Abstract
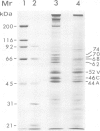
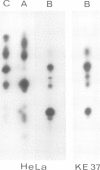
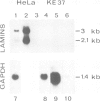
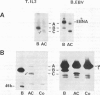
Lamins A, B and C, the three major proteins of nuclear envelope, constitute a class of intermediate filament polypeptides. We have compared the amount of these polypeptides in two human cell lines, epithelial HeLa cells and T lymphoblasts KE 37. It was found that the three lamins were present in roughly equimolar stoichiometry in HeLa cells, while lamin B was the unique lamin component in T lymphoblasts. Moreover, 3-kb mRNA of lamin A and 2.1-kb mRNA of lamin C were detected with a human cDNA probe in HeLa cells but not in T lymphoblasts. These results suggest that (i) lamin B can build up the lamina structure in actively dividing somatic cells by itself, and (ii) lamin expression in lymphoid cells may be subject to important quantitative variations. Comparison of the lamin composition of human cloned T lymphocytes and Epstein-Barr virus-transformed human B lymphocytes confirmed this statement. The lamin B level was nearly equivalent in both cells but the content of lamins A and C varied to a large extent, being low in T cells and high in B cells.
Full text
PDF
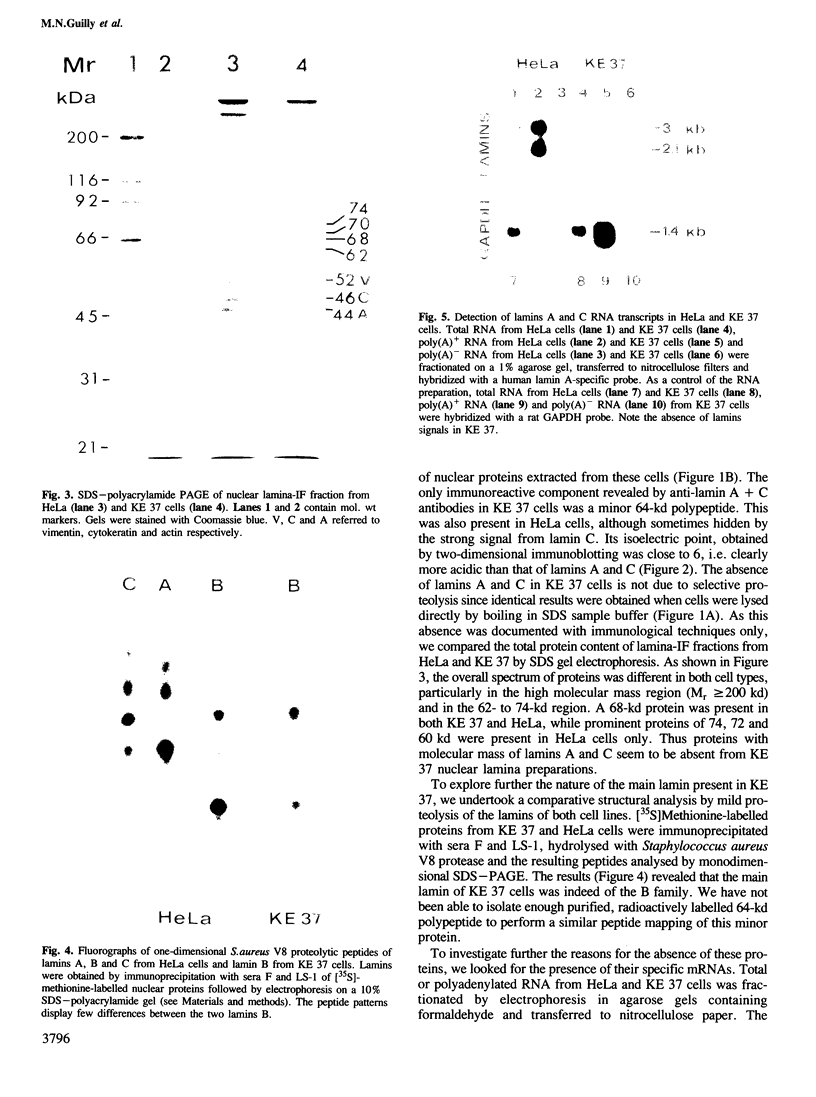


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
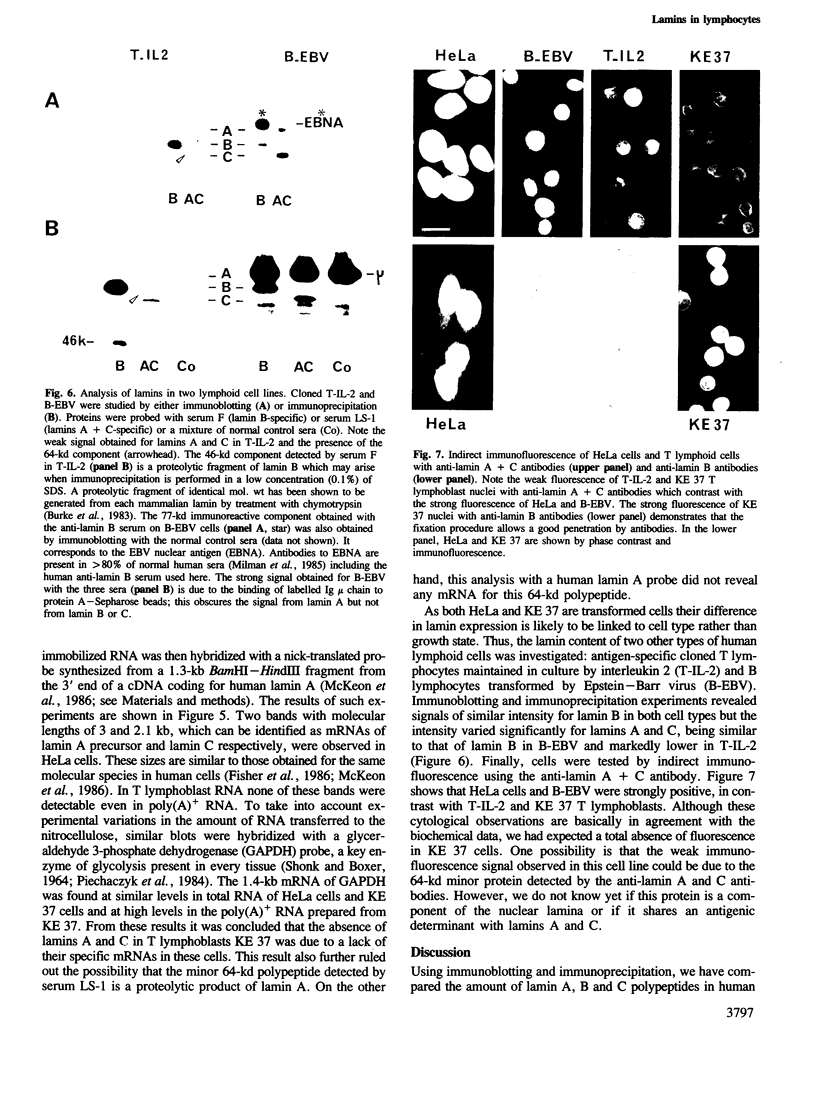
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Franke W. W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985 May;41(1):177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Burke B., Tooze J., Warren G. A monoclonal antibody which recognises each of the nuclear lamin polypeptides in mammalian cells. EMBO J. 1983;2(3):361–367. doi: 10.1002/j.1460-2075.1983.tb01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen L. K., Tourvieille B., Burns G. F., Bach F. H., Mathieu-Mahul D., Sasportes M., Bensussan A. Interferon: a cytotoxic T lymphocyte differentiation signal. Eur J Immunol. 1986 Jul;16(7):767–770. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Portier M. M., Lenoir G. M. Abnormal expression of vimentin intermediate filaments in human lymphoid cell lines with deletion or translocation of the distal end of chromosome 8. J Natl Cancer Inst. 1984 Jul;73(1):95–100. [PubMed] [Google Scholar]
- Dellagi K., Vainchenker W., Vinci G., Paulin D., Brouet J. C. Alteration of vimentin intermediate filament expression during differentiation of human hemopoietic cells. EMBO J. 1983;2(9):1509–1514. doi: 10.1002/j.1460-2075.1983.tb01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Goldman A. E., Maul G., Steinert P. M., Yang H. Y., Goldman R. D. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilly M. N., Danon F., Brouet J. C., Bornens M., Courvalin J. C. Autoantibodies to nuclear lamin B in a patient with thrombopenia. Eur J Cell Biol. 1987 Apr;43(2):266–272. [PubMed] [Google Scholar]
- Krohne G., Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986 Jan;162(1):1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Krohne G., Dabauvalle M. C., Franke W. W. Cell type-specific differences in protein composition of nuclear pore complex-lamina structures in oocytes and erythrocytes of Xenopus laevis. J Mol Biol. 1981 Sep 5;151(1):121–141. doi: 10.1016/0022-2836(81)90224-2. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Scheer U. The major polypeptides of the nuclear pore complex. Exp Cell Res. 1978 Oct 1;116(1):85–102. doi: 10.1016/0014-4827(78)90067-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Electrophoretic analysis of three major nuclear envelope polypeptides. Topological relationship and sequence homology. J Biol Chem. 1979 Nov 25;254(22):11713–11720. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., Kurer V., Eppenberger H. M., Nigg E. A. The nuclear lamin protein family in higher vertebrates. Identification of quantitatively minor lamin proteins by monoclonal antibodies. J Biol Chem. 1986 Oct 5;261(28):13293–13301. [PubMed] [Google Scholar]
- Lilienbaum A., Legagneux V., Portier M. M., Dellagi K., Paulin D. Vimentin gene: expression in human lymphocytes and in Burkitt's lymphoma cells. EMBO J. 1986 Nov;5(11):2809–2814. doi: 10.1002/j.1460-2075.1986.tb04572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Kunkel H. G. Human T cell hybridomas secreting factors for IgA-specific help, polyclonal B cell activation, and B cell proliferation. J Exp Med. 1982 Dec 1;156(6):1860–1865. doi: 10.1084/jem.156.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- McTavish C. F., Nelson W. J., Traub P. Synthesis of vimentin in a reticulocyte cell-free system programmed by poly(A)-rich RNA from several cell lines and rat liver. Eur J Biochem. 1983 Jan 17;130(1):211–221. doi: 10.1111/j.1432-1033.1983.tb07138.x. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Milman G., Scott A. L., Cho M. S., Hartman S. C., Ades D. K., Hayward G. S., Ki P. F., August J. T., Hayward S. D. Carboxyl-terminal domain of the Epstein-Barr virus nuclear antigen is highly immunogenic in man. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6300–6304. doi: 10.1073/pnas.82.18.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]