Abstract
Myeloid leucocytes mediate host protection against infection and critically regulate inflammatory responses in body tissues. Pattern recognition receptor signalling is crucial for myeloid cell responses to pathogens, but growing evidence suggests an equally potent role for Calcineurin–NFAT signalling in control of myeloid cell function. All major subsets of myeloid leucocytes employ Calcineurin–NFAT signalling during immune responses to pathogens and/or tissue damage, but the influence this pathway exerts on pathogen clearance and host susceptibility to infection is not fully understood. Recent data from experimental models indicate that Calcineurin‐NFAT signalling is essential for infection control, and calcineurin inhibitors used in transplantation medicine (including cyclosporine A and tacrolimus) are now being tested for efficacy in a diverse range of inflammatory conditions and autoimmune pathologies. Efforts to repurpose calcineurin inhibitor drugs for new therapeutic applications may yield rapid improvements in clinical outcomes, but the potential impact of these compounds on myeloid cell function in treated patients is largely unknown. Here we discuss Calcineurin–NFAT control of myeloid leucocyte function in the context of recent therapeutic developments and ongoing clinical studies.
Keywords: cyclosporine A, Dectin‐1, immunosuppression, tacrolimus, TLR4
Subject Categories: Haematology, Immunology
Glossary
- Adaptive immune response
Branch of immune system evolved in vertebrates to provide more specific recognition of dangerous antigens. Lymphocytes adapt to specific pathogen and confer lifelong protective immunity due to immunologic memory of adaptive response. In addition to specificity, the immunologic memory is the major benefit of adaptive immunity as it activates rapid and robust protective response in case of re‐exposure to the same antigen.
- Calcineurin inhibitors
Drugs that prevent calcineurin‐driven dephosphorylation and activation of nuclear factor of activated T cells (NFAT). The archetypal drugs in this class are cyclosporine A and tacrolimus which have revolutionized the field of organ transplantation. The main purpose of these inhibitors is to suppress T‐cell responses to allografts.
- Drug repurposing
Strategy of testing approved agents for new therapeutic applications. This is an important approach used to accelerate the discovery of new treatment strategies and reduce costs associated with development of novel compounds. Drug repurposing is typically guided by detailed molecular knowledge of the target pathologies.
- Fungal morphotypes
Some fungi species including Aspergillus fumigatus develop through different morphotypic stages; this includes conidia, swollen conidia and fully germinated hyphae. This process has important consequence for pattern recognition receptor stimulation as Aspergillus morphotypes express different amounts of β‐glucans on the surface.
- Immune synapse
Cell–cell communication used by immune cells. The formation of immune synapses is the initial event leading to adaptive immunity response and is characterized by membrane rearrangements in both cell types involved.
- Immunomodulatory chemokines and cytokines
Signalling compounds secreted by various cells to orchestrate immune response to a desired level, that is immunopotentiation, immunosuppression or induction of immunologic tolerance.
- Immunosuppressive therapy
Therapeutic administration of drugs that inhibit immune responses. Immunosuppressive drugs target several different mechanisms and are primarily used to prevent rejection of tissue grafts and transplants as well as suppress autoimmune diseases and inflammatory disorders.
- Innate response
Branch of the immune system that allows direct and immediate responses to pathogens. In vertebrates, this evolutionarily conserved host protection strategy is complemented by the adaptive immune system.
- Lupus nephritis
Inflammatory disease affecting the kidney and, more specifically, glomeruli. It is caused by systemic lupus erythematosus and can lead to kidney failure. Systemic lupus erythematosus is an autoimmune disease caused by production of autoimmune antibodies against nuclear antigens associated with chronic inflammation.
- Myeloid immunity
Immune response mediated by cells of myeloid origin such as granulocytes, macrophages, monocytes and some dendritic cell subsets. Upon pathogen invasion, myeloid cells are rapidly recruited to the site of infection, where they exert their effector functions (cytokine secretion, phagocytosis, etc.).
- Ontogeny
All developmental changes occurring throughout the existence of an individual organism. In cell biology, ontogeny refers specifically to developmental and differentiation processes within a cell lineage.
- Pattern recognition receptors
Set of innate immune receptors that recognize molecular patterns associated with pathogens or tissue damage. Advances in understanding of their molecular mechanisms of action are leading to the development of new therapies.
- Physical form of the antigen
Immunogenicity of soluble and particulate form of antigens is different, for example pattern recognition receptor binds the two forms of β‐glucan differently. Differences in this process following ligation by soluble versus particulate ligands enable receptor‐driven distinction between the two forms of the same antigen.
- Plasmacytoid DCs
Subset of dendritic cells specialized in rapid type I interferon production mainly in response to viruses or nucleic acids. pDCs have been implicated in pathogenesis of autoimmune diseases characterized by type I IFN signature.
- Rheumatoid arthritis
Chronic autoimmune disorder associated with tissue inflammation of the joints. Several clinical trials have already shown beneficial effects of immunosuppression in patients affected by this condition.
- Sjogren's syndrome
Autoimmune disorder characterized by chronic inflammation, infiltration of immune cells to exocrine organs and progressive destruction of moisture‐producing glands.
- T‐cell receptor
Complex of integral membrane proteins, which recognizes specific antigen and activate T cells. The variety of TCR is established by developmentally regulated TCR gene rearrangements followed by predominantly intra‐thymic selection processes.
Introduction
During an adaptive immune response, engagement of the T‐cell receptor stimulates an increase in intracellular calcium that promotes calmodulin binding to the serine/threonine protein phosphatase enzyme calcineurin. Once activated in this way, calcineurin can dephosphorylate nuclear factor of activated T‐cell (NFAT) transcription factors, which modify gene expression and regulate immune responses. The Calcineurin–NFAT pathway was initially described in T cells, where NFAT acts as a master regulator of lymphocyte development, expression of interleukin (IL)‐2, and controls major effector T‐cell functions. Accordingly, drugs developed to inhibit Calcineurin–NFAT binding such as cyclosporine A and tacrolimus have proven highly effective at suppressing T‐cell responses and preventing allograft rejection in transplantation medicine. While long regarded as a specific regulator of T‐cell activity, NFAT signalling was later also identified in other cell types including B cells (Muller & Rao, 2010), and was reported to mediate embryogenic development of multiple tissues including the hematopoietic system (Muller et al, 2009). These data suggested that the influence of Calcineurin–NFAT on immunity was not restricted to T cells alone and that potent effects outside the adaptive immune response were likely.
Despite widespread use in clinical settings, the mechanisms by which Calcineurin–NFAT inhibitors suppress host immunity are not as specific as was initially thought. Various studies of cyclosporine A and tacrolimus treatment have revealed a surprising ability of these drugs to disrupt T‐cell activation without impacting on Ca2+ flux (Metcalfe et al, 1994), and impair lymphocyte responses via effects on the mitogen‐activated protein kinase (MAPK) pathway (Su et al, 1994; Matsuda et al, 1998) in NFAT‐independent manner. Given that NFAT can also cooperate with transcription factors such as AP‐1 and NF‐kB to modify immune responses (Crabtree, 1989), it is perhaps not surprising that calcineurin inhibitors are now thought to mediate wide‐ranging effects that can also impact on myeloid cell function. The key roles of Calcineurin–NFAT signalling in myeloid cell biology have recently been discussed elsewhere (Muller & Rao, 2010; Fric et al, 2012), and myeloid lineages not directly involved in host immunity to pathogens will not be discussed in detail here. The current review instead focuses on Calcineurin–NFAT control of myeloid immunity because the growing number of clinical studies is now seeking to manipulate this pathway for therapeutic benefit in diverse human pathologies. Indeed, all five members of the NFAT family are known to be involved in regulation of immune responses (Macian, 2005), and a major risk of immunosuppressive therapy in human patients is increased susceptibility to opportunistic infections. Consequently, investigators seeking to repurpose calcineurin inhibitor drugs for new disease indications must consider recent reports that NFAT signalling exerts a critical influence on innate immune function, as well as emerging evidence that these therapies disrupt pathways known to be essential for myeloid cell defence against pathogens.
Pattern recognition receptors initiate Calcineurin–NFAT signalling in myeloid leucocytes
NFAT activation in dendritic cells (DCs) and macrophages was first observed upon cell activation through Dectin‐1 (Goodridge et al, 2007), and was soon followed by reports that NFAT activation could also be triggered by other pattern recognition receptors (PRRs) including TLR4 and CD14 (Fig 1).
Figure 1. PRR signal integration with the Calcineurin–NFAT pathway.
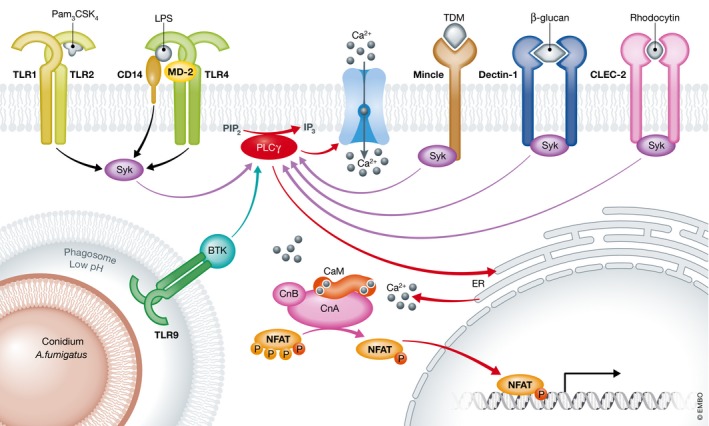
Multiple different pattern recognition receptors (PRRs) have been reported to trigger Calcineurin–NFAT signalling upon ligand binding. In particular, TLRs and C‐type lectin receptors (CLR) play critical roles in NFAT activation during innate immune responses. The observation that LPS exposure can stimulate NFAT‐mediated IL‐2 expression focused the majority of early research on the role of TLR4 (Granucci et al, 2001, 2003). The LPS co‐receptor CD14 was later also reported to promote Calcineurin–NFAT activation via recruitment of Syk (Zanoni et al, 2009). In mast cells, heterodimers of TLR1‐TLR2 recognize Pam3 CSK 4 and drive the recruitment of Fc‐γ receptor which contains ITAM motifs (Jin et al, 2016). TLR9 can also promote NFAT activation upon exposure to Aspergillus fumigatus conidia in acidified endosomes, which results in recruitment of BTK, activation of PLCγ followed by increase in intracellular calcium concentration and NFAT translocation to the nucleus (Herbst et al, 2015). Alternatively, Syk recruitment, PLCγ activation and nuclear localization of NFAT can instead be induced by ligand binding of ITAM‐containing CLRs such as Dectin‐1 (Goodridge et al, 2007), macrophage‐inducible Ca2+‐dependent lectin (Mincle) (Yamasaki et al, 2008) and CLEC‐2 (Mourao‐Sa et al, 2011; Severin et al, 2011).
Initial mechanistic studies sought to understand how NFAT regulates gene expression in DCs exposed to various types of pathogen (Granucci et al, 2001, 2003). In particular, much early research focused on the role of the CD14‐MD2‐TLR4 pathway in modulating NFAT signalling (Zanoni et al, 2009). This critical signalling axis is now known to exert complex effects on DC function via a range of different mechanisms, including LPS binding to CD14, which can induce calcium flux without the involvement of TLR4 (Zanoni et al, 2009), and internalization of the entire CD14‐MD2‐TLR4 complex which instead promotes interferon production (Kagan et al, 2008; Zanoni et al, 2011). Subsequent work has suggested that a large number of innate cell functions could be controlled by protein complexes such as these termed “signalling organelles” which cooperate to alter intracellular calcium levels and regulate gene expression (Zanoni et al, 2011; Chiang et al, 2012; Kagan, 2012). While it is now widely accepted that calcineurin can dephosphorylate NFAT to facilitate transcription factor translocation to the nucleus in myeloid cells as well as in lymphocytes, two important questions remain unresolved (i) which PRRs are involved in mediating this process? and (ii) why are NFAT‐driven expression programs highly specific to different cells and tissues?
Calcineurin–NFAT activity has now been reported in almost all subsets of myeloid cells (Muller & Rao, 2010; Fric et al, 2012). Most recently, an important role of NFAT1 was identified in microglia subjected to chronic LPS stimulation (Ma et al, 2015). Other investigators have dissected the translocation/turnover kinetics of the dephosphorylated nuclear fraction of NFAT3 and 4 in monocytes activated through TLR2/4 (Minematsu et al, 2011). In human mast cells, calcium mobilization is activated by triggering of TLR‐2, which has been linked with NFAT‐mediated transcriptional responses to Leishmania (Zaidi et al, 2006; Bhattacharjee et al, 2016). Calcineurin–NFAT signalling has also been shown to regulate mast cell activation (Walczak‐Drzewiecka et al, 2008), survival (Ulleras et al, 2008) and cytokine expression (Monticelli et al, 2004; Klein et al, 2006). Furthermore, recognition of bacterial or fungal entry into host cells via cytoplasmic PRRs known as NOD‐like receptors (NLRs) has also been shown to influence Calcineurin–NFAT activation (Tourneur et al, 2013; Vandewalle et al, 2014). The various PRR‐dependent mechanisms of Calcineurin–NFAT activation identified in myeloid leucocytes are summarized in Fig 1.
Features of the Calcineurin–NFAT signalling cascade in myeloid cells
A key advance in understanding how PRR ligation triggers Calcineurin–NFAT signalling was the discovery that cytoplasmic calcium levels are the sole determinant of calcineurin activity following leucocyte exposure to LPS (Zanoni et al, 2009) or the Dectin‐1 ligand curdlan (Xu et al, 2009). While originally described in signalling downstream of the T‐cell receptor, immune receptor tyrosine‐based activation motifs (ITAM) are now also known to contribute to signal transduction in myeloid cells upon ligation of Dectin‐1 and other C‐type lectin receptors including CLEC‐2 (Mourao‐Sa et al, 2011; Severin et al, 2011).
In a signalling cascade comparable with that displayed by T cells, myeloid cells undergo dephosphorylation of ITAM motifs by Src kinases to create a binding site for spleen tyrosine kinase (Syk), which is the major integrator of PRR signals that induce intracellular calcium flux. These shared early signalling events suggest that Dectin‐1 initiation of phagocytic activity in myeloid cells is the functional equivalent of immune synapse formation for T‐cell stimulation (Goodridge et al, 2011). Syk subsequently cooperates with Src kinases to activate phospholipase γ (PLCγ), which represents the major point of convergence between PRR signalling and Calcineurin–NFAT activation by stimuli including Dectin‐1 ligands (Xu et al, 2009). Evidence that PLCγ plays a major role in DC biology was first reported by Tassi et al, who observed that bone marrow‐derived DCs (BMDCs) derived from PLCγ‐deficient mice were unable to prime T‐cell expression of IL‐17 in response to Dectin‐1 binding (Tassi et al, 2009). PLCγ also contributes to myeloid cell ontogeny due to activation by the key growth factors M‐CSF and G‐CSF in lineage progenitor cells (Barbosa et al, 2014).
An important signalling event in NFAT activation is ligand internalization and/or intense clustering of signalling molecules to lipid rafts. This process can be readily observed in response to molecules in particulate form such as β‐glucan or zymosan (Goodridge et al, 2007), but has also been shown to occur following a LPS challenge. CD14‐mediated internalization of LPS into signalling compartments activates Syk/PLCγ and promotes intracellular calcium flux in monocytes, macrophages and DCs (Zanoni et al, 2011; Vigano et al, 2015). We and others have also reported that signalling through PRRs can induce calcium flux and activate Calcineurin–NFAT signalling upon recognition of complex particulate antigens including whole bacteria and fungal conidia (Fric et al, 2014b). Indeed, while zymosan binding to Dectin‐1 alone is sufficient to activate Calcineurin–NFAT signalling, Aspergilus fumigatus can also trigger TLR9 and Bruton's tyrosine kinase (Btk) in addition to Dectin‐1, leading to further upregulation of Calcineurin–NFAT activity (Strijbis et al, 2013; Herbst et al, 2015).
Calcineurin–NFAT control of bacterial and viral infections
Engagement of the Calcineurin–NFAT pathway has now been detected in myeloid cell responses to bacteria (Zanoni et al, 2009; Minematsu et al, 2011; Ranjan et al, 2014), parasitic infections (Kayama et al, 2009) and viruses (Miskin et al, 1998, 2000). In human DC, exposure to cyclosporine A reduces interferon (IFN)‐α responses to Sendai virus (Tajima et al, 2003), while macrophages from NFAT4 knockout mice display reduced iNOS expression and attenuated bactericidal activity in a sepsis model, consistent with the ability of calcineurin inhibitors to block NFAT4 binding to the iNOS promoter (Ranjan et al, 2014). Given that the NFAT family can also cooperate with transcription factors such as NF‐κB to modulate gene expression (Bronk et al, 2014), the Calcineurin–NFAT pathway is also likely to impact on antimicrobial immune responses via additional mechanisms that have yet to be identified. Indeed, previous studies of NFAT‐dependent genes have relied mainly on global gene expression analyses in genetically engineered mouse models; hence, there are currently only limited data available on NFAT binding sites in myeloid cells. However, using an alternative chip‐on‐chip approach, Yu et al performed genome‐wide mapping of potential target sites in human DCs to identify that NFAT1 can modulate expression of critical immunomodulatory chemokines and cytokines including IL‐2, IL‐12p40 and IL‐23 (Yu et al, 2015). Similar strategies have also been used to identify cooperation between NFAT4 and transcription factor IRF7 in binding to IFN promoters in plasmacytoid DCs (Bao et al, 2016).
So far there are only limited data available from studies of human tissues and patient samples to support a role for NFAT in myeloid cell responses to major pathogens in vivo. However, signalling through TLR4 and NOD1, which impact on the Calcineurin–NFAT pathway, correlate with phagocytic activity in human renal transplant recipients who exhibit increased susceptibility to E. coli infection (Tourneur et al, 2013). When combined with extensive data available from experimental models, these findings provide clear evidence that Calcineurin–NFAT signalling in myeloid cells is critically involved in host protection against infection. It is therefore highly likely that drug inhibition of Calcineurin–NFAT signalling in myeloid cells will disrupt numerous mechanisms of innate immune protection in treated human patients.
Calcineurin–NFAT‐regulated genes in myeloid cells control fungal infection
The first NFAT‐regulated genes to be identified included IL‐2 expression induced by triggering of the T‐cell receptor (Shaw et al, 1988). An important observation in this context was that stimulation of DCs with microbial compounds can also induce NFAT‐dependent production of IL‐2 (Granucci et al, 2001, 2003; Zanoni et al, 2009). While both NFAT‐ and MAPK‐dependent effects of calcineurin inhibition on myeloid cell development were also identified, these were not initially linked with altered host susceptibility to infection (Miranda & Johnson, 2007; Fric et al, 2014a). Indeed, Calcineurin–NFAT‐driven IL‐2 expression can also be triggered by DC phagocytosis of sterile adjuvants including alum, SiO2 particles and monosodium urate crystals (Khameneh et al, 2017).
A role for calcium‐NFAT signalling in innate immunity was initially identified in NK cells (Aramburu et al, 1995), and later reported in macrophages and DCs stimulated through Dectin‐1 using either Candida albicans or zymosan, which modulated expression of key transcription factors (Egr2, Egr3) and pro‐inflammatory mediators including Cox‐2, IL‐2, IL‐10 and IL‐12p70 (Goodridge et al, 2007; Xu et al, 2009). These data suggest that a wide range of effects can be mediated by calcium‐NFAT signalling, especially via DC‐derived IL‐2 which has been identified as a key mediator of NK cell activation (Granucci et al, 2004, 2006) and regulatory T‐cell (T‐reg) maintenance (Guiducci et al, 2005). Indeed, recent work using an intranasal Aspergillus fumigatus infection model has demonstrated an important role for DC‐derived IL‐2 in mucosal immune responses in vivo (Zelante et al, 2015). In this report, conditional knockout of IL‐2 expression in CD11c+ cells was sufficient to disrupt fungus recognition, inhibit phagocytosis and impair Th17 responses to live conidia. Correlation of NFAT signalling with DC production of the antifungal protein pentraxin‐3 has been also reported in a model of intravenous A. fumigatus infection (Zelante et al, 2017). Consequently, host deficiency in calcineurin signalling or lack of IL‐2 expression in myeloid cells confers a significant increase in A. fumigatus‐associated mortality in rodents (Zelante et al, 2015).
While IL‐2 cytokine clearly has a major role to play in regulating cross talk between innate and adaptive immune cells (Malek & Castro, 2010; Yuan et al, 2015), other NFAT‐regulated genes involved in host protection against infection include Cox‐2, PGE‐2 and various immunomodulatory cytokines (Zanoni et al, 2012). For example, tacrolimus treatment leads to a significant decrease in TNF expression by murine macrophages infected with A. fumigatus (Herbst et al, 2015). These data demonstrate that Calcineurin–NFAT signalling not only represents a central component of adaptive immunity but also plays important roles in innate responses. Consequently, the increased risk of bacterial and fungal infections observed in transplant patients treated with calcineurin inhibitors is likely due to defects in innate pathogen recognition in addition to suppression of T‐cell responses.
Myeloid Calcineurin–NFAT function in immunopathology
Myeloid cells accumulate in host tissues and organs in a range of different pathologies including chronic inflammatory disorders, malignancies and autoimmune diseases (Nicholson et al, 2009). PRR signalling in myeloid cells has been the target of rheumatoid arthritis (RA) experimental therapy (Kim et al, 2014). Escolano et al (2014) showed that calcineurin impairment in macrophages drives the development of an anti‐inflammatory phenotype, with potential beneficial effects in some pathologies. Critical to many of these disease processes is antigen presentation by monocytes, macrophages and DCs, which stimulate T cells to produce pro‐inflammatory cytokines and mediate potent effector functions. While myeloid cell induction of T‐cell‐mediated immunity is critical for eliminating pathogens from infected tissues, these responses can also damage host tissues if directed against self‐antigens or not subsequently resolved. The range of clinical settings in which calcineurin inhibitor drugs might prove efficacious is therefore extremely broad, and the potential side effects likely include elevated risk of a wide variety of infections (Table 1).
Table 1.
Overview of experimental models demonstrating a role for NFAT in innate immunity
| Model Disease | Cell type | Host | Pathogen Trigger | Study | Inhibitor Gene KO | Genes, Phenotype | References | |
|---|---|---|---|---|---|---|---|---|
| FUNGI | Invasive fungal infection | BMDM BMDC | Ms | C. albicans | In vitro | CsA VIVIT | Egr2, Egr3, Cox‐2, IL‐2, IL‐10, IL‐12p70 | Goodridge et al (2007) |
| Neut MF | Ms | C. albicans | In vivo In vitro | CsA CnB NFAT2 NFAT4 | Mortality | Greenblatt et al (2010) | ||
| Al. MF | Ms | A. fumigatus | In vivo | FK506 | Hyperinflammation Mortality | Herbst et al (2013) | ||
| DC | Ms | A. fumigatus | In vivo In vitro | CsA FK506 CnB | Mortality, IL‐2, Th17 regulation | Zelante et al (2015) | ||
| Neut MF |
Ms Hu Zf |
A. fumigatus | In vivo In vitro | FK506 | Mortality | Herbst et al (2015) | ||
| DC Neut MF | Ms | A. fumigatus | In vivo In vitro | CnB | Mortality, Ptx‐3 | Zelante et al (2017) | ||
| Neut | Hu | A. fumigatus | Ex vivo In vitro | CsA FK506 | A.f. growth control | Imbert et al (2016) | ||
| MF |
Hu Zf |
A. fumigatus | In vivo In vitro | FK506 | Inflammatory response A.f. growth control | Shah et al (2016) | ||
| YEAST | Opportunistic infection | MF | Ms | S. cerevisiae | In vivo, In vitro | CsA FK506 | CCR2 chemokines, clearance of infection | Busch et al (2016) |
| BACTERIA/PAMPs | Sepsis | MF | Ms | LPS | In vivo In vitro | CsA, VIVIT |
iNOS Bactericidal activity |
Ranjan et al (2014) |
| Colitis IBD | MF | Ms | LPS | In vivo In vitro | FK506 VIVIT |
IL‐12p40 IL‐12p70 IL‐23 |
Elloumi et al (2012) | |
| Acute pyelonephritis |
Neut DC MF |
Ms Hu | E. coli | In vivo In vitro | CsA VIVIT | Chemokines Susceptibility | Tourneur et al (2013) | |
| Bacterial infection |
Mo MF DC |
Ms | LPS | In vivo In vitro | FK506 CnB | LPS tolerance |
Jennings et al (2009) Kang et al (2007) |
|
| PARASITES | Chagas' disease |
BMDC RAW 264 |
Ms | T. cruzi | In vivo In vitro | FK506 NFAT2 | TLR‐independent, calcium‐dependent, IFN‐g production | Kayama et al (2009) |
| VIRUSES | African swine fever | MF | Pig | African swine fever virus | In vitro | CnA deletion | Virus protein inhibits CnB activity |
Miskin et al (2000) Miskin et al (1998) |
| Sendai virus | DC | Hu | Sendai virus | In vitro | CsA | IFN‐α | Tajima et al (2003) | |
| STERILE PARTICLES | Vaccination | DC | Ms | Alum adjuvants | In vivo In vitro | CsA FK506 | IL‐2, CD4 proliferation, Humoral response | Khameneh et al (2017) |
Species abbreviations: mouse (Ms), human (Hu), Candida (C.), Aspergillus fumigatus (A.f.), trypanosoma (T.). Cell types: bone marrow‐derived macrophages (BMDMs), bone marrow‐derived dendritic cells (BMDCs), neutrophils (Neut), alveolar macrophages (Al. MF), monocytes (Mo), cyclosporine A (CsA), tacrolimus (FK506), calcineurin (Cn).
Invasive fungal infections are a major cause of morbidity and mortality in immunocompromised patients (Pappas et al, 2010), and both experimental and clinical studies have demonstrated that the Calcineurin–NFAT pathway collaborates with NF‐κB to coordinate macrophage TNF responses to Aspergillus (Herbst et al, 2015). Similarly, administration of calcineurin inhibitors is associated with defects in neutrophil/macrophage control of fungal germination and hyphal growth that likely increase pathology in transplant recipients (Imbert et al, 2016; Shah et al, 2016). The discovery that defective Calcineurin–NFAT signalling in myeloid cells confers increased susceptibility to C. albicans infection in mice (Greenblatt et al, 2010) has also led to the development of new models of infection in immunocompromised hosts that more closely resemble human clinical scenarios. In one such model of pulmonary aspergillosis, disruption of Calcineurin–NFAT signalling impaired fungal killing in alveolar macrophages, leading to sustained inflammatory responses in the lung, enhanced tissue destruction and increased rates of mortality (Herbst et al, 2013, 2015). An alternative model of invasive aspergillosis has also been used to demonstrate that NFAT‐dependent expression of IL‐2 in DCs is required for optimal Th17 responses in the lung, which decreases inflammatory pathology and reduces risk of mortality following infection (Zelante et al, 2015, 2017). In the peritoneal cavity, calcineurin inhibition during opportunistic Saccharomyces cerevisiae infection was reported to impair macrophage expression of CCR2 chemokines, impair neutrophil mobilization and delay pathogen clearance (Busch et al, 2016). The process of phagocytosis itself has also been identified as a driver of NFAT activation in macrophages, which may employ the TLR9‐BTK‐Calcineurin–NFAT (MyD88‐independent) pathway as a mechanism of intracellular pathogen detection (Herbst et al, 2015). There is now substantial evidence that Calcineurin–NFAT signalling in myeloid cells affects the outcome of fungal infections in particular, although only limited data are available on how myeloid cell responses to fungi are altered in human patients receiving immunosuppressive therapy. Indeed, drug effects on non‐immune lineages including epithelial cells that also express fungus‐sensing PRRs may further modify patient outcomes. Indeed, a recent study demonstrated that upregulation of Dectin‐1 in airway epithelial cells enhanced pro‐inflammatory responses to A. fumigatus, increased neutrophil recruitment to the lungs and improved rates of Aspergillus clearance and host survival (Liu et al, 2015). However, it remains unconfirmed whether this Dectin‐1‐dependent ability of epithelial cells to protect against fungal infection is mediated by NFAT.
Autoimmune disorders as targets for immunosuppressive therapies
The discovery that drug inhibition of the Calcineurin–NFAT pathway potently suppresses T‐cell responses led to a revolution in transplant medicine (Yeh & Markmann, 2013). While clinical usage of calcineurin inhibitors began in the 1980s, additional effects of these drugs on myeloid cell biology were not appreciated until almost 20 years later. Extensive efforts are now being made to better understand the full range of effects induced by calcineurin inhibitors and to identify new therapeutic applications for these drugs in a wide range of disease indications. While there is huge potential to improve therapy options for several major human disorders, these strategies will likely also confer increased infection risk due to disruption of myeloid cell responses to commensal organisms and pathogenic microbes.
In a previous study of calcineurin inhibitor effects on macrophage function in murine colitis, LPS‐induced expression of IL‐12p40, IL‐12p70 and IL‐23 was significantly reduced by treatment with tacrolimus or the calcineurin inhibitory peptide 11R‐VIVIT (Elloumi et al, 2012). These data suggest that selective blockade of NFAT might be a promising therapeutic strategy in inflammatory bowel diseases. These findings are consistent with an earlier report that inactivation of calcineurin in myeloid cells can induce a form of LPS tolerance that inhibits inflammation (Jennings et al, 2009). Administration of calcineurin inhibitors may therefore also provide effective protection against LPS toxicity in disorders such as sepsis. Indeed, the range of pathologies in which calcineurin inhibition is thought to be potentially beneficial is now extensive, and the number of clinical studies initiated to test the efficacy of cyclosporine or tacrolimus has seen a corresponding increase in recent years (Fig 2A).
Figure 2. Clinical testing of calcineurin inhibitors cyclosporine A and tacrolimus in autoimmune disorders.
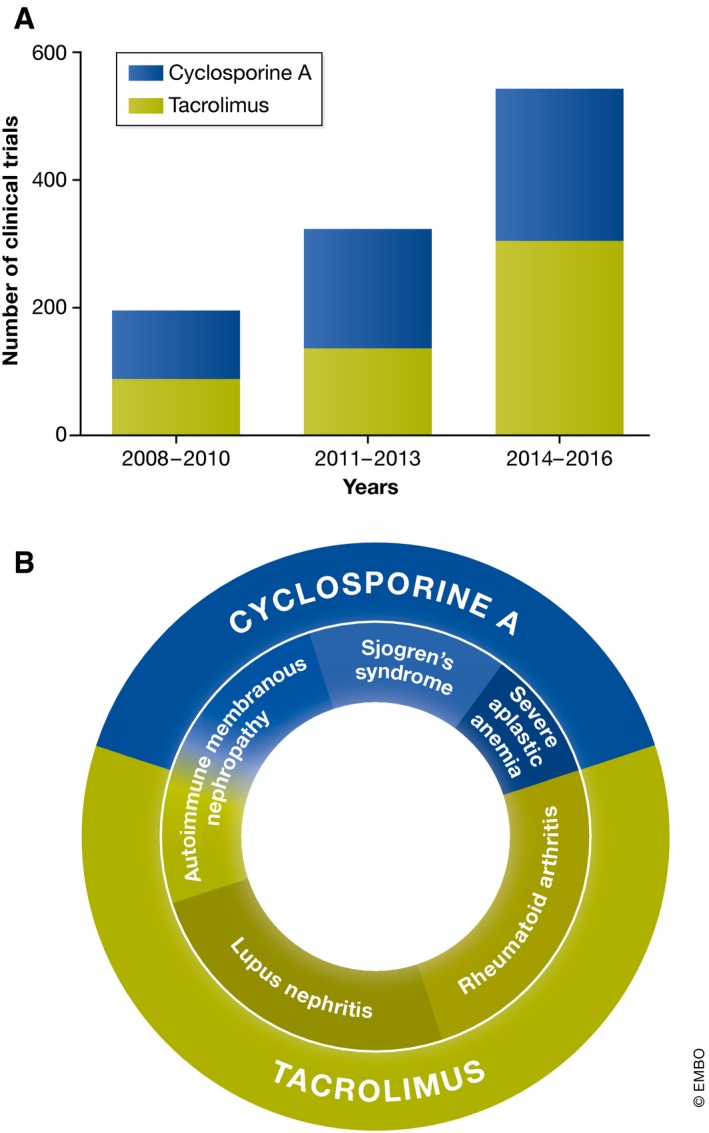
(A) Increasing number of clinical studies testing “off‐label” calcineurin inhibitor use for the treatment of autoimmune disorders. Histograms show studies of tacrolimus and cyclosporine A as well as the year each study was completed or last updated (clinicaltrials.gov). (B) Graphical overview of the major autoimmune disorders targeted using calcineurin inhibitors.
A potential new therapeutic application for calcineurin inhibitor drugs is the treatment of autoimmune disorders (Fig 2B and Table 2). Several studies have now reported that cyclosporine A can inhibit pathological IL‐17 expression in patients with RA (Zhang et al, 2008). While some studies have failed to show any improvement of advanced RA with tacrolimus treatment (Schiff et al, 2010), recent clinical studies using tacrolimus as part of a multiple drug treatment in RA have reported very promising results (Takahashi et al, 2015; Hirai et al, 2017; Naniwa et al, 2017). However, since clinical studies often exclude patients who develop infections of unconfirmed origin, it will be important to determine whether treated individuals exhibit increased infection risk as reported elsewhere in immunosuppressed arthritis patients (Misra et al, 2014).
Table 2.
Overview of clinical studies using calcineurin inhibitors to treat autoimmune disorders
| Pathology | Inhibitor | Monotherapy | In combination |
|---|---|---|---|
| Rheumatoid arthritis | FK506 |
NCT02837978 NCT01511003 |
Methotrexate—NCT02837978 Methotrexate—NCT01746680 Sarilumab—NCT02373202 Biolagens—NCT01870908 |
| Membranous nephropathy | FK506 |
Rituximab—NCT00843856 Mycophenolate—NCT01955187 |
|
| CsA |
NCT01282073 NCT01180036 |
Rituximab NCT00977977 | |
| Lupus nephritis | FK506 |
NCT01410747 NCT01316133 NCT02457221 NCT01580865 NCT02630628 |
|
| Severe aplastic anaemia | CsA |
Eltrombopag + hATG NCT01623167 hATG or rAGT NCT00260689 Alemtuzumab—NCT00195624 |
|
| Sjogren's syndrome | CsA |
NCT02004067 NCT02370550 |
Both cyclosporine A and tacrolimus have proven effective for treatment of autoimmune membranous nephropathy, which is increasingly prevalent in older patients who already exhibit high risk of opportunistic infections (Yamaguchi et al, 2014; Alfaadhel & Cattran, 2015). For other autoimmune pathologies including lupus nephritis, clinical studies are ongoing to demonstrate the efficacy of tacrolimus treatment which is currently used only on an “off‐label” basis (Kraaij et al, 2016). Similarly, the chronic autoimmune condition known as Sjogren's syndrome is reportedly associated with abnormal activation of Th17 cells and has also been shown to respond well to combination therapy including cyclosporine A. Together, these data demonstrate that cyclosporine and tacrolimus can provide therapeutic benefit in human rheumatoid disorders and potentially other diseases in which myeloid cell function has been implicated.
Numerous in vitro studies and investigations in animal models have indicated that calcineurin inhibition can potently inhibit inflammatory cytokine expression without inducing severe side effects. While the range of potential drug effects on host susceptibility to infection appears extensive, additional effects of calcineurin inhibitors on myeloid cell activity in autoimmune disease are also likely to be identified in future. Indeed, the potent immunosuppressive properties of myeloid cells have already been confirmed in studies of tolerogenic DCs in RA (Hilkens et al, 2010; Hilkens & Isaacs, 2013) and via development of novel tissue‐specific DC‐based immunotherapies (Mbongue et al, 2014). Further investigations will therefore be required to better understand the role of Calcineurin–NFAT signalling in control of myeloid cell function in human patients, to target this pathway more effectively in future and achieve greater therapeutic benefit for patients with a range of increasingly common pathologies.
Concluding remarks
In the current review, we have summarized recent advances in understanding the role played by calcineurin/NFAT‐regulated genes in the control of myeloid leucocyte responses to infection. Due to decades of clinical use in transplant settings, there is little concern regarding the safety of calcineurin inhibitor drug use in human patients. While most of the clinical studies discussed in this review carefully assessed infection risk associated with immunosuppressive therapies, the contribution of myeloid cell dysfunction in treated patients has been largely overlooked. To our knowledge, no previous study has reported a link between immunosuppressive therapy and dysregulation of myeloid immunity. However, data from infection models and studies in genetically engineered animals clearly indicate a major role for Calcineurin–NFAT signalling in myeloid cell protection against pathogens.
Pending issues.
How far are drug effects on myeloid cells responsible for the increased infection risk observed in patients treated with calcineurin inhibitors?
How can new knowledge of the roles played by Calcineurin–NFAT signalling be effectively translated into novel therapeutic strategies?
How do calcineurin inhibitors impact on development and maintenance of different myeloid cells populations in immunosuppressed patients?
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We wish to thank Dr. Neil McCarthy of Insight Editing London for critical review of the manuscript. Authors are supported by European Social Fund and European Regional Development Fund—Project MAGNET (No. CZ.02.1.01/0.0/0.0/15_003/0000492).
EMBO Mol Med (2017) 9: 990–999
See the Glossary for abbreviations used in this article.
References
- Alfaadhel T, Cattran D (2015) Management of membranous nephropathy in western countries. Kidney Dis 1: 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Azzoni L, Rao A, Perussia B (1995) Activation and expression of the nuclear factors of activated T cells, NFATp and NFATc, in human natural killer cells: regulation upon CD16 ligand binding. J Exp Med 182: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Wang Y, Liu Y, Shi P, Lu H, Sha W, Weng L, Hanabuchi S, Qin J, Plumas J et al (2016) NFATC3 promotes IRF7 transcriptional activity in plasmacytoid dendritic cells. J Exp Med 213: 2383–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa CM, Bincoletto C, Barros CC, Ferreira AT, Paredes‐Gamero EJ (2014) PLCgamma2 and PKC are important to myeloid lineage commitment triggered by M‐SCF and G‐CSF. J Cell Biochem 115: 42–51 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Majumder S, Das S, Ghosh S, Biswas S, Majumdar S (2016) Leishmania donovani‐induced prostaglandin E2 generation is critically dependent on host toll‐like receptor 2‐cytosolic phospholipase A2 signaling. Infect Immun 84: 2963–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk CC, Yoder S, Hopewell EL, Yang S, Celis E, Yu XZ, Beg AA (2014) NF‐kappaB is crucial in proximal T‐cell signaling for calcium influx and NFAT activation. Eur J Immunol 44: 3741–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R, Murti K, Liu J, Patra AK, Muhammad K, Knobeloch KP, Lichtinger M, Bonifer C, Wortge S, Waisman A et al (2016) NFATc1 releases BCL6‐dependent repression of CCR2 agonist expression in peritoneal macrophages from Saccharomyces cerevisiae infected mice. Eur J Immunol 46: 634–646 [DOI] [PubMed] [Google Scholar]
- Chiang CY, Veckman V, Limmer K, David M (2012) Phospholipase Cgamma‐2 and intracellular calcium are required for lipopolysaccharide‐induced Toll‐like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J Biol Chem 287: 3704–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR (1989) Contingent genetic regulatory events in T lymphocyte activation. Science 243: 355–361 [DOI] [PubMed] [Google Scholar]
- Elloumi HZ, Maharshak N, Rao KN, Kobayashi T, Ryu HS, Muhlbauer M, Li F, Jobin C, Plevy SE (2012) A cell permeable peptide inhibitor of NFAT inhibits macrophage cytokine expression and ameliorates experimental colitis. PLoS ONE 7: e34172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolano A, Martinez‐Martinez S, Alfranca A, Urso K, Izquierdo HM, Delgado M, Martin F, Sabio G, Sancho D, Gomez‐del Arco P et al (2014) Specific calcineurin targeting in macrophages confers resistance to inflammation via MKP‐1 and p38. EMBO J 33: 1117–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fric J, Zelante T, Wong AY, Mertes A, Yu HB, Ricciardi‐Castagnoli P (2012) NFAT control of innate immunity. Blood 120: 1380–1389 [DOI] [PubMed] [Google Scholar]
- Fric J, Lim CX, Mertes A, Lee BT, Vigano E, Chen J, Zolezzi F, Poidinger M, Larbi A, Strobl H et al (2014a) Calcium and calcineurin‐NFAT signaling regulate granulocyte‐monocyte progenitor cell cycle via Flt3‐L. Stem Cells 32: 3232–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fric J, Zelante T, Ricciardi‐Castagnoli P (2014b) Phagocytosis of particulate antigens – all roads lead to calcineurin/NFAT signaling pathway. Front Immunol 4: 513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, Simmons RM, Underhill DM (2007) Dectin‐1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol 178: 3107–3115 [DOI] [PubMed] [Google Scholar]
- Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME et al (2011) Activation of the innate immune receptor Dectin‐1 upon formation of a “phagocytic synapse”. Nature 472: 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi‐Castagnoli P (2001) Inducible IL‐2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol 2: 882–888 [DOI] [PubMed] [Google Scholar]
- Granucci F, Feau S, Angeli V, Trottein F, Ricciardi‐Castagnoli P (2003) Early IL‐2 production by mouse dendritic cells is the result of microbial‐induced priming. J Immunol 170: 5075–5081 [DOI] [PubMed] [Google Scholar]
- Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi‐Castagnoli P (2004) A contribution of mouse dendritic cell‐derived IL‐2 for NK cell activation. J Exp Med 200: 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Zanoni I, Ricciardi‐Castagnoli P (2006) Natural killer (NK) cell functions can be strongly boosted by activated dendritic cells (DC). Eur J Immunol 36: 2819–2820 [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Aliprantis A, Hu B, Glimcher LH (2010) Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med 207: 923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Valzasina B, Dislich H, Colombo MP (2005) CD40/CD40L interaction regulates CD4+ CD25+ T reg homeostasis through dendritic cell‐produced IL‐2. Eur J Immunol 35: 557–567 [DOI] [PubMed] [Google Scholar]
- Herbst S, Shah A, Carby M, Chusney G, Kikkeri N, Dorling A, Bignell E, Shaunak S, Armstrong‐James D (2013) A new and clinically relevant murine model of solid‐organ transplant aspergillosis. Dis Model Mech 6: 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S et al (2015) Phagocytosis‐dependent activation of a TLR9‐BTK‐calcineurin‐NFAT pathway co‐ordinates innate immunity to Aspergillus fumigatus . EMBO Mol Med 7: 240–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens CM, Isaacs JD, Thomson AW (2010) Development of dendritic cell‐based immunotherapy for autoimmunity. Int Rev Immunol 29: 156–183 [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Isaacs JD (2013) Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol 172: 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Ikeda K, Fujishiro M, Tsushima H, Hayakawa K, Suzuki S, Yamaguchi A, Nozawa K, Morimoto S, Takasaki Y et al (2017) The effectiveness of new triple combination therapy using synthetic disease‐modifying anti‐rheumatic drugs with different pharmacological function against rheumatoid arthritis: the verification by an in vitro and clinical study. Clin Rheumatol 36: 51–58 [DOI] [PubMed] [Google Scholar]
- Imbert S, Bresler P, Boissonnas A, Gauthier L, Souchet L, Uzunov M, Leblond V, Mazier D, Nguyen S, Fekkar A (2016) Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol 138: 860–868 [DOI] [PubMed] [Google Scholar]
- Jennings C, Kusler B, Jones PP (2009) Calcineurin inactivation leads to decreased responsiveness to LPS in macrophages and dendritic cells and protects against LPS‐induced toxicity in vivo . Innate immunity 15: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Yu B, Zhang W, Zhang W, Xiao Z, Mao Z, Lai Y, Lin D, Ma Q, Pan E et al (2016) Toll‐like receptor 2‐mediated MAPKs and NF‐kappaB activation requires the GNAO1‐dependent pathway in human mast cells. Integr Biol 8: 968–975 [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R (2008) TRAM couples endocytosis of Toll‐like receptor 4 to the induction of interferon‐beta. Nat Immunol 9: 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC (2012) Signaling organelles of the innate immune system. Cell 151: 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Kusler B, Otsuka M, Hughes M, Suzuki N, Suzuki S, Yeh WC, Akira S, Han J, Jones PP (2007) Calcineurin negatively regulates TLR‐mediated activation pathways. J Immunol 179: 4598–4607 [DOI] [PubMed] [Google Scholar]
- Kayama H, Koga R, Atarashi K, Okuyama M, Kimura T, Mak TW, Uematsu S, Akira S, Takayanagi H, Honda K et al (2009) NFATc1 mediates Toll‐like receptor‐independent innate immune responses during Trypanosoma cruzi infection. PLoS Pathog 5: e1000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khameneh HJ, Ho AW, Spreafico R, Derks H, Quek HQ, Mortellaro A (2017) The Syk‐NFAT‐IL‐2 pathway in dendritic cells is required for optimal sterile immunity elicited by alum adjuvants. J Immunol 198: 196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Chen Z, Chamberlain ND, Essani AB, Volin MV, Amin MA, Volkov S, Gravallese EM, Arami S, Swedler W et al (2014) Ligation of TLR5 promotes myeloid cell infiltration and differentiation into mature osteoclasts in rheumatoid arthritis and experimental arthritis. J Immunol 193: 3902–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Klein‐Hessling S, Palmetshofer A, Serfling E, Tertilt C, Bopp T, Heib V, Becker M, Taube C, Schild H et al (2006) Specific and redundant roles for NFAT transcription factors in the expression of mast cell‐derived cytokines. J Immunol 177: 6667–6674 [DOI] [PubMed] [Google Scholar]
- Kraaij T, Bredewold OW, Trompet S, Huizinga TW, Rabelink TJ, de Craen AJ, Teng YK (2016) TAC‐TIC use of tacrolimus‐based regimens in lupus nephritis. Lupus Sci Med 3: e000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZC, Wang M, Sun WK, Xia D, Tan MM, Ding Y, Qian Q, Su X, Shi Y (2015) Up‐regulation of Dectin‐1 in airway epithelial cells promotes mice defense against invasive pulmonary aspergillosis. Int J Clin Exp Med 8: 17489–17497 [PMC free article] [PubMed] [Google Scholar]
- Ma B, Yu J, Xie C, Sun L, Lin S, Ding J, Luo J, Cai H (2015) Toll‐like receptors promote mitochondrial translocation of nuclear transcription factor nuclear factor of activated T‐cells in prolonged microglial activation. J Neurosci 35: 10799–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F (2005) NFAT proteins: key regulators of T‐cell development and function. Nat Rev Immunol 5: 472–484 [DOI] [PubMed] [Google Scholar]
- Malek TR, Castro I (2010) Interleukin‐2 receptor signaling: at the interface between tolerance and immunity. Immunity 33: 153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Moriguchi T, Koyasu S, Nishida E (1998) T lymphocyte activation signals for interleukin‐2 production involve activation of MKK6‐p38 and MKK7‐SAPK/JNK signaling pathways sensitive to cyclosporin A. J Biol Chem 273: 12378–12382 [DOI] [PubMed] [Google Scholar]
- Mbongue J, Nicholas D, Firek A, Langridge W (2014) The role of dendritic cells in tissue‐specific autoimmunity. J Immunol Res 2014: 857143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe S, Alexander D, Turner J (1994) FK506 and cyclosporin A each inhibit antigen‐specific signaling in the T cell line 171 in the absence of a calcium signal. Cell Immunol 158: 46–58 [DOI] [PubMed] [Google Scholar]
- Minematsu H, Shin MJ, Celil Aydemir AB, Kim KO, Nizami SA, Chung GJ, Lee FY (2011) Nuclear presence of nuclear factor of activated T cells (NFAT) c3 and c4 is required for Toll‐like receptor‐activated innate inflammatory response of monocytes/macrophages. Cell Signal 23: 1785–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MB, Johnson DE (2007) Signal transduction pathways that contribute to myeloid differentiation. Leukemia 21: 1363–1377 [DOI] [PubMed] [Google Scholar]
- Miskin JE, Abrams CC, Goatley LC, Dixon LK (1998) A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science 281: 562–565 [DOI] [PubMed] [Google Scholar]
- Miskin JE, Abrams CC, Dixon LK (2000) African swine fever virus protein A238L interacts with the cellular phosphatase calcineurin via a binding domain similar to that of NFAT. J Virol 74: 9412–9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra DP, Parida JR, Chowdhury AC, Agarwal V (2014) Pulmonary co‐infection with Nocardia and Aspergillus in a patient with adult‐onset Still's disease receiving steroids and tacrolimus. BMJ Case Rep 2014 doi:10.1136/bcr‐2014‐207335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli S, Solymar DC, Rao A (2004) Role of NFAT proteins in IL13 gene transcription in mast cells. J Biol Chem 279: 36210–36218 [DOI] [PubMed] [Google Scholar]
- Mourao‐Sa D, Robinson MJ, Zelenay S, Sancho D, Chakravarty P, Larsen R, Plantinga M, Van Rooijen N, Soares MP, Lambrecht B et al (2011) CLEC‐2 signaling via Syk in myeloid cells can regulate inflammatory responses. Eur J Immunol 41: 3040–3053 [DOI] [PubMed] [Google Scholar]
- Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, Gelinas C, Rossi DJ, Pipkin ME, Rajewsky K et al (2009) Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA 106: 7034–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MR, Rao A (2010) NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol 10: 645–656 [DOI] [PubMed] [Google Scholar]
- Naniwa T, Iwagaitsu S, Kajiura M (2017) Efficacy of add‐on tacrolimus on methotrexate to maintain clinical remission after rediscontinuation of a tumor necrosis factor inhibitor in rheumatoid arthritis patients who relapsed shortly after discontinuation of the same tumor necrosis factor inhibitor due to clinical remission. Mod Rheumatol 27: 29–34 [DOI] [PubMed] [Google Scholar]
- Nicholson LB, Raveney BJ, Munder M (2009) Monocyte dependent regulation of autoimmune inflammation. Curr Mol Med 9: 23–29 [DOI] [PubMed] [Google Scholar]
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J et al (2010) Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50: 1101–1111 [DOI] [PubMed] [Google Scholar]
- Ranjan R, Deng J, Chung S, Lee YG, Park GY, Xiao L, Joo M, Christman JW, Karpurapu M (2014) The transcription factor nuclear factor of activated T cells c3 modulates the function of macrophages in sepsis. J Innate Immun 6: 754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Beaulieu A, Scott DL, Rashford M (2010) Mycophenolate mofetil in the treatment of adults with advanced rheumatoid arthritis: three 24‐week, randomized, double‐blind, placebo‐ or cyclosporin‐controlled trials. Clin Drug Invest 30: 613–624 [DOI] [PubMed] [Google Scholar]
- Severin S, Pollitt AY, Navarro‐Nunez L, Nash CA, Mourao‐Sa D, Eble JA, Senis YA, Watson SP (2011) Syk‐dependent phosphorylation of CLEC‐2: a novel mechanism of hem‐immunoreceptor tyrosine‐based activation motif signaling. J Biol Chem 286: 4107–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Kannambath S, Herbst S, Rogers A, Soresi S, Carby M, Reed A, Mostowy S, Fisher MC, Shaunak S et al (2016) Calcineurin orchestrates lateral transfer of Aspergillus fumigatus during macrophage cell death. Am J Respir Crit Care Med 194: 1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR (1988) Identification of a putative regulator of early T cell activation genes. Science 241: 202–205 [DOI] [PubMed] [Google Scholar]
- Strijbis K, Tafesse FG, Fairn GD, Witte MD, Dougan SK, Watson N, Spooner E, Esteban A, Vyas VK, Fink GR et al (2013) Bruton's Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1‐dependent phagocytosis of Candida albicans in macrophages. PLoS Pathog 9: e1003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben‐Neriah Y (1994) JNK is involved in signal integration during costimulation of T lymphocytes. Cell 77: 727–736 [DOI] [PubMed] [Google Scholar]
- Tajima K, Amakawa R, Ito T, Miyaji M, Takebayashi M, Fukuhara S (2003) Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology 108: 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Fujibayashi T, Kida D, Hirano Y, Kato T, Kato D, Saito K, Kaneko A, Yabe Y, Takagi H et al (2015) Concomitant methotrexate and tacrolimus augment the clinical response to abatacept in patients with rheumatoid arthritis with a prior history of biological DMARD use. Rheumatol Int 35: 1707–1716 [DOI] [PubMed] [Google Scholar]
- Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M (2009) Requirement of phospholipase C‐gamma2 (PLCgamma2) for Dectin‐1‐induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol 39: 1369–1378 [DOI] [PubMed] [Google Scholar]
- Tourneur E, Ben Mkaddem S, Chassin C, Bens M, Goujon JM, Charles N, Pellefigues C, Aloulou M, Hertig A, Monteiro RC et al (2013) Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)‐mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog 9: e1003152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulleras E, Karlberg M, Moller Westerberg C, Alfredsson J, Gerondakis S, Strasser A, Nilsson G (2008) NFAT but not NF‐kappaB is critical for transcriptional induction of the prosurvival gene A1 after IgE receptor activation in mast cells. Blood 111: 3081–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle A, Tourneur E, Bens M, Chassin C, Werts C (2014) Calcineurin/NFAT signaling and innate host defence: a role for NOD1‐mediated phagocytic functions. Cell Commun Signal 12: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A (2015) Human caspase‐4 and caspase‐5 regulate the one‐step non‐canonical inflammasome activation in monocytes. Nat Commun 6: 8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak‐Drzewiecka A, Ratajewski M, Wagner W, Dastych J (2008) HIF‐1alpha is up‐regulated in activated mast cells by a process that involves calcineurin and NFAT. J Immunol 181: 1665–1672 [DOI] [PubMed] [Google Scholar]
- Xu S, Huo J, Lee KG, Kurosaki T, Lam KP (2009) Phospholipase Cgamma2 is critical for Dectin‐1‐mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem 284: 7038–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Ando M, Yamamoto R, Akiyama S, Kato S, Katsuno T, Kosugi T, Sato W, Tsuboi N, Yasuda Y et al (2014) Patient age and the prognosis of idiopathic membranous nephropathy. PLoS ONE 9: e110376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T (2008) Mincle is an ITAM‐coupled activating receptor that senses damaged cells. Nat Immunol 9: 1179–1188 [DOI] [PubMed] [Google Scholar]
- Yeh H, Markmann JF (2013) Transplantation: are calcineurin inhibitors safer than mTOR inhibitors? Nat Rev Nephrol 9: 11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HB, Yurieva M, Balachander A, Foo I, Leong X, Zelante T, Zolezzi F, Poidinger M, Ricciardi‐Castagnoli P (2015) NFATc2 mediates epigenetic modification of dendritic cell cytokine and chemokine responses to dectin‐1 stimulation. Nucleic Acids Res 43: 836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Dee MJ, Altman NH, Malek TR (2015) IL‐2Rbeta‐dependent signaling and CD103 functionally cooperate to maintain tolerance in the gut mucosa. J Immunol 194: 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi AK, Thangam ER, Ali H (2006) Distinct roles of Ca2+ mobilization and G protein usage on regulation of Toll‐like receptor function in human and murine mast cells. Immunology 119: 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G et al (2009) CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460: 264–268 [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC (2011) CD14 controls the LPS‐induced endocytosis of Toll‐like receptor 4. Cell 147: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Barresi S, Di Gioia M, Broggi A, Costa B, Marzi R, Granucci F (2012) CD14 and NFAT mediate lipopolysaccharide‐induced skin edema formation in mice. J Clin Invest 122: 1747–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Wong AY, Ping TJ, Chen J, Sumatoh HR, Vigano E, Hong Bing Y, Lee B, Zolezzi F, Fric J et al (2015) CD103(+) dendritic cells control Th17 cell function in the lung. Cell Rep 12: 1789–1801 [DOI] [PubMed] [Google Scholar]
- Zelante T, Wong AY, Mencarelli A, Foo S, Zolezzi F, Lee B, Poidinger M, Ricciardi‐Castagnoli P, Fric J (2017) Impaired calcineurin signaling in myeloid cells results in downregulation of pentraxin‐3 and increased susceptibility to aspergillosis. Mucosal Immunol 10: 470–480 [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang J, Yang B, Wu C (2008) Cyclosporin A inhibits the production of IL‐17 by memory Th17 cells from healthy individuals and patients with rheumatoid arthritis. Cytokine 42: 345–352 [DOI] [PubMed] [Google Scholar]


