Abstract
Although Syrian hamsters are thought to be naturally solitary, recent evidence from our laboratory demonstrates that hamsters may actually prefer social contact. Hamsters increase their preference for a location associated with an agonistic encounter regardless of whether they have “won” or “lost”. It has also been reported that social housing as well as exposure to intermittent social defeat or a brief footshock stressor increase food intake and body mass in hamsters. By contrast, it has also been suggested that housing hamsters in social isolation causes anxiety-induced anorexia and reductions in body mass selectively in females. The purpose of this study was to determine the physiological consequences of housing hamsters in social isolation versus in social groups. Male and female hamsters were housed singly or in stable groups of 5 for 4 weeks after which they were weighed and trunk blood was collected. In addition, fat pads and thymus and adrenal glands were extracted and weighed. Serum and fecal cortisol were measured using an enzyme-linked immunoassay. Housing condition had no effect on serum or fecal cortisol, but socially housed hamsters displayed modest thymus gland involution. Socially housed females weighed more than did any other group, and socially housed females and males had more fat than did socially isolated hamsters. No wounding or tissue damage occurred in grouped hamsters. Overall, these data suggest that Syrian hamsters tolerate both stable social housing and social isolation in the laboratory although social housing is associated with some alteration in stress-related and bioenergetic measures.
Keywords: Cortisol, Thymus, Obesity, Social Stress, Social Defeat, Aggression
1. Introduction
Since their introduction to the laboratory in the 1930’s, Syrian, or Golden, hamsters have been used as an animal model in a wide variety of research areas. This is partly because many aspects of their physiology and their regulation of energy balance recapitulate more closely that of humans than do many other rodents [1]. For instance, hamsters exhibit high cholesterol ester transport protein and low hepatic low-density lipoprotein receptor activity [2] as well as a high glycemic response to dietary fructose [3], none of which are exhibited by rats and mice. This leaves hamsters and humans more susceptible to cardiovascular diseases, including cardiomyopathy and atherosclerosis, as well as to diabetes [4, 5]. In addition, hamsters, like humans, are dual secretors of cortisol and corticosterone with cortisol being the glucocorticoid that is more labile in response to circadian influences and to the effects of stress [6–10]. This pattern of response is in marked contrast to most laboratory rodents, which secrete primarily corticosterone, and suggests that hamsters are an ideal species for stress research.
Although Syrian hamsters have been studied only sparsely in the wild, they are generally viewed as largely solitary animals that live alone in burrows and that defend home territories [11–14]. This information has contributed to the widely held, but probably erroneous, impression that hamsters are asocial; however, despite this pervasive belief, it has usually been the case that hamsters are group housed in the laboratory [15–21]. In fact, it is also well known that hamsters of both sexes display rich social, agonistic, and communicative behaviors [22–24] and are capable of complex social discriminations including kin and individual recognition [25–28]. There is also some recent evidence that Syrian hamsters not only tolerate but may actually prefer social contact [19, 29]. We have demonstrated that social conflict, regardless of outcome (i.e, win or lose) increases Fos-immunoreactivity in the ventral tegmental area as well as increases the time that a hamster will spend in the location where the social interaction occurred, both of which suggest that social interactions are rewarding.
This preference for a location where one has fought, even lost, is perhaps surprising, given that it has long been known that being on the losing end of a social encounter, in particular, is stressful for hamsters as evidenced by neuroendocrine [8, 30, 31] and behavioral [32, 33] responses to social defeat. Indeed, the effects of social defeat stress are pervasive and are similar across species and even taxa [33–36]. These effects include hypothalamic-pituitary-adrenocortical (HPA) activation, changes in sleep and food intake, as well as marked increases in social avoidance, responses that are observed in species with a wide variety of social structures (e.g., solitary, colony-living). Interestingly, it has also long been known that group housing leads to marked weight gain in female hamsters [15, 16], a result that was hypothesized to be due to social stress experienced in group housed females. Consistent with this hypothesis, we have demonstrated that repeated social defeat stress is associated with an increase in food intake, body mass, and adiposity in male Syrian hamsters [37]. This increase in body mass and adiposity in hamsters can be observed after as few as four exposures to social defeat and is mimicked by exposure to a footshock stressor, further implying that the change in energy regulation is a stress effect [38] and supporting the hypothesis that the early observations of greater weight gain in socially housed females was a social stress effect. Thus, the data on social housing and social stress in hamsters are mixed, but overall suggest that social housing is more stressful to hamsters than is social isolation.
Conversely, it has recently been proposed that social isolation, or social separation, of hamsters causes reductions in food intake, body mass, and adiposity and increases in anxiety-like behavior, particularly among female hamsters [18]. Shannonhouse et al. (2014) maintain that the long-held interpretation that social housing or social interactions are stressful for hamsters may be incorrect and that it is instead social separation that causes an anxiety-like response, particularly after hamsters have been stably group-housed during adolescence. It is certainly the case that hamsters are almost always socially housed in the laboratory after weaning and that experimenters rarely take into account the possibility that this change in housing may have important effects when they singly house hamsters for a few weeks before an experiment begins. Given that hamsters have such wide utility in biomedical research, and because they are housed both singly and in groups in the laboratory, it is important to document clearly the effect of housing on a number of different stress and bioenergetic endpoints. Because both sexes display such a wide variety of social behaviors, hamsters are also very useful in studies examining possible sex differences and the mechanics underlying them (e.g., [20]). Because there is a suggestion in the literature that there is an important sex difference in the effect of housing condition on body weight in hamsters, it is also important to examine housing effects in both male and female hamsters. In the current experiment we measured both basal serum and fecal cortisol concentrations, in addition to measuring body, fat pad, thymus, and adrenal masses, following 4 weeks of social housing or social isolation in male and female hamsters. Finally, in a separate experiment, we also exposed both group and single housed males and females to a social defeat stressor in order to determine if the glucocorticoid response to defeat varies by sex or housing condition. The overarching purpose of the study was to determine if social housing or social isolation appears more stressful for, or alters the neuroendocrine response to social stress, in hamsters.
2. Material and methods
2.1 Animals
Male and female Syrian hamsters (Experiment 1 N=40; Experiment 2 N=36) were bred from Charles River stock and were born in-house in the Georgia State University vivarium. The hamsters were weaned into groups of 5 on postnatal day 25 (P25), and littermates were evenly assigned across cages and groups; post-weaning group housing was used because this is the common housing of hamsters at commercial breeders and in laboratory settings. For Experiment 1, on P60, half of the animals moved to single housing while the grouped animals were moved into new group cages with 5 animals per cage, resulting in four groups: female socially housed (n=10), female socially isolated (n=10), male socially housed (n=10), and male socially isolated (n=10). For Experiment 2, on P42, 8 males and 8 females were moved to single housing while the others were transferred into new group housed cages (5 animals per cage), again resulting in four groups: female socially housed (n=10), female socially isolated (n = 8), male socially housed (n=10), and male socially isolated (n=8). Group housed animals in both experiments were carefully observed daily to verify that no wounding had occurred. All hamsters were housed in polycarbonate cages (23 × 43 × 20 cm) with wire grid lids, corn cob bedding and cotton nesting material. Chow (LabDiet 5001, St. Louis, MO) and water were available ad libitum. The room was maintained on a 14:10 light:dark cycle, as is customary with hamsters to maintain gonadal patency. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Tissue collection
Animals were not handled except for standard cage changes, which occurred at the same time for group and single housed animals. For Experiment 1, after 4 weeks of single or group housing, animals were moved into another room and weighed. Eleven hours later, at the time of lights on, the nadir of the circadian cortisol rhythm [6], the hamsters were quickly removed from their home cages, rapidly decapitated, and trunk blood was collected (~10 seconds between handling and blood collection). After coagulation, blood was centrifuged to collect serum. Epididymal (males) and perimetrial (females) fat pads, from here on referred to as gonadal fat (GWAT), mesenteric (MWAT), retroperitoneal (RWAT), and inguinal (IWAT) fat pads, and thymus and adrenal glands were removed and weighed. In addition, fecal boli were collected from the colon. For Experiment 2, after 2.5 weeks of single or group housing, all animals were transported to the behavioral testing suite in the vivarium 1 hr before lights off. A shorter housing manipulation was used in Experiment 2 to determine if habituation of serum cortisol response would occur in less than 4 weeks. At lights off, half of each group of hamsters was subjected to a 15 min social defeat in the home cage of a larger, same-sex opponent (resident aggressor) as described below. After the defeat, hamsters were placed in a holding cage for 5 min and then decapitated. Trunk blood was collected and processed as described in Experiment 1 to obtain serum. Trunk blood was also collected and processed for serum from the remaining hamsters that were not defeated. All behavioral testing and blood collection was done within the first 2 hr after lights off and the order of collection was counterbalanced for sex, housing, and defeat status.
2.3 Behavior (Experiment 2)
Social defeat occurred in the home cage of a same-sex, larger resident aggressor that reliably attacked and defeated the intruder (subject). All defeats were 15 min in duration. A trained observer watched all defeats. The 15 min defeat was timed from the first attack by the resident, which occurred within 30 sec in all pairings. The observer determined a dominance hierarchy based on the behavior of the hamsters. The dominant hamster (always the resident in this study) exhibited side and upright attacks and chasing and the subordinate hamsters produced submissive behaviors such as flight, tail lift, and side and upright submission (for a detailed description of behaviors observed see [23]). Male and female hamsters display identical behaviors in resident-intruder pairings. In our studies, we include a caveat that any agonistic pairing in which the skin is broken be stopped immediately, but no cases of tissue damage occurred in this study.
2.4 Cortisol Assays
Serum cortisol was measured with an enzyme-linked immunosorbent assay (ELISA; Enzo, Farmingdale, NY) following manufacturer’s instructions. Samples were first diluted (1:100) with a steroid displacement reagent, then diluted again (1:10) with assay buffer before they were loaded onto the assay plate. Samples were run in duplicate across 2 plates. Absorbance was measured at 405 nm with a correction at 580 nm. The average intra-assay coefficient of variability (CV) was 8.6, and the average inter-plate CV was 7.4. Fecal cortisol was extracted with ethanol and measured with an ELISA (Immuno-Biological Laboratories, Minneapolis, MN) following manufacturer’s instructions. Samples were diluted (1:10) with assay buffer before they were loaded onto the assay plate. Samples were run in duplicate across 2 plates. Absorbance was measured at 450 nm. The average intra-assay CV was 9.0 and the average inter-plate CV was 5.0.
2.5 Statistical Analysis
All data were stored and analyzed with Microsoft Excel, Version 14.7.1 and Statistical Package for the Social Sciences (SPSS), Version 23. A two-way analysis of variance (ANOVA) and Tukey post hoc tests were performed to determine if there were differences between male and female and group versus single housed hamsters for Experiment 1. In addition, thymus and adrenal gland masses were corrected for body mass before the ANOVA was conducted. A three-way ANOVA and post hoc Tukey and a priori t-tests were performed for Experiment 2 to determine if there were significant effects of sex, housing condition, and social stress on serum cortisol concentrations. Effect sizes were calculated using eta squared for ANOVAs and Cohen’s d for pairwise comparisons.
We were unable to obtain enough serum to analyze cortisol in one female hamster that was group housed in Experiment 1; therefore, this hamster was not included in the serum cortisol analysis. In addition, in Experiment 2, three hamsters (1 male, 2 females) were not defeated by the resident aggressor (one male and one female was not attacked by the RA, and the second female displayed only lordosis during the pairing) thus were not included in the analysis.
3. Results
It is important to note that no wounding or tissue damage was observed in group housed males or females in either experiment. There was also no wounding during the social defeat stress in Experiment 2; therefore, no hamsters were excluded from the statistical analysis based on wounding.
In Experiment 1, there was a significant interaction between housing condition and sex on body mass [F(1, 36) = 4.44, p < 0.05, η2 = 0.11], where females socially housed groups exhibited significantly higher body mass than did all other groups (Fig 1). In addition, in all fat pads measured, socially housed hamsters had increased fat compared to socially isolated hamsters, regardless of sex: GWAT [F(1, 36) = 23.6, p < 0.05, η2 = 0.40], MWAT [F(1, 36) = 10.5, p < 0.05, η2 = 0.23], RWAT [F(1, 36) = 9.35, p < 0.05, η2 = 0.21], and IWAT [F(1, 36) = 7.92, p < 0.05, η2 = 0.18] (Fig 2A–D).
Figure 1.
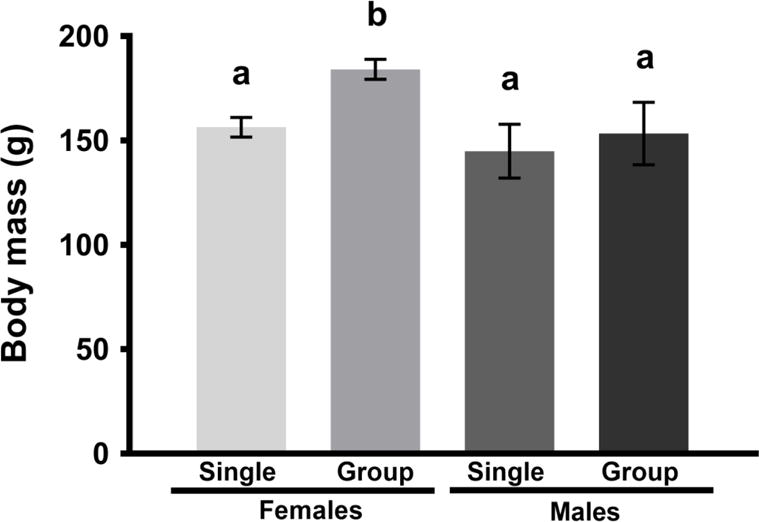
Body mass (g; mean ± SE) of male and female Syrian hamsters after 4 weeks of single or group housing in Experiment 1. Group housed females gained more body mass compared to all other groups (p < 0.05, η2 = 0.11; unshared letters denote statistical significance).
Figure 2.
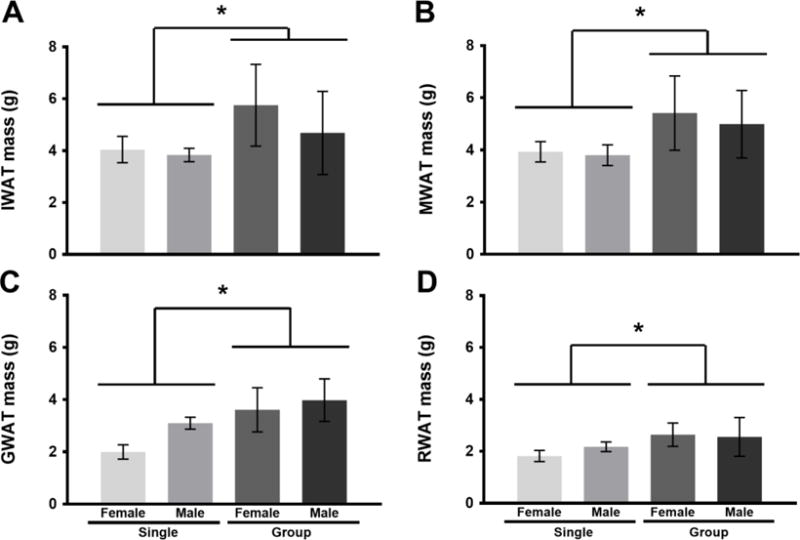
Fat pad mass (g; mean ± SE) of male and female Syrian hamsters after 4 weeks of single or group housing in Experiment 1. Inguinal white adipose tissue (IWAT) is depicted in panel A); B) mesenteric white adipose tissue (MWAT); C) gonadal white adipose tissue; D) retroperitoneal white adipose tissue (RWAT). Fat pads were significantly increased in grouped compared to single housed animals (*p < 0.05) regardless of sex (p > 0.05).
There was a significant effect of both sex [F(1, 36) = 8.49, p < 0.05, η2 = 0.19] and housing [F(1, 36) = 11.72, p < 0.05, η2 = 0.25] on thymus gland mass (Fig 3). Thymus glands, after correction for body mass, were significantly lighter in males compared to females, and, importantly, significantly lighter in socially housed compared to socially isolated hamsters. Unfortunately, due to a procedural error, we were unable to obtain accurate weights of adrenal glands.
Figure 3.
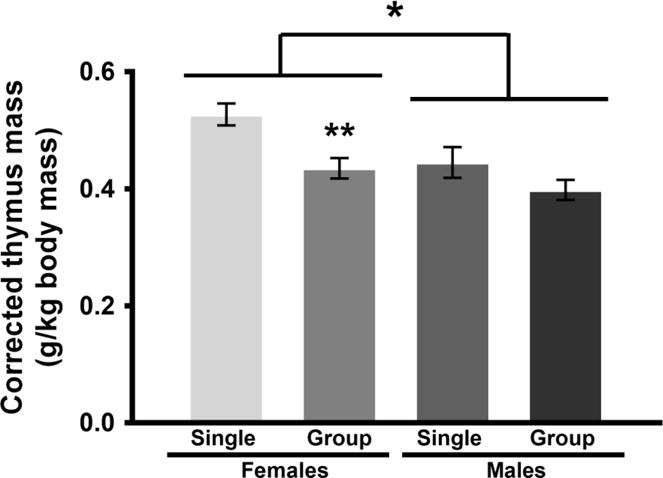
Thymus mass corrected for body mass (g/kg body mass; means ± SE) of male and female Syrian hamsters after 4 weeks of single or group housing in Experiment 1. An ANOVA revealed significant main effects of both sex and housing on thymus mass. Thymus masses were significantly lighter in males compared to females (*p < 0.05, η2 = 0.19). In addition, thymus masses were significantly lighter in group housed animals compared to single housed animals (**p < 0.05, η2 = 0.25).
In Experiment 1, serum cortisol (Fig 4A) was significantly higher in males compared to females [F(1, 35) = 5.57, p < 0.05, η2 = 0.14], but there was no significant effect of housing condition on serum cortisol [F(1, 35) = 0.24, p > 0.05, η2 = 0.01]. Similarly, there was also no significant effect of housing on fecal cortisol [F(1, 36), = 1.38, p > 0.05, η2 = 0.04]. Finally, although there still appeared to be a pattern wherein cortisol was higher in males, there was also no significant effect of sex on fecal cortisol [F(1, 36) = 0.60, p > 0.05, η2 = 0.02] (Fig 4B).
Figure 4.
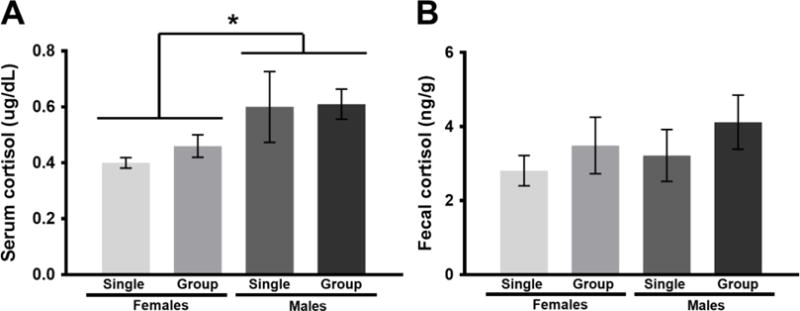
Cortisol concentrations (means ± SE) of male and female Syrian hamsters after 4 weeks of single or group housing in Experiment 1. (A) Serum cortisol was significantly higher in males compared to females (*p < 0.05, η2 = 0.14). There was no significant effect of housing condition on serum cortisol (p > 0.05, η2 = 0.01). (B) There was no significant effect of sex (p > 0.05, η2 = 0.02) or housing condition (p > 0.05, η2 = 0.04) on fecal cortisol.
In Experiment 2, there was a significant interaction between sex and social stress on serum cortisol [F(1, 25) = 14.51, p < 0.05, η2 = 0.37], where defeated males exhibited significantly higher cortisol compared to all other groups (Fig 5). In addition, t-tests revealed higher serum cortisol in defeated females [t(14) = −5.97, p < 0.05, d = 2.87] and defeated males [t(15) = −5.14, p < 0.05, d = 2.56] compared to non-defeated females and males, respectively.
Figure 5.
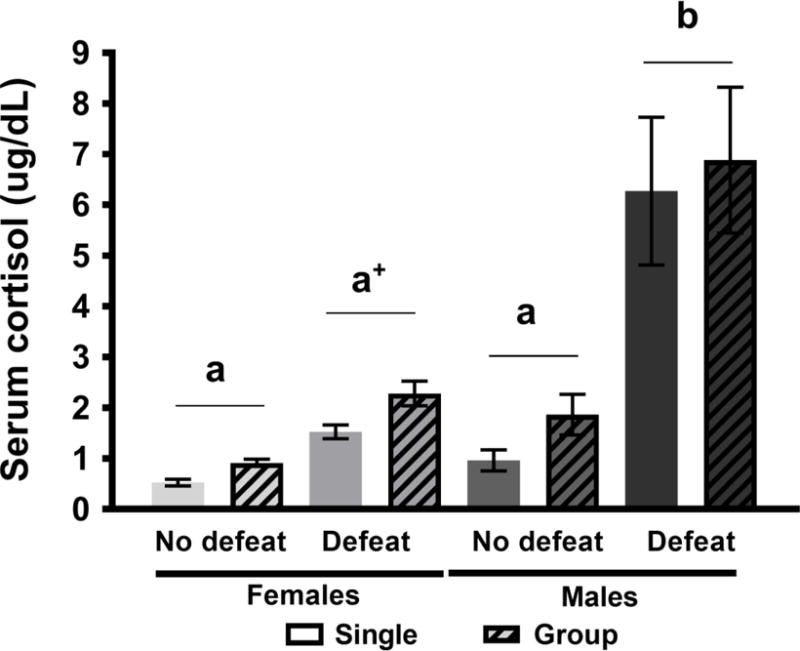
Serum cortisol (mean ± SE) of defeated or non-defeated male and female Syrian hamsters after 2.5 weeks of single or group housing in Experiment 2. ANOVA revealed that there was no significant effect of housing on serum cortisol in non-defeated or defeated hamsters (p > 0.05, η2 = 0.06). Tukeys posthoc comparisons indicated that cortisol was highest in defeated males compared to all other groups (p < 0.05, η2 = 0.37; unshared letters denote statistical significance). An a priori t-test also revealed higher cortisol in defeated compared to non-defeated females (+p < 0.05, d = 2.87).
4. Discussion
Perhaps the most surprising conclusion that can be drawn from the present data is that male and female Syrian hamsters adapt quite well to both stable social housing and social isolation. The socially housed hamsters did not incur any wounding or tissue damage, and the grouped cages were thus maintained stably for the duration of the study. There were, however, large effects of housing condition on body and thymus masses as well as adiposity between groups that may be relevant to the controversy over whether either social housing or social isolation is stressful to Syrian hamsters.
The most compelling evidence supporting the possibility that male and female hamsters tolerate both social housing and social isolation is the finding that there were no significant effects of the housing manipulation in either sex on circulating or fecal cortisol. Similarly, Chelini et al. (2011) found no effect of stable pair housing from weaning on fecal cortisol metabolites in adult female Syrian hamsters [21]. It is important to emphasize, however, that the absence of HPA activation was observed after four weeks of stable social housing or social isolation in Experiment 1 and in stable pair housing in the Chelini study. It is certainly possible, perhaps even likely given our past research on the effects of social stress on glucocorticoid release [8, 30, 31], that there were at least some animals, presumably the subordinates, within grouped cages that mounted significant hormonal stress responses during the initial formation of dominance hierarchies. Interestingly, when we reduced the duration of the initial housing manipulation in Experiment 2 to only two and a half weeks, we still did not observe differences in serum cortisol among groups, suggesting that the presumed habituation of the hormonal stress response to social stress had already occurred within this shortened time frame.
Conversely, the thymus gland measures suggest that social housing was at least somewhat stressful for hamsters. Thymus involution is a well-accepted hallmark of stress, and we obtained a modest reduction in thymus mass in group housed animals. This finding is consistent with previously reported reductions in thymus mass in hamsters repeatedly exposed to a predator odor [39] and in rats subjected to cat odor or chronic, unstable group housing [40]. Our results are obviously just a single measure obtained after four weeks of stable housing conditions, and additional research would be necessary to determine a time course for the effect of housing or the effect of changing group composition in grouped animals on the thymus gland.
It is less clear what the increase in body mass and adiposity observed in the current study means in terms of stress. The predominant interpretation in past studies has been that this increase results from social stress experienced in socially housed female hamsters [15, 16, 41]. We have also previously reported that male hamsters exposed to social defeat stress display increased body mass and adiposity compared to unstressed controls [37, 38]. Males that fight but become dominant also gain more weight than do unstressed controls but significantly less than do their subordinate opponents [38]. Dominant males, by contrast, do not exhibit significant increases in adiposity. Finally, the increase in body mass and adiposity observed in subordinate males is recapitulated by brief exposure to a footshock stressor, supporting the contention that fighting and losing is stressful. Given these data, it thus seems possible that the increased body mass and adiposity observed in the current study and in Gattermann et al. (2002) is driven by the subordinate hamsters in group housed cages, but this possibility was not assessed directly in the current study. It does appear clear, however, that the effect of social housing on body mass is greater in females; however, the current study also reveals that increases in fat mass occurs in socially housed hamsters of both sexes. Finally, the current results indicate that sustained cortisol release is not necessary to maintain the effects of housing on bioenergetic measures.
5. Conclusions
Overall, interpretation of these data suggests that even though Syrian hamsters may be naturally solitary animals, they are able to tolerate both social housing and social isolation in the laboratory. If group housing is used, however, we suggest that it is important that the groups be maintained stably (i.e., wherein hamsters are not added to or removed from groups) and that sufficient time is given for the animals to exhibit HPA axis habituation. Similarly, if hamsters are separated into individual cages after previous stable group housing during development, it has been suggested that animals may also experience some stress due to the change in housing [18] and thus should also be given adequate time to habituate. The current data indicate that this habituation occurs within 2.5 weeks. Finally, it is clear that both socially housed males and females gain more weight and/or fat than do their socially isolated conspecifics and this phenomenon also needs to be taken into consideration when designing experiments using Syrian hamsters.
Highlights.
Socially housed and socially isolated Syrian hamsters had similar cortisol levels. Socially housed hamsters displayed modest thymus gland involution.
Socially housed females weighed more than did any other group.
Socially housed females and males had increased adiposity.
Acknowledgments
We would like to thank Mary Karom for her assistance with this research and the GSU Department of Animal Resources for taking excellent care of the hamsters.
Funding
This work was supported by the National Institutes of Health [grant numbers R01MH062044 to KLH, R01MH110212 to HEA, and NS092545 to JCW]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao M, Zhang B, Liu J, Guo X, Li H, Wang T, et al. Generation of transgenic golden Syrian hamsters. Cell Res. 2014;24:380–2. doi: 10.1038/cr.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briand F, Treguier M, Andre A, Grillot D, Issandou M, Ouguerram K, et al. Liver X receptor activation promotes macrophage-to-feces reverse cholesterol transport in a dyslipidemic hamster model. J Lipid Res. 2010;51:763–70. doi: 10.1194/jlr.M001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naples M, Baker C, Lino M, Iqbal J, Hussain MM, Adeli K. Ezetimibe ameliorates intestinal chylomicron overproduction and improves glucose tolerance in a diet-induced hamster model of insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1043–52. doi: 10.1152/ajpgi.00250.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jove M, Ayala V, Ramirez-Nunez O, Serrano JC, Cassanye A, Arola L, et al. Lipidomic and metabolomic analyses reveal potential plasma biomarkers of early atheromatous plaque formation in hamsters. Cardiovasc Res. 2013;97:642–52. doi: 10.1093/cvr/cvs368. [DOI] [PubMed] [Google Scholar]
- 5.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62:373–81. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers HE, Yogev L, Todd RB, Goldman BD. Adrenal corticoids in hamsters: role in circadian timing. Am J Physiol. 1985;248:R434–8. doi: 10.1152/ajpregu.1985.248.4.R434. [DOI] [PubMed] [Google Scholar]
- 7.Ottenweller JE, Tapp WN, Pitman DL, Natelson BH. Adrenal, thyroid, and testicular hormone rhythms in male golden hamsters on long and short days. Am J Physiol. 1987;253:R321–8. doi: 10.1152/ajpregu.1987.253.2.R321. [DOI] [PubMed] [Google Scholar]
- 8.Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiology & behavior. 1990;47:949–56. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- 9.Solomon MB, Sakai RR, Woods SC, Foster MT. Differential effects of glucocorticoids on energy homeostasis in Syrian hamsters. Am J Physiol Endocrinol Metab. 2011;301:E307–16. doi: 10.1152/ajpendo.00009.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler WJ, Knigge KM. Adrenal cortical secretion by the golden hamster. Endocrinology. 1959;65:739–47. doi: 10.1210/endo-65-5-739. [DOI] [PubMed] [Google Scholar]
- 11.Nowack BM, Paradiso JL. Walker’s Mammals of the World. 4th. Baltimore: The Johns Hopkins Univeristy Press; 1983. [Google Scholar]
- 12.Murphy MR. Intraspecific sexual preferences of female hamsters. J Comp Physiol Psychol. 1977;91:1337–46. [Google Scholar]
- 13.Siegel HI. The hamster - reproduction and behavior. New York: Plenum; 1985. [Google Scholar]
- 14.Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, et al. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) Journal of Zoology. 2001;154:359–65. [Google Scholar]
- 15.Borer KT, Pryor A, Conn CA, Bonna R, Kielb M. Group housing accelerates growth and induces obesity in adult hamsters. Am J Physiol. 1988;255:R128–33. doi: 10.1152/ajpregu.1988.255.1.R128. [DOI] [PubMed] [Google Scholar]
- 16.Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiology & behavior. 1990;47:815–7. doi: 10.1016/0031-9384(90)90002-l. [DOI] [PubMed] [Google Scholar]
- 17.Gattermann R, Fritzsche P, Weinandy R, Neumann K. Comparative studies of body mass, body measurements and organ weights of wild-derived and laboratory golden hamsters (Mesocricetus auratus) Lab Anim. 2002;36:445–54. doi: 10.1258/002367702320389125. [DOI] [PubMed] [Google Scholar]
- 18.Shannonhouse JL, Fong LA, Clossen BL, Hairgrove RE, York DC, Walker BB, et al. Female-biased anorexia and anxiety in the Syrian hamster. Physiology & behavior. 2014;133:141–51. doi: 10.1016/j.physbeh.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–72. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terranova JI, Song Z, Larkin TE, 2nd, Hardcastle N, Norvelle A, Riaz A, et al. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U S A. 2016;113:13233–8. doi: 10.1073/pnas.1610446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chelini MO, Palme R, Otta E. Social stress and reproductive success in the female Syrian hamster: endocrine and behavioral correlates. Physiology & behavior. 2011;104:948–54. doi: 10.1016/j.physbeh.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Hormones and behavior. 2012;61:283–92. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Albers HE, Huhman KL, Meisel RL. Hormonal basis of social conflict and communication. Hormones, brain and behavior. 2002;1:393–433. [Google Scholar]
- 24.Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1962;21:246–51. [Google Scholar]
- 25.Mateo JM, Johnston RE. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc Biol Sci. 2000;267:695–700. doi: 10.1098/rspb.2000.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann KE, Huhman KL. The effect of escapable versus inescapable social defeat on conditioned defeat and social recognition in Syrian hamsters. Physiology & behavior. 2012;105:493–7. doi: 10.1016/j.physbeh.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai WS, Johnston RE. Individual recognition after fighting by golden hamsters: a new method. Physiology & behavior. 2002;76:225–39. doi: 10.1016/s0031-9384(02)00721-7. [DOI] [PubMed] [Google Scholar]
- 28.Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–57. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- 29.Gil M, Nguyen NT, McDonald M, Albers HE. Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. The European journal of neuroscience. 2013;38:2308–18. doi: 10.1111/ejn.12216. [DOI] [PubMed] [Google Scholar]
- 30.Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Hormones and behavior. 1991;25:206–16. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- 31.Huhman KL, Moore TO, Mougey EH, Meyerhoff JL. Hormonal responses to fighting in hamsters: separation of physical and psychological causes. Physiology & behavior. 1992;51:1083–6. doi: 10.1016/0031-9384(92)90097-l. [DOI] [PubMed] [Google Scholar]
- 32.Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- 33.Huhman KL. Social conflict models: can they inform us about human psychopathology? Hormones and behavior. 2006;50:640–6. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 35.Solomon MB. Evaluating social defeat as a model for psychopathology in adult female rodents. J Neurosci Res. 2017;95:763–76. doi: 10.1002/jnr.23971. [DOI] [PubMed] [Google Scholar]
- 36.Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–18. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 37.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–93. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 38.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–90. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JX, Cao C, Gao H, Yang ZS, Sun L, Zhang ZB, et al. Effects of weasel odor on behavior and physiology of two hamster species. Physiology & behavior. 2003;79:549–52. doi: 10.1016/s0031-9384(03)00123-9. [DOI] [PubMed] [Google Scholar]
- 40.Seetharaman S, Fleshner M, Park CR, Diamond DM. Influence of daily social stimulation on behavioral and physiological outcomes in an animal model of PTSD. Brain Behav. 2016;6:e00458. doi: 10.1002/brb3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fritzsche P, Riek M, Gattermann R. Effects of social stress on behavior and corpus luteum in female golden hamsters (Mesocricetus auratus) Physiology & behavior. 2000;68:625–30. doi: 10.1016/s0031-9384(99)00211-5. [DOI] [PubMed] [Google Scholar]


