 – 1.06 | 0.16 |
| log C6 | 0.98 | 0.86 – 1.12 | 0.77 |
| log C8 | 1.05 | 0.93 – 1.18 | 0.45 |
| log C8:1 | 0.88 | 0.79 – 0.99 | 0.45 |
| log C10 | 0.82 | 0.73 – 0.93 | 0.002 |
| log C10:1 | 0.95 | 0.85 – 1.06 | 0.38 |
| log C12 | 0.69 | 0.60 – 0.78 | <.001 |
| log C14 | 0.87 | 0.74 – 1.10 | 0.06 |
| log C14:1 | 0.75 | 0.65 – 0.85 | <.001 |
| log C16 | 0.61 | 0.52 – 0.73 | <.001 |
| log C16:1 | 0.77 | 0.68 – 0.88 | <.001 |
| log C18 | 0.84 | 0.69 – 1.03 | 0.09 |
| log C18:1 | 0.69 | 0.56 – 0.84 | <.001 |
| log C18:1OH | 0.94 | 0.82 – 1.09 | 0.40 |
| log FC | 1.26 | 1.07 – 1.48 | 0.01 |
| log FC/(C16+C18:1) | 1.76 | 1.51 – 2.06 | <.001 |
| log C3/C2 | 1.25 | 1.04 – 1.57 | 0.02 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for race/ethnicity, total parenteral nutrition, day at test (by grouping), and gestational age by birth weight grouping.
Combined Risk of NEC Acylcarnitines model
When patient characteristics, acylcarnitines, and acylcarnitine ratios were evaluated together, five acylcarnitines (log C5, log C5:1, log C8:1, log C12, log C14:1), one acylcarnitine ratio (log FC/(C16+C18:1)), gestational age by birth weight grouping, and use of total parenteral nutrition were found to significantly associate with NEC at p < .05 (Table 4). This combination of factors was able to correctly group preterm infants with and without NEC 89.8% of the time (area under the curve=0.8983, 95% confidence interval 0.8895 – 0.9072) in the model-development cohort and 90.8% of the time (area under the curve=0.9078, 95% confidence interval 0.8903 – 0.9253) in the validation cohort (Table 5).
Table 4.
Final acylcarnitinea (AC) necrotizing enterocolitis (AC-NEC) model.
| AC log | C-5 | C-5:1 | C-8:1 | C-12 | C-14:1 | FC/(C16+C18:1) | GA by BW groupingb | TPN |
|---|---|---|---|---|---|---|---|---|
| Odds Ratioa | 1.75 | 1.25 | 0.72 | 0.74 | 0.66 | 1.38 | 1.84 | 1.35 |
| 95% CI | 1.33 – 2.30 | 1.01 – 1.55 | 0.61 – 0.86 | 0.59 – 0.93 | 0.52 – 0.83 | 1.04 – 1.85 | 1.68 – 2.01 | 1.03 – 1.77 |
| P Value | <.001 | 0.04 | <.001 | 0.01 | <.001 | 0.03 | <.001 | 0.03 |
Abbreviations: AC, acylcarnitine; BW, birth weight; CI, confidence interval; GA, gestational age; TPN, total parenteral nutrition.
Odds ratios are per log unit increase in acylcarnitines, change in group for GA by BW, and TPN yes and no adjusted for all other factors in the model.
< 32 weeks by < 1500 grams, 1500 to 2499 grams, and ≥ 2500 grams and 32 to 36 weeks by < 1500 grams, 1500 to 2499 grams, and ≥ 2500 gr
Table 5.
Receiver operating characteristic curves (ROCs) for AC-NEC modela.
| 2005-2008 Model Development | 2009 Validation | |||
|---|---|---|---|---|
|
| ||||
| AUC | 95% CI | AUC | 95% CI | |
|
| ||||
| All | 0.8983 | 0.8895 – 0.9072 | 0.9078 | 0.8903 – 0.9253 |
| Weeks Gestation | ||||
| < 32 | 0.7406 | 0.7248 – 0.7564 | 0.7410 | 0.7021 – 0.7798 |
| 32 – 36 | 0.8600 | 0.8380 – 0.8819 | 0.9030 | 0.8725 – 0.9334 |
| Hours/ Days at Testing | ||||
| 12 hours – 2 days | 0.9339 | 0.9238 – 0.9440 | 0.9518 | 0.9380 – 0.9655 |
| 3 – 4 days | 0.8573 | 0.8361 – 0.8785 | 0.8898 | 0.8558 – 0.9239 |
| 5 – 6 days | 0.7421 | 0.7057 – 0.7789 | 0.7329 | 0.6613 – 0.8045 |
| 7 – 8 days | 0.7821 | 0.6928 – 0.8676 | 0.7512 | 0.5111 – 0.9913 |
Abbreviations: AC-NEC model, acylcarnitine necrotizing enterocolitis model; AUC, Area under the curve.
log C:5, log C5:1, log C:8:1, log C12, log C14:1, log FC/(C16+C18:1), Gestational age by birth weight grouping, TPN.
Acylcarnitines, Prematurity and Biologic Vulnerability
Model performance was the best among those with newborn screening obtained between 12 hours and 2 days of life (area under the curve=0.9339, 95% confidence interval 0.9236 – 0.9440 in the model development cohort and area under the curve=0.9518, 95% confidence interval 0.9380 – 0.9655 in the validation cohort) (Table 5). When characteristics and acylcarnitines were considered in isolation, both sets of factors were associated with AUCs > 85% overall, > 70% in newborns with gestational ages < 32 weeks, and > 80% in newborns with gestational ages between 32 and 36 weeks in both the model development and validation cohorts (Table 6). In both the development and validation cohorts, increased AUCs were observed when characteristics and acylcarnitines were considered together, although in general, increases were modest.
Table 6.
Receiver operating characteristic curves (ROCs) for AC-NEC a model by characteristics and acylcarnitines only and combined overall and by gestational age groupings.a
| Development | Validation | |||
|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | |
|
|
|
|
|
|
| All | ||||
| GA by Birth Weight +TPN Only | 0.8850 | 0.8751 – 0.8949 | 0.8914 | 0.8693 – 0.9135 |
| Acylcarnitines (ACs) Only | 0.8545 | 0.8429 – 0.8661 | 0.8784 | 0.8569 – 0.8999 |
| GA by Birth Weight +TPN+ACs | 0.8983 | 0.8895 – 0.9072 | 0.8983 | 0.8895 – 0.9072 |
| <32 Weeks | ||||
| GA by Birth Weight +TPN Only | 0.7239 | 0.7095 – 0.7382 | 0.7258 | 0.6975 – 0.7542 |
| Acylcarnitines (ACs) Only | 0.7188 | 0.7013 – 0.7363 | 0.7108 | 0.6688 – 0.7528 |
| GA by Birth Weight +TPN+ACs | 0.7406 | 0.7248 – 0.7564 | 0.7410 | 0.7021 – 0.7798 |
| 32 to 36 Weeks | ||||
| GA by Birth Weight +TPN Only | 0.8263 | 0.8014 – 0.8511 | 0.8547 | 0.8037 – 0.9058 |
| Acylcarnitines (ACs) Only | 0.8121 | 0.7860 – 0.8382 | 0.8735 | 0.8302 – 0.9168 |
| GA by Birth Weight +TPN+ACs | 0.8600 | 0.8380 – 0.8819 | 0.9030 | 0.8725 – 0.9334 |
Abbreviations: AC, acylcarnitine; AC-NEC model, acylcarnitine necrotizing enterocolitis model; AUC, Area under the curve; CI, confidence interval; GA, gestational age; TPN, total parenteral nutrition.
log C:5, log C5:1, log C:8:1, log C12, log C14:1, log FC/(C16+C18:1), Gestational age by birth weight grouping, TPN.
Discussion
Herein we provide the first report of an observed association between fatty acid metabolism (acylcarnitine profiles) and NEC in premature newborns. These observations demonstrate that metabolic profiles obtained at birth reflect biologic vulnerability prior to any alteration by clinical care. These data provide important pathophysiologic insights into newborn systemic metabolic function that predisposes the vulnerable premature to acquired disease like NEC and could therefore support the development and testing of prevention strategies. This potential novel application of newborn screening data demonstrates a widely available vehicle for further development as a risk stratification mechanism.
Prior biomarker studies have attempted to identify high-risk populations early in the course of disease and to differentiate NEC from other neonatal inflammatory conditions.(18-27) These reports have largely focused on inflammatory pathways and have therefore used combinations of non-specific markers that have failed to identify high-risk infants in a timeframe that would allow implementation of disease prevention strategies. Our results introduce the concept of utilizing NBS at birth to identify metabolic dysfunction and the link to the acquired disease of prematurity NEC. Accordingly, acylcarnitine levels measured within the first several days of life may provide an opportunity for early risk stratification and a method for testing various metabolism based prevention strategies including probiotics or modified feeding protocols that have shown some promise in prior clinical studies.(28, 29)
Metabolism and NEC
The underlying pathophysiology of NEC remains incompletely understood and is likely multifactorial. The combination of prematurity, variable feeding practices and bacterial colonization are consistently implicated as the major predisposing factors.(1, 2) Although the proximal event leading to mucosal injury is not well defined, it is conceivable that premature newborns are predisposed to NEC as a result of compromised fatty acid metabolism. Since acylcarnitines are derived from the metabolism of fatty and organic acids, it is plausible that abnormal systemic fatty acid oxidation predisposes to gut specific toxicity following the introduction of a metabolic challenge as occurs with enteral feedings. It has been previously reported in animal models of prematurity that exposure of the intestinal mucosa to fatty acid derivatives causes mucosal necrosis.(12, 13, 30)
NEC is most commonly diagnosed after the initiation of enteral feeding and may be related to both the timing (early or late) of initiation of enteral feedings and rate of feeding advancement, thus implying that increased exposure of the premature gastrointestinal lumen to gut fermentation products including fatty and organic acids produces NEC inciting injury.(31-33) It is intriguing that total parenteral nutrition appears to be a risk factor for the development of NEC both in this study and others.(34) It is unclear whether total parenteral nutrition is exacerbating metabolic dysfunction or is simply a surrogate for sicker preterm infants who begin enteral feeding in a delayed manner. Importantly, the acylcarnitine-NEC association described in this study was most accurate for infants who underwent screening within the first 48 hours of life perhaps suggesting that metabolic profiling may reflect development dependent metabolic dysfunction. Additional studies evaluating the gastrointestinal toxicity of dysfunctional metabolism involving fatty acid oxidation in both laboratory and clinical studies of NEC is warranted to confirm these speculations.
Clinical Utility and Insights
Despite good overall model sensitivity, the utility of the current acylcarnitine-based model as a clinical tool requires additional consideration. The addition of other routinely measured metabolic parameters (e.g. amino acids) and serial testing may both improve on the statistical performance and positive predictive value of metabolic profiling as a clinical prediction tool as well as account for clinical care confounding and influence on metabolic risk longitudinally. A reasonable objective may be to utilize the present metabolic model to facilitate the development of novel management and prevention strategies based upon metabolic profiling. The potential risks and benefits of promising NEC prevention strategies (including monitored feeding protocols and/or probiotics) are subject to ongoing study.(32, 35) However, given the substantial NEC related mortality as well as the possibility of significant life-long gastrointestinal and neurologic impairment in survivors, the potential benefit from the prevention of any case of NEC should be viewed as highly significant relative to the potential for harm of perceived low risk interventions.
Strengths and Limitations
The strengths of the present study include the use of population-based metabolic screening data linked to a comprehensive neonatal outcomes database. This combination has expanded the novel application of linking newborn screening results to acquired newborn disease.(36, 37) Our study population included preterm infants from across the broad geographic and socioeconomic regions of California and the results remained robust while controlling for multiple patient demographic factors. In light of these strengths it should also be recognized that there are important limitations to the present study. The use of a population-based dataset meant that we relied exclusively on hospital discharge records for NEC diagnosis and were therefore limited in our ability to stratify results by severity of disease (progressive and non-progressive NEC) through the examination of clinical records. Further, since the validation cohort was also derived from California births (albeit in a different year) may have led to some over fitting of the model. These issues point to the importance of testing the relationships observed in the current study in other populations where tighter phenotypic description is possible. Accordingly, subsequent efforts will benefit from a focus on targeted age and size cohorts (e.g. <32, or 32-26 weeks) exclusively given the discussed etiologic and clinical implications.
Summary
The observed association between acylcarnitine profile and NEC offers the potential for early identification of high-risk newborns based upon metabolism utilizing an available testing platform and represents an important first step towards directing preventive measures and developing improved therapeutic strategies. The present findings suggest that NEC may be the manifestation of a predisposing systemic metabolic dysfunction thus providing new insights to the pathophysiology of NEC.
Conclusions
There is an association between abnormal acylcarnitine profiles measured during newborn screening in premature infants and risk for NEC. Replication and external validation of the findings may lead to the development of novel prevention strategies.
Acknowledgments
Data from the California Newborn Screening Program were obtained through the California Biobank Program (Screening Information System request no. 476). Data were obtained with an agreement that the California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication.
Funding Sources: This article was supported by FDA Grant Number 1UO1FD004194-01 (KGS); AHRQ Grant Number HS000028 (ZJK); The Jack and Marion Euphrat Fellowship in Pediatric Translational Medicine and Stanford CTSA Grant Number UL1 RR025744 (ZJK). The Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R00HD065786 (KKR). The March of Dimes Foundation and Stanford University School of Medicine provided some grant support for the March of Dimes Prematurity Research Center at Stanford University School of Medicine (GMS and DKS). Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of the AHRQ.
Abbreviations
- NEC
necrotizing enterocolitis
- NBS
newborn screening
- AUC
area under the curve
Footnotes
The authors of this manuscript have no conflicts of interest to disclose. Dr. Sylvester wrote the first draft of the manuscript. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Contribution: KGS, ZJK, RLM, GME, TMC, GMS, DKS, TJS, CS, KKR: Study conception and design, analysis and interpretation of data, drafting and revising the article for critically important intellectual content, final approval of the version to be published. LJP: Study conception and design, analysis and interpretation of data, drafting and revising the article for critically important intellectual content, final approval of the version to be published. LJP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Citations
- 1.Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Seminars in perinatology. 2008;32(2):70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Hull MA, Fisher JG, Gutierrez IM, Jones BA, Kang KH, Kenny M, et al. Mortality and Management of Surgical Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Prospective Cohort Study. Journal of the American College of Surgeons. 2013 doi: 10.1016/j.jamcollsurg.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clinics in perinatology. 2013;40(1):135–48. doi: 10.1016/j.clp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Kelleher AS, Chace DH, Spitzer AR. Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics. 2014;134(1):e37–46. doi: 10.1542/peds.2014-0329. [DOI] [PubMed] [Google Scholar]
- 7.Gucciardi A, Zaramella P, Costa I, Pirillo P, Nardo D, Naturale M, et al. Analysis and interpretation of acylcarnitine profiles in dried blood spot and plasma of preterm and full-term newborns. Pediatric research. 2015;77(1-1):36–47. doi: 10.1038/pr.2014.142. [DOI] [PubMed] [Google Scholar]
- 8.Jelliffe-Pawlowski LL, Norton ME, Baer RJ, Santos N, Rutherford GW. Gestational dating by metabolic profile at birth: a California cohort study. American journal of obstetrics and gynecology. 2016;214(4):511 e1–e13. doi: 10.1016/j.ajog.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryckman KK, Berberich SL, Dagle JM. Predicting gestational age using neonatal metabolic markers. American journal of obstetrics and gynecology. 2016;214(4):515 e1–e13. doi: 10.1016/j.ajog.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson K, Hawken S, Potter BK, Chakraborty P, Walker M, Ducharme R, et al. Accurate prediction of gestational age using newborn screening analyte data. American journal of obstetrics and gynecology. 2016;214(4):513 e1–9. doi: 10.1016/j.ajog.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Clark DA, Thompson JE, Weiner LB, McMillan JA, Schneider AJ, Rokahr JE. Necrotizing enterocolitis: intraluminal biochemistry in human neonates and a rabbit model. Pediatric research. 1985;19(9):919–21. doi: 10.1203/00006450-198509000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo M, Bass J, Krantis A. An intraluminal model of necrotizing enterocolitis in the developing neonatal piglet. Journal of pediatric surgery. 1995;30(8):1138–42. doi: 10.1016/0022-3468(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 13.Gollin G, Marks WH. Elevation of circulating intestinal fatty acid binding protein in a luminal contents-initiated model of NEC. Journal of pediatric surgery. 1993;28(3):367–70. doi: 10.1016/0022-3468(93)90233-b. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, Ling XB, Zhao Y, Hu Z, Zheng X, Xu Z, et al. A data-driven algorithm integrating clinical and laboratory features for the diagnosis and prognosis of necrotizing enterocolitis. PloS one. 2014;9(2):e89860. doi: 10.1371/journal.pone.0089860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J. Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Medical hypotheses. 2004;62(2):291–3. doi: 10.1016/S0306-9877(03)00333-5. [DOI] [PubMed] [Google Scholar]
- 16.Feuchtbaum L, Lorey F, Faulkner L, Sherwin J, Currier R, Bhandal A, et al. California's experience implementing a pilot newborn supplemental screening program using tandem mass spectrometry. Pediatrics. 2006;117(5 Pt 2):S261–9. doi: 10.1542/peds.2005-2633E. [DOI] [PubMed] [Google Scholar]
- 17.Gallant NM, Leydiker K, Tang H, Feuchtbaum L, Lorey F, Puckett R, et al. Biochemical, molecular, and clinical characteristics of children with short chain acyl-CoA dehydrogenase deficiency detected by newborn screening in California. Molecular genetics and metabolism. 2012;106(1):55–61. doi: 10.1016/j.ymgme.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361(9354):310–1. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 19.Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. The Journal of pediatrics. 2010;157(5):757–61. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmrath MA, Shin CE, Fox JW, Erwin CR, Warner BW. Epidermal growth factor in saliva and serum of infants with necrotising enterocolitis. Lancet. 1998;351(9098):266–7. doi: 10.1016/S0140-6736(05)78271-4. [DOI] [PubMed] [Google Scholar]
- 21.Ng PC. Biomarkers of necrotising enterocolitis. Seminars in fetal & neonatal medicine. 2014;19(1):33–8. doi: 10.1016/j.siny.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Pourcyrous M, Korones SB, Yang W, Boulden TF, Bada HS. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics. 2005;116(5):1064–9. doi: 10.1542/peds.2004-1806. [DOI] [PubMed] [Google Scholar]
- 23.Rabinowitz SS, Dzakpasu P, Piecuch S, Leblanc P, Valencia G, Kornecki E. Platelet-activating factor in infants at risk for necrotizing enterocolitis. The Journal of pediatrics. 2001;138(1):81–6. doi: 10.1067/mpd.2001.110132. [DOI] [PubMed] [Google Scholar]
- 24.Sylvester KG, Ling XB, Liu GY, Kastenberg ZJ, Ji J, Hu Z, et al. A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut. 2014;63(8):1284–92. doi: 10.1136/gutjnl-2013-305130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sylvester KG, Ling XB, Liu GY, Kastenberg ZJ, Ji J, Hu Z, et al. Urine protein biomarkers for the diagnosis and prognosis of necrotizing enterocolitis in infants. The Journal of pediatrics. 2014;164(3):607–12. e1–7. doi: 10.1016/j.jpeds.2013.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Annals of surgery. 2010;251(6):1174–80. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 27.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. The Journal of pediatrics. 2007;150(4):358–63. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122(4):693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 29.Raval MV, Hall NJ, Pierro A, Moss RL. Evidence-based prevention and surgical treatment of necrotizing enterocolitis-a review of randomized controlled trials. Seminars in pediatric surgery. 2013;22(2):117–21. doi: 10.1053/j.sempedsurg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Gollin G, Stadie D, Mayhew J, Slater L, Asmerom Y, Boskovic D, et al. Early detection of impending necrotizing enterocolitis with urinary intestinal fatty acid-binding protein. Neonatology. 2014;106(3):195–200. doi: 10.1159/000362497. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DM, Kliegman RM. The relationship of neonatal alimentation practices to the occurrence of endemic necrotizing enterocolitis. American journal of perinatology. 1991;8(1):62–7. doi: 10.1055/s-2007-999344. [DOI] [PubMed] [Google Scholar]
- 32.Group TSI. Early enteral feeding strategies for very preterm infants: current evidence from Cochrane reviews. Archives of Diseases of Childhood Fetal and Neonatal. 2013;98(6):F470–F2. doi: 10.1136/archdischild-2012-303260. [DOI] [PubMed] [Google Scholar]
- 33.McCallie KR, Lee HC, Mayer O, Cohen RS, Hintz SR, Rhine WD. Improved outcomes with a standardized feeding protocol for very low birth weight infants. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(Suppl 1):S61–7. doi: 10.1038/jp.2010.185. [DOI] [PubMed] [Google Scholar]
- 34.Moss RL, Kalish LA, Duggan C, Johnston P, Brandt ML, Dunn JC, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: a multi-institutional study. Journal of perinatology : official journal of the California Perinatal Association. 2008;28(10):665–74. doi: 10.1038/jp.2008.119. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. 2013;132(6):1055–62. doi: 10.1542/peds.2013-1339. [DOI] [PubMed] [Google Scholar]
- 36.Ryckman KK, Dagle JM, Shchelochkov OA, Ehinger N, Poole SD, Berberich SL, et al. Association of amino acids with common complications of prematurity. Pediatric research. 2013;73(6):700–5. doi: 10.1038/pr.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryckman KK, Spracklen CN, Dagle JM, Murray JC. Maternal factors and complications of preterm birth associated with neonatal thyroid stimulating hormone. Journal of pediatric endocrinology & metabolism : JPEM. 2014 doi: 10.1515/jpem-2013-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]