Abstract
Background
The adverse effects of alcohol on brain function result, in part, from inflammatory processes. The sex-specific neuropsychiatric consequences and inflammatory status of active alcohol dependence and early remission from dependence have not been investigated.
Methods
Neuropsychiatric symptoms, inflammatory factors, and liver enzymes were compared in a prospective cohort study of adults with (n = 51) or without (n = 31) a current or recent history of alcohol dependence.
Results
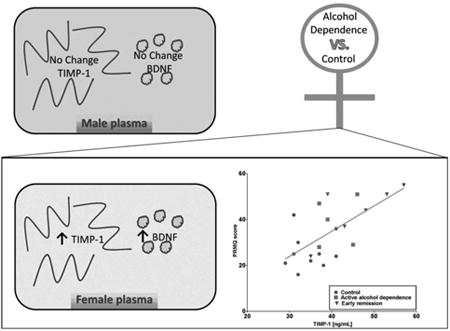
Neuropsychiatric profiles were similar in adults with current or recent alcohol dependence regardless of sex. In male and female participants measures of depression (female p < 0.05, male p < 0.001), anxiety (female p < 0.001, male p < 0.001), and memory complaints (female p < 0.001, male p < 0.05) were elevated, relative to non-dependent controls. Significant sex × alcohol dependence history interactions were observed for plasma levels of tissue inhibitor of metalloproteinase 1 (TIMP-1) and brain derived neurotrophic factor (BDNF), with women in the alcohol dependent group exhibiting increased levels of both analytes (p < 0.05) relative to controls. Positive correlations between TIMP-1 levels and measures of depression (r2 = 0.35, p < 0.01), anxiety (r2 = 0.24, p < 0.05) and memory complaints (r2 = 0.44, p < 0.01) were found in female, but not male, participants.
Conclusions
Though neuropsychiatric profiles were similar for men and women with current or recent alcohol dependence, plasma factors associated with increases in depression, anxiety, and memory impairment differed and support the need to tailor treatments based on sex.
Keywords: Alcohol use disorder, sex differences, anxiety, depression, cognitive impairment, inflammation
Graphical abstract
1. Introduction
Sexual dimorphism is observed in many pathological processes including brain diseases and responses to a variety of drugs, including alcohol. For instance, women are more vulnerable to alcohol-induced organ damage in the periphery, with higher rates or greater severity of cardiomyopathy, peripheral neuropathy, some types of cancer and liver cirrhosis (Ammendola et al., 2000; Fernandez-Sola and Nicolas-Arfelis, 2002; Kovacs and Messingham, 2002; Praud et al., 2016). Due to differences in body mass composition, as well as other still unknown factors, women are more susceptible to the harmful effects of alcohol than men, as reflected by the lower alcohol consumption recommendations for women vs. men provided by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Sex differences in alcohol-induced brain damage and cognitive impairment have rarely been addressed in clinical studies, and a consensus of whether increased damage or dysfunction is observed in women is lacking (reviewed in Nixon et al., 2014). In general, studies suggest that women exhibit brain damage with less total alcohol consumption, or more damage with similar levels of intake than men, though not all studies have observed this phenomenon (Hommer, 2003). Women may recover from alcohol-induced white matter damage more quickly than men (Ruiz et al., 2013). Sex differences have also been observed in the relationship between circulating inflammatory factors such as C-reactive protein and the quantity of alcohol consumed (Oliveira et al., 2010). Mortality, the most devastating consequence of alcohol abuse, is higher in women who have a greater-than two-fold increased risk of premature death than men with alcohol dependence (John et al., 2013), a finding that has been confirmed by systematic meta-analysis of mortality risks in alcohol use disorders (Roerecke and Rehm, 2013). Despite accumulating evidence, little effort has been extended to identify the mechanisms underlying sex-differences in alcohol responses.
About half of the nearly 20 million people with an alcohol use disorder in the United States develop neuropsychiatric symptoms that adversely impact addiction treatment outcomes. The course of alcohol use disorders appears to vary between the sexes, with women showing higher levels of alcohol craving associated with nicotine use (Hitschfeld et al., 2015) and increased vulnerability to alcohol relapse (Abulseoud et al., 2013). Further, women exhibit stronger associations between alcohol abuse and depression or anxiety than men (King et al., 2003), and women with depression report engaging in heavy episodic alcohol drinking more frequently than women without depression (Pedrelli et al., 2016).
A strong link between depression and inflammatory signaling has been established, and mounting evidence suggests that inflammation may play a similar role in anxiety and memory dysfunction (Huckans et al., 2015b; Vogelzangs et al., 2013). Women experience higher rates of depression than men (reviewed in Albert, 2015), and inflammation appears to be a key contributor to depression in women (reviewed in Derry et al., 2015). Chronic alcohol consumption also induces inflammatory responses that contribute to the drug's adverse neuropsychiatric effects. For example, neuroinflammation is evident in the brains of adults with a history of alcohol abuse, with increased activation of microglia and elevated expression of central and peripheral inflammatory factors (Achur et al., 2010; He and Crews, 2008). Studies using animal models of alcohol abuse observe greater inflammation following alcohol exposure in females than males both in the brain (Alfonso-Loeches et al., 2013; Wilhelm et al., 2014) and in the periphery (Fulham and Mandrekar, 2016). Thus, we hypothesize that men and women with alcohol dependence may exhibit differences in the symptom severity or inflammatory factors associated with neuropsychiatric impairments.
In this study, self-report measures of depression, anxiety, and memory complaints were used to explore associations among neuropsychiatric symptoms and a panel of immune factors to identify potential sex-specific effects of alcohol dependence in adults. We focused on cytokines and chemokines commonly associated with inflammation-related depression including interferon-γ (IFN- γ) and interleukin-6 (IL-6) (Dahl et al., 2016; Huckans et al., 2015a), and emerging factors that we and others have recently found to contribute to mood and cognitive impairments including matrix metalloproteinase-3 (MMP-3), tissue inhibitor of metalloproteinases-1 (TIMP-1), and brain derived neurotrophic factor (BDNF) (Hoyo-Becerra et al., 2013; Hoyo-Becerra et al., 2015; Huckans et al., 2015b; Rybakowski et al., 2013; Zhang et al., 2016). A hypothesis of this study was that relationships between inflammatory markers and neuropsychiatric symptoms may be sex-specific, thus associations were examined within each sex and not across the entire sample population. Our results identify distinct plasma profiles linked with alcohol dependence and neuropsychiatric impairment in men and women. These findings emphasize the need to develop sex-specific treatments for alcohol and other substance use disorders, particularly interventions (e.g., immunotherapies) targeting neuropsychiatric symptoms that hinder early recovery efforts.
2. Material and methods
2.1. Participants
Participants were categorized into the following study groups: 1) control group (n = 31; n = 10 female; n = 21 male): adults with no lifetime history of dependence on any substance other than nicotine or caffeine and 2) alcohol dependent group (n = 51): adults actively using alcohol and currently meeting criteria for alcohol dependence (n = 21; n = 5 female; n = 16 male) and adults in early remission from alcohol dependence (defined as ≥ 1 month and ≤ 9 months) (n = 30; n = 6 female; n = 24 male). Research participants in the early remission group were recruited from Portland, Oregon area addiction treatment centers. Participants who were active users or controls were recruited from the Portland, Oregon community through word of mouth and via study advertisements posted in clinics, websites, and newspapers.
General exclusion criteria included history of a major medical illness or current use of medications that are likely to be associated with serious neurological or immune dysfunction [e.g., stroke, traumatic brain injury, human immunodeficiency virus (HIV) infection, hepatitis C virus (HCV) infection, primary psychotic disorder, immunosuppressants, antivirals, or antitumor necrosis factor (TNF) agents]. Additional exclusion criteria for the control group included: 1) meets criteria for lifetime history of dependence on any substance (other than nicotine or caffeine dependence) based on Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) (American Psychiatric Association, 2000) and confirmed by the Mini International Neuropsychiatric Interview questionnaire (MINI) (Sheehan et al., 1998), 2) for men, average recent alcohol use > 6 standard drinks/day for ≥ 1 year, and 3) for women, average recent alcohol use > 4 standard drinks/day for ≥ 1 year, and 4) a positive test on a urine drug analysis for any drug of abuse on the day of the study. The urine drug analysis tested for the following drugs: amphetamine, tetrahydrocannabinol/cannabis, opiates, cocaine, and methamphetamine. Additional inclusion criteria for the alcohol dependent groups included: 1) meets DSM-IV (American Psychiatric Association, 2000) criteria for current or recent dependence on alcohol, confirmed by the MINI (Sheehan et al., 1998), 2) for men, average use > 6 standard drinks/day for ≥ 1 year during active dependence, and 3) for women, average use > 4 standard drinks/day for ≥ 1 year during active dependence. Participants in the alcohol dependent group did not test positive on a urine drug analysis for any drug of abuse on the day of the study. Other reported drug usage by control and alcohol groups is reported in Supplemental Table 1 and indicates that use of other drugs (with the exception of nicotine and alcohol) was not recent.
2.2. Ethical approval
The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the Institutional Review Boards at the VA Portland Health Care System and Oregon Health & Science University. Research participants gave informed consent after the procedures of the study were explained in full.
2.3. Procedures
Participants were compensated with grocery store vouchers ($50) to complete the following procedures: clinical interview, urine drug analysis, HCV and HIV antibody screening, blood sample collection for immune factor analysis and liver function measurements, and questionnaires to assess depression, anxiety, and memory. All procedures were carried out by a certified phlebotomist/retired licensed practical nurse that was trained and supervised by a licensed psychologist and clinical neuropsychologist (MH). All measures were entered into a database and double-checked by separate study personnel prior to analysis.
2.3.1. Questionnaires
1) Patient Health Questionaire-9 (PHQ-9) (Kroenke et al., 2001), a nine-item measure of depression severity. 2) Generalized Anxiety Disorder-7 Scale (GAD-7) (Spitzer et al., 2006), a seven-item measure of anxiety. 3) Prospective-Retrospective Memory Questionnaire (PRMQ) (Smith et al., 2000), a 16-item measure of self-reported memory complaints.
2.4. Multiplex assessment of inflammatory factors
Following all other study procedures, blood was drawn in the afternoon (mean time was 1:28 PM, SD = 2:35 hours) by one-time venipuncture into cell preparation tubes (BD Vacutainer Systems, Franklin Lakes, NJ, USA) containing 1 mL of 0.1M sodium citrate solution. The blood was then centrifuged at 1500 × g for 20 minutes at room temperature (22-25° C). Plasma was separated, collected and immediately aliquoted into polypropylene tubes (Phenix Research Products, Hayward, CA, USA) and frozen at -80° C until assayed. Plasma levels of IFN-γ, IL-6, TIMP-1, MMP-3, and BDNF were determined by Myriad Rules Based Medicine, Inc., a CLIA certified laboratory, using a Human Inflammation Multi-Analyte Profile (v 1.0) panel designed to discern inflammatory patterns in biological samples (Austin, TX, USA). Levels of IFN-γ (lowest detected concentration = 1.3 pg/ml) and IL-6 (lowest detected concentration = 8.1 pg/ml) were below detection limits for the majority of participants in all groups and therefore were not further analyzed.
2.5. Liver function tests
Whole blood was collected in BD Vacutainer tubes with lithium heparin (Fisher Scientific, Pittsburgh, PA, USA). Liver function tests were conducted by the VA Portland Health Care System Phlebotomy Lab per standard operating procedures.
2.6. Statistical analysis
Data analyses and graphs were carried out or created using Prism 6.05 (GraphPad Software, Inc., La Jolla, CA, USA) and IBM SPSS Statistics (IBM Corporation, Armonk, NY, USA). Given the limited number of women in the alcohol dependent groups (n = 5 with current alcohol dependence; n = 6 in early remission from alcohol dependence), participants with active alcohol dependence and those in early remission from dependence were combined into a single group for statistical analyses. Analyses were conducted using analysis of variance (ANOVA) and analysis of covariance (ANCOVA) to compare across groups. Data were examined for normality and transformed as needed to conform to the assumptions of ANOVA. Last alcohol use was natural log transformed prior to analysis. If normality could not be achieved, the Kruskal-Wallis non-parametric test was employed. Following significant main effects or interactions, post hoc tests were carried out with Sidak corrections for multiple comparisons. Statistical significance was maintained at p < 0.05. Correlations were carried out using Pearson's correlation as the data conformed to assumptions of normality. Outliers were identified using Prism analysis, with Q = 1% (Motulsky and Brown, 2006) (n = 2 data points excluded, 1 PRMQ score from the male alcohol group and 1 plasma MMP-3 value from the male alcohol group). Fisher's exact test of independence was carried out (http://www.quantpsy.org/fisher/fisher.htm) to determine whether groups differed in race, or nicotine status. Data are presented as mean ± standard deviation (S.D.) unless otherwise indicated.
3. Results
3.1. Sample characteristics
Two-way ANOVAs with sex (male vs. female) and study group (control vs. alcohol) as factors indicated that participants did not differ in body mass index (BMI) and women did not differ in years of education (Table 1). Men in the alcohol group had fewer years of education than men in the control group (p < 0.05). Women were younger overall than men (ANOVA: F1,78 = 4.87, p < 0.05; women: 32.3 ± 2.3 years old vs. men: 38.0 ± 12 years old) with no differences between control and alcohol groups. Women in the alcohol remission group had last consumed alcohol 91 ± 83 days ago compared with 209 ± 573 days ago for the control group. Men in the alcohol remission group had last consumed alcohol 99 ± 66 days ago compared with 83 ± 245 days ago for men in the control group. In a two-way ANOVA with sex and alcohol group as factors, there were no effects of sex, alcohol, or sex × alcohol interaction comparing the days since last alcohol between the control and alcohol remission groups. Women in the alcohol group averaged 15 ± 4 drinks per day during alcohol dependence, with men averaging 18 ± 10 drinks per day. There was no significant difference in drinks per day between men and women in the alcohol groups. Women in the alcohol group averaged 14 ± 9 years of dependence, while men averaged 18 ±11 years of dependence, with a t-test indicating no significant difference between the sexes in length of dependence.
Table 1. Group demographics, clinical characteristics, and neuropsychiatric measures.
| Female | Male | ||||||
|---|---|---|---|---|---|---|---|
| Demographics | Control (n = 10) | Alcohol (n =11) | Control vs. Alcohol | Control (n = 21) | Alcohol (n = 40) | Control vs. Alcohol | Male vs. Female |
| Age | 29.7 (10.3) | 34.7 (10.6) | p = 0.28 | 41.3 (13.9) | 36.2 (10.6) | p = 0.12 | p < 0.05 |
| White, % | 80% | 82% | 81% | 70% | |||
| White, n | 8 | 9 | a | 17 | 28 | a | a |
| Nonwhite, n | 2 | 2 | p = 1.00 | 4 | 11 | p = 0.54 | p = 0.77 |
| Ethnicity, n | |||||||
| Black | 0 | 2 | 1 | 4 | |||
| Native American | 1 | 0 | 2 | 2 | |||
| Bi-racial | 1 | 0 | 1 | 5 | |||
| Years of education | 14.4 (1.5) | 13.7 (2.3) | p = 0.45 | 14 (1.4) | 13.1 (1.5) | p < 0.05 | p = 0.21 |
| Clinical characteristics | |||||||
| Body mass index | 30.5 (7.5) | 27.2 (4.1) | p = 0.22 | 28.3 (4.9) | 28.1 (4.2) | p = 0.88 | p = 0.61 |
| Self-reported current or past nicotine dependence, % | 60% | 91% | p = 0.15a | 57% | 93% | p < 0.01a | p = 0.76a |
| Years of nicotine use | 8 (14) | 13 (7) | p = 0.37 | 7 (11) | 19 (13) | p < 0.01 | p = 0.64 |
| Cigarettes per day | 4 (6) | 8 (3) | p < 0.05 | 5 (13) | 13 (10) | p < 0.05 | |
| Last alcohol use, remission groups only, d | 209 (573) | 91 (83) | p = 0.63 | 83 (245) | 99 (66) | p = 0.76 | p = 0.66 |
| Alcohol drinks per day | 15 (4) | 18 (10) | p = 0.09 | ||||
| Neuropsychiatric measures | |||||||
| Depression (PHQ-9) | 2.5 (2.6) | 9.0 (2.9) | p < 0.01 | 2.7 (3.4) | 8.8 (5.2) | p < 0.01 | p = 0.83 |
| Anxiety (GAD-7) | 2.3 (2.6) | 12.4 (1.7) | p < 0.001 | 2.5 (3.5) | 10.4 (7.1) | p < 0.001 | p = 0.23 |
| Memory Complaints (PRMQ) | 26.1 (7.9) | 39.0 (10.4) | p < 0.05 | 30.1 (7.3) | 40.2 (13.7) | p < 0.05 | p = 0.89 |
Fisher's exact tests were conducted for statistical comparison. For Fisher's exact test comparison of males vs. females, data were collapsed across treatment group. Other analyses were carried out using ANOVA with sex and alcohol group as factors. No significant interactions were observed in two-way ANOVAs. Significant comparisons are highlighted in bold. Data are presented as mean (SD), unless otherwise defined. Abbreviations: d = day, PHQ-9 = Patient Health Questionnaire, GAD-7 = Generalized Anxiety Disorder 7-item, PRMQ = prospective and retrospective memory questionnaire
For nicotine use, women in the alcohol group averaged 13 ± 7 years of use and 8 ± 3 cigarettes per day compared with 8 ± 14 years of use and 4 ± 6 cigarettes per day for women in the control group. Men in the alcohol group averaged 19 ± 13 years of nicotine use and 13 ± 10 cigarettes per day compared with 7 ± 11 years of use and 5 ± 7 cigarettes per day for men in the control group. ANOVA indicated that the alcohol group had a longer history of nicotine use than the control group (ANOVA: F1,78 = 5.25, p < 0.05), with no other significant effects or interactions. The data for the number of cigarettes per day were non-normal and could not be normalized via transformation, therefore, independent samples Kruskal-Wallis tests were used and indicated no difference in the number of cigarettes per day by sex, but that participants in the alcohol group smoked more cigarettes per day than those in the control group (p < 0.001).
Fisher's exact test found a greater prevalence of nicotine usage in the alcohol group relative to the control group for men with a similar trend (non-significant) observed in women.
3.2. Neuropsychiatric symptoms
Individuals in the alcohol dependent group reported greater neuropsychiatric symptom severity compared to the control group, as evidenced by higher scores on measures of depression (PHQ-9: F1,78 = 19.7, p < 0.001), anxiety (GAD-7: F1,78 = 27.3, p < 0.001), and memory complaints (PRMQ: F1,77 = 22.4, p < 0.001). Post hoc tests indicated that female and male alcohol dependent groups had increased scores on all neuropsychiatric measures relative to their same-sex control groups (Table 1). Scores on the PHQ-9 and GAD-7 by participants in the alcohol dependent groups were also elevated in comparison to normative scores for women (PHQ-9: 3.1 ± 3.5; GAD-7: 3.2 ± 3.5) and men (PHQ-9: 2.7 ± 3.5; GAD-7: 2.7 ± 3.2) (Kocalevent et al., 2013; Lowe et al., 2008). Sex specific normative scores for the PRMQ were not available (Crawford et al., 2003). The presence of co-morbid mood disorders was also assessed (Table 2). Women in the alcohol group exhibited increased prevalence of major depressive disorder (MDD), panic disorder, and GAD relative to the same-sex control group. Men in the alcohol group exhibited increased prevalence of agoraphobia and GAD relative to their same-sex control group.
Table 2. Comparison of mental health diagnoses for female and male participants.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Control (n = 10) | Alcohol (n = 11) | Control vs. Alcohol | Control (n = 21) | Alcohol (n = 40) | Control vs. Alcohol | |
| MDD, n (%) | 1 (10%) | 9 (82%) | p < 0.01 | 4 (19%) | 11 (28%) | p = 0.55 |
| Bipolar disorder, n (%) | 0 (0%) | 2 (18%) | p = 0.48 | 0 (0%) | 5 (13%) | p = 0.15 |
| Mood disorder (other), n (%) | 0 (0%) | 0 (0%) | p = 1.00 | 0 (0%) | 0 (0%) | p = 1.00 |
| PTSD, n (%) | 2 (20%) | 5 (46%) | p = 0.36 | 2 (10%) | 7 (18%) | p = 0.48 |
| Panic disorder, n (%) | 0 (0%) | 5 (46%) | p < 0.05 | 1 (5%) | 4 (10%) | p = 0.65 |
| Agoraphobia, n (%) | 1 (10%) | 5 (46%) | p = 0.15 | 0 (0%) | 11 (28%) | p < 0.05 |
| Social phobia, n (%) | 0 (0%) | 3 (27%) | p = 0.24 | 0 (0%) | 4 (10%) | p = 0.29 |
| GAD, n (%) | 0 (0%) | 5 (46%) | p < 0.05 | 1 (5%) | 12 (30%) | p < 0.05 |
| Anxiety disorder (other), n (%) | 0 (0%) | 1 (9%) | p = 1.00 | 0 (0%) | 1 (3%) | p = 1.00 |
Fisher's exact tests were used to compare rates of diagnoses between: i) female control and alcohol groups, and ii) male control and alcohol groups. The p-values for these comparisons are reported in the Control vs. Alcohol columns. Statistically significant comparisons are highlighted in bold. Abbreviations: MDD = major depressive disorder, PTSD = post-traumatic stress disorder, GAD = generalized anxiety disorder
3.3. Plasma inflammatory factors
Significant sex effects were observed for plasma MMP-3 levels (ANOVA: F1,77 = 10.96, p < 0.01), with women having lower plasma concentrations relative to men (Fig. 1A; Table 3). MMP-3 levels increase with age (Komosinska-Vassev et al., 2011), so with age included as a covariate, women still had significantly lower plasma concentrations of MMP-3 relative to men (ANCOVA: F1,76 = 10.74, p < 0.01). A main effect of study group (ANOVA: F1,78 = 4.79, p < 0.05) and a significant sex × study group interaction (ANOVA: F1,78 = 7.31, p < 0.01) was observed for plasma levels of TIMP-1 (Fig. 1B). Inclusion of age as a covariate did not change the results (main effect of study group: F1,77 = 5.02, p < 0.05; sex × study group interaction: F1,77 = 5.34, p < 0.05). The main effect of group was driven by a significant increase in plasma TIMP-1 in the female alcohol dependent group compared to the female control group, with no such effect observed in the male alcohol dependent group. A similar pattern was observed for plasma levels of BDNF (Fig. 1C) with a main effect of study group (ANOVA: F1,78 = 4.36, p < 0.05) and a sex × study group interaction (ANOVA: F1,78 = 4.57, p < 0.05). When age was included as a covariate, the main effect of study group remained (ANCOVA: F1,77 = 4.38, p < 0.05); however, the sex × study group interaction became marginal (ANCOVA: F1,77 = 3.54, p = 0.06). As with TIMP-1, post hoc comparisons indicated that the effect of study group was driven by a significant increase in plasma BDNF in women, but not in men, with current or recent alcohol dependence.
Fig. 1.
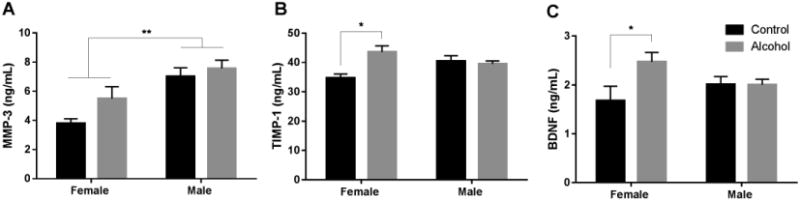
Alcohol dependence is associated with increased plasma TIMP-1 and BDNF in women. Two-way ANOVAs, followed by post hoc tests as appropriate, were conducted comparing plasma levels of MMP-3, TIMP-1, and BDNF in control and alcohol dependent groups. Female participants had significantly lower levels of MMP-3 than male participants, regardless of their alcohol use disorder history (A). Female and male participants had similar levels of TIMP-1 and BDNF, but compared to their same-sex control group, women with a current or recent history of alcohol dependence had increased plasma levels of TIMP-1 (B) and BDNF (C). *p < 0.05, **p < 0.01. Graphs depict mean ± standard error of the mean (S.E.M)
Table 3. Plasma inflammatory factors and liver function tests by participant sex and study group.
| Female | Male | |||
|---|---|---|---|---|
| Analyte (units; normal range) | Control (n = 10) | Alcohol (n = 11) | Control (n = 21) | Alcohol (n = 40) |
| Plasma inflammatory factors | ||||
| MMP-3** (ng/mL) | 4.4 (1.9) | 5.5 (2.7) | 7.0 (2.7) | 7.6 (3.6) |
| TIMP-1 (ng/mL) | 34.7 (4.3)# | 43.5 (7.1) | 40.4 (8.7) | 39.5 (6.6) |
| BDNF (ng/mL) | 1.7 (0.9)# | 2.47 (0.64) | 2.01 (0.72) | 2.00 (0.71) |
| Liver factors | ||||
| Aspartate aminotransferase (AST)*,$ (U/L; 8 – 48 U/L) | 16 (3) | 23 (6) | 29 (13) | 29 (24) |
| Alanine aminotransferase (ALT)*,$ (U/L; 7 – 55 U/L) | 16 (5) | 26 (14) | 39 (28) | 31 (23) |
| Alkaline phosphatase (ALP) (U/L; 45 – 115 U/L) | 78 (28) | 73 (14) | 68 (17) | 76 (28) |
| Total bilirubin* (mg/dL; 0.1 – 1.2 mg/dL) | 0.3 (0.1) | 0.3 (0.1) | 0.4 (0.2) | 0.4 (0.3) |
| Protein (g/dL; 6.3 – 7.9 g/dL) | 7.4 (0.6) | 7.3 (0.8) | 7.6 (0.6) | 7.4 (0.5) |
| Albumin*,$ (g/dL; 3.5 – 5.0 g/dL) | 4.4 (0.3) | 4.5 (0.5) | 4.7 (0.3) | 4.6 (0.3) |
ANOVA main effect of sex
p < 0.05,
p < 0.01.
denotes p < 0.05 ANCOVA main effect of sex with age included as a covariate.
denotes p < 0.05 post-hoc comparison with Sidak corrections. All data are presented as mean (SD). Normal range values for plasma inflammatory factors were not available.
3.4. Relationship between neuropsychiatric symptom severity and inflammatory factors
Previous studies from our lab (Huckans et al., 2015b; Loftis and Hauser, 2004) and others (reviewed in Byrne et al., 2016) indicate significant associations between mood and cognitive impairments and circulating immune factors. Therefore, correlations between neuropsychiatric symptom severity and plasma factors impacted by alcohol dependence (i.e., TIMP-1 and BDNF) were investigated. In women, TIMP-1 was positively correlated with all three neuropsychiatric measures (Fig. 2A-C). Correlations are also shown for men (Fig. 2D-F), where TIMP-1 was not correlated with any of the neuropsychiatric outcomes and in some cases exhibited a trend toward a negative correlation. No statistically significant correlations were observed for BDNF (data not shown).
Fig. 2.
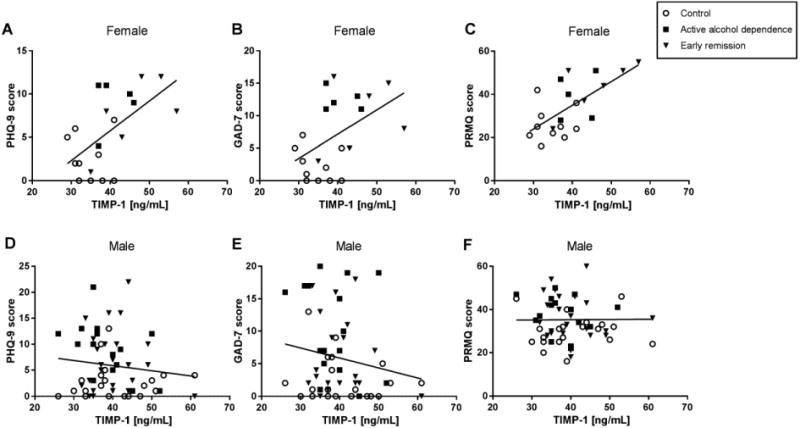
Plasma TIMP-1 correlates with depression, anxiety, and memory complaints in women. Self-report measures of depression (PHQ-9; r2 = 0.35, n = 21, p < 0.01) (A), anxiety (GAD-7; r2 = 0.24, n = 21, p < 0.05) (B), and memory complaints (PRMQ; r2 = 0.44, n = 21, p < 0.01) (C) were positively correlated with plasma TIMP-1 levels in female participants. No significant correlations were present in men (D-F).
3.5. Liver function assessment
The liver is a major target of alcohol's effects and a substantial contributor to alcohol-induced inflammatory signaling. Previous studies demonstrate that women are more susceptible to alcoholic liver disease than men both in human (Kovacs and Messingham, 2002) and animal studies (Fulham and Mandrekar, 2016). To evaluate the potential contribution of alcohol-induced liver damage to neuropsychiatric function and inflammatory responses, blood liver function tests were conducted. All measures of liver function were within the normal range (Staff, 2015) in men and women (Table 3).
4. Discussion
Neuropsychiatric profiles were similar in men and women with a current or recent history of alcohol dependence. Participants with a history of alcohol dependence exhibited increases in measures of depression, anxiety, and memory impairment relative to non-dependent controls. These results support and extend the findings of other studies (e.g., Ling et al., 2003) by examining sex × alcohol dependence interaction effects. In contrast to the similar mood and cognitive effects, inflammatory factors putatively involved in neuropsychiatric function differed between men and women. Women with a current or recent history of alcohol dependence had higher plasma levels of TIMP-1 and BDNF relative to controls. Increased plasma BDNF in recently abstinent alcohol-dependent subjects relative to controls has been reported in a largely male cohort (Costa et al., 2011), but sex was not included as a factor in between-group comparisons. Previous studies examining inflammatory factors associated with liver fibrosis and alcoholic hepatitis have not observed increases in TIMP-1 associated with alcohol abuse (reviewed in Parkes et al., 2012). Women in both groups exhibited lower levels of plasma MMP-3 than men, which is consistent with previous observations (Komosinska-Vassev et al., 2011). A growing body of literature now supports sex-specific responses to alcohol and indicates that women are more likely to exhibit a pro-inflammatory response to the effects of alcohol (e.g. Pascual et al., 2016).
Reductions in BDNF are often associated with mood disorders (reviewed in Hashimoto, 2010; Moonat et al., 2011) and cognitive impairments (Levada et al., 2016); however, elevations in BDNF are also reported in bipolar disorder (Barbosa et al., 2013) as well as in inflammatory conditions with a high prevalence of co-morbid depression and anxiety such as fibromyalgia (Epstein et al., 1999; Haas et al., 2010; Okifuji et al., 2000). Thus, alcohol-related neuropsychiatric symptoms may share common pathways with neuropsychiatric symptoms observed in fibromyalgia and in mental health disorders potentially mediated via inflammatory mechanisms.
Women in the alcohol dependent group had increased TIMP-1 relative to the control group, and TIMP-1 was correlated with measures of depression, anxiety, and memory impairment in female participants. These sex-dependent findings with alcohol dependence are consistent with other studies which show that elevations in TIMP-1 are associated with mild cognitive impairment in a predominantly female population (Hanzel et al., 2014), disruptions in long-term potentiation (Okulski et al., 2007) and learning and memory (Jourquin et al., 2005). TIMP-1 levels in the cerebrospinal fluid are also elevated in several neurodegenerative diseases including: Alzheimer's disease, Parkinson's disease, Huntington's disease, and Amyotrophic Lateral Sclerosis (Lorenzl et al., 2003). A preclinical rodent model of IFN-α-induced depression found elevations in Timp-1 mRNA expression in the hippocampus and prefrontal cortex of animals exhibiting depression-like behavior (Hoyo-Becerra et al., 2015), and in humans, administration of IFN-α as an antiviral therapy leads to significant behavioral alterations, including increases in anxiety, depression, and fatigue (Huckans et al., 2015a; Loftis et al., 2013). Elevations in TIMP-1 have also been observed in the hippocampus of suicidal individuals and in primary neuronal cultures treated with IFN-α and the toll-like receptor 3 (TLR3) agonist poly(I:C) (Hoyo-Becerra et al., 2013). TIMP-1 may be a sex-specific marker of neuropsychiatric impairment associated with alcohol dependence in women.
These results are limited by the relatively non-diverse sample of subjects available in this study, the modest sample size of women, and the range of detection for IFN-γ and IL-6. Due to the sample size, results are considered preliminary, thus subsequent replication and elaboration of results will be important. Men were also older than women, though inclusion of age as a co-variate in analyses did not change the statistical outcomes. Observations were carried out in a cross-sectional manner, thereby restricting interpretations to associations. In addition, nicotine usage differed somewhat between control and alcohol dependent groups, making it difficult to attribute group differences purely to alcohol dependence. The study is strengthened by the use of comprehensive clinical assessments (including detailed substance use histories and liver function tests), validated neuropsychiatric tests and well-characterized and matched samples minimizing the likelihood that other unmeasured factors contribute to the observations. Though subjects in the alcohol groups had experience with a wider range of drugs of abuse, the majority of these experiences occurred several years prior to the current study (Supplemental Table 1). Nevertheless, it is possible that some of these other drugs of abuse induced long-term behavioral or biochemical changes that influenced the results of the current study.
Peripheral immune factors can influence nervous system function and neuropsychiatric status with several reports documenting the association between inflammatory cytokine signaling and depression (e.g. Lotrich, 2015). Nevertheless, the connection between peripheral markers of inflammation and subsequent neuropsychiatric dysfunction is still not fully understood. The existence of sexually dimorphic factors associated with alcohol misuse has been observed previously, with correlations reported between brain shrinkage and hematocrit in males and brain shrinkage and AST levels in females (Chen et al., 2012). Circulating factors such as TIMP-1 or BDNF may directly compromise the blood-brain barrier via dysregulation of the extracellular matrix, may interact with the central nervous system through cells lining or sampling from the cerebral vasculature (e.g. active transport), or via areas of the nervous system with less restrictive blood-brain-barrier permeability (Abdul Muneer et al., 2012; Denes et al., 2011).
Conclusions
Neuropsychiatric symptoms were similar for male and female participants with alcohol dependence (including those with active diagnoses and in early remission). The significant, positive correlations between TIMP-1 and measures of depression, anxiety, and memory complaints in women identify TIMP-1 as a potential marker of alcohol-induced damage which may also be important therapeutically. Reliable biomarkers of tissue damage and neuropsychiatric dysfunction remain lacking for alcohol use disorders. Regulators of the extracellular matrix such as TIMP-1 are novel, inflammatory factors associated with dependence and neuropsychiatric dysfunction.
Supplementary Material
Highlights.
Affective symptoms and memory problems are increased in alcohol dependent men and women.
Increases in tissue inhibitor of metalloproteinase 1 (TIMP-1) and brain derived neurotrophic factor (BDNF) are associated with alcohol dependence, but only in women.
TIMP-1 correlates with depression, anxiety, and memory complaints in women.
Acknowledgments
Role of Funding Source Nothing declared
Author Disclosures: Conflict of Interest This work was supported in part by VA Merit Review Award #1I01BX002061 (JML) from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development, by NIH/NIDA grant #RC1DA028537 (JML, MH) and the Methamphetamine Abuse Research Center #P50DA018165 (JML, MH). Drs. Clare Wilhelm, Bret Fuller, Marilyn Huckans, and Jennifer Loftis are employees of the Department of Veterans Affairs. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper are solely those of the authors. The contents do not represent the views of the United States (U.S.) Department of Veterans Affairs or the U.S. Government.
Footnotes
Contributors Drs Loftis and Huckans had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Loftis, Huckans. Acquisition of data: Huckans, Loftis. Analysis and interpretation of data: All authors. Drafting of manuscript: All authors. Critical revision of the manuscript for intellectual content: All authors. Statistical analysis: Wilhelm, Fuller. Administrative, technical, or material support: Loftis, Huckans. Approval of the final manuscript before submission: All authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol. 2012;32:1167–1177. doi: 10.1161/ATVBAHA.112.247668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Karpyak VM, Schneekloth T, Hall-Flavin DK, Loukianova LL, Geske JR, Biernacka JM, Mrazek DA, Frye MA. A retrospective study of gender differences in depressive symptoms and risk of relapse in patients with alcohol dependence. Am J Addict. 2013;22:437–442. doi: 10.1111/j.1521-0391.2013.12021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40:219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. doi: 10.1016/j.tox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic And Statistical Manual For Mental Disorders. American Psychiatric Association; Washington, DC: p. 2000. [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F. Gender and peripheral neuropathy in chronic alcoholism: A clinical-electroneurographic study. Alcohol Alcohol. 2000;35:368–371. doi: 10.1093/alcalc/35.4.368. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Rocha NP, Miranda AS, Huguet RB, Bauer ME, Reis HJ, Teixeira AL. Increased BDNF levels in long-term bipolar disorder patients. Rev Bras Psiquiatr. 2013;35:67–69. doi: 10.1016/j.rbp.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Byrne ML, Whittle S, Allen NB. The role of brain structure and function in the association between inflammation and depressive symptoms: A systematic review. Psychosom Med. 2016;78:389–400. doi: 10.1097/PSY.0000000000000311. [DOI] [PubMed] [Google Scholar]
- Chen CH, Walker J, Momenan R, Rawlings R, Heilig M, Hommer DW. Relationship between liver function and brain shrinkage in patients with alcohol dependence. Alcohol Clin Exp Res. 2012;36:625–632. doi: 10.1111/j.1530-0277.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MA, Girard M, Dalmay F, Malauzat D. Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal. Alcohol Clin Exp Res. 2011;35:1966–1973. doi: 10.1111/j.1530-0277.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Smith G, Maylor EA, Della Sala S, Logie RH. The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory. 2003;11:261–275. doi: 10.1080/09658210244000027. [DOI] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Sandvik L, Malt UF, Andreassen OA. Recovery from major depressive disorder episode after non-pharmacological treatment is associated with normalized cytokine levels. Acta psychiatrica Scandinavica. 2016;134:40–47. doi: 10.1111/acps.12576. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Kovacs KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflamm. 2011;8:164. doi: 10.1186/1742-2094-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt-Glaser JK. Sex differences in depression: Does inflammation play a role? Curr Psychiatry Rep. 2015;17:78. doi: 10.1007/s11920-015-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SA, Kay G, Clauw D, Heaton R, Klein D, Krupp L, Kuck J, Leslie V, Masur D, Wagner M, Waid R, Zisook S. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation Psychosomatics. 1999;40:57–63. doi: 10.1016/S0033-3182(99)71272-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Nicolas-Arfelis JM. Gender differences in alcoholic cardiomyopathy. J Gender Specific Med. 2002;5:41–47. [PubMed] [Google Scholar]
- Fulham MA, Mandrekar P. Sexual dimorphism in alcohol induced adipose inflammation relates to liver injury. PloS one. 2016;11:e0164225. doi: 10.1371/journal.pone.0164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas L, Portela LV, Bohmer AE, Oses JP, Lara DR. Increased plasma levels of brain derived neurotrophic factor (BDNF) in patients with fibromyalgia. Neurochem Res. 2010;35:830–834. doi: 10.1007/s11064-010-0129-z. [DOI] [PubMed] [Google Scholar]
- Hanzel CE, Iulita MF, Eyjolfsdottir H, Hjorth E, Schultzberg M, Eriksdotter M, Cuello AC. Analysis of matrix metallo-proteases and the plasminogen system in mild cognitive impairment and Alzheimer's disease cerebrospinal fluid. J Alzheimers Dis. 2014;40:667–678. doi: 10.3233/JAD-132282. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitschfeld MJ, Schneekloth TD, Ebbert JO, Hall-Flavin DK, Karpyak VM, Abulseoud OA, Patten CA, Geske JR, Frye MA. Female smokers have the highest alcohol craving in a residential alcoholism treatment cohort. Drug Alcohol Depend. 2015;150:179–182. doi: 10.1016/j.drugalcdep.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hoyo-Becerra C, Huebener A, Trippler M, Lutterbeck M, Liu ZJ, Truebner K, Bajanowski T, Gerken G, Hermann DM, Schlaak JF. Concomitant interferon alpha stimulation and TLR3 activation induces neuronal expression of depression-related genes that are elevated in the brain of suicidal persons. PloS one. 2013;8:e83149. doi: 10.1371/journal.pone.0083149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo-Becerra C, Liu Z, Yao J, Kaltwasser B, Gerken G, Hermann DM, Schlaak JF. Rapid regulation of depression-associated genes in a new mouse model mimicking interferon-alpha-related depression in hepatitis C virus infection. Mol Neurobiol. 2015;52:318–329. doi: 10.1007/s12035-014-8861-z. [DOI] [PubMed] [Google Scholar]
- Huckans M, Fuller B, Wheaton V, Jaehnert S, Ellis C, Kolessar M, Kriz D, Anderson JR, Berggren K, Olavarria H, Sasaki AW, Chang M, Flora KD, Loftis JM. A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C. J Psychosom Res. 2015a;78:184–192. doi: 10.1016/j.jpsychores.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma inflammatory factors are associated with anxiety, depression, and cognitive problems in adults with and without methamphetamine dependence: An exploratory protein array study. Front Psychiatry. 2015b;6:178. doi: 10.3389/fpsyt.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Rumpf HJ, Bischof G, Hapke U, Hanke M, Meyer C. Excess mortality of alcohol-dependent individuals after 14 years and mortality predictors based on treatment participation and severity of alcohol dependence. Alcohol Clin Exp Res. 2013;37:156–163. doi: 10.1111/j.1530-0277.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Jourquin J, Tremblay E, Bernard A, Charton G, Chaillan FA, Marchetti E, Roman FS, Soloway PD, Dive V, Yiotakis A, Khrestchatisky M, Rivera S. Tissue inhibitor of metalloproteinases-1 (TIMP-1) modulates neuronal death, axonal plasticity, and learning and memory. Eur J Neurosci. 2005;22:2569–2578. doi: 10.1111/j.1460-9568.2005.04426.x. [DOI] [PubMed] [Google Scholar]
- King AC, Bernardy NC, Hauner K. Stressful events, personality, and mood disturbance: Gender differences in alcoholics and problem drinkers. Addict Behav. 2003;28:171–187. doi: 10.1016/s0306-4603(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Kocalevent RD, Hinz A, Brahler E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2013;35:551–555. doi: 10.1016/j.genhosppsych.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Komosinska-Vassev K, Olczyk P, Winsz-Szczotka K, Kuznik-Trocha K, Klimek K, Olczyk K. Age- and gender-dependent changes in connective tissue remodeling: Physiological differences in circulating MMP-3, MMP-10, TIMP-1 and TIMP-2 level. Gerontol. 2011;57:44–52. doi: 10.1159/000295775. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Messingham KA. Influence of alcohol and gender on immune response. Alcohol Res Health. 2002;26:257–263. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levada OA, Cherednichenko NV, Trailin AV, Troyan AS. Plasma brain-derived neurotrophic factor as a biomarker for the main types of mild neurocognitive disorders and treatment efficacy: A preliminary study. Dis Markers. 2016;2016:4095723. doi: 10.1155/2016/4095723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Heffernan TM, Buchanan T, Rodgers J, Scholey AB, Parrott AC. Effects of alcohol on subjective ratings of prospective and everyday memory deficits. Alcohol Clin Exp Res. 2003;27:970–974. doi: 10.1097/01.ALC.0000071741.63467.CB. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Dis. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Patterson AL, Wilhelm CJ, McNett H, Morasco BJ, Huckans M, Morgan T, Saperstein S, Asghar A, Hauser P. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: A 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Lotrich FE. Inflammatory cytokine-associated depression. Brain Res. 2015;1617:113–125. doi: 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B, Decker O, Muller S, Brahler E, Schellberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46:266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky HM, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA, N.I..A.A.A. Drinking Levels Defined. [accessed on May 20 2016 2016]; http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Nixon SJ, Prather R, Lewis B. Sex differences in alcohol-related neurobehavioral consequences. Handb Clin Neurol. 2014;125:253–272. doi: 10.1016/B978-0-444-62619-6.00016-1. [DOI] [PubMed] [Google Scholar]
- Okifuji A, Turk DC, Sherman JJ. Evaluation of the relationship between depression and fibromyalgia syndrome: Why aren't all patients depressed? J Rheumatol. 2000;27:212–219. [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sanchez-Capelo A, Mallet J, Kaczmarek L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol Psychiatry. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Rodriguez-Artalejo F, Lopes C. Alcohol intake and systemic markers of inflammation--shape of the association according to sex and body mass index. Alcohol Alcohol. 2010;45:119–125. doi: 10.1093/alcalc/agp092. [DOI] [PubMed] [Google Scholar]
- Parkes J, Guha IN, Harris S, Rosenberg WM, Roderick PJ. Systematic review of the diagnostic performance of serum markers of liver fibrosis in alcoholic liver disease. Comp Hepatol. 2012;11:5. doi: 10.1186/1476-5926-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ, Guerri C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol. 2016 doi: 10.1111/adb.12461. [DOI] [PubMed] [Google Scholar]
- Pedrelli P, Borsari B, Lipson SK, Heinze JE, Eisenberg D. Gender differences in the relationships among major depressive disorder, heavy alcohol use, and mental health treatment engagement among college students. J Stud Alcohol Drugs. 2016;77:620–628. doi: 10.15288/jsad.2016.77.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud D, Rota M, Rehm J, Shield K, Zatonski W, Hashibe M, La Vecchia C, Boffetta P. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138:1380–1387. doi: 10.1002/ijc.29890. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. Alcohol use disorders and mortality: A systematic review and meta-analysis. Addiction. 2013;108:1562–1578. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol Clin Exp Res. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Remlinger-Molenda A, Czech-Kucharska A, Wojcicka M, Michalak M, Losy J. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J Affect Dis. 2013;146:286–289. doi: 10.1016/j.jad.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smith G, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Staff MC. Liver function tests. [accessed on May 9 2016];2015 http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/PRC-20012602.
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Sonmez MK, Wiren KM. Understanding the addiction cycle: A complex biology with distinct contributions of genotype vs. sex at each stage. Neurosci. 2014;279C:168–186. doi: 10.1016/j.neuroscience.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14:721–731. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


