Abstract
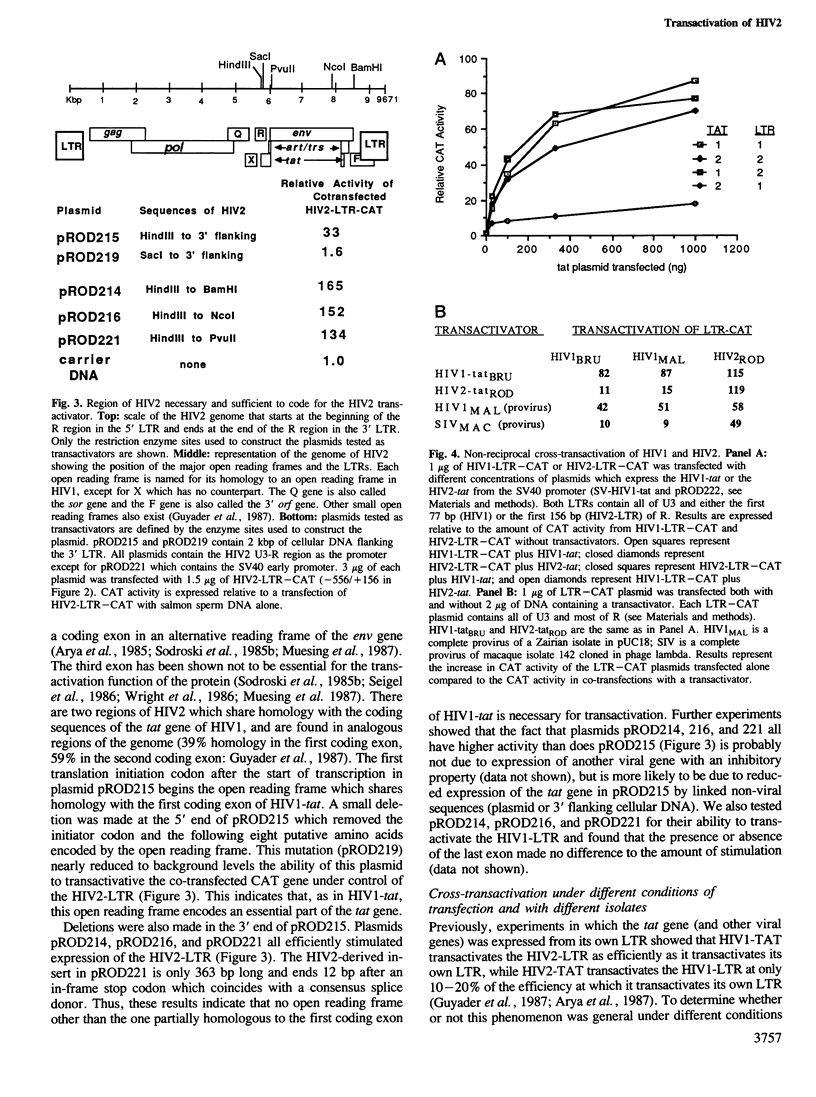
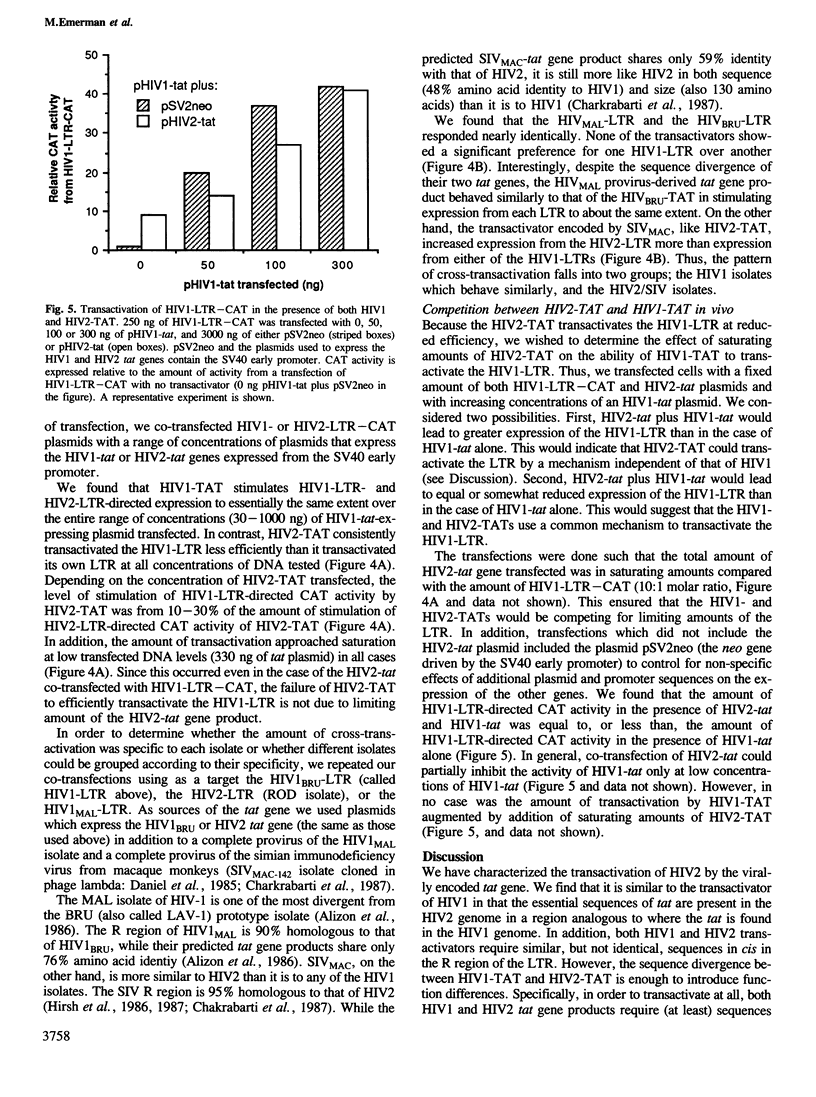
The recently described human immunodeficiency virus type 2 (HIV2) is significantly divergent in sequence from the more frequently isolated human immunodeficiency virus type 1 (HIV1). Both HIV1 and HIV2 encode a transactivator that is capable of strongly stimulating expression directed by the viral long terminal repeat (LTR). Here, we define the region of the HIV2 genome encoding the transactivator and show that the specificity of the transactivator differs from that of HIV1. By deletion analysis of the HIV2-LTR, we show that both HIV1 and HIV2 transactivators require sequences within 35 to 53 bp downstream of the start of transcription. However, in order to stimulate expression at full efficiency, the HIV2 transactivator further requires sequences unique to the HIV2-LTR between nucleotides +53 and +99. Hence, HIV2 poorly transactivates the LTR of HIV1, while two divergent isolates of HIV1 will efficiently transactivate the LTR of either HIV1 or HIV2. Nonetheless, in vivo competition between the transactivators of HIV1 and HIV2 suggests that they use a common mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Beaver B., Jagodzinski L., Ensoli B., Kanki P. J., Albert J., Fenyo E. M., Biberfeld G., Zagury J. F., Laure F. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature. 1987 Aug 6;328(6130):548–550. doi: 10.1038/328548a0. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chen I. S. Regulation of AIDS virus expression. Cell. 1986 Oct 10;47(1):1–2. doi: 10.1016/0092-8674(86)90359-4. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guyader M., Guétard D., Sallé M., Montagnier L., Alizon M. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature. 1986 Dec 18;324(6098):691–695. doi: 10.1038/324691a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Mansinho K., Chamaret S., Guetard D., Favier V., Nina J., Santos-Ferreira M. O., Champalimaud J. L., Montagnier L. Human immunodeficiency virus type 2 infection associated with AIDS in West Africa. N Engl J Med. 1987 May 7;316(19):1180–1185. doi: 10.1056/NEJM198705073161903. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Rosen C., Sodroski J., Ho D. D., Haseltine W. A. Identification of a protein encoded by the trans activator gene tatIII of human T-cell lymphotropic retrovirus type III. J Virol. 1986 Jul;59(1):181–184. doi: 10.1128/jvi.59.1.181-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Kornfeld H., Kanki P. J., Essex M., Mullins J. I. Cross-reactivity to human T-lymphotropic virus type III/lymphadenopathy-associated virus and molecular cloning of simian T-cell lymphotropic virus type III from African green monkeys. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9754–9758. doi: 10.1073/pnas.83.24.9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., M'Boup S., Ricard D., Barin F., Denis F., Boye C., Sangare L., Travers K., Albaum M., Marlink R. Human T-lymphotropic virus type 4 and the human immunodeficiency virus in West Africa. Science. 1987 May 15;236(4803):827–831. doi: 10.1126/science.3033826. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Seigel L. J., Ratner L., Josephs S. F., Derse D., Feinberg M. B., Reyes G. R., O'Brien S. J., Wong-Staal F. Transactivation induced by human T-lymphotropic virus type III (HTLV III) maps to a viral sequence encoding 58 amino acids and lacks tissue specificity. Virology. 1986 Jan 15;148(1):226–231. doi: 10.1016/0042-6822(86)90419-8. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wright C. M., Felber B. K., Paskalis H., Pavlakis G. N. Expression and characterization of the trans-activator of HTLV-III/LAV virus. Science. 1986 Nov 21;234(4779):988–992. doi: 10.1126/science.3490693. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]



