Abstract
The pericentriolar stacks of Golgi cisternae undergo extensive reorganization during mitosis in mammalian cells. GM130 and GRASP65 (Golgi reassembly stacking protein of 65 kDa) are Golgi-associated proteins that are targets of mitotic kinases, and they have also been implicated in the reorganization of the Golgi structure during cell division. Previous studies have reported that mitogen-activated protein kinase kinase-1 (MEK1) and Cdc2 protein kinases are involved in these dynamic changes in the Golgi structure. More recently, the mitotic polo-like kinase (Plk) has been shown to interact with and phosphorylate GRASP65. Here, we provide evidence that Plk is involved in the mitosis-specific fragmentation of the Golgi apparatus. The addition of kinase-defective Plk or immunodepletion of Plk disrupts the fragmentation process. Furthermore, Golgi fragmentation is inhibited by the addition of either full-length or truncated GRASP65. These findings suggest that phosphorylation of GRASP65 by Plk may be a critical event in the reorganization of the Golgi structure during mitosis.
The stacks of Golgi cisternae undergo extensive fragmentation during mitosis in mammalian cells; thus, fragmentation into smaller units is proposed to allow partitioning of the Golgi apparatus into the daughter cells (1). It has been shown that mitosis-specific reactions catalyze the process of Golgi fragmentation. But two major issues remain unresolved: the nature of the molecular machinery that mediates the fragmentation process, and the enzymes that regulate the cell cycle-specific functions of the fragmentation machinery. Thus far, two protein kinases, Cdc2 and mitogen-activated protein kinase kinase-1 (MEK1), have been implicated in mitosis-specific Golgi fragmentation (2, 3). Cdc2 phosphorylates GM130, a Golgi-associated protein (3). MEK1, on the other hand, phosphorylates and activates the mitogen-activated protein kinase ERK (extracellular signal-regulated kinase), and ERK was shown to phosphorylate the peripheral Golgi protein GRASP55 (Golgi reassembly stacking protein of 55 kDa) in vitro (4). It is, however, not clear whether these phosphorylation events contribute directly or indirectly to the fragmentation and dispersal of Golgi membranes at the onset of mitosis. More recently, we have shown that GRASP65, another peripheral Golgi protein related to GRASP55 and with a proposed role in the postmitotic reassembly of Golgi cisternae, is phosphorylated by a mitotic kinase of the polo-like kinase (Plk) family (5, 6).
Members of the Plk family of kinases influence multiple events during cell division, including centrosome maturation, spindle function, chromosome segregation, regulation of the anaphase-promoting complex, and execution of cytokinesis (7). The mammalian mitotic polo-like kinase, Plk, is a serine/threonine protein kinase whose activity peaks at the onset of M-phase (8–10). Plk activity is regulated by cell cycle-specific changes in its abundance, as well as by phosphorylation (9). Moreover, Plk undergoes a remarkable intracellular redistribution as cells progress through the cell cycle (8). During interphase, Plk appears to be mostly cytosolic; early in mitosis, Plk associates with centrosomes and spindle poles; then, it is found at the equatorial plane of the mitotic spindle at later stages of mitosis. These properties of Plk and the observation that it phosphorylates GRASP65 (6) prompted us to examine whether Plk is involved in the fragmentation of the pericentriolar Golgi apparatus during mitosis.
To address this issue, we used an assay in which the fragmentation of the pericentriolar Golgi apparatus is reconstituted in samples containing mitotic cytosol and an ATP-regenerating system (2). Our results show that kinase-defective Plk disrupts Golgi fragmentation, and that Plk is required for the mitotic cytosol-specific fragmentation of the Golgi apparatus. We also provide evidence that Plk by itself, in the absence of other mitotic activities, is not sufficient to cause Golgi fragmentation. These findings indicate a new role for Plk in mitosis-specific events and add to the list of kinases that regulate the dynamics of the Golgi apparatus in a mitosis-specific manner. Furthermore, we show that GRASP65 is phosphorylated by recombinant Plk and by mitotic extract and that it may also be involved in mitotic Golgi fragmentation.
Materials and Methods
Cell Culture, Preparation of Mitotic and Interphase Cytosol, and Golgi Fragmentation Assay.
Normal rat kidney (NRK) cells were grown in complete medium consisting of α-MEM (GIBCO/BRL) containing 10% FCS, 10 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 incubator. The cytosol from NRK cells arrested either in mitosis or in interphase, at a concentration of 12–14 mg/ml, was prepared as described (2). Permeabilization of cells grown on coverslips and the mitotic Golgi fragmentation assay were performed as described (2). In brief, NRK cells grown on coverslips were treated with 2 mM thymidine for 8–14 h. Cells were then permeabilized on ice with 30 μg/ml digitonin in KHM buffer [25 mM Hepes-KOH, pH 7.4/125 mM potassium acetate/2.5 mM magnesium acetate], washed with KHM containing 1 M KCl, and incubated with the reaction mixture containing an ATP-regenerating system for 60 min at 32°C. Incubations were carried out in a volume of 50 μl. For each experiment, 200 cells per coverslip were analyzed for their Golgi morphology.
In experiments analyzing the effect of recombinant Plk on Golgi morphology, cells grown on coverslips were permeabilized with digitonin and washed with 1 M KCl-containing buffer as described above. Semi-intact cells were then incubated with an ATP-regenerating system and 20 μg/ml constitutively active glutathione S-transferase (GST)-tagged Plk in the absence or presence of interphase cytosol for 60 min at 32°C. The Golgi morphology was visualized by staining the samples with anti-mannosidase II antibody.
Expression and Purification of Recombinant Proteins.
Recombinant Plk was purified from SF9 insect cells (kindly provided by Dr. James Kadonaga, University of California at San Diego) infected with baculovirus encoding GST-tagged Plk-K82M (kinase inactive) or GST-tagged Plk-T210D (constitutively active). The recombinant proteins were isolated by adsorption to glutathione Sepharose beads (Amersham Pharmacia) and eluted from the beads with 30 mM glutathione. The eluate was dialyzed against KHM containing 5% glycerol. During the purification process, Plk was followed by Western blot analysis using a commercial monoclonal anti-Plk antibody (Zymed).
GRASP65 constructs were generated by PCR using a full-length FLAG-tagged cDNA construct in pBSK as template (6). The PCR fragments, full-length (upstream primer 1, 5′-GGAGGCATGCGGGGCTAGGGGCAAGCAGCGAG; downstream primer 1, 5′-GGAGAAGCTTCTACTTGTCGTCCTTGTAG), 1–134 (upstream primer 1, downstream primer 2, 5′-GGAGAAGCTTCTACTTGTCATCGTCGTCCTTGTAGTCGTAGTCTGTGTAAGG), and Δ129 (upstream primer 2, 5′-GGAGGCATGCCCCTTACACAGACTACATTG, downstream primer 2), were subcloned into the SphI and HindIII restriction sites of the pQE31 bacterial expression vector (Qiagen, Chatsworth, CA). Recombinant proteins were obtained by expression in bacteria and subsequent purification with Ni-agarose. Purified proteins were concentrated with Microcon spin columns (Millipore) and dialyzed against KHM, 5% glycerol. It is important to note that all recombinant proteins were purified in the complete absence of detergent.
Immunodepletion of Plk from Mitotic Cytosol.
The crude rabbit antiserum 8847, directed against amino acids 6–24 of murine Plk (9), was coupled to protein G-Sepharose beads (Amersham Pharmacia) in PBS for 2 h at 4°C. The antibody–protein G complex was washed once with PBS and three times with KHM and incubated with mitotic cytosol. After 25 min, the beads were pelleted, and the cytosol was incubated with fresh antibody–protein G complex for another 25 min. Mock incubations were performed for the same periods of time in the absence of Plk antibody.
Microinjections.
NRK cells were grown on coverslips and arrested in S-phase with 2.5 μg/ml aphidicolin for 4 h before nuclear injections of plasmid encoding hemagglutinin (HA)-tagged constitutively active Plk (pCI-neo PlkT210D; ref. 6). Cells were kept in the presence of aphidicolin for 6 h postinjection and were then processed for immunofluorescence. Cells were double-stained by using monoclonal anti-HA and polyclonal anti-mannosidase antibody to visualize injected cells and Golgi morphology, respectively.
Results
Disruption of Golgi Fragmentation by Kinase-Defective Plk.
We have reconstituted mitosis-specific fragmentation of the Golgi apparatus in normal rat kidney (NRK) cells. NRK cells are permeabilized, washed with 1 M KCl-containing buffer, and incubated with mitotic cytosol prepared from NRK cells. After incubation for 60 min at 32°C in the presence of an ATP-regenerating system, the Golgi apparatus is found in the form of small punctate structures dispersed throughout the cytoplasm (2). Electron microscopy analysis of these cells revealed that the small structures are composed of tubulo-reticular Golgi membranes (11). Fractionation of mitotic cytosol on a phosphocellulose column revealed that the Golgi fragmentation activity was in the flow through fractions. Although Cdc2 is bound by the phosphocellulose and absent from the flow through fractions, both MEK1 (2) and Plk (data not shown) were found in the flow through fractions containing the fragmentation activity.
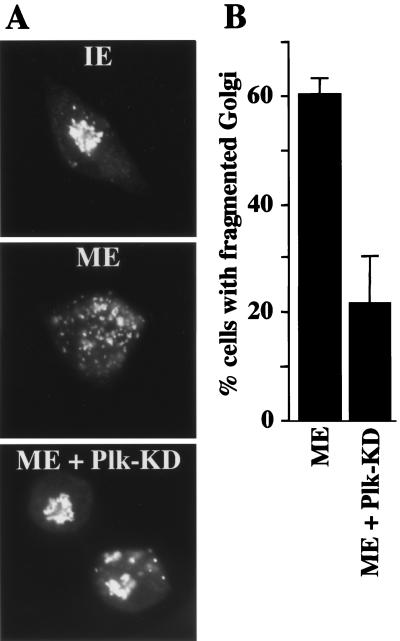
To examine the role of Plk in mitotic Golgi fragmentation, permeabilized NRK cells were incubated in the presence of an ATP-regenerating system with either interphase or mitotic cytosol and in the absence or presence of 20 μg/ml of a kinase-inactive form of Plk (Plk-KD; Fig. 1A). Plk-KD, which is unable to phosphorylate known Plk substrates in vitro, has been shown to disrupt Plk function and the cell cycle when overexpressed in mammalian cells (10, 12). After incubation with cytosol and recombinant Plk, the cells were processed for fluorescence microscopy with anti-mannosidase II antibody, a marker of the cis/medial Golgi cisternae (2, 13). The experiment was quantitated (see Materials and Methods) and revealed that, in the presence of Plk-KD, only 21% of cells showed a fragmented Golgi apparatus compared with 60% in control incubations (Fig. 1 A and B). The ability of some cells to undergo Golgi fragmentation (Fig. 1B) even in the presence of Plk-KD indicates that, under these assay conditions, either the amount of Plk-KD used is not sufficient for completely abrogating the functions of endogenous Plk or other Plk-independent mechanisms may also function in mitotic Golgi fragmentation.
Figure 1.
Kinase inactive Plk inhibits Golgi fragmentation by mitotic cytosol. (A) NRK cells were grown on coverslips and treated with 2 mM thymidine for 8 to 14 h. Cells were subsequently permeabilized with digitonin, washed with 1 M KCl-containing buffer, and incubated with either 7 mg/ml interphase cytosol (IE), 7 mg/ml mitotic extract (ME), or mitotic extract to which 20 μg/ml kinase inactive Plk (ME + Plk-KD) was added. After a 60-min incubation at 32°C, cells were fixed and stained with anti-mannosidase II antibody to visualize the Golgi apparatus by fluorescence microscopy. (B) Percentage of cells with fragmented Golgi after incubation with mitotic extract (ME) in the absence or the presence of kinase inactive Plk (ME + Plk-KD). The histogram represents the average of four independent experiments.
Plk Is Required for Golgi Fragmentation.
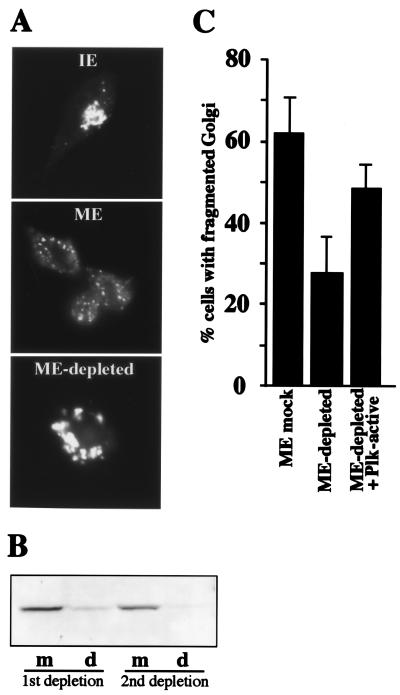
To determine the requirement for Plk in the fragmentation process, an antibody recognizing the N-terminal 6–24 aa of murine Plk was conjugated to protein G-Sepharose and used for immunodepletion of Plk from mitotic cytosol. As can be seen in Fig. 2B, two rounds of incubation with anti-Plk antibody coupled to protein G-Sepharose were necessary to deplete greater than 90% of Plk from the mitotic cytosol (d = depleted). Control depletions (m = mock depletion) were carried out by incubating mitotic extract with protein G-Sepharose beads only. The same blot was also analyzed for the presence of MEK1, and we found that immunodepletion of Plk did not reduce the level of MEK1 in the cytosol (data not shown).
Figure 2.
Immunodepletion of Plk from mitotic cytosol inhibits Golgi fragmentation. (A) Semi-intact, salt-washed NRK cells were incubated in the presence of an ATP-regenerating system with either 7 mg/ml interphase cytosol (IE), 7 mg/ml mitotic extract (ME), or 7 mg/ml mitotic extract depleted of Plk (ME-depleted). After a 60-min incubation at 32°C, cells were fixed and stained with anti-mannosidase II antibody to visualize the Golgi apparatus by fluorescence microscopy. (B) Immunoblot of mock (m) and Plk-depleted (d) mitotic extracts. Greater than 90% depletion of Plk was achieved by two consecutive rounds of incubation of mitotic extract with anti-Plk antibody conjugated to protein G-Sepharose beads. (C) Percentage of cells with fragmented Golgi after incubation of semi-intact cells with either mock (ME mock) or Plk-depleted (ME-depleted) mitotic cytosol or Plk-depleted cytosol to which 20 μg/ml constitutively active recombinant Plk (ME-depleted + Plk-active) was added. The data represent the average of four independent experiments.
Testing mock and Plk-depleted extract in the mitotic assay revealed that depletion of Plk prevented Golgi fragmentation (Fig. 2 A and C). To further assure that this loss of fragmentation was due to specific removal of Plk, we tested the ability of purified constitutively active, recombinant Plk to restore the activity of the Plk-depleted extract. As shown in Fig. 2C, the added recombinant Plk substantially restored the Golgi fragmenting activity of the Plk-depleted extract. Similar to the dominant-negative experiment using Plk-KD (Fig. 1), the inhibition of Golgi fragmentation was not complete when Plk has been immunodepleted from the mitotic cytosol (Fig. 2C). This finding may reflect the limitations of the experimental system where a residual amount of Plk, undetectable by Western analysis, still remains even after two rounds of incubation with Plk antibody and is able to drive the fragmentation process. Alternatively, as noted previously, other Plk-independent mechanisms may also potentiate the fragmentation process. In light of the partial inhibition of Golgi fragmentation by the dominant-negative enzyme or by immunodepletion of Plk, it should be noted that the extent of the inhibition by disrupting Plk function is similar to that observed when MEK1 or Cdc2 has been disrupted by drugs or immunodepletion (2, 3).
Plk Alone Is Not Sufficient To Cause Fragmentation of the Pericentriolar Golgi Apparatus.
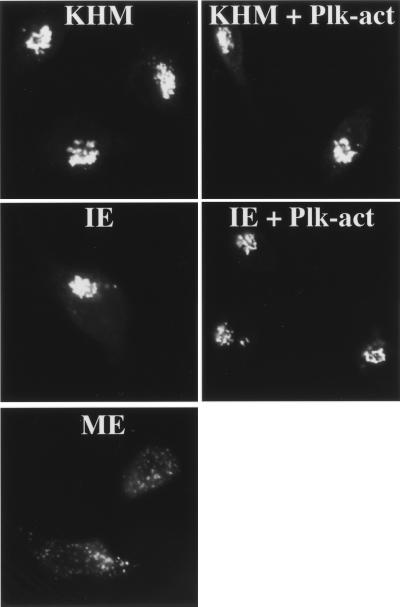
We next conducted experiments to determine whether Plk activity alone is sufficient to drive the fragmentation process. Permeabilized NRK cells were incubated with an ATP-regenerating system and with constitutively active Plk in the presence or absence of interphase cytosol. Immunofluorescence microscopy was carried out to visualize the organization of Golgi membranes in treated and control cells. Our results show that Plk by itself or when combined with interphase cytosol had no appreciable effect on the Golgi membranes, as revealed by light microscopy (Fig. 3).
Figure 3.
Constitutively active Plk is not sufficient to cause Golgi fragmentation. Semi-intact NRK cells were incubated with an ATP-regenerating system and KHM buffer (KHM), 7 mg/ml interphase cytosol (IE), 7 mg/ml mitotic cytosol (ME), 20 μg/ml constitutively active GST-tagged Plk in KHM (KHM + Plk-act), or 20 μg/ml constitutively active GST-tagged Plk added to 7 mg/ml interphase extract (IE + Plk-act). After a 60-min incubation at 32°C, cells were processed for immunofluorescence by using anti-mannosidase II antibody to monitor Golgi morphology.
We also addressed this issue by using a different approach. NRK cells were arrested in interphase (S-phase) by treatment with aphidicolin as described (11). A plasmid encoding hemagglutinin-tagged constitutively active Plk was injected into the nucleus of interphase-arrested NRK cells, and the expression of recombinant protein and the Golgi morphology was determined by immunofluorescence microscopy. Expression of constitutively active Plk had no apparent effect on the organization of Golgi membranes, which remained in the pericentriolar region (data not shown). These results support our findings in vitro (Fig. 3) that Plk alone or when combined with interphase cytosol does not cause any detectable change in the organization of the pericentriolar Golgi apparatus at the light microscopy level. There are two possible explanations for this result: (i) other activities in addition to Plk are required for mitosis-specific Golgi fragmentation; (ii) Plk has to undergo a specific change that occurs only in mitosis and this change (e.g., phosphorylation) is necessary to target injected active mutant Plk for Golgi fragmentation. This is a strong possibility because we have found that MEK1 undergoes a specific change by phosphorylation, in a mitosis-specific manner, and this change is necessary for Golgi fragmentation (11).
GRASP65 Is a Potential Downstream Target of Plk on the Golgi Membranes.
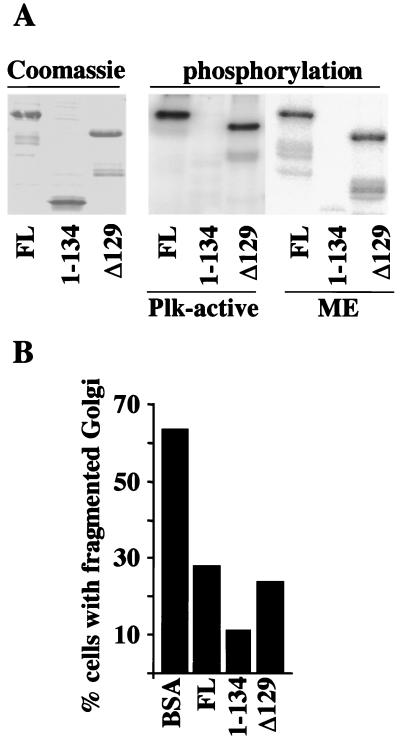
Our results provide strong evidence that Plk is required for the fragmentation of the Golgi apparatus, but only in the presence of other mitosis-specific factors. The Golgi-associated protein GRASP65 is a major mitotic phosphoprotein (5), and GRASP65 can be coimmunoprecipitated with Plk when coexpressed with Plk, and is phosphorylated by Plk (6). Based on two-hybrid analysis, the binding site of Plk to GRASP65 has been mapped to residues 7–120; however, the exact sites of GRASP65 phosphorylation by Plk remain to be determined (6). To further characterize the interaction between GRASP65 and Plk, we expressed various recombinant GRASP65 fragments in bacteria and tested whether they could be phosphorylated either by Plk or by mitotic extract (Fig. 4A). The recombinant proteins, full-length GRASP65 (FL), the N-terminal Plk-binding domain of GRASP65 (1), and the C terminus of GRASP65 (Δ129) were incubated with either constitutively active Plk (Plk-act) or mitotic cytosol in the presence of [γ-32P]ATP. The products of the reaction were separated by SDS/PAGE and analyzed by autoradiography. Whereas both full-length GRASP65 and the C-terminal region are phosphorylated by mitotic cytosol and by constitutively active Plk (Fig. 4A), the N-terminal Plk-binding domain is not a substrate for phosphorylation. This result confirms our previous findings that GRASP65 is phosphorylated by Plk (6) and suggests that the phosphorylation sites are located in the C-terminal region of the protein.
Figure 4.
GRASP65 is involved in mitotic Golgi fragmentation. (A) Recombinant 6-His-tagged GRASP65 proteins [full-length (FL), the Plk-binding domain (1), and the C-terminal domain (Δ129)] were generated in bacteria and purified as described in Materials and Methods. These proteins were incubated with either recombinant constitutively active Plk (Plk-active) or mitotic cytosol (ME) in the presence of [γ-32P]ATP, and analyzed by SDS/PAGE. An autoradiograph of the gel is shown. (B) Recombinant GRASP65 proteins were preincubated for 10 min with mitotic cytosol before the Golgi fragmentation assay. After a 60-min incubation at 32°C, cells were fixed and stained with anti-mannosidase II antibody. A histogram of these experiments is shown; experiments were done in duplicate.
To analyze whether GRASP65 is involved in Plk-mediated Golgi fragmentation, we tested the effect of addition of GRASP65 fragments on mitotic Golgi fragmentation. NRK cells were treated as described previously and incubated with mitotic cytosol and an ATP-regenerating system in the presence of 100 μg/ml BSA as a control, full-length GRASP65, the Plk-binding domain (1), or the C-terminal domain of GRASP65 (Δ129). Immunofluorescence microscopy revealed that, in the presence of excess recombinant GRASP65, the Golgi apparatus was not found in small fragments, in contrast to the case with mitotic cytosol only. Instead, large structures were discernible similar to those observed on blocking Plk activity. The percentage of cells with fragmented Golgi is shown (Fig. 4B). Our results indicate that addition of exogenous GRASP65 inhibits the fragmentation of the pericentriolar Golgi apparatus by mitotic cytosol. Both the Plk-binding domain and the C-terminal domain of GRASP65, which is phosphorylated by Plk, also inhibit Golgi fragmentation by mitotic extract, most likely by sequestering the endogenous Plk. These results suggest that Plk-mediated binding and phosphorylation of GRASP65 may be involved in Golgi fragmentation during mitosis.
Discussion
The stacks of Golgi apparatus undergo extensive fragmentation during mitosis. Analysis in vitro thus far has suggested the involvement of two kinases in this process: MEK1 and Cdc2. The downstream target for MEK1 in this reaction is not known, although a recent analysis has revealed that GRASP55, a GRASP65-related protein, is phosphorylated by the MEK1 substrate ERK2 (4, 14). Cdc2, on the other hand, phosphorylates GM130 both in vitro and in vivo (3). It is not known, however, whether these phosphorylation events have direct or indirect roles in Golgi fragmentation. Plk was recently found to bind and phosphorylate GRASP65 (6). In this report, we provide evidence that Plk and GRASP65 are involved in mitotic Golgi fragmentation.
The data from our dominant-negative and immunodepletion experiments suggest that Plk is required for the fragmentation process. It is less clear, however, whether GRASP65 is directly involved in Golgi fragmentation. The inhibition of Golgi fragmentation by the addition of full-length and truncated GRASP65 may be due to blocking the interactions between endogenous Plk and Golgi components (GRASP65 or other Golgi proteins). The identification of the Plk phosphorylation sites in GRASP65 and the determination of the functional relevance of the N-terminal region of GRASP65, which appears to be involved in binding Plk, but which is not phosphorylated by Plk or other mitotic kinases, should help clarify the role of GRASP65 in Golgi fragmentation. It is also important to note that Cdc2 has been reported to phosphorylate GRASP65 in vitro (6), although it phosphorylates a limited number of sites compared with Plk. The interaction between Cdc2 and GRASP65 is less well characterized, however, and our Golgi fragmentation assay does not require Cdc2 activity (2). Additional experiments are required to obtain a more definitive picture regarding the role of GRASP65 in Plk-mediated fragmentation of pericentriolar Golgi stacks.
Based on analyses in vitro, GRASP65, as well as its relative GRASP55, has been implicated with a role in the stacking of Golgi cisternae (5, 15). It is possible that phosphorylation of GRASP55 and GRASP65 by MEK1-MAPK and Plk, respectively, destabilizes the Golgi stacks at the onset of mitosis and thus triggers the subsequent fragmentation. An alternative hypothesis is that GRASP55 and GRASP65 are also involved in the anchoring of Golgi membranes to the pericentriolar region. Phosphorylation of the GRASP proteins by the MAPK pathway and by Plk could disrupt the attachment of Golgi membranes, which then undergo further reorganization and dispersal throughout the cell. It is unlikely that Cdc2, MEK1, Plk, and their substrates are the only molecules involved in the fragmentation and partitioning of Golgi membranes during the cell cycle.
The stacks of Golgi cisternae are connected to each other and protein complexes that keep the stacks in the pericentriolar vicinity. It is most likely that a large number of proteins are involved in maintaining such a large and dynamic structure in the pericentriolar region. During mitosis, the Golgi stacks have to be separated from each other and from the pericentriolar region. There is also the issue of whether the smaller Golgi fragments undergo further processing to generate small vesicles or fuse with the endoplasmic reticulum (11, 16, 17). It is therefore premature to conclude that the kinases and their potential substrates that have been identified so far constitute the minimal machinery required for Golgi fragmentation. While we await the identification of additional mitosis-specific factors, the most obvious challenges at present are as follows: (i) demonstrate a role for GRASP65 and its phosphorylation by Plk in the Golgi fragmentation process in vivo; and (ii) identify downstream target(s) of MEK1 on the Golgi to strengthen its proposed role in mitosis-specific Golgi dynamics. Moreover, although GRASP55 is phosphorylated by the MEK1 substrate ERK2 in vitro, it remains to be determined whether this phosphorylation event is important for mitotic Golgi fragmentation. Addressing these issues should further illuminate the mechanisms involved in the reorganization of the Golgi structure at the onset of mitosis.
Acknowledgments
We thank the members of the Malhotra lab for their support, helpful discussions, and comments on this manuscript. We thank Carolan Buckmaster and Dr. James Feramisco at the Microinjection Facility at the University of California at San Diego for doing the microinjections, and Eleanor Erikson for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (NIH; to V.M.). C.S. was supported by a long-term EMBO postdoctoral fellowship and by postdoctoral fellowships from the Novartis Foundation, Basel, Switzerland, and the Roche Foundation, Basel, Switzerland. These studies were also supported by NIH Grants CA62580 and GM59172 (to R.L.E.). R.L.E. is the John F. Drum American Cancer Society Professor of Cellular and Developmental Biology at Harvard University.
Abbreviations
- Plk
polo-like kinase
- GRASP65
Golgi reassembly stacking protein of 65 kDa
- MEK1
mitogen-activated protein kinase kinase-1
- ERK
extracellular signal-regulated kinase
- NRK
normal rat kidney
- GST
glutathione S-transferase
References
- 1.Warren G. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- 2.Acharya U, Mallabiabarrena A, Acharya J K, Malhotra V. Cell. 1998;92:183–192. doi: 10.1016/s0092-8674(00)80913-7. [DOI] [PubMed] [Google Scholar]
- 3.Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jamsa E, Rahman D, Pappin D J, Warren G. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 4.Lewis T S, Hunt J B, Aveline L D, Jonscher K R, Louie D F, Yeh J M, Nahreini T S, Resing K A, Ahn N G. Mol Cell. 2000;6:1343–1354. doi: 10.1016/s1097-2765(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 5.Barr F A, Puype M, Vandekerckhove J, Warren G. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 6.Lin C Y, Madsen M L, Yarm F R, Jang Y J, Liu X, Erikson R L. Proc Natl Acad Sci USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. . (First Published October 24, 2000; 10.1073/pnas.220423497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigg E A. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- 8.Golsteyn R M, Mundt K E, Fry A M, Nigg E A. J Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamanaka R, Smith M R, O'Connor P M, Maloid S, Mihalic K, Spivak J L, Longo D L, Ferris D K. J Biol Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- 10.Lee K S, Erikson R L. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colanzi A, Deerinck T J, Ellisman M H, Malhotra V. J Cell Biol. 2000;149:331–339. doi: 10.1083/jcb.149.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundt K E, Golsteyn R M, Lane H A, Nigg E A. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 13.Saraste J, Bronson M, Palade G E, Farquhar M G. Prog Clin Biol Res. 1988;270:129–139. [PubMed] [Google Scholar]
- 14.Jesch S A, Lewis T S, Ahn N G, Linstedt A D. Mol Biol Cell. 2001;12:1811–1817. doi: 10.1091/mbc.12.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shorter J, Watson R, Giannakou M E, Clarke M, Warren G, Barr F A. EMBO J. 1999;18:4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shima D T, Haldar K, Pepperkok R, Watson R, Warren G. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaal K J, Smith C L, Polishchuk R S, Altan N, Cole N B, Ellenberg J, Hirschberg K, Presley J F, Roberts T H, Siggia E, Phair R D, Lippincott-Schwartz J. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]