Abstract
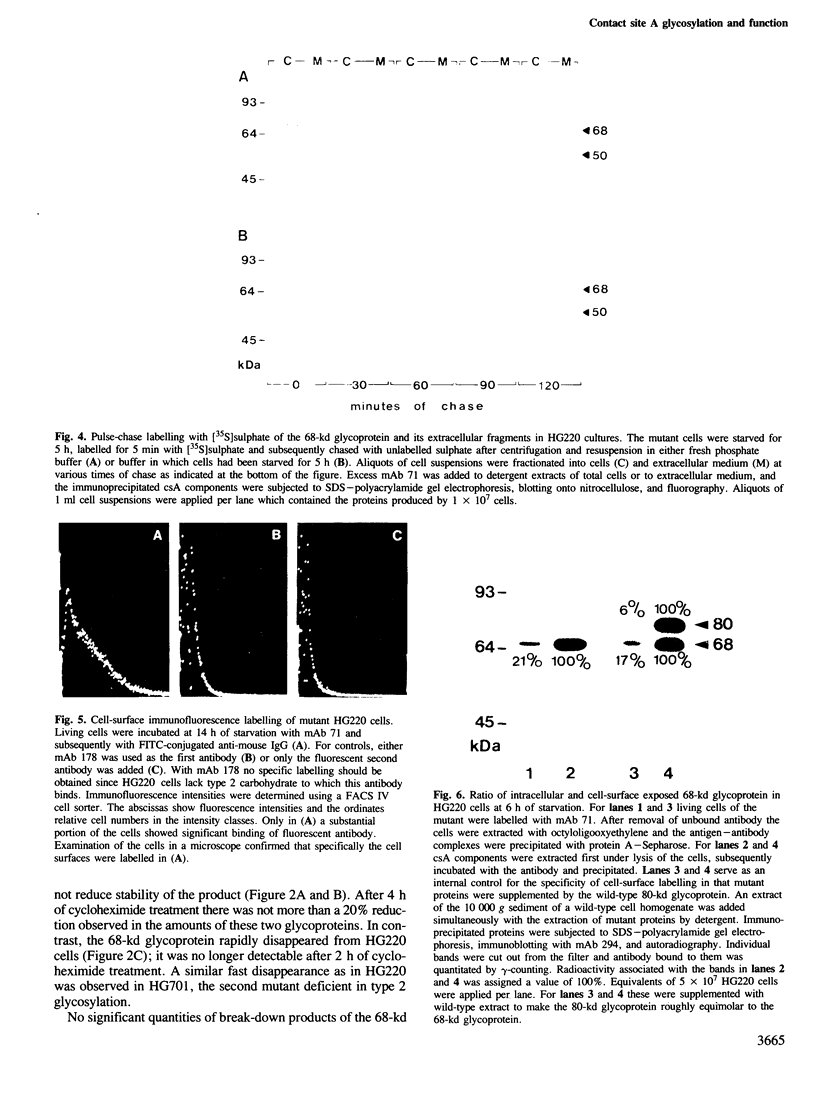
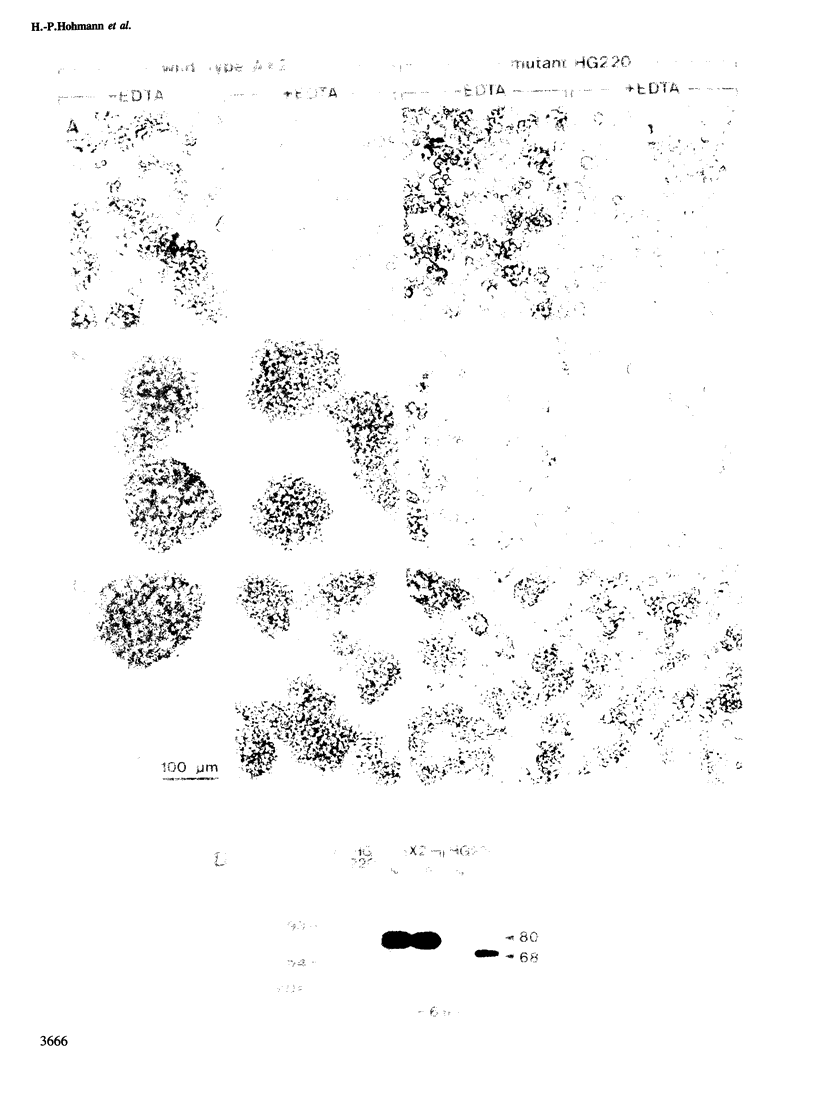
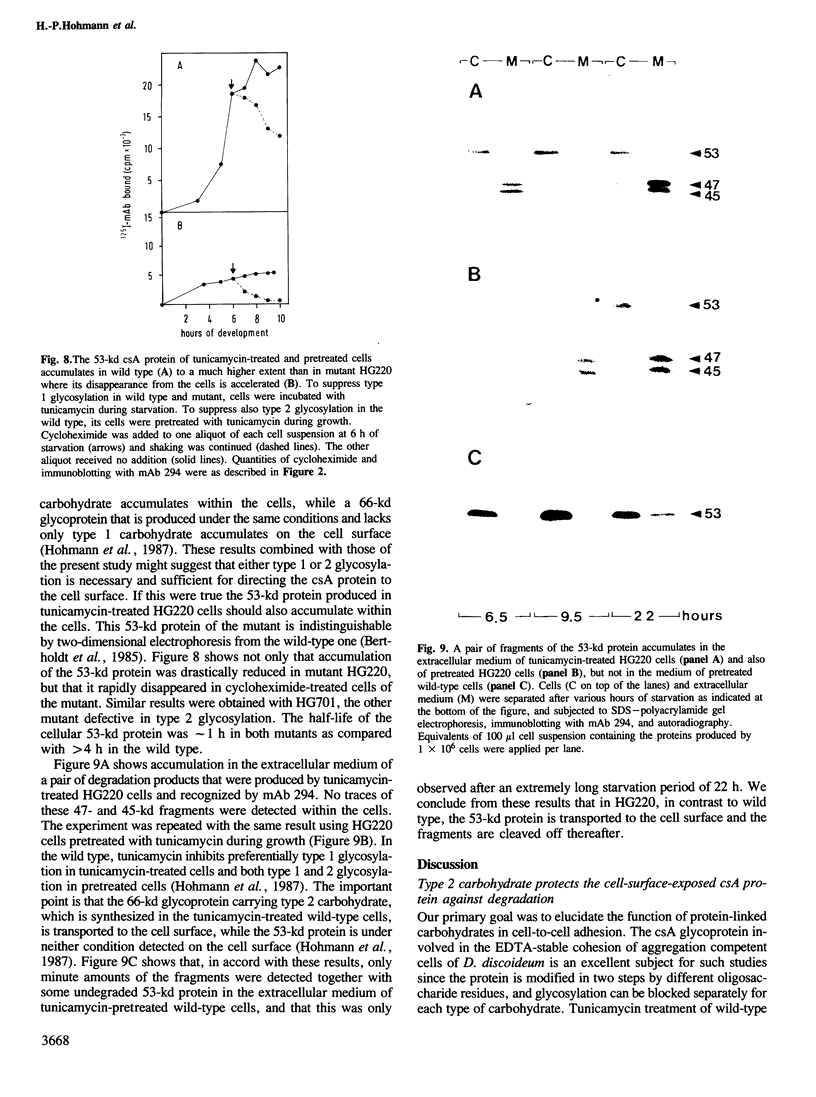
The functions of type 1 and 2 carbohydrates of the contact site A (csA) glycoprotein of Dictyostelium discoideum have been investigated using mutants lacking type 2 carbohydrate. In two mutant strains, HG220 and HG701, a 68-kd glycoprotein was synthesized as the final product of csA biosynthesis. This glycoprotein accumulated to a much lower extent on the surfaces of mutant cells than the mature 80-kd glycoprotein did in wild-type cells. There was also no accumulation of the 68-kd glycoprotein observed within the mutant cells nor was a precursor of lower molecular mass detected, in accordance with previous findings that indicated cotranslational linkage of type 1 carbohydrate by N-glycosylation. Pulse-chase labelling showed that a 50-kd glycopeptide was cleaved off from the mutant 68-kd glycoprotein and released into the medium, while the fully glycosylated 80-kd glycoprotein of the wild type was stable. These results assign a function to type 2 carbohydrate in protecting the cell-surface-exposed csA glycoprotein against proteolytic cleavage. HG220 cells were still capable of forming EDTA-stable contacts to a reduced extent, consistent with the low amounts of the 68-kd glycoprotein present on their surfaces. Thus type 1 rather than type 2 carbohydrate appears to be directly involved in intercellular adhesion that is mediated by the csA glycoprotein. Tunicamycin-treated wild-type and mutant cells produce a 53-kd protein that lacks both type 1 and 2 carbohydrates. While this protein is stable and not transported to the cell surface in the wild type, it is cleaved in the mutants and fragments of it are released into the extracellular medium. These results suggest that the primary defect in the two mutants studied is relief from a restriction in protein transport to the cell surface, and that the defect in type 2 glycosylation is secondary.
Keywords: cell adhesion, contact sites A, Dictyostelium, post-translational glycosylation, carbohydrate function
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholdt G., Stadler J., Bozzaro S., Fichtner B., Gerisch G. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 1985 May;16(3):187–202. doi: 10.1016/0045-6039(85)90516-0. [DOI] [PubMed] [Google Scholar]
- Bozzaro S., Merkl R., Gerisch G. Cell adhesion: its quantification, assay of the molecules involved, and selection of defective mutants in Dictyostelium and Polysphondylium. Methods Cell Biol. 1987;28:359–385. doi: 10.1016/s0091-679x(08)61657-x. [DOI] [PubMed] [Google Scholar]
- Bozzaro S., Merkl R. Monoclonal antibodies against Dictyostelium plasma membranes: their binding to simple sugars. Cell Differ. 1985 Aug;17(2):83–94. doi: 10.1016/0045-6039(85)90474-9. [DOI] [PubMed] [Google Scholar]
- Francis D., Toda K., Merkl R., Hatfield T., Gerisch G. Mutants of Polysphondylium pallidum altered in cell aggregation and in the expression of a carbohydrate epitope on cell surface glycoproteins. EMBO J. 1985 Oct;4(10):2525–2532. doi: 10.1002/j.1460-2075.1985.tb03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Hagmann J., Hirth P., Rossier C., Weinhart U., Westphal M. Early Dictyostelium development: control mechanisms bypassed by sequential mutagenesis. Cold Spring Harb Symp Quant Biol. 1985;50:813–822. doi: 10.1101/sqb.1985.050.01.099. [DOI] [PubMed] [Google Scholar]
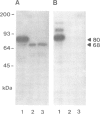
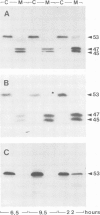
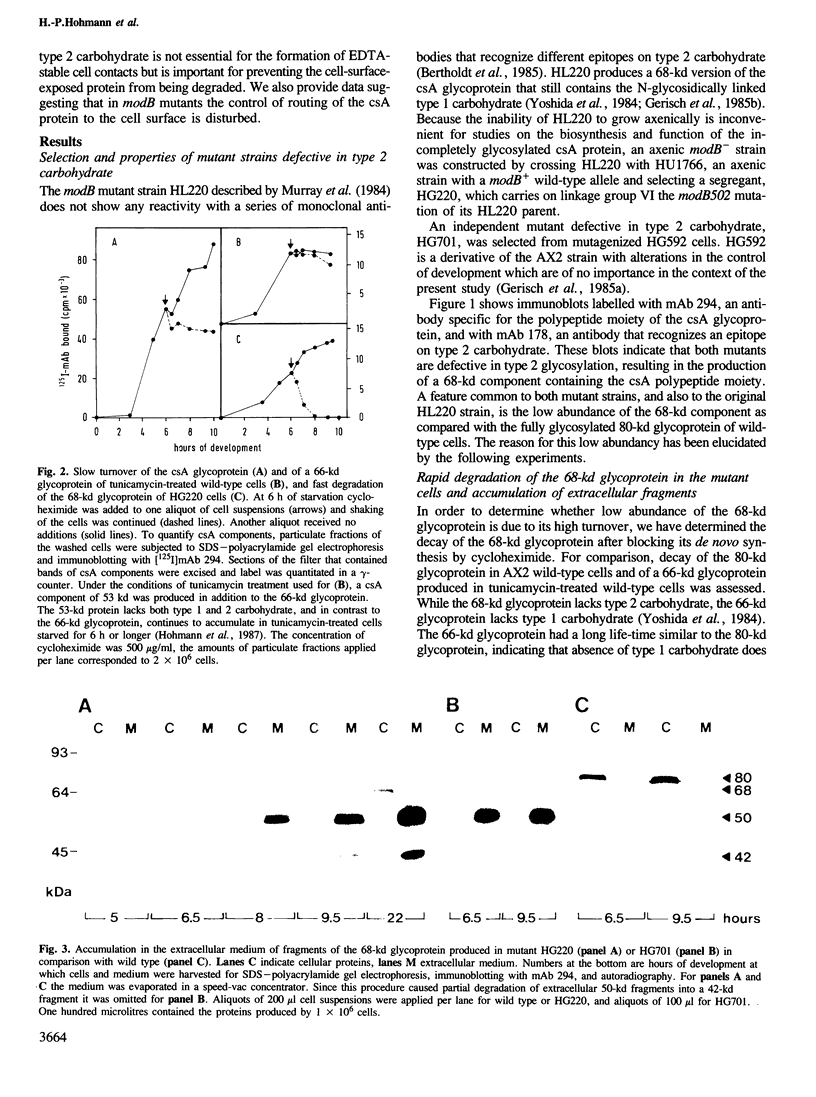
- Gerisch G., Weinhart U., Bertholdt G., Claviez M., Stadler J. Incomplete contact site A glycoprotein in HL220, a modB mutant of Dictyostelium discoideum. J Cell Sci. 1985 Feb;73:49–68. doi: 10.1242/jcs.73.1.49. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Gerisch G., Lee R. W., Huttner W. B. Cell-free sulfation of the contact site A glycoprotein of Dictyostelium discoideum and of a partially glycosylated precursor. J Biol Chem. 1985 Nov 5;260(25):13869–13878. [PubMed] [Google Scholar]
- Lam T. Y., Siu C. H. Inhibition of cell differentiation and cell cohesion by tunicamycin in Dictyostelium discoideum. Dev Biol. 1982 Aug;92(2):398–407. doi: 10.1016/0012-1606(82)90185-3. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A., Springer W. R., Barondes S. H. Adhesion mutants of Dictyostelium discoideum lacking the saccharide determinant recognized by two adhesion-blocking monoclonal antibodies. Dev Biol. 1985 May;109(1):111–117. doi: 10.1016/0012-1606(85)90351-3. [DOI] [PubMed] [Google Scholar]
- Malchow D., Nägele B., Schwarz H., Gerisch G. Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur J Biochem. 1972 Jun 23;28(1):136–142. doi: 10.1111/j.1432-1033.1972.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Wheeler S., Jongens T., Loomis W. F. Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol. 1984 Mar;4(3):514–519. doi: 10.1128/mcb.4.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Noegel A., Gerisch G., Stadler J., Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986 Jul;5(7):1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H., Stadler J., Westphal M., Wagle G., Merkl R., Gerisch G. Monoclonal antibodies against contact sites A of Dictyostelium discoideum: detection of modifications of the glycoprotein in tunicamycin-treated cells. EMBO J. 1982;1(8):1011–1016. doi: 10.1002/j.1460-2075.1982.tb01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Cell adhesion molecules: detection with univalent second antibody. J Cell Biol. 1980 Dec;87(3 Pt 1):703–707. doi: 10.1083/jcb.87.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Protein-linked oligosaccharide implicated in cell-cell adhesion in two Dictyostelium species. Dev Biol. 1985 May;109(1):102–110. doi: 10.1016/0012-1606(85)90350-1. [DOI] [PubMed] [Google Scholar]
- West C. M., Loomis W. F. Absence of a carbohydrate modification does not affect the level or subcellular localization of three membrane glycoproteins in modB mutants of Dictyostelium discoideum. J Biol Chem. 1985 Nov 5;260(25):13803–13809. [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Williams K. L., Newell P. C. A genetic study of aggregation in the cellular slime mould Dictyostelium discoideum using complementation analysis. Genetics. 1976 Feb;82(2):287–307. doi: 10.1093/genetics/82.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Identification of carbohydrate moieties involved in EDTA-stable or EDTA-sensitive cell contact of Dictyostelium discoideum. J Biochem. 1987 May;101(5):1233–1245. doi: 10.1093/oxfordjournals.jbchem.a121987. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Stadler J., Bertholdt G., Gerisch G. Wheat germ agglutinin binds to the contact site A glycoprotein of Dictyostelium discoideum and inhibits EDTA-stable cell adhesion. EMBO J. 1984 Nov;3(11):2663–2670. doi: 10.1002/j.1460-2075.1984.tb02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]