Abstract
Purpose
The primary aim was to compare the diagnostic performance of PET/MRI (performed with basic anatomical MRI sequences) in detecting sites of disease in adult patients with lymphoma compared with the current standard of care, PET/CT. Secondary aims were to assess the additional value of Diffusion Weighted Imaging (DWI) to PET/MRI in disease detection and to evaluate the relationship between the Standarised Uptake Value on PET/MR with the Apparent Diffusion Coefficient on DWI.
Methods
68 studies in 66 consecutive patients with histologically proven Hodgkins or non-Hodgkins lymphoma were prospectively evaluated. Each patient had whole body PET/CT, followed by whole body PET/MR. Two experienced readers independently evaluated the PET/MRI studies, and two other experienced readers independently evaluated PET/CT. Site of lymphoma involvement and SUVmax at all nodal sites more avid than background liver were recorded. Readers provided stage (in baseline cases) and disease status (remission vs active disease). The ADCmean value corresponding to the most avid PET site of disease was recorded.
Results
Ninety-five nodal and 8 extranodal sites were identified on both PET/CT and PET/MRI. In addition, 3 nodal and 1 extranodal sites were identified on PET/MRI. For positive lesion detection, reader agreement in PET/MR was perfect between the two readers and almost perfect between PET/CT and PET/MR (k>0.978). Intermodality agreement between PET/CT and PET/MRI was also near-perfect to perfect for staging/disease status (0.979 – 1.000). SUVmax from PET/CT and PET/MRI correlated significantly, (Spearman’s rho correlation coefficient 0.842, p<0.001). DWI did not alter lesion detection or staging in any case. A negative correlation was demonstrated between ADC mean and SUVmax (Spearman’s rho correlation coefficient r −0.642, p<0.001).
Conclusion
PET/MRI is a reliable alternative to PET/CT in the evaluation of patients with lymphoma. DWI did not alter diagnostic accuracy. With comparable accuracy in detection of disease sites and added benefit of radiation dose reduction, PET/MRI has a potential to become part of routine lymphoma imaging.
Keywords: PET/MRI, PET/CT, Lymphoma
Introduction
Lymphoma is a common haematologic malignancy with 386,000 new cases of Non-Hodgkin lymphoma (NHL) and 66,000 new cases of Hodgkin lymphoma diagnosed worldwide in 2012. In the same year, lymphoma accounted for 2.3% of all cancer deaths worldwide [1]. Therapeutic developments as well as advances in diagnostic imaging have lead to a trend of increasing survival.
PET/CT use has increased considerably over recent years with extensive evidence in existence to support positive impact on staging and response assessment in Hodgkins and Non Hodgkins lymphoma [2,3,4,5].
Patients with lymphoma may undergo multiple CT and PET/CT examinations during the course of investigation and treatment.
There has been recent interest in the potential application of whole body MRI in lymphoma, as a radiation free comparable modality to PET/CT, including the measurement of the Apparent Diffusion Coefficient (ADC) with some promising results [6,7].
However, FDG PET/CT remains the reference standard for lymphoma staging as there is a lack of larger-scale studies demonstrating unequivocal benefit of whole-body MRI-DWI [8].
Hybrid PET/MRI imaging systems have been recently introduced to allow simultaneous acquisition of PET and MRI data. In these scanners, MRI sequences (Two-point Dixon) are used for attenuation correction instead of CT and other MRI sequences are acquired for anatomic correlation [9].
Several feasibility studies have evaluated patients with various tumour types when comparing PET/CT and PET/MR [10,11]. Generally, PET/MR has been shown to be equivalent to PET/CT in localising tumour and metastases, with a variable proportion of additional lesions detected with the benefit of superior soft tissue resolution on PET/MRI. Catalano et al studied 134 patients with cancer who had PET/CT and PET/MR on the same day and found additional findings on PET/MR affecting clinical management in 17.4% of cases [12].
There is a lack of published data regarding ability of PET/MRI to evaluate adult patients with lymphoma. The purpose of this study was to specifically evaluate the diagnostic performance of lesion detection and disease status in adult lymphoma patients on PET/MRI, compared to the current standard of care of PET/CT. The relationship between SUV and ADC was also evaluated.
Subjects and methods
This study underwent approval from the local institutional ethics review board. Consecutive patients undergoing clinically indicated whole body 18F FDG PET/CT (for staging or response assessment of lymphoma) were identified and invited to have the additional PET/MRI study.
Patients received an intravenous injection of 18F FDG and underwent PET/CT, followed by PET/MR with residual signal from the initial injection.
Between May 2012 and January 2014, 68 scans were performed in 66 patients (45males and 21 females, with two men being studied twice during the study period) who underwent PET/CT and additional PET/MRI. The mean age of the final study group was 42.8 years (range 18–87). The indications for scanning were mixed (pretreatment (n=18), and post treatment). Types of lymphoma studied were Hodgkin (n=27), DLBCL (n= 25), FL (n= 1), Mixed DLBCL and FL (n=1) and others (n=14, composed of mantle cell (n=4), plasmablastic (n=1), splenic marginal zone (n=1), T cell (n=6), lymphoblastic (n=1) and Burkitts (n=1).
Image acquisition
PET/CT examination
The PET/CT scan was performed in fasted patients using a Discovery VCT 64-slice PETCT scanner (GE Healthcare, Milwaukee, WI) 60 min after intravenous injection of 5.5 MBq/kg of 18F-FDG (mean 64 minutes, range 50–90, +/− 7.8). Emission scan was acquired as per our usual clinical protocol to cover vertex to midthighs. Mean injected FDG dose was 369.3MBql, (range 160–241, +/− 42.5). PET images were reconstructed with iterative reconstruction with 2.5minutes per bed position.
CT was performed according to a standard protocol with the following parameters: 140 keV, modulated 30 – 300 mAs, pitch 1.375, and section thickness 2.5 mm. The mean duration of the PET CT studies was 20.4minutes (15.5 – 22.5, +/− 1.4).
PET/MRI examination
Patients were then transferred to the PET/MR scanner (3 T Biograph mMR; Siemens, Erlangen, Germany) located in a different building, where a integrated PET and MR acquisition was performed, as soon as possible after the PET/CT examination, mean interval 136 min (range, 90 – 243min, +/− 28.6) after original FDG injection. Four tissue class (soft tissue, fat, lung, air) attenuation correction maps were calculated from two-point Dixon sequences. The MR protocol for each patient included axial T2-weighted HASTE, axial diffusion (DWI, b=0,400,800) and coronal T1-weighted 3-D interpolated breath-hold examination (VIBE). Studies were performed at 3minutes per bed position. The scan duration ranged between 20–30minutes, depending on time for shimming. Thirteen minutes of additional scan time for the acquisition of DWI.
Image interpretation
Two radiologist readers, (10 years and 4 years experience in reporting PET/CT) independently evaluated the PET/CTs, with access to clinical history but without access to the PET/MRI study.
Two other radiologist readers (both with 3 years experience in reporting PET/MRI) independently read the PET/MRI studies, also with access to clinical history, but without access to the PET/CT performed on the same day.
Nodal stations were divided in to the following groups; right and left cervical, supraclavicular, subpectoral, axillary, and also mediastinal, liver hilum, splenic hilum, mesenteric, retroperitoneal, right and left iliac and inguinal. Sites of extranodal disease were also recorded.
Lesion uptake was determined as significant if higher than liver background except in those regions where high physiological tracer activity is expected, e.g. tonsils in which cases abnormal uptake was defined as specific for those regions.
Tumour staging was determined for baseline PET/CT and PET/MRI scans. For post treatment scans disease status was divided into metabolic remission and metabolic active disease.
Although PET/CT was the reference standard, review in consensus and review of follow up imaging was performed for any discrepant cases.
PET/CT
Images of a Maximum Intensity Projection of the attenuation corrected PET study, and multiplanar images of the AC PET, whole body CT and free-breathing lung CT data were viewed on a GE advantage workstation, Adw 4.4. A volume of interest was drawn within the right hepatic lobe for liver SUVmax. Sites of nodal disease were recorded if FDG uptake was greater than liver SUVmax.
PET/MRI
Images of a Maximum Intensity Projection of the attenuation corrected PET study, and multiplanar images of the AC and MRI sequences were reviewed on a workstation using OsiriX (http://www.osirix-viewer.com/) software. Both readers reviewed all scans in a sequential manner (summarised in Fig 1), initially viewing the PET images together with T2 HASTE, T1 VIBE and fused PET/T2 HASTE sequences. After this, each reader reviewed the DWI sequences (b0,400,800 and ADC images) alongside the PET and T1 and T2 sequences. Sites of nodal disease were recorded if FDG uptake was greater than liver SUVmax. Any change based on review of DWI was also recorded. Regions of interest were drawn within the right hepatic lobe for liver SUVmax. Regions of interest were drawn around FDG avid nodes on single axial slices, ensuring the SUVmax was recorded.
Figure 1.
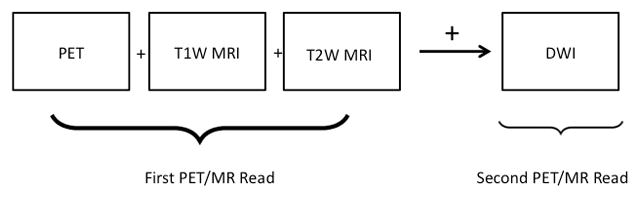
Summary of image interpretation pathway for readers of PET/MRI studies
Quantitative evaluation of ADC was performed on the single lesion, (if convincingly visible on the DWI) which demonstrated the greatest SUVmax value in each study. A single reader displayed the T2HASTE image, PET AC image, b800 and ADCmap. Using all data combined, a region of interest was drawn on the ADC map to provide a value of ADC mean for the most avid lesion on the PET study.
Statistical analysis
Statistical analysis was performed using dedicated software (SPSS version 21, Chicago, IL).
The generalized kappa statistic (κ) was used to determine PET/MR interobserver agreement and intermodality agreement (PET/CT vs. PET/MR) for staging and sites of nodal involvement, considering: κ <0.2 as slight agreement, κ 0.21 – 0.40 as fair agreement, κ 0.41 – 0.60 as moderate agreement, κ 0.61 – 0.80 as substantial agreement, and κ 0.81 – 1.00 as almost perfect to perfect agreement [13].
SUV results were evaluated by nonparametric correlation analysis, using the Spearman’s rank correlation coefficient to examine the association between SUVmax from PET/CT and PET/MRI.
SUV and ADC results were evaluated by nonparametric correlation analysis, using the Spearman’s rank correlation coefficient to examine the association between SUVmax and ADC value from PET/MRI.
A Bland Altman plot was used to assess inter modality agreement on SUVmax values.
Statistical significance was accepted for P values lower than 0.05.
Results
Nodal and extranodal disease
Nodal disease
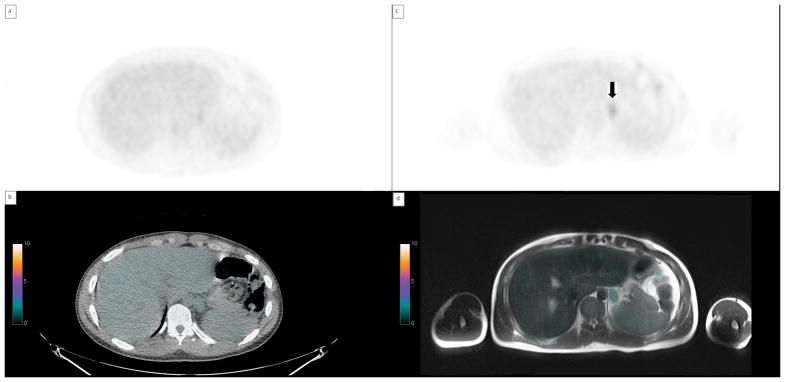
A total of 95 sites of nodal disease were reported as positive on both PET/CT and PET/MRI. In two cases (of NHL), there were a total of three nodal disease sites reported as called positive by both PET/MRI readers but not reported by either PET/CT reader. In the first case the nodal sites were in the mesentery and retroperitoneum (figure 2) and in the second case the positive nodes were in the axilla.
Figure 2.
Retroperitoneal lymph nodes apparent on PET/MRI but not PET/CT a) MIP image from PET/MRI AC data, arrows indicate FDG avid abdominal nodes. b) MIP from PET/CT showing no avid abdominal nodes c)axial image from AC from PET/CT showing no FDG avid nodes d) axial image from AC from PET/MRI with avid abdominal nodes (arrow).
Fifteen of the 27 patients with Hodgkins lymphoma had FDG avid disease (44 nodal and 2 extra nodal).
Extranodal Disease
There were 4 studies identifying splenic involvement on PET/CT and PET/MRI (2 in Hodgkins lymphoma). Two cases of marrow involvement were called positive on both modalities (both were in patients with Hodgkins lymphoma).
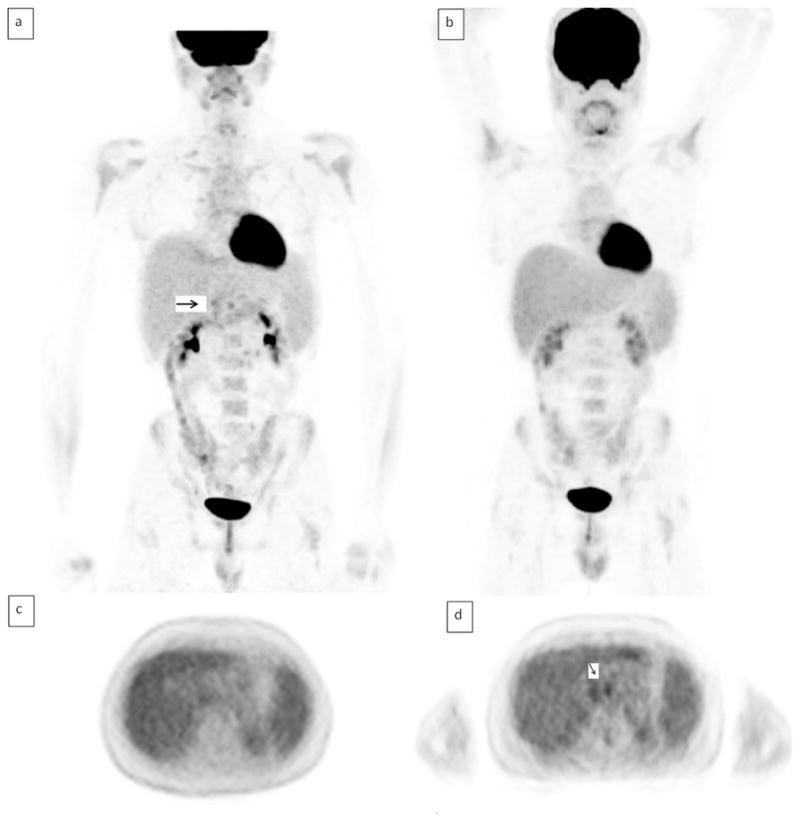
In 8 studies, there were 8 sites of extranodal disease on PET/CT (2 were in patients with Hodgkins lymphoma) and 9 sites of extranodal disease in 8 studies on PET/MRI. In one case, where a patient had lung and adrenal involvement, both PET/CT readers and both PET/MRI readers called lung involvement positive. However, neither PET/CT reader called positive disease in the adrenal gland whereas both PET/MRI readers identified disease at this site (Figure 3). Review of follow up imaging confirmed the presence of extranodal adrenal disease. (increased size and metabolic activity). Table 1 shows nodal and extranodal sites identified by each reader and Table 2 shows the kappa analysis for agreement for detection of nodal sites between readers and between PET/CT and PET/MRI.
Figure 3.
Left adrenal nodule positive on PET/MRI but not PET/CT. a)PET/CT AC axial image, b)PET/CT fused axial image, c) PET/MR AC axial image d)PET/MR axial fused –arrow indicates FDG avid adrenal nodule. FDG avid diaphragmatic node also shown.
Table 1.
Nodal and extranodal sites of disease identified by each reader
| Reader | Positive nodal sites identified | Extranodal sites identified |
|---|---|---|
| PET/CT Reader 1 | 95 | 8 |
| PET/CT Reader 2 | 95 | 8 |
| PET/MRI Reader 1 | 98 | 9 |
| PET/MRI Reader 2 | 98 | 9 |
Table 2.
Analysis for agreement for detection of nodal sites between readers and between PET/CT and PET/MRI.
| kappa | SE | P | |
|---|---|---|---|
| For all nodal sites 68studies Reader 1 CT vs MR |
0.978 | 0.011 | .000 |
| For all nodal sites 68 studies Reader 2 CT vs MR |
0.984 | 0.009 | .000 |
| For all nodal sites 68 studies CT Reader 1 vs Reader 2 |
1.000 | 0.000 | .000 |
| For all nodal sites 68 studies MR Reader 1 vs. Reader 2 |
0.994 | 0.006 | .000 |
Staging of baseline studies and disease status post treatment
Staging was concordant between PET/CT readers and between PET/MRI readers in all 18 baseline cases. A single case where right axillary nodes were called positive on PET/MRI but not on PET/CT was deemed a true positive but did not alter staging in this patient. In the post treatment cohort, all cases were concordant with the exception of one case where mesenteric and retroperitoneal nodes were called positive resulted in a discrepancy where both PET/MRI readers recorded both sites (metabolically active disease) and both PET/CT readers recorded no disease (metabolic remission).
Table 3 shows the kappa analysis of agreement in staging/response assessment between readers and between PET/CT and PET/MRI.
Table 3.
Analysis of agreement of disease status combined (baseline staging and post treatment response) between readers and between PET/CT and PET/MRI.
| Kappa | SE | p | |
|---|---|---|---|
| 68 studies Reader 1 CT vs MR |
0.979 | 0.021 | .000 |
| 68 studies Reader 2 CT vs. MR |
0.979 | 0.021 | .000 |
| 68 studies CT Reader 1 vs. Reader 2 |
1.000 | 0.000 | .000 |
| 68 studies MR Reader 1 vs. Reader 2 |
1.000 | 0.000 | .000 |
DWI
None of the 68 cases had additional nodal or extranodal sites identified with DWI. The staging categories with each modality is shown in table 4.
Table 4.
Lymphoma stage (Modified Ann Arbor) using PET/CT and PET/MRI
| Stage | Number of cases (PET/CT) | Number of cases (PET/MRI) | Number of cases (PET/MRI + DWI) |
|---|---|---|---|
| 0 | 2 | 2 | 2 |
| I | 5 | 5 | 5 |
| II | 7 | 7 | 7 |
| III | 4 | 4 | 4 |
| IV | 0 | 0 | 0 |
| Total | 18 | 18 | 18 |
A moderate inverse, correlation was found between the ADCmean and SUVmax. Correlation coefficient Spearman’s rho correlation coefficient r −0.642, p<0.001 (95% Confidence interval for r −0.339 to −0.824). This was evaluated in 27 studies where the most avid lesion on PET corresponded to a measurable lesion on the ADC map (Figure 4).
Figure 4.
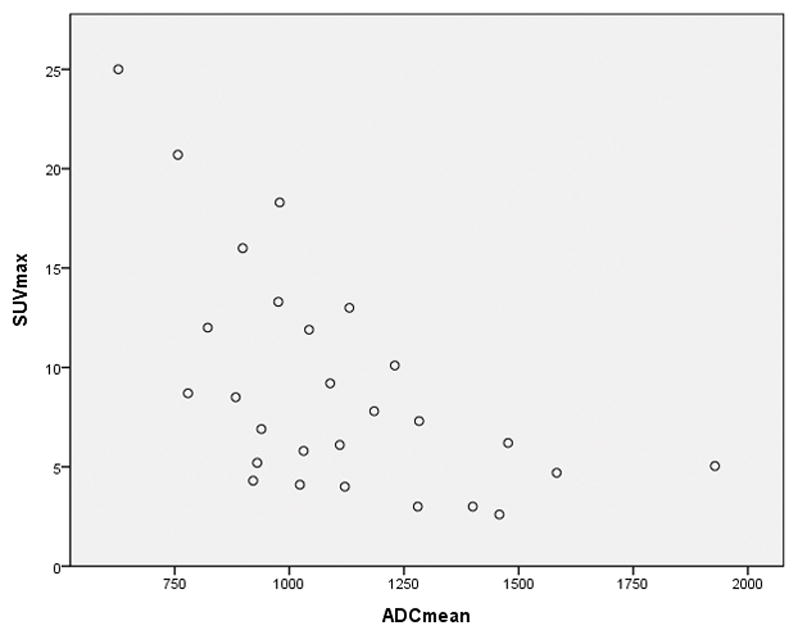
The relationship between SUVmax and ADCmean (x10−3) on PET/MRI. Correlation coefficient rho= −0.642, p=0.001, (95% Confidence interval −0.339 to −0.824).
SUVmax analysis
There was a strongly positive correlation between SUVmax on PET/CT and on PET/MRI (figure 5), with Spearman’s rho correlation coefficient of 0.842 (p<0.001).
Figure 5.
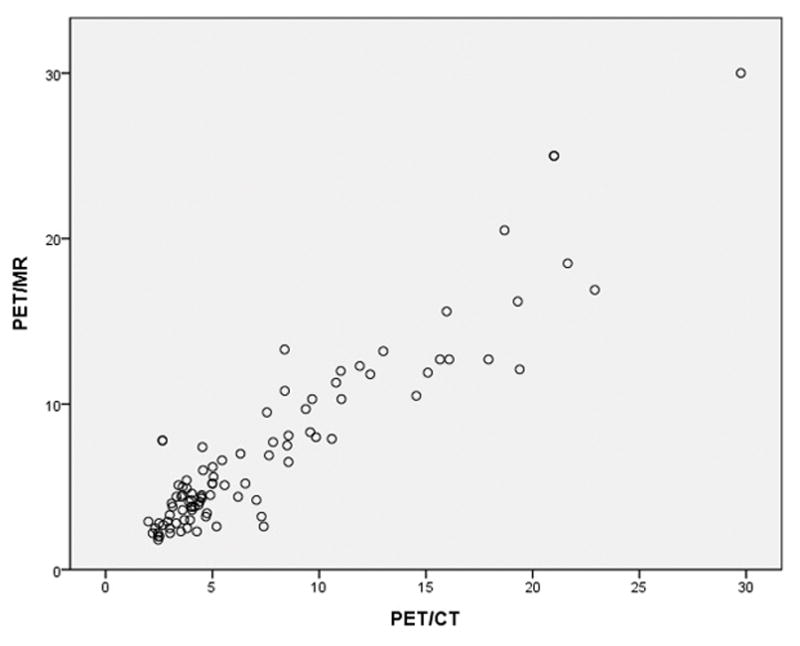
SUVmax of all nodal sites of disease on PET/CT and PET/MRI. A significantly positive correlation, Spearman’s rho correlation coefficient = 0.842 (p<0.001).
The Bland-Altman analysis (Figure 6) showed a mean difference between SUVmax from PET/CT and PET/MRI 0f 0.3157 (95% confidence interval −0.1197 to 0.7512), standard deviation of the difference as 2.13, upper limit 4.48 (95% CI 3.74 to 5.23), lower limit −3.85 (95% CI −4.60 to −3.11). This demonstrated the mean of the difference between SUVmax from PET/CT and PET/MRI is not significantly different from 0 (p = 0.15).
Figure 6.
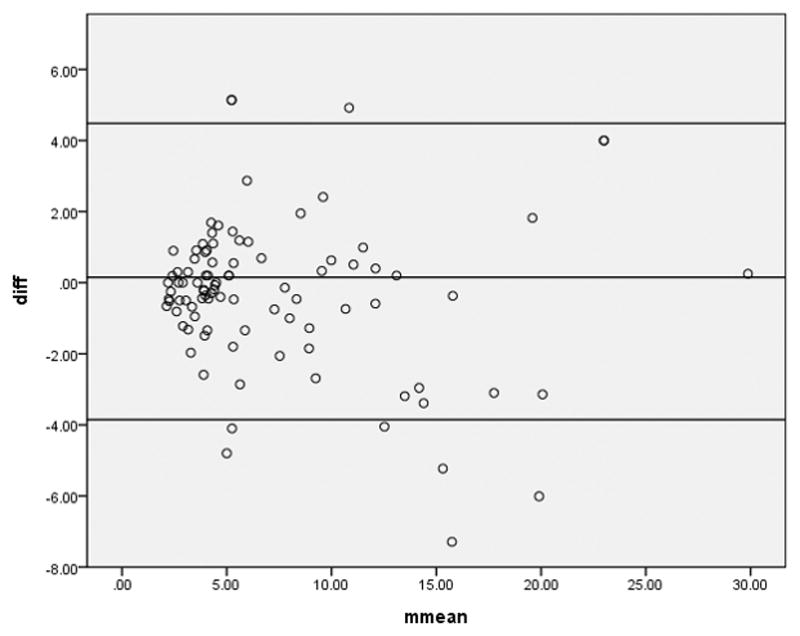
Bland-Altman analysis of mean difference of SUVmax from PET/CT and PET/MRI. (p=0.211). The mean of the difference between SUVmax from PET/CT and PET/MRI is not significantly different from 0.
Discussion
This study demonstrated that PET/MR had comparable ability to detect nodal and extranodal disease in a range of lymphomas and was equivalent in providing the overall stage and disease status when compared with the reference standard of PET/CT.
There were a small number of discrepant cases where PET/MR detected additional lesions. As these areas were not subjected to further biopsy it is not possible to give a definitive answer as to whether these were genuine sites of disease. Three nodal sites detected in the abdomen and axilla on PET/MRI and not on PET/CT may have been due to increased tracer accumulation with delayed acquisition. However, an additional site of extranodal (adrenal) disease reported on PET/MRI had the benefit of follow up PET/CT imaging (showing a continued increase in size and metabolic activity) which suggested this was a true site of disease.
This adrenal lesion may have been identified due to a combination of better anatomic detail on MRI as well as increased tracer accumulation within the lesion from the delayed acquisition. Therefore, a definite benefit from the modality alone cannot be confirmed.
Inter reader agreement was near perfect to perfect for nodal, extranodal and staging for readers in PET/MRI and for readers of PET/CT.
Semiquantitative assessment on PET/MRI (measurement of SUVmax) demonstrated excellent correlation with the same data from PET/CT. These two features are essential components for validation of PET/MRI prior to adoption in to clinical trials and routine clinical use.
The findings from this study are in agreement with those from a pilot study of 28 patients with lymphoma by Heacock et al, where PET/CT and PET/MRI staging was concordant in 96.4% [14]. The one case of discrepancy in that study involved PET/MRI detecting bone marrow involvement not identified on PET/CT. In our study, only two patients had bone marrow involvement, both correctly identified on PET/CT and PET/MRI. Our study also evaluated inter-reader agreement in this population which had not previously been reported.
Diffusion weighted imaging when assessed in a qualitative manner provided no additional value to PET in detecting sites of disease or in altering staging or disease status. Similar findings of a lack of benefit of DWI in lesion localisation in addition to routine PET/MRI sequences has been reported in a range of tumours. In 25 oncology patients, Buchbender et al identified 49 lesions both with PET/MRI alone and with PET/MRI combined with DWI [15,16]. There may be a potential role for DWI improving diagnostic accuracy in lymphomas with variable FDG uptake, although such types e.g. MALT lymphoma were not represented in our study population [17].
Only the most avid lesions in each PET/MRIstudy were chosen and the results demonstrated a negative correlation between these two imaging biomarkers. In a small study of patients with DLBCL, ADCmean correlated inversely with the SUVmax (r=−0.74, P<0.05) (18). In a mixed population of patient with DLBCL and FL, Wu et al found significant correlations between ADCmin and SUVmax (19). The relationship was also demonstrated in paediatric Hodgkin lymphoma (20). However, other studies have found no significant correlation between ADC and SUVmetrics [21,22].
The lack of an exact and consistent correlation can be explained by the different physiological mechanisms which each biomarker examines. Although SUV is well recognised as a marker of tumour glucose metabolism and therefore cellular proliferation, the components contributing to an ADC value are more complex, and include cellular density, extracellular fibrosis, the shape and size of the intercellular spaces, and other microscopic tissue/tumour organizational characteristics. These ADC components can be considered to reflect tumour aggressiveness and a relationship between tumour proliferation and tumour aggressiveness is widely recognised.
Although the DWI did not alter disease detection, there is potential for this to provide prognostic information in patients with lymphoma. A recent study in Hodgkins lymphoma found pre-treatment ADC was predictive of site specific interim response to chemotherapy [23].
Aside from the comparable diagnostic performance as shown in this study, there are several other major issues to consider with regards to implementing PET/MRI into clinical practice. Recently described in a paediatric population, PET/MRI can offer significant ionising dose reduction; 73% (± 11%) reduction in total effective dose in one study [24]. The length of scanning time is comparable for PET/MRI and PET/CT when anatomic sequences alone are performed. DWI significantly increased scan time for PET/MRI, although this study showed no additional benefit of acquiring these images. There are considerable start-up costs for introducing a PET/MR service and the cost of a PET/MR scan is greater than PET/CT, for example, £1300 Vs £950 in the UK.
Limitations and further considerations
Due to the acquisition of PET/MRI from the radiotracer injected for the preceding PET/CT and the location of the PET/MRI being in a different building within our site, there was a variable delay of acquisition time for the PET. This could have increased the uptake in some sites of disease relative to background due to tracer accumulation. An overestimation of FDG uptake has been reported when PET/MRI is performed after PET/CT [10], although others have described a decrease in SUVmax within lesions [25].
A reduced time interval between the two studies may have minimised this effect, but although two more nodal and one extranodal sites were deemed positive on PET/MRI, in only one case did this result in altered response status. The finding of additional nodal stations being called positive with PET/MRI was not described by Heacock et al, possibly related to a shorter duration between injection and uptake time for PET/MRI when compared with our study [14].
Patients were selected on the basis of consecutive clinically indicated PET/CTs for lymphoma assessment. These were not limited to baseline or particular types of lymphoma and therefore there was heterogeneity in the patient population. This was in order to create a large enough cohort to generate meaningful results and reflect clinical workload within the department. None the less this could have created inhomogeneity and thus bias in the study population.
Clinically, a five point score has been widely implemented to quantify response assessment by measuring SUVmax relative to mediastinal blood pool and background liver uptake [26] with good inter reader agreement. [27,28,29,]. The score is based on the most avid site of disease with reference to mediastinal blood pool and liver SUVmax. In this study, only uptake greater than liver background (equivalent to a score of 4 or more) was used to indicate positive sites of disease. A score of 4 is accepted as indicating active metabolic disease whereas uptake greater than mediastinum background but less than liver background (score of 3) is in many cases linked with good outcome and probable remission. SUVmax is a more robust comparator over time which was useful given extra delay with imaging the PET/MRI studies. Prolonged uptake times lead to an increased washout in normal structures, which appear more marked in the mediastinal blood pool. In 99 patients, Chin et al [30] demonstrated the mean SUVs on 3- versus 1-hour images were significantly lower for aortic blood pool 13% (p < 0.0001) but found no significant difference in the liver (1%, p=0.85).
PET/MRI allows an opportunity for numerous MRI sequences to be acquired for diagnostic purposes. At the most basic level, the MRI component can provide anatomic localisation of FDG uptake. However, there is potential for more advanced, functional sequences to improve upon PET/CT in lesion detection. We did not find DWI to be of help, but did not investigate other MRI sequences e.g. STIR for bone marrow involvement in the interest of reducing scan time. Despite this, both cases with marrow involvement on PET/CT were also correctly identified as such on PET/MRI.
Therefore we have shown compelling data that PET/MRI is at least equivalent to PET/CT in detection of nodal disease, extranodal disease and in providing staging/disease status. Future studies may include additional MR sequences to localise disease and study the predictive and prognostic benefits of using PET/MRI.
Conclusion
PET/MRI is a reliable alternative to PET/CT in the evaluation of patients with lymphoma. A small number of additional sites of disease were detected with PET/MRI, although this may have been due to delayed acquisition time.
There is scope to gain additional prognostic information from PET/MRI not available on PET/CT, although this data does not influence lesion detection or staging - DWI offered no diagnostic benefit in this population. A weakly negative but significant correlation between SUVmax and ADCmean was compatible with some other published studies. Given the inherent benefits of ionising radiation dose reduction, and the fact that patients will often require multiple scans, PET/MRI has the potential to become part of routine lymphoma imaging.
Acknowledgments
Funding received from the National Institute for Health Research University College London Hospitals Biomedical Research Centre and UCL Experimental Cancer Medicine Centre.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [accessed October 3rd 2015]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 3.Zinzani PL, Rigacci L, Stefoni V, et al. Early interim 18F-FDG PET in Hodgkin’s lymphoma: Evaluation on 304 patients. Eur J Nucl Med Mol Imaging. 2012;39:4–12. doi: 10.1007/s00259-011-1916-8. [DOI] [PubMed] [Google Scholar]
- 4.Safar V, Dupuis J, Itti E, et al. Interim[18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 5.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Littooij AS, Kwee TC, de Keizer B, et al. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: A prospective multicenter study. J Magn Reson Imaging. 2015 May 8; doi: 10.1002/jmri.24938. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Mayerhoefer ME, Karanikas G, Kletter K, et al. Evaluation of Diffusion-Weighted Magnetic Resonance Imaging for Follow-up and Treatment Response Assessment of Lymphoma: Results of an 18F-FDG-PET/CT-Controlled Prospective Study in 64 Patients. Clin Cancer Res. 2015;21:2506–13. doi: 10.1158/1078-0432.CCR-14-2454. [DOI] [PubMed] [Google Scholar]
- 8.van Ufford HM, Kwee TC, Beek FJ, et al. Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18F-FDG PET/CT. AJR Am J Roentgenol. 2011;196:662–9. doi: 10.2214/AJR.10.4743. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Möller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–526. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 10.Al-Nabhani KZ, Syed R, Michopoulou S, et al. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. J Nucl Med. 2014 Jan;55:88–94. doi: 10.2967/jnumed.113.123547. [DOI] [PubMed] [Google Scholar]
- 11.Grueneisen J, Schaarschmidt BM, Beiderwellen K, et al. Diagnostic value of diffusion-weighted imaging in simultaneous 18F-FDG PET/MR imaging for whole-body staging of women with pelvic malignancies. J Nucl Med. 2014 Dec;55:1930–5. doi: 10.2967/jnumed.114.146886. [DOI] [PubMed] [Google Scholar]
- 12.Catalano OA, Rosen BR, Sahani DV, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients--a hypothesis-generating exploratory study. Radiology. 2013;269:857–69. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 13.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–74. [PubMed] [Google Scholar]
- 14.Heacock L, Weissbrot J, Raad R, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015;204:842–8. doi: 10.2214/AJR.14.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaarschmidt BM, Grueneisen J, Heusch P, et al. Oncological whole-body staging in integrated (18)F-FDG PET/MR: Value of different MR sequences for simultaneous PET and MR reading. Eur J Radiol. 2015;84:1285–92. doi: 10.1016/j.ejrad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Buchbender C, Hartung-Knemeyer V, Beiderwellen K, et al. Diffusion-weighted imaging as part of hybrid PET/MRI protocols for whole-body cancer staging: does it benefit lesion detection? Eur J Radiol. 2013 May;82(5):877–82. doi: 10.1016/j.ejrad.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Mayerhoefer ME, Karanikas G, Kletter K, et al. Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin Cancer Res. 2014;20:2984–93. doi: 10.1158/1078-0432.CCR-13-3355. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Kellokumpu-Lehtinen PL, Pertovaara H, et al. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B-cell lymphoma – a pilot study: comparison with 2-deoxy-2-fluoro-D-glucose-positron emission tomography/computed tomography. NMR Biomed. 2011;24:1181–1190. doi: 10.1002/nbm.1689. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Pertovaara H, Korkola P, et al. Correlations between functional imaging markers derived from PET/CT and diffusion-weighted MRI in diffuse large B-cell lymphoma and follicular lymphoma. PLoS One. 2014;9:e84999. doi: 10.1371/journal.pone.0084999. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punwani S, Prakash V, Bainbridge A, et al. Quantitative diffusion weighted MRI: a functional biomarker of nodal disease in Hodgkin lymphoma? Cancer Biomark. 2010;7(4):249–59. doi: 10.3233/CBM-2010-0197. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Korkola P, Pertovaara H, Eskola H, Järvenpää R, Kellokumpu-Lehtinen PL. No correlation between glucose metabolism and apparent diffusion coefficient in diffuse large B-cell lymphoma: A PET/CT and DW-MRI study. Eur J Radiol. 2011;79:e117–e121. doi: 10.1016/j.ejrad.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 22.de Jong A, Kwee TC, de Klerk JM, et al. Relationship between pretreatment FDG-PET and diffusion-weighted MRI biomarkers in diffuse large B-cell lymphoma. Am J Nucl Med Mol Imaging. 2014;4:231–8. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- 23.Punwani S, Taylor SA, Saad ZZ, et al. Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging. 2013 Feb;40(3):373–85. doi: 10.1007/s00259-012-2293-7. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273:220–231. doi: 10.1148/radiol.14131732. [DOI] [PubMed] [Google Scholar]
- 25.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience with integrated whole-body PET/MRI: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 26.Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 27.Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–1833. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 28.Le Roux PY, Gastinne T, Le Gouill S, et al. Prognostic value of interim FDG PET/CT in Hodgkin’s lymphoma patients treated with interim response-adapted strategy: Comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011;38:1064–1071. doi: 10.1007/s00259-011-1741-0. [DOI] [PubMed] [Google Scholar]
- 29.Furth C, Amthauer H, Hautzel H, et al. Evaluation of interim PET response criteria in paediatric Hodgkin’s lymphoma-results for dedicated assessment criteria in a blinded dual-centre read. Ann Oncol. 2011;22:1198–1203. doi: 10.1093/annonc/mdq557. [DOI] [PubMed] [Google Scholar]
- 30.Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2009 Mar-Apr;11(2):118–22. doi: 10.1007/s11307-008-0177-9. Epub 2008 Nov 27. [DOI] [PubMed] [Google Scholar]