Abstract
Objective
There is limited investigation into the use of bio-absorbable antibiotic beads for the treatment of prosthetic vascular graft infections. Our goal was to investigate the rates of infection eradication, graft preservation, and limb salvage in patients who are not candidates for graft explant or extensive reconstruction.
Methods
A retrospective review of patients implanted with antibiotic impregnated bio-absorbable calcium sulfate beads at a major university center was conducted.
Results
Six patients with prosthetic graft infections were treated with bio-absorbable antibiotics beads from 2012–2014. Grafts included an aortobifemoral, an aorto-hepatic/superior mesenteric artery, and four extra-anatomic bypasses. Pathogens included Gram-positive and Gram-negative bacteria. Half of the patients underwent graft explant with reconstruction and half debridement of the original graft, all with antibiotic bead placement around the graft. Mean follow-up was 7.3±8.3 months; all patients had infection resolution, healed wounds, and 100% graft patency, limb salvage, and survival.
Conclusion
This report details the successful use of bio-absorbable antibiotic beads for the treatment prosthetic vascular graft infections in patients at high risk for graft explant or major vascular reconstruction. At early follow-up, we demonstrate successful infection suppression, graft preservation, and limb salvage with the use of these beads in a subset of vascular patients.
Keywords: Prosthesis-related infections, anti-bacterial agents, drug implants
Introduction
Prosthetic vascular graft infections occur in approximately 1–10% of patients and are associated with a high rate of morbidity and mortality.1,2 The clinical presentation is variable and depends on the vasculature involved. Aortic graft infections can present with gastrointestinal hemorrhage from an aortoenteric fistula, rupture from a pseudoaneurysm, and sepsis; these are associated with a 20% mortality rate and 5–25% amputation rate.1 Peripheral vascular graft infections are also associated with significant morbidity including sepsis, anastomotic disruption, thrombosis, limb loss, and up to 22% mortality.1,2
Traditionally, management of prosthetic graft infections included complete graft explant with extra-anatomic or in situ revascularization.3,4 However, some patients are unable to tolerate vascular reconstruction or have limited bypass options. Graft salvage or in situ replacement with autogenous tissue coverage and local wound debridement has been investigated in such situations with varying rates of success.5–7
Non-absorbable antibiotic polymethylmethacrylate (PMMA) beads have been routinely used in orthopedic surgery for the treatment of chronic osteomyelitis and prosthetic joint infections.8–11 Recently, studies have assessed the use of antibiotic PMMA beads for the treatment of prosthetic vascular grafts for both graft salvage and in situ reconstruction, with acceptable graft preservation and limb salvage rates.12,13 Unfortunately, these beads are associated with an intense local inflammatory response and require explant, which might cause challenges in deep cavitary infections. Bio-absorbable calcium sulfate antibiotic beads are gaining clinical use in orthopedic surgery for the treatment of osteomyelitis given the decrease in wound drainage, higher local antibiotic concentration, decreased inflammatory response, and absorbability.10,11,14–16 Given these potential advantages, we chose to investigate the use of bio-absorbable antibiotic impregnated beads in infection eradication, graft preservation, and limb salvage in the setting of prosthetic graft infection in patients who are not candidates for graft explant or extensive vascular reconstruction. We present a series of intra-abdominal and extra-cavitary prosthetic graft infections treated with antibiotic impregnated calcium sulfate beads.
Material and methods
A retrospective review at a major university center was conducted on all patients who had an implantation of Stimulan (Biocomposites Ltd, Wilmington, NC) bio-absorbable, calcium sulfate antibiotic beads for a prosthetic vascular graft infection. This study was approved by the Institutional Review Board of the University of Pittsburgh. Just as the treatment of prosthetic graft infections with antibiotic impregnated PMMA (non-absorbable) beads12,13 is off-label and not the standard of care, the use of Stimulan beads in this setting is also an off-label use of bio-absorbable impregnated beads and all patients gave their informed consent prior to implant. Stimulan beads were implanted in six patients between 2012 and 2014. Data on patient demographics, preoperative comorbidities, previous procedures, clinical presentation, postoperative adverse events, reinterventions, infection resolution, graft preservation, and long-term outcomes were collected for each patient. Follow-up data was collected through 1 August 2014. Basic summary statics, such as percentage, mean, and ranges, were used.
Patient selection
Patients were selected for Stimulan bead implantation if they had a prosthetic graft infection that required either preservation of the original graft, or explant of the initially infected graft with in situ reconstruction with prosthetic graft material. Half of the patients required graft preservation due to lack of further bypass options or inability to tolerate further major procedures (see Table 1, patients four to six). The remaining patients underwent graft explant and subsequent reconstruction with prosthetic material (Table 1, patients one to three). At initial presentation, all patients were placed on intravenous broad-spectrum antibiotics, and wound cultures were obtained preoperatively based on bedside cultures or ultrasound-guided aspiration if applicable. Operative exploration and debridement of necrotic and infected tissues were then performed, with or without in situ reconstruction, and the antibiotic beads were placed in the surrounding tissues. Care was taken to sharply debride all the material, including the biofilm layer surrounding the graft. Stimulan beads were prepared per Biocomposites Ltd protocol17 (see Figure 1). Ten mL of calcium sulfate was mixed with 1 g of vancomycin, 80–400 mg of gentamycin, and one patient had an additional 600 mg rifampin for an intra-abdominal infection associated with bowel ischemia. Tissue coverage was performed based on the location of the graft; all extra-cavitary infections had a rotational muscle flap. A six-week course of antibiotics was prescribed based on cultures, in consultation with the infectious disease service, followed by long-term suppressive oral antibiotics.
Table 1.
Demographics, risk factors, and presentation of vascular graft infections.
| Cases | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Gender | Female | Male | Female | Male | Female | Male |
| Age | 68 | 60 | 70 | 67 | 72 | 68 |
| Infected prosthetic graft | Aortobifemoral bypass graft | Aorto-SMA graft | Femoral–femoral bypass | Jump graft from left limb of ABF to profunda | Right external iliac artery-SFA bypass | Right axillary-bifemoral bypass |
| Graft type | Dacron | Dacron, propaten | Bovine pericardial patch and PTFE | Dacron | Dacron | Dacron and bovine pericardial patch |
| Infection location | Intra-abdominal and bilateral groins | Intra-abdominal | Right groin | Left groin | Right groin | Right groin |
| Prior graft revisions | ||||||
| Total | 1 | 4 | 7 | 5 | 2 | 4 |
| 3 months prior | 0 | 2 | 3 | 2 | 2 | 4 |
| 1 month prior | 0 | 1 | 1 | 2 | 0 | 4 |
| Time to infection | 11 years | 2.5 months | 1 month | 10 days | 2 months | 3 weeks |
| Other infection risk factors | Gangrenous cholecystitis Bowel ischemia |
Urostomy Multiple recent femoral revisions |
Multiple open thrombectomies of prior fem-pop bypass | Colostomy midline abscess ex-lap for SBO | Aortoenteric fistula Thrombectomy of RLE 24 h after ax-fem |
|
| Signs and symptoms | Abdominal and back pain No fevers, leukocytosis, or bacteremia |
Abdominal pain, sepsis, leukocytosis, lactic acidosis, bowel ischemia | Fevers and chills, erythema and purulent drainage from right groin | Left groin erythema and purulent drainage No fevers, leukocytosis, or bacteremia |
Right groin erythema and pain No fevers, leukocyteosis, or bacteremia |
Right groin erythema and purulent drainage No fevers, leukocytosis, or bacteremia |
| Diagnosis | CT scan: fluid collection at all anastomoses | Ex lap: thrombosed graft with purulence | CT scan: fluid surrounding R femoral artery/fem–fem anastomosis | Clinical exam | CT scan: fluid collection surrounding bypass graft | CT scan: fluid and air around right femoral anastomosis |
| Initial antibiotics | Vancomycin aztreonam metronidazole | Tigecycline aztreonam metronidazole | Vancomycin rifampin | Piperacillin-tazobactam | Vancomycin piperacillin-tazobactam | Vancomycin piperacillin-tazobactam |
| Cultures | Wound: no growth | Wound: VRE, Pseudomonas | Wound: MRSA Blood: MRSA |
Wound: Serratia, Candida albicans | Wound: S. epidermidis | Wound: VRE, E. coli, |
| Graft management plan | Explant with in situ reconstruction | Explant with in situ reconstruction | Explant with extra-anatomic bypass | Graft preservation (no further extra-anatomic options) | Graft preservation (metastatic ovarian CA) | Graft preservation (no further extra-anatomic options, unable to tolerate further bypass) |
SMA: superior mesenteric artery; VRE: vancomycin-resistant Enterococci faecalis; MRSA: methicillin-resistant Staphylococcus aureus; CT: computed tomopgraphy; SBO: small bowel obstruction.
Figure 1.
Preparation of Stimulan antibiotic beads: bead mixture is applied to bead mat for 3–5 min (left) to produce the antibiotic beads (right).
Results
Baseline patient characteristics
Six patients between 2012 and 2014 presented with prosthetic vascular graft infections requiring the use of Stimulan bio-absorbable antibiotic beads. Demographics are presented in Table 1. All patients had multiple medical comorbidities, including coronary artery disease (n=4), hypertension (n=5), hyperlipidemia (n=5), diabetes (n=3), smoking (n=5), and previous stroke or transient ischemic attack (n=2). Two patients had hypercoagulable states at baseline, one of which was secondary to metastatic ovarian cancer; both were on long-term anticoagulation. One patient presented with baseline end-stage renal disease on hemodialysis.
Patient presentation
All patients presented early, within one to three months of the last graft intervention, except for one patient who required explant and in situ reconstruction 11 years after an aortobifemoral (ABF) bypass; these patients had anywhere from two to four vascular reconstructions within three months of their prosthetic graft infection (Table 1). Other risk factors for prosthetic graft infection included gangrenous cholecystitis, urosotomy, colostomy, lower midline incision abscess unroofed during lysis of adhesions for a small bowel obstruction, and an aortoenteric fistula.
The locations of the prosthetic graft infections were both intra-abdominal and extra-cavitary. Specifically, graft infections included an ABF bypass infection with fluid collections at all anastomoses, an aorto-superior mesenteric artery (SMA)/hepatic bypass graft infection, and multiple femoral graft infections (Table 1). Infected graft material included Dacron, bovine pericardial patches, and polytetrafluoroethylene (PTFE).
All patients were stable on presentation with no signs of systemic infection except Case 2, who presented in septic shock from a thrombosed aorto-SMA/hepatic artery bypass bowel ischemia three days post robotic cholecystectomy. Those with extra-cavitary infections presented with groin erythema, fullness, pain, and drainage. CT scans were obtained to assess the extent of the infection and to assist in operative planning.
Initial patient management
All patients were immediately started on broad-spectrum intravenous antibiotics to cover Gram-positive, Gram-negative, and anaerobic bacteria (Table 1). Bedside cultures were obtained on patients with active drainage and patients without drainage underwent sterile, ultrasound-guided aspiration. Patients with intra-abdominal infections had cultures obtained intra-operatively. We obtained blood cultures on all of the patients as well. Bacteriology is presented in Table 1. Half of the patients in this case series underwent explant of their originally infected graft with in situ reconstruction with a prosthetic graft; the other had debridement and washout with preservation of the original graft.
Graft explant with in situ reconstruction/extra-anatomic reconstruction
Case 1 underwent explant of an ABF bypass graft for expanding fluid collections around all anastomoses. In situ reconstruction was done with a rifampin-soaked bifurcated Dacron graft, with antibiotic beads placed at the proximal anastomosis with omental flap coverage, and at the distal femoral anastomoses with sartorius flap coverage. She had an uncomplicated postoperative course, with no evidence of reinfection to date (see Table 2).
Table 2.
Vascular graft infection management and final outcomes.
| Cases | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Operative intervention | Explant ABF wound debridement | Explant of infected/thrombosed SMA graft small bowel resection | Explant of fem-fem bypass and R bovine patch R femoral patch angioplasty with SFA |
Left groin debridement Explant proximal thrombosed fem-pop bypass |
Right groin debridement | Right groin debridement |
| In situ reconstruction | ABF with rifampin soaked Dacron |
Iliac-SMA with ringed propaten | L ax-fem with ringed propaten | N/A | N/A | N/A |
| Antibiotic bead location | Proximal and bilateral distal anastomoses | Previous graft tunnel Perigraft |
Bilateral groins | Right groin | Right groin | Right groin |
| Antibiotic bead | 1 gm vancomycin 120 mg gentamycin |
1 gm vancomycin 80 mg gentamycin |
1 gm vancomycin 120 mg gentamycin 600 mg rifampin |
1 gm vancomycin 400 mg gentamycin |
1 gm vancomycin 240 mg gentamycin |
1 gm vancomycin 400 mg gentamycin |
| Tissue Coverage | Proximal: omental flap Distal: sartorius flaps |
Retroperitoneal coverage | Bilateral sartorius flaps JP drains |
Left sartorius flap | Right sartorius flap JP drain |
Right sartorius flap JP drain |
| Time to follow up | 3 months | 6 months | 6 months | 9 months | 2 years | 2 months |
| Complications | None | Prolonged intubation, temporary dialysis | Left axillary hematoma requiring evacuation | Infection of remaining distal fem-pop bypass, requiring explant | None | Anemia |
| Infection status | Resolution of infection Incisions healed |
CT scan: no evidence of infection Incisions well healed, small midline seroma |
Resolution of infection Incisions well healed |
Resolution of infection Incision well healed |
CT: scan: no evidence of infection Incision well healed |
Resolution of infection Good granulation tissue |
| Graft status | Replaced aortobifemoral graft patent and preserved No graft thrombosis, bleeding or PSA |
Duplex: Replaced iliac-SMA bypass patent & preserved CT scan: no evidence of PSA or hematoma |
Left axillary-femoral bypass patent and preserved CT scan: no fluid collections or PSA in either groin |
Left jump graft from aortobifemoral to profunda patent and preserved No fluid collection or PSA |
Duplex: right iliac-femoral bypass patent and preserved No fluid collection or PSA |
Left axillary-bifemoral bypass patent and preserved |
| Major amputation | None | None | None | None | None | None |
| Death | No | No | No | No | No | No |
N/A: not applicable; PSA: pseudoaneurysm; SMA: superior mesenteric artery CT: computed tomography; SFA: superficial femoral artery.
Case 2 presented with sepsis secondary to bowel ischemia and gross infection around a thrombosed aorto-SMA/hepatic artery bypass. He underwent bowel resection followed by explant of the SMA limb, along with an iliac-SMA bypass with 8 mm ringed propaten. During the third look operation, the replaced graft was copiously irrigated and covered with antibiotics beads and retroperitoneal tissue. At six months, a CT scan did not demonstrate any fluid collections suggestive of active infection and duplex demonstrated graft patency.
Case 3 presented with an infected femoral–femoral bypass after multiple femoral explorations, and required an axillary-femoral bypass in close proximity to the infected field after explant of the femoral–femoral bypass. On six-month follow-up, her axillary-femoral bypass was patent without evidence of infection, pseudoaneurysm, or bleeding.
Original graft preservation
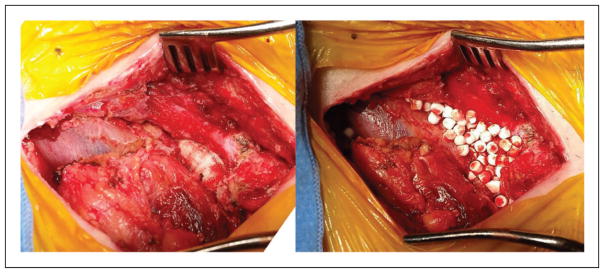
All the patients treated with graft preservation (Cases 4–6) presented with groin infections and had multiple medical comorbidities and deconditioning that precluded them further extra-anatomic bypasses; in addition, Cases 4 and 6 had no further bypass options available. In all of these cases, extensive debridement and washout of necrotic and infected tissue were performed, including the biofilm around the graft. This was followed by implantation of Stimulan antibiotic impregnated beads around the graft and sartorius flap coverage, see Figure 2. An appropriate six-week course of intravenous antibiotics was given, followed by lifelong suppressive oral antibiotics.
Figure 2.
Infected prosthetic femoral graft: after debridement (left) and after placement of Stimulan antibiotic beads (right).
In the perioperative period, one patient required a return to the operating room for completion explant of the distal portion of a previously, partially explanted, thrombosed prosthetic femoral-popliteal bypass (Case 4). Another patient had a protracted hospital course for ileus and anemia that resulted in deconditioning; however, these were not a direct complication of his groin reconstruction (Case 6).
Mean follow-up was 7.3±8.3 months (2–24 months) for all six patients. All patients had resolution or ongoing suppression of their prosthetic graft infection. All grafts were preserved without recurrent infection, thrombosis, pseudoaneurysm development, or bleeding events. There were no major amputations or mortality in the follow-up period.
Discussion
Infections of prosthetic vascular grafts, both intra-abdominal and peripheral bypasses, are associated with high rates of morbidity and mortality.1,2 Infections associated with prosthetic bypasses are particularly pathogenic given the biofilm that promotes adherence of the bacteria to the prosthetic material; this impairs not only the host defense mechanisms, but also the antimicrobial activity of intravenous antibiotics.1 Historically, pathogens causing early graft infection were mainly coagulase-positive staphylococci, whereas late graft infections were mainly caused by coagulase-negative staphylococci. More recently, graft infection is predominately caused by S. aureus, particularly methicillin-resistant Staphylococcus aureus (MRSA), S. epidermidis, and E. coli, and even more frequently mixed infections including a variety of Gram negative bacteria.1,12,13 Early infecting organisms such as S. aureus, E. coli, Proteus, and P. aeruginosa are typically more virulent, seen in extra-cavitary infections, and associated with higher rates of anastomotic disruption and worse outcomes.1,6,13 As in recent studies, the pathogens in our series included multiple organisms, including MRSA, vancomycin-resistant Enterococci faecalis (VRE), P. aeruginosa, and E. coli.
Traditional management of prosthetic graft infections includes complete graft explant with extra-anatomic revascularization.3,4 This may not be possible for some critically ill patients who cannot tolerate major revascularization or those with no further revascularization options. Studies of attempted graft salvage with aggressive tissue debridement alone have high rates of persistent infection (up to 82%) with the need for complete explant and an associated amputation rate of 40%.3
Early studies suggested that in patients with hemostatic and patent grafts with limited further revascularization options, graft preservation could be achieved with aggressive tissue debridement in addition to muscle flap coverage and prolonged antibiotic therapy.5 Muscle flap coverage of a graft provides improved obliteration of dead space, improve systemic antibiotic and host immune system delivery, and improved drainage control.5 However, conflicting outcomes with muscle flap coverage alone have been reported. Some reports indicate high rates of survival and graft salvage of 85–90% at one year,5 while others demonstrate suboptimal overall long-term graft salvage rates of only 50%.6,7
Given its success in the orthopedic field with the treatment of chronic osteomyelitis and prosthetic joint infections,8–11 there have been several studies that have recently investigated the use of non-absorbable antibiotic PMMA beads for the treatment of prosthetic vascular grafts for both graft salvage and in situ reconstruction.12,13 Stone et al.,13 have documented a zero 30-day morality rate; however, they also report a 66% graft preservation rate at 17 months follow-up, 21% limb loss, and 20% reinfection rate. Slightly more promising results were reported by Poi et al.,12 with 86% graft preservation at 36 months, with a 13% limb loss, and 12% reinfection rate. While these studies suggest that antibiotic beads have improved infection control and graft preservation compared to muscle flap coverage alone, the PMMA beads have a major disadvantage of requiring explant and are associated with a high inflammatory reaction. Bead explant may be particularly challenging in cavitary infections as it may require extensive re-exploration.10,11
Recent orthopedic literature has noted good clinical efficacy in the treatment of chronic osteomyelitis with bio-absorbable, antibiotic impregnated calcium sulfate beads, with no recurrent episodes of infection and a low rate of self-limiting, wound drainage.10,11,14–16 Moreover, studies have demonstrated that the concentration of antibiotic released from calcium sulfate beads was three times that of the concentration released from the PMMA beads in vitro,18 with more consistent, prolonged levels of local antibiotic delivery.15,16 These beads provide adequate local antibiotic levels for approximately 6–10 weeks, at which time they dissolve.14
We have treated six patients with both intra-abdominal and extra-cavitary prosthetic graft infections with antibiotic impregnated calcium sulfate Stimulan beads. Three patients underwent in situ reconstruction with prosthetic graft, two intra-abdominal; one had an extra-anatomical reconstruction within an infected field. All three of these patients had control of their infection, maintained patency of the new graft, and had 100% limb salvage and survival. The other three patients required primary graft preservation; this was performed with extensive tissue debridement, bio-absorbable antibiotics bead placement, and rotational muscle flap coverage. At follow-up, these patients also had suppression of their infection, maintained patency of the primary graft, and had 100% limb salvage and survival. All patients who had an extra-cavitary component to the infection had an associated sartorius flap to aid in infection clearance and wound healing. Patients were also placed on an appropriate course of intravenous antibiotics for six weeks, followed by a suppressive regimen.
This report has several limitations. This series is a retrospective review of a rare, but morbid vascular surgery complication. This patient population has multiple, advanced comorbidities, with a limited life expectancy. As a result, patient follow-up is limited to the short and mid-term time frame given the inherent poor prognosis of this cohort. Moreover, this study is limited by the small sample size but does, nonetheless, demonstrate the potential clinical efficacy of antibiotic impregnated, bio-absorbable, calcium phosphate beads for selective patients with prosthetic vascular graft infection, with maintained graft patency and limb salvage. Other applications of bio-absorbable antibiotics beads in the vascular surgical patient population, such as infected dialysis grafts, have not been explored in this series and we limited our application of this therapy to the setting prosthetic material that is required to remain in an infected field.
Conclusion
This report describes the novel use of bio-absorbable antibiotic impregnated beads for the treatment prosthetic vascular grafts infections in patients at high risk for graft explant or major vascular reconstruction. Both antibiotic impregnated, non-absorbable PMMA beads and bio-absorbable, calcium sulfate beads are not the standard of care for prosthetic graft infections and represent an off-label use of these products. However, bio-absorbable antibiotic beads have multiple advantages, such as higher concentrations of local antibiotic delivery, decreased inflammation, and decreased dead space production with gradual bead absorption. The results presented here demonstrate a high rate of graft preservation, infection suppression, and limb salvage with the bio-absorbable antibiotic bead treatment and should be considered part of the treatment algorithm for patients at high risk for graft explant. This series calls for further clinical investigation and longer term follow-up to determine their definitive role in the management of prosthetic vascular graft infections.
Acknowledgments
This article was presented at the 2014 Joint Annual Meeting of the New England Society for Vascular Surgery/Eastern Vascular Society, Boston, MA; Winner of the Resident Award Competition for EVS Oral Presentation, 15 September 2014.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported, in part, by a NIH T32 Post-Doctoral Vascular Surgery Research Grant (5T32HL098036-05) awarded to Elizabeth Genovese MD, MS.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Herscu G, Wilson SE. Prosthetic infection: lessons from treatment of the infected vascular graft. Surg Clin North Am. 2009;89:391–401. doi: 10.1016/j.suc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Legout L, Sarraz-Bournet B, D’Elia PV, et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: a prospective observational cohort study. Clin Microbiol Infect. 2012;18:352–358. doi: 10.1111/j.1469-0691.2011.03618.x. [DOI] [PubMed] [Google Scholar]
- 3.Mertens RA, O’Hara PJ, Hertzer NR, et al. Surgical management of infrainguinal arterial prosthetic graft infections: review of a thirty-five-year experience. J Vasc Surg. 1995;21:782–790. doi: 10.1016/s0741-5214(05)80009-6. (discussion 90–91) [DOI] [PubMed] [Google Scholar]
- 4.Calligaro KD, Veith FJ, Schwartz ML, et al. Differences in early versus late extracavitary arterial graft infections. J Vasc Surg. 1995;22:680–685. doi: 10.1016/s0741-5214(95)70058-7. (discussion 5–8) [DOI] [PubMed] [Google Scholar]
- 5.Illig KA, Alkon JE, Smith A, et al. Rotational muscle flap closure for acute groin wound infections following vascular surgery. Ann Vasc Surg. 2004;18:661–668. doi: 10.1007/s10016-004-0105-7. [DOI] [PubMed] [Google Scholar]
- 6.Seify H, Moyer HR, Jones GE, et al. The role of muscle flaps in wound salvage after vascular graft infections: the Emory experience. Plast Reconstr Surg. 2006;117:1325–1333. doi: 10.1097/01.prs.0000204961.32022.ab. [DOI] [PubMed] [Google Scholar]
- 7.Herrera FA, Kohanzadeh S, Nasseri Y, et al. Management of vascular graft infections with soft tissue flap coverage: improving limb salvage rates – a veterans affairs experience. Am Surg. 2009;75:877–881. doi: 10.1177/000313480907501003. [DOI] [PubMed] [Google Scholar]
- 8.Walenkamp GH, Kleijn LL, de Leeuw M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop Scand. 1998;69:518–522. doi: 10.3109/17453679808997790. [DOI] [PubMed] [Google Scholar]
- 9.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 10.Kluin OS, van der Mei HC, Busscher HJ, et al. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10:341–351. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 11.Gogia JS, Meehan JP, Di Cesare PE, et al. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23:100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poi MJ, Pisimisis G, Barshes NR, et al. Evaluating effectiveness of antibiotic polymethylmethacrylate beads in achieving wound sterilization and graft preservation in patients with early and late vascular graft infections. Surgery. 2013;153:673–682. doi: 10.1016/j.surg.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Stone PA, Mousa AY, Hass SM, et al. Antibiotic-loaded polymethylmethacrylate beads for the treatment of extra-cavitary vascular surgical site infections. J Vasc Surg. 2012;55:1706–1711. doi: 10.1016/j.jvs.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson JY, Dudareva M, Riley ND, et al. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J. 2014;96-B:829–836. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 15.Aiken SS, Cooper JJ, Florance H, et al. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect. 2015;16:54–61. doi: 10.1089/sur.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConoughey SJ, Howlin RP, Wiseman J, et al. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res Part B Appl Biomater. 2015;103:870–877. doi: 10.1002/jbm.b.33247. [DOI] [PubMed] [Google Scholar]
- 17.Biocomposites Ltd. [accessed 8 February 2016];STIMULAN antibiotic mixing guide. 2014 http://www.biocomposites.com/media/1344/stimulan-eu-mixing-guide-v2.pdf.
- 18.Udomkusonsri P, Kaewmokul S, Arthitvong S, et al. Elution profiles of cefazolin from PMMA and calcium sulfate beads prepared from commercial cefazolin formulations. J Vet Med Sci. 2012;74:301–305. doi: 10.1292/jvms.11-0095. [DOI] [PubMed] [Google Scholar]