Abstract
Purpose
To compare a novel multicoil compressed sensing technique with flexible temporal resolution, golden-angle radial sparse parallel (GRASP), to conventional fat-suppressed spoiled three-dimensional (3D) gradient-echo (volumetric interpolated breath-hold examination, VIBE) MRI in evaluating the conspicuity of benign and malignant breast lesions.
Materials and Methods
Between March and August 2015, 121 women (24–84 years; mean, 49.7 years) with 180 biopsy-proven benign and malignant lesions were imaged consecutively at 3.0 Tesla in a dynamic contrast-enhanced (DCE) MRI exam using sagittal T1-weighted fat-suppressed 3D VIBE in this Health Insurance Portability and Accountability Act-compliant, retrospective study. Subjects underwent MRI-guided breast biopsy (mean, 13 days [1–95 days]) using GRASP DCE-MRI, a fat-suppressed radial “stack-of-stars” 3D FLASH sequence with golden-angle ordering. Three readers independently evaluated breast lesions on both sequences. Statistical analysis included mixed models with generalized estimating equations, kappa-weighted coefficients and Fisher’s exact test.
Results
All lesions demonstrated good conspicuity on VIBE and GRASP sequences (4.28 ± 0.81 versus 3.65 ± 1.22), with no significant difference in lesion detection (P = 0.248). VIBE had slightly higher lesion conspicuity than GRASP for all lesions, with VIBE 12.6% (0.63/5.0) more conspicuous (P < 0.001). Masses and nonmass enhancement (NME) were more conspicuous on VIBE (P < 0.001), with a larger difference for NME (14.2% versus 9.4% more conspicuous). Malignant lesions were more conspicuous than benign lesions (P < 0.001) on both sequences.
Conclusion
GRASP DCE-MRI, a multicoil compressed sensing technique with high spatial resolution and flexible temporal resolution, has near-comparable performance to conventional VIBE imaging for breast lesion evaluation.
Dynamic contrast-enhanced (DCE) breast MRI requires an inherent tradeoff between spatial and temporal resolution, which usually results in loss of important temporal wash-in kinetic information.1 Conventional DCE sequences are, therefore, optimized for high spatial resolution but low temporal resolution based on the limitations of current DCE-MRI. As invasive carcinomas and high-grade ductal carcinoma in situ (DCIS) are more likely to demonstrate fast initial uptake of contrast immediately after contrast injection,1–4 sequences that can simultaneously optimize spatial and temporal resolution may have higher detection of these rapidly enhancing and potentially biologically significant lesions.5–7
A previously described DCE-MRI method known as golden-angle radial sparse parallel (GRASP) uses a combination of compressed sensing and parallel imaging to acquire simultaneous high spatial and temporal resolution.8–10 The GRASP technique exploits joint multicoil sparsity techniques to allow continuous acquisition of dynamic information before, during, and after contrast agent injection. During image reconstruction, data are sorted into sequential timeframes and then reconstructed using highly undersampled data in a multicoil compressed sensing iterative method.8–10 The same dataset can be grouped after acquisition into time frames at any desired temporal reconstruction, to a resolution of as low as 2.5 s per frame, resulting in highly flexible retrospective analysis that offers higher robustness to respiratory motion and flow
Previous use of GRASP DCE-MRI has resulted in high image quality for body, breast and head/neck imaging,8–14 with similar or slightly lesser lesion conspicuity noted in many of these.11,12,14 Kim et al evaluated breast image quality for benign and malignant lesions, demonstrating high image quality as assessed both qualitatively and quantitatively.9 In this study, semi-quantitative analysis of breast lesions demonstrated increased Ktrans values for malignant lesions at high temporal resolutions, suggesting that while general quantitative parameters (such as initial enhancement ratio) are preserved, some pharmacokinetic model-derived values may be less accurate in low temporal reconstructions.9 Breast GRASP DCE-MRI potentially offers both high spatial resolution and semi-quantitative analysis after minimal offline processing time.
GRASP thus shows promise as one of the few compressed sensing techniques reported in the literature for breast evaluation8,9,15–18; none of these have been used to clinically evaluate breast lesions. Although other high temporal and spatial resolution sequences (TWIST19,20, UF-MRI21 have been used in assessing breast lesions, GRASP has not yet been tested in clinical practice for evaluation of breast lesion conspicuity, a necessary precursor to incorporating it into diagnostic protocols. The purpose of our study was, therefore, to evaluate conspicuity between a fat-suppressed spoiled three-dimensional (3D) gradient-echo sequence (volumetric interpolated breath-hold examination, VIBE) and GRASP DCE-MRI for benign and malignant lesions.
Materials and Methods
Patients
This Health Insurance Portability and Accountability Actcompliant retrospective study was performed with approval from our Institutional Review Board and waived informed consent. One hundred twenty-one women (ages, 24–84 years; mean, 49.7 years) with 180 biopsy-proven benign and malignant lesions were consecutively imaged between March and August 2015. All women underwent initial diagnostic breast MRI on followed by MRI-guided breast biopsy using GRASP within approximately 30 days (average 13 days; range, 1–95 days; median 9 days) of the initial study. Final surgical excisional pathology with tumor markers was available on all malignant lesions. All benign lesions were either core needle biopsy-proven or benign at final surgical excision.
MRI Technique
Diagnostic MRI was performed in all patients on 3.0 Tesla (T) clinical systems (TimTrio, Siemens Medical Solutions, Erlangen, Germany) imaging both breasts using seven-channel breast coils. The routine breast protocol included the following sequences: sagittal T1-weighted gradient echo, sagittal T2-weighted, sagittal T1-weighted gradient echo fat suppressed VIBE pre- and four postcontrast acquisitions beginning at 90 s and ending at 7 min postinjection of a single dose of Gd-DTPA (Magnevist, Bayer Healthcare, Leverkusen, Germany) at 0.1 mM/kg body weight at a rate of 2mL/s. T1-weighted imaging parameters included: repetition time/echo time (TR/TE) = 4.01/1.52 ms, flip angle 12 °, slice thickness 1 mm, matrix 202 × 384, FOV 242 × 270 mm2, spatial resolution 1.2 × 0.7 × 1.0 mm, VIBE acquisition time 120 s. Subtraction images were automatically generated at the workstation.
Breast biopsy images were acquired of the targeted and compressed breast with a continuously acquired sagittal GRASP sequence before, during and after injection of a single dose of Gd-DTPA (Magnevist, Bayer Healthcare, Leverkusen, Germany) at 0.1 mM/kg body weight at a rate of 2 mL/s. GRASP imaging parameters included: TR/TE: 3.6/1.47 ms, flip angle 12 °, matrix 256 × 256, slice thickness 2 mm, FOV 280 × 280 mm2, bandwidth = 710 Hz/pixel, spatial resolution 1.1 × 1.1 × 2.0 mm. A total of 2280 spokes were acquired for each of the 35 partitions during free breathing to cover one breast planned for biopsy, with two-fold readout oversampling (512 sample points/spoke) to avoid aliasing. The reconstructed image matrix size per frame was 256 × 256 × 72. After baseline acquisition of 57 s (380 spokes), a single dose of Gd-DTPA (Magnevist, Bayer Healthcare, Leverkusen, Germany) at 0.1 mM/kg body weight was injected at 2 mL/s intravenously while the scan continued for another 4 min 45 s (1900 spokes). Total imaging time was approximately 5 min 50 s.9
Image Reconstruction
VIBE DCE-MRI was reconstructed in-line using standard image reconstruction methodology. GRASP images were reconstructed as previously described, using a radial version of the multicoil k-t SPARSE-SENSE method for radial k-space data.8–10 Coil sensitivity maps are first estimated from the multicoil static image that results from applying a nonuniform Fast Fourier Transform (NUFFT) to all acquired spokes. An undersampled image time series is the formed by grouping consecutive spokes into temporal frames. The final step in reconstruction is to run an iterative algorithm, which enforces joint multicoil sparsity constrained by data consistency. At each iteration, the reconstruction algorithm will enforce sparsity to the temporal difference between consecutive frames and make sure that is operation is consistent with the data acquisition model. Temporal differences are expected to be sparse because only the regions with contrast enhancement will show distinct intensities between consecutive frames and most of the pixels will have similar intensity. The sparsity enforcement operation is applied to the coil combined image, and thus the name of multicoil sparsity. This effectively exploits correlations between coils and thus improves performance. This process is initiated by the technologist at the workstation; images are processed offline by a custom-developed C + + program which reads the raw k-space data and sends DICOM images directly to our picture archive and communications system (PACS). High or low temporal resolution can be chosen at any time after acquisition using the standalone program. For this approach, 55 consecutive spokes were grouped into each dynamic frame, providing a total of 35 frames with temporal resolution of 8.3 s.9 These images are processed and available for the radiologists’ review within 20–25 min of acquisition. Higher temporal resolutions require 30–90 min reconstruction time.
Image Analysis
Three breast imagers (R1, 5 years; R2, 2 years; R3, 6 years) independently evaluated all lesions at the second postcontrast time point on VIBE and the 21st frame of GRASP images (i.e., both images for 180 s post injection) while blinded to lesion pathology. Readers rated VIBE or GRASP images in randomized order independently for each lesion, with at least 2 weeks separation between reads. When evaluating images, readers rated conspicuity on a 1–5 point scale (from 1 = poor to 5 = excellent), and shape, morphology, and internal enhancement for masses and nonmass enhancement (NME) per MRI Breast Imaging Reporting and Data System (BI-RADS) criteria,22 including BI-RADS assessment. As readers were aware all lesions underwent biopsy, they gave a BI-RADS assessment limited to BI-RADS Category 4: Suspicious or BIRADS Category 5: Highly suggestive of malignancy. BI-RADS 4 categories were divided further into 4A (>2% to ≤ 10% likelihood of malignancy), 4B (>10% to ≤ 50% likelihood of malignancy) and 4C (>50% to < 95% likelihood of malignancy.22 Due to the limitations of assessing temporal kinetic data during breast biopsy compression, kinetic curve washout data were reviewed retrospectively for lesions on VIBE images alone.
Statistical Analysis
To account for the complex correlation structure resulting from some patients having multiple lesions and all lesions being evaluated by three independent readers using both sequences, a mixed model approach was used to compare sequences or lesion types in terms of reader conspicuity scores. The model included sequence (GRASP, VIBE) and reader as fixed classification factors. Interaction terms were tested to assess whether the difference between lesion types in terms of conspicuity scores depended on the sequence; equivalently, whether the difference between sequences depended on the lesion type. Logistic regression for correlated data was used to compare sequences in terms of the percentage of lesions classified as having each level of the features of shape, margin, internal enhancement and BI-RADS assessment. Specifically, a generalized estimating equation (GEE) based on binary logistic regression was used to model the binary indicator of whether or not a lesion was assigned to each level of each feature as a function of reader and sequence. For the mixed model and GEE analyses, the correlation structure was modeled by incorporating indicator variables identifying the patient and lesion within patients associated with each score into the model as random classification factors.
As a result, model errors were assumed to be independent when associated with different patients and correlated when associated with the same patient with the correlation stronger among errors associated with the same lesion in patients with multiple lesions. All statistical tests were conducted at the two-sided 5% significance level using SAS 9.3 (SAS Institute, Cary, NC). When the analysis involved the pairwise comparison of multiple levels of a given feature, P values adjusted according to the Tukey HSD multiple correction procedure are also provided. Fisher’s exact test was used to assess binary outcomes per reader.
Simple and linear weighted kappa coefficients were used to assess reader agreement with respect to the binary measure (margin) and ordinal outcomes (conspicuity, BI-RADS), respectively. The percentage of times two readers provided concordant assessments for the same patient was provided as a measure of agreement for all measures. Kappa (κ) was interpreted as an indication of poor agreement when less than zero, as slight agreement when 0 ≤ κ ≤ 0.2, as fair agreement when 0.2 < κ ≤ 0.4, as moderate agreement when 0.4 < κ ≤ 0.6 and as substantial agreement when κ > 0.6.
Results
The average patient age was 49.7 years (range, 24–84 years). Fifty-four of 121 (45%) were postmenopausal, 5/121 (4%) were on continuous oral or intrauterine contraceptives, and 62/121 (51%) were premenopausal. Of the 62 premenopausal women, 19/62 (31%) had both the original diagnostic MRI and biopsy MRI at optimal menstrual cycle time (i.e., week 2), 15/62 (24%) had both outside of week 2, and 23/62 (37%) had one MRI exam outside of week 2. Five of 62 (8%) did not have a recorded last menstrual period for at least one exam. Patients underwent MRI for determining extent of disease (72/121; 60%), elevated risk (30/121; 25%), problem-solving (12/121; 10%), or follow-up of BI-RADS three lesion on prior MRI (7/121; 6%).
Ninety-one lesions were benign (91/180; 50.6%; Table 1) and 89/180 (49.4%) were malignant. The predominant malignant pathology was intermediate grade invasive ductal carcinoma (Table 2). The median BI-RADS assessment for both GRASP and VIBE lesions was 4B.
TABLE 1.
Benign Lesion Pathology (n = 91)
| Benign lesions (n = 91) | |
|---|---|
| ADHa | 2/91 (2.2%) |
| Benign changes | 10/91 (10.9%) |
| Lactational changes | 3/91 (3.3%) |
| Fibroadenoma/fibroadenomatous change | 16/91 (17.6%) |
| Fat necrosis | 4/91 (4.4%) |
| FCC | 51/91 (54.9%) |
| FEAa | 1/91 (1.1%) |
| Inflammatory changes | 1/91 (1.1%) |
| PASH | 2/91 (2.2%) |
| Sclerosing papillomaa | 1/91 (1.1%) |
No upgrade at final excisional pathology.
ADH = atypical ductal hyperplasia; FEA = flat epithelial atypia; PASH = pseudoangiomatous stromal hyperplasia.
TABLE 2.
Malignant Lesion Pathology by Pathology Type and Grade (n = 89)
| Type | N = 89 |
|---|---|
| Invasive carcinoma | |
| IDC | 46/89 (51.6%) |
| Low grade | 13/46 (28.2%) |
| Intermediate grade | 23/46 (50.0%) |
| High grade | 10/46 (21.7%) |
| ILC | 13/89 (14.6%) |
| Low grade | 0/13 (0.0%) |
| Intermediate grade | 12/13 (92.3%) |
| High grade | 1/13 (7.7%) |
| Papillary | 1/89 (1.1%) |
| Intermediate grade | 1/1 (100%) |
| Carcinoma in situ | |
| DCIS | 29/89 (32.6%) |
| Low grade | 2/29 (7%) |
| Intermediate grade | 7/29 (21.1%) |
| High grade | 20/29 (69%) |
IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma.
Readers found both GRASP and VIBE to have overall adequate lesion conspicuity, with reader 1 identifying all lesions on both sequences, reader 2 identifying all but one benign (fibrocystic change, FCC) and one malignant lesion (intermediate grade DCIS) on GRASP, and reader 3 identifying all but one benign lesion (FCC) on GRASP. This was not statistically significant between sequences (P = 0.24). One additional lesion (low grade DCIS) was not seen by reader 3 on either GRASP or VIBE. However, VIBE had slightly higher lesion conspicuity (4.28 ± 0.81) than GRASP (3.65 ± 1.22) for all lesions and all readers (Figs. 1 and 2), with lesions rated 12.6% (0.63/5.0) more conspicuous on average (P < 0.001) (Table 3). This association held true when evaluating benign lesions alone, malignant lesions alone, masses alone, and NME alone for each reader (P ≤ 0.023). Although both masses and NME were more conspicuous on VIBE than on GRASP (P < 0.001), this difference was more apparent for NME (14.2% more conspicuous versus 9.4% more conspicuous). Overall, masses were 16.8% more conspicuous than NME (P < 0.001) and malignant lesions were more conspicuous than benign lesions (P < 0.001) (Table 4).
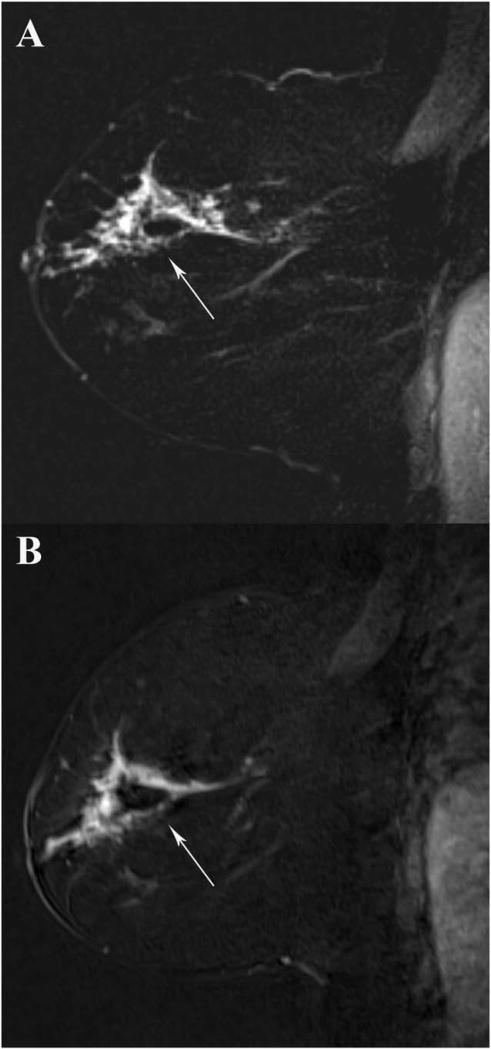
FIGURE 1.
A 52-year-old woman undergoing MRI for evaluation of known cancer. A 2.0cm segmental nonmass enhancement in the central breast corresponds to biopsy-proven invasive lobular carcinoma (arrow). All three readers rated this this lesion 5/5 on both GRASP (A) and VIBE (B) T1-weighted second postcontrast subtracted images.
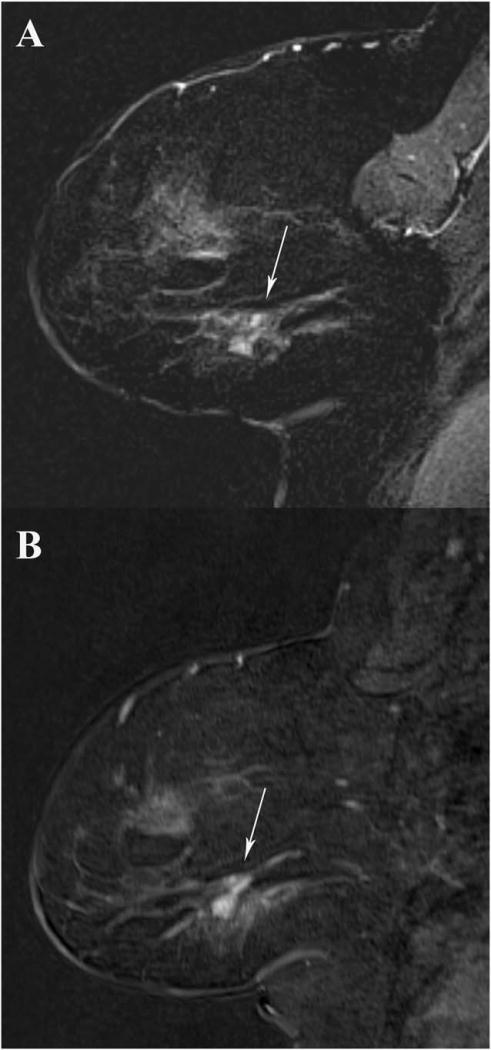
FIGURE 2.
A 53-year-old woman undergoing MRI for evaluation of known cancer. Subtle 1.0cm mass in the right breast (arrow) was biopsy-proven FCC. Reader 1 noted this mass was less conspicuous (3/5) on GRASP imaging compared with VIBE (5/5). Readers 2 and 3 rated the mass the same on GRASP (A) and VIBE (B) T1-weighted second postcontrast subtracted images.
TABLE 3.
Lesion Conspicuity Scores for Both GRASP and VIBE Sequences by Reader, Including Masses, NME, Benign and Malignant Lesions
| GRASP | VIBE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Lesion type | Reader | Mean | SD | Median | IQR | Mean | SD | Median | IQR | P-Value |
| All | 1 | 3.63 | 1.25 | 4 | 2 | 4.31 | 0.88 | 5 | 1 | <0.001 |
|
| ||||||||||
| All | 2 | 3.41 | 1.23 | 3 | 3 | 4.25 | 0.84 | 4 | 1 | <0.001 |
|
| ||||||||||
| All | 3 | 3.92 | 1.15 | 4 | 2 | 4.28 | 0.72 | 4 | 1 | <0.001 |
|
| ||||||||||
| Benign | 1 | 3.29 | 1.24 | 3 | 2 | 4.09 | 0.95 | 4 | 2 | <0.001 |
|
| ||||||||||
| Benign | 2 | 3.1 | 1.2 | 3 | 2 | 3.99 | 0.77 | 4 | 2 | <0.001 |
|
| ||||||||||
| Benign | 3 | 3.59 | 1.16 | 4 | 1 | 4.11 | 0.72 | 4 | 1 | <0.001 |
|
| ||||||||||
| Malignant | 1 | 3.98 | 1.16 | 4 | 2 | 4.54 | 0.74 | 5 | 1 | <0.001 |
|
| ||||||||||
| Malignant | 2 | 3.73 | 1.17 | 4 | 2 | 4.52 | 0.83 | 5 | 1 | <0.001 |
|
| ||||||||||
| Malignant | 3 | 4.25 | 1.04 | 5 | 1 | 4.46 | 0.68 | 5 | 1 | 0.023 |
|
| ||||||||||
| Mass | 1 | 3.99 | 1.28 | 5 | 2 | 4.52 | 0.83 | 5 | 1 | 0.003 |
|
| ||||||||||
| Mass | 2 | 4.1 | 0.97 | 4 | 2 | 4.62 | 0.61 | 5 | 1 | <0.001 |
|
| ||||||||||
| Mass | 3 | 4.34 | 0.94 | 5 | 1 | 4.54 | 0.57 | 5 | 1 | 0.007 |
|
| ||||||||||
| NME | 1 | 3.35 | 1.15 | 3 | 2 | 4.1 | 0.88 | 4 | 1.5 | <0.001 |
|
| ||||||||||
| NME | 2 | 2.96 | 1.15 | 3 | 2 | 3.86 | 0.87 | 4 | 1.75 | <0.001 |
|
| ||||||||||
| NME | 3 | 3.63 | 1.14 | 4 | 2 | 4.06 | 0.77 | 4 | 1 | <0.001 |
IQR = interquartile range.
TABLE 4.
Mean Conspicuity Scores for Malignant Compared to Benign Lesions, and Masses Compared to NME, Including Both GRASP and VIBE*
| Lesion type | Mean | SD | Median | IQR | P-Value |
|---|---|---|---|---|---|
| Mass | 4.25 | 1.00 | 5 | 1 | <0.001 |
| NME | 3.69 | 1.10 | 4 | 2 | |
| Malignant | 4.37 | 0.91 | 5 | 1 | <0.001 |
| Benign | 3.64 | 1.09 | 4 | 2 |
Values reported for all readers.
IQR = interquartile range.
As BI-RADS assessment increased from 4A to 5, there was a significant increase in overall lesion conspicuity scores (P < 0.001), VIBE conspicuity scores (P < 0.001) and GRASP conspicuity scores (P < 0.001). A similar relationship was seen with kinetic curve type as assessed on VIBE (from Type 1 to Type 3) and increasing lesion conspicuity (P < 0.015). For enhancement type, both masses and NME demonstrated increased lesion conspicuity for heterogeneously enhancing lesions compared with homogenously enhancing lesions (P < 0.001), but not for any other enhancement type (P = 0.106–0.805). There was no association between tumor grade and lesion conspicuity (P = 0.324–0.706).
Readers had fair agreement for margin (κ = 0.36), moderate agreement for BI-RADS (weighted κ = 0.44), and moderate agreement for conspicuity (κ = 0.41) analysis when looking at all sequences. When reader agreement was analyzed for each sequence separately, agreement was higher for GRASP than VIBE for BI-RADS (κ = 0.41 versus 0.38) and conspicuity (κ = 0.43 versus 0.32) but lower for GRASP for margin (κ = 0.34 versus 0.38).
Discussion
Although GRASP has been previously used to evaluate breast image quality,9 no prior studies have evaluated the use of this or any other compressed sensing technique in a clinical breast assessment. In this study, we compared GRASP lesion conspicuity as evaluated by experienced breast imagers to that of conventional VIBE imaging, and found no statistical difference in lesion detection, with a small but statistically significant difference in lesion conspicuity between the two sequences. This held true for both masses and NME, which may reflect the expected technical limitation of using compressed biopsy images to test GRASP.23 Benign lesions display a slower wash-in of contrast,2,4,7 which was reflected by an overall lower lesion conspicuity compared with malignant lesions. Although lesions were more conspicuous as both BI-RADS assessment and temporal kinetic curve increased, there was no association between tumor grade and conspicuity on either sequence.
Our results are similar to those seen in the evaluation by Chandarana et al of liver image quality and morphology using GRASP,11 which demonstrated lower image quality in the early arterial and portal venous phases and decreased liver morphology conspicuity compared with VIBE. In the study by Chandarana et al, image quality of GRASP images was nonetheless considered to be sufficient diagnostic quality for routine use, with overall decreased motion artifact compared with VIBE. In our study, readers similarly found a small but significant decrease in lesion conspicuity in breast GRASP images with no significant difference in lesion detection between GRASP and VIBE, suggesting that GRASP has sufficient image quality for diagnostic breast imaging. This is an important preliminary step before optimization of the reconstruction algorithm for GRASP and introduction of routine semi-quantitative analysis. The initial assessment by Kim et al of varying GRASP temporal resolutions and regularization factors demonstrated good to excellent breast image quality at the same parameters used in our study.9 Additional abdominal and head/neck studies12,14 have also demonstrated equal or higher image quality for GRASP compared with VIBE.
While we did not compare semi-quantitative kinetics due to the low temporal resolution of the VIBE sequence used and the effects of biopsy compression, other studies of high temporal resolution sequences have shown that adding semi-quantitative analysis to diagnostic breast MRI at the time of interpretation increases sensitivity and specificity.19,21,24 Prior high temporal resolution studies have shown that malignant lesions have early arterial enhancement that occurs before parenchymal enhancement and is not captured at standard temporal resolutions.21,25 Notably, Pineda et al demonstrated that initial slope was six times steeper for malignant lesions in the first 60 s postcontrast injection,21 Mann et al showed that high temporal resolution kinetic curves were more accurate than standard resolution temporal kinetic curves in distinguishing malignant from benign lesions,19 and Tudorica et al demonstrated that using a high temporal resolution Ktrans cutoff value had 91% specificity and 100% sensitivity in evaluating mammographically visible lesions.20 These prior studies suggest that use of high temporal and spatial resolution sequences will allow simultaneous morphologic and semi-quantitative analysis of breast lesions, potentially compensating for any minimal and possibly artifactual difference in lesion conspicuity.
There are several limitations to this study. Like Kim et al, we did not directly compare image quality between GRASP and VIBE sequences9 because lesion conspicuity is more relevant to clinical workflow. Our decreased lesion conspicuity using GRASP likely reflects the increased slice thickness used in our biopsy protocol (2 mm versus 1 mm in VIBE) and the use of compression during biopsy, which can reduce both size and conspicuity.23 A 1 mm slice thickness is technically feasible using GRASP and could be easily incorporated into a full GRASP diagnostic protocol in the future; a 2 mm slice thickness was used for biopsy images to adhere to American College of Radiology MRI-guided biopsy guidelines.26 An inherent limitation of our study was evaluating lesions at the same postcontrast imaging time point given the difference in technique. As VIBE acquires the center of k-space at the midpoint of the total acquisition time (180 s postinjection) but GRASP acquires the center of k-space in every radial spoke, we evaluated the higher temporal resolution GRASP images at the time point that corresponds to the VIBE acquisition midpoint.
The use of a compressed sensing technique also limited objective measurement of image quality, as both signal-to-noise and contrast-to-noise ratios can be arbitrarily changed by the regularization method used. Due to this and the lack of consensus on image quality measures for lesion conspicuity, we chose to evaluate quality subjectively using three experienced breast imagers.
A final limitation of our study was the inability to image patients same day with both GRASP and VIBE sequences. However, although breast lesions may be less conspicuous when imaged outside of week two of the menstrual cycle,27–29 the majority of our patients were either postmenopausal, on continuous hormonal contraceptives, or imaged within the optimal second week after the last menstrual period for both exams.
In conclusion, GRASP DCE-MRI, a multicoil compressed sensing technique, has lower lesion conspicuity than VIBE DCE-MRI imaging for characterization of breast lesions but no significant difference in lesion detection. Given the similar image quality between these two sequences demonstrated in prior studies, this minor difference likely reflects the limitations of the GRASP biopsy protocol rather than a true clinical disadvantage. This study is, therefore, an important step closer to incorporating GRASP into real-time clinical imaging. We anticipate that future use of GRASP DCE-MRI in clinical practice will allow for diagnostic quality high spatial resolution with flexible temporal resolution and semi-quantitative analysis.
References
- 1.Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology. 2005;236:789–800. doi: 10.1148/radiol.2363040811. [DOI] [PubMed] [Google Scholar]
- 2.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263:374–382. doi: 10.1148/radiol.12111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardanelli F, Rescinito G, Giordano GD, Calabrese M, Parodi RC. MR dynamic enhancement of breast lesions: high temporal resolution during the first-minute versus eight-minute study. J Comput Assist Tomogr. 2000;24:724–731. doi: 10.1097/00004728-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238:42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]
- 5.Buadu LD, Murakami J, Murayama S, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology. 1996;200:639–649. doi: 10.1148/radiology.200.3.8756909. [DOI] [PubMed] [Google Scholar]
- 6.Leong LC, Gombos EC, Jagadeesan J, Fook-Chong SM. MRI kinetics with volumetric analysis in correlation with hormonal receptor subtypes and histologic grade of invasive breast cancers. AJR Am J Roentgenol. 2015;204:W348–W356. doi: 10.2214/AJR.13.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo BK, Aspelin P, Kristoffersen Wiberg M, Tot T, Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol. 2003;13:2425–2435. doi: 10.1007/s00330-003-2000-y. [DOI] [PubMed] [Google Scholar]
- 8.Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72:707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SG, Feng L, Grimm R, et al. Influence of temporal regularization and radial undersampling factor on compressed sensing reconstruction in dynamic contrast enhanced MRI of the breast. J Magn Reson Imaging. 2016;43:261–269. doi: 10.1002/jmri.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64:767–776. doi: 10.1002/mrm.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013;48:10–16. doi: 10.1097/RLI.0b013e318271869c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenkrantz AB, Geppert C, Grimm R, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. J Magn Reson Imaging. 2015;41:1365–1373. doi: 10.1002/jmri.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi Espagnet MC, Bangiyev L, Haber M, et al. High-resolution DCE-MRI of the pituitary gland using radial k-space acquisition with compressed sensing reconstruction. AJNR Am J Neuroradiol. 2015;36:1444–1449. doi: 10.3174/ajnr.A4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Raz E, Block TK, et al. Contrast-enhanced radial 3D fat-suppressed T1-weighted gradient-recalled echo sequence versus conventional fat-suppressed contrast-enhanced T1-weighted studies of the head and neck. AJR Am J Roentgenol. 2014;203:883–889. doi: 10.2214/AJR.13.11729. [DOI] [PubMed] [Google Scholar]
- 15.Chan RW, Ramsay EA, Cheung EY, Plewes DB. The influence of radial undersampling schemes on compressed sensing reconstruction in breast MRI. Magn RESON med. 2012;67:363–377. doi: 10.1002/mrm.23008. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves BA, Saranathan M, Sung K, Daniel BL. Accelerated breast MRI with compressed sensing. Eur J Radiol. 2012;81(Suppl 1):S54–S55. doi: 10.1016/S0720-048X(12)70020-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith DS, Welch EB, Li X, et al. Quantitative effects of using compressed sensing in dynamic contrast enhanced MRI. Phys Med Biol. 2011;56:4933–4946. doi: 10.1088/0031-9155/56/15/018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HY, Miao YW, Zhou K, et al. Feasibility of high temporal resolution breast DCE-MRI using compressed sensing theory. Med Phys. 2010;37:4971–4981. doi: 10.1118/1.3483094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49:579–585. doi: 10.1097/RLI.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 20.Tudorica LA, Oh KY, Roy N, et al. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn Reson Imaging. 2012;30:1257–1267. doi: 10.1016/j.mri.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pineda FD, Medved M, Wang S, et al. Ultrafast bilateral DCE-MRI of the breast with conventional fourier sampling: preliminary evaluation of semi-quantitative analysis. Acad Radiol. 2016;23:1137–1144. doi: 10.1016/j.acra.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris EA, Comstock C, Lee CH. ACR BI-RADS S® atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. ACR BI-RADS® Magnetic resonance imaging. [Google Scholar]
- 23.El Khouli RH, Macura KJ, Kamel IR, Bluemke DA, Jacobs MA. The effects of applying breast compression in dynamic contrast material-enhanced MR imaging. Radiology. 2014;272:79–90. doi: 10.1148/radiol.14131384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schabel MC, Morrell GR, Oh KY, Walczak CA, Barlow RB, Neumayer LA. Pharmacokinetic mapping for lesion classification in dynamic breast MRI. J Magn Reson Imaging. 2010;31:1371–1378. doi: 10.1002/jmri.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced MRI: a sensitivity and specificity study. Magn Reson Med. 2008;59:747–754. doi: 10.1002/mrm.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moy L, Newell M, Barke L, et al. ACR practice parameter for the performance of magnetic resonance imaging-guided breast interventional procedures. American College of Radiology; 2016. [Accessed October 3, 2016]. Available at: http://www.acr.org/~/media/FFD2D1CA57ED479DBB6DD0DA0D9E3A87.pdf. [Google Scholar]
- 27.Kang SS, Ko EY, Han BK, Shin JH, Hahn SY, Ko ES. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. J Magn Reson Imaging. 2014;39:526–534. doi: 10.1002/jmri.24185. [DOI] [PubMed] [Google Scholar]
- 28.Muller-Schimpfle M, Ohmenhauser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145–149. doi: 10.1148/radiology.203.1.9122383. [DOI] [PubMed] [Google Scholar]
- 29.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–144. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]