Abstract
Inflammation is a defensive mechanism for pathogen clearance and maintaining tissue homeostasis. In the skeletal system, inflammation is closely associated with many bone disorders including fractures, nonunions, periprosthetic osteolysis (bone loss around orthopedic implants), and osteoporosis. Acute inflammation is a critical step for proper bone-healing and bone-remodeling processes. On the other hand, chronic inflammation with excessive proinflammatory cytokines disrupts the balance of skeletal homeostasis involving osteoblastic (bone formation) and osteoclastic (bone resorption) activities. NF-κB is a transcriptional factor that regulates the inflammatory response and bone-remodeling processes in both bone-forming and bone-resorption cells. In vitro and in vivo evidences suggest that NF-κB is an important potential therapeutic target for inflammation-associated bone disorders by modulating inflammation and bone-remodeling process simultaneously. The challenges of NF-κB-targeting therapy in bone disorders include: (1) the complexity of canonical and noncanonical NF-κB pathways; (2) the fundamental roles of NF-κB-mediated signaling for bone regeneration at earlier phases of tissue damage and acute inflammation; and (3) the potential toxic effects on nontargeted cells such as lymphocytes. Recent developments of novel inhibitors with differential approaches to modulate NF-κB activity, and the controlled release (local) or bone-targeting drug delivery (systemic) strategies, have largely increased the translational application of NF-κB therapy in bone disorders. Taken together, temporal modulation of NF-κB pathways with the combination of recent advanced bone-targeting drug delivery techniques is a highly translational strategy to reestablish homeostasis in the skeletal system.
1. INTRODUCTION
Bone is the major component of the skeletal system and provides physical support and protection of the body, calcium metabolism, and endocrine regulation, and it facilitates the hematopoietic system in bone marrow. Bone remodeling is a dynamic process that continues throughout life and involves bone formation and bone resorption activities. The common path-ophysiological event in bone disorders is the disruption of bone homeostasis (Theoleyre et al., 2004). Bone homeostasis depends on the functional balance between bone-forming cells (osteoblasts, OBs) and bone-resorptive cells (osteoclasts, OCs). A functional imbalance between these two arms determines either osteosclerotic bone-forming diseases (i.e., osteopetrosis) or osteolytic bone-resorptive diseases (Theoleyre et al., 2004).
Inflammation is a protective mechanism involving the activation of innate and adaptive immune systems in response to exogenous (bacteria, virus, etc.) or endogenous (necrotic cells) stimuli. Immune cells recognize the inflammatory stimuli to activate several cellular signaling including nuclear factor-κB (NF-κB) (Cordova et al., 2014). NF-κB is a master transcriptional factor in regulation of the inflammatory response and bone-remodeling process (Lin, Tamaki, et al., 2014; Novack, 2011). The proinflammatory cytokines driven by NF-κB are powerful signals to modulate OB and OC activities (Purdue, Koulouvaris, Potter, Nestor, & Sculco, 2007). Activation of NF-κB signaling in OCs is crucial for their differentiation and activation (Boyle, Simonet, & Lacey, 2003), whereas the activation in OBs inhibits bone formation (Chang et al., 2009). These unique characteristics imply the great potential of NF-κB as a therapeutic target for the treatment of inflammatory-associated bone disorders.
Acute inflammation is an essential step to initiate tissue repair processes including bone healing (Alexander et al., 2011; Raggatt et al., 2014). Unresolved inflammation progresses into chronic inflammation and leads to pathological conditions in affected organs. This review will focus on the biological significance and therapeutic potential of NF-κB in bone disorders with acute (fracture healing) or chronic (fracture nonunion (FNU), per-iprosthetic osteolysis (see Section 2.3.2), and senile osteoporosis) inflammation. Tumor, osteoarthritis, rheumatoid arthritis, bone infection, and metabolic bone disorders are excluded because of their complicated pathogenesis involving (in some instances) systemic factors, the adaptive immune system, and factors beyond innate immunity and NF-κB signaling.
2. INFLAMMATION AND BONE DISORDERS
2.1 Inflammation
The major functions of inflammation are clearance of pathogens and reestablishment of tissue homeostasis. In addition to pathogen infection, sterile inflammation is defined as inflammatory responses induced by trauma, ischemia-reperfusion injury, or chemical-induced injury (Chen & Nunez, 2010). The acute inflammatory response in damaged tissue initiates the release of chemical mediators that increase vascular permeability and leukocyte infiltration via activation of the local endothelium. The infiltrated leukocytes, including neutrophils and macrophages, can recognize necrotic cell debris and secrete proinflammatory cytokines and chemokines to further enhance immune cell infiltration. The infiltrated cells engulf the damaged tissue and cell debris, and secrete proteinases and growth factors to facilitate tissue remodeling and reconstruction. Successful clearance of inflammatory stimuli is accompanied by increased antiinflammatory and reparative cytokines to resolve the inflammatory response and reestablish tissue homeostasis (Serhan & Savill, 2005). However, if unresolved, these events may progress to chronic inflammation when inflammatory stimuli persist in damaged tissue. This results in continuous secretion of cytokines that enhance tissue destruction and impair the homeostasis.
2.1.1 Acute vs Chronic Inflammation
Acute inflammation is initiated by recognition of inflammatory stimuli including microorganisms or damaged cell debris via the pattern-recognition receptors (PRRs). There are several classes of PRRs that recognize a variety of stimuli and trigger downstream inflammatory responses, including toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), C-type lectin receptors (CLRs), and absence in melanoma 2 (AIM2)-like receptors. PRRs can recognize pathogen-associated molecular patterns (PAMPs) from microorganisms. In sterile inflammation, PRRs can recognize damage-associated molecular patterns (DAMPs) from damaged tissue. The recognition of PAMPs and DAMPs and the activation of proinflammatory responses have been well documented by Takeuchi and Akira (2010) and Chen and Nunez (2010). DAMPs are intracellular proteins such as heat-shock proteins (Quintana & Cohen, 2005), nucleic acid (Cavassani et al., 2008; Imaeda et al., 2009), and intracellular cytokines (Eigenbrod, Park, Harder, Iwakura, & Nunez, 2008). Release of DAMPs from necrotic cells with impaired membrane integrity is an indicator of tissue damage. Recognition of DAMPs via PRRs in tissue macrophages leads to the secretion of chemoattractants and local recruitment of neutrophils and circulating monocytes/macrophages.
The inflammatory response may switch from acute to chronic inflammation when the stimuli persist (tissue damage, infection, etc.). Chronic inflammation is marked by infiltration of macrophages and lymphocytes, as well as ongoing attempts at repair. Excessive production of inflammatory cytokines from macrophages during chronic inflammation may cause tissue damage and fibrosis. For example, in patients with joint replacement surgery, continued release of wear particles from implanted biomaterials induce chronic inflammation and periprosthetic osteolysis in confined regions (Lin, Tamaki, et al., 2014).
2.1.2 Proinflammatory and Antiinflammatory Functions of Macrophages
Macrophages are crucial regulators of initiation, progression, and resolution of inflammation (Martinez, Sica, Mantovani, & Locati, 2008). Polarized macrophages may acquire distinct phenotypes with proinflammatory (M1) or antiinflammatory (M2) behaviors (Mantovani, Biswas, Galdiero, Sica, & Locati, 2013; Martinez et al., 2008). Classical activation of macrophages with interferon-γ and/or lipopolysaccharide leads to M1 macrophage polarization. M1 macrophages secrete proinflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), etc.) and chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), etc.) in a NF-κB-dependent pathway, which can result in tissue damage with additional leukocytes infiltration. Alternatively, macrophages exposed to IL-4 or IL-13 are polarized into M2 macrophages, marked by increased arginase-1 and antiinflammatory cytokines such as IL-10 and IL-1 receptor antagonist (IL-1Ra). The interaction between the NF-κB pathway and M2 macrophage polarization remains unclear. M2 or M2-like macrophages are capable of modulating and terminating the inflammatory response and are crucial for tissue remodeling and repair. Crosstalk between polarized macrophages and bone remodeling has been reviewed comprehensively elsewhere (Loi et al., 2016).
Thus, the biological roles of inflammation are to eliminate pathogens and foreign bodies, as well as initiate tissue repair and remodeling. The crosstalk of macrophages and others cells in the tissue microenvironment via modulation of inflammatory status and tissue remodeling may determine the status of inflammatory-associated conditions such as bone remodeling and repair.
2.2 Acute Inflammation-Associated Bone Disorders
Traumatic fracture and fragility fracture secondary to osteoporosis are closely associated with acute inflammatory responses. Infiltration of immune cells, especially macrophages, is critical for bone-healing processes (Loi et al., 2016). Fracture healing involves four consecutive phases: (a) acute inflammation, (b) cell proliferation and progenitor recruitment, (c) a stabilization step characterized by the consecutive formation of a fibrous, cartilaginous, and immature bony callus, and then (d) remodeling of the immature callus (Loi et al., 2016). The acute inflammation phase is driven by phagocytic cells including macrophages and polymorphonuclear neutrophils (PMNs), which are recruited from the hematopoietic niche and then attracted to the fractured site (Purdue et al., 2007). Macrophages and PMNs recognize DAMPs and PAMPs through TLRs and other receptors (see Section 2.1.1) and mediate NF-κB-dependent induction of proinflammatory and pro-osteoclastogenic cytokine secretion including TNF-α and IL-1β (Lin et al., 2015; Lin, Tamaki, et al., 2014). Transient elevated TNF-α levels during the acute inflammatory stage (d1–d3) are critical to the mediation of mesenchymal stem cell (MSC) migration into the fracture site and the differentiation into osteoblastic lineage cells (Karnes, Daffner, & Watkins, 2015). Supplementation of TNF-α (1 ng/ml) accelerates fracture healing rate and mineralization of callus (Glass et al., 2011). Nevertheless, persisted proinflammatory responses at the callus formation stage (days 3–7) could impair fracture healing. Martensson et al. reported that TNF-α and IL-1β synergistically decrease chondrocyte proliferation and survival at fracture callus, leading to reduction of bone formation in a rat model (Martensson, Chrysis, & Savendahl, 2004).
2.3 Chronic Inflammation-Associated Bone Disorders
2.3.1 Fracture Nonunion
FNU consists in incomplete consolidation of the fracture, with an absence of progressive radiographic signs of healing over three consecutive months (Loi et al., 2016; Panteli, Pountos, Jones, & Giannoudis, 2015). Based on the radiographic findings, FNU can be classified into either hypertrophic, exhibiting an oversized soft callus around the fracture site; or atrophic, featured by the absence of visible soft callus (Marsell & Einhorn, 2011). While hypertrophic FNU is associated with mechanical instability, atrophic FNU involves an intrinsic deficit of host immune and/or bone-healing responses (Karnes et al., 2015). Histologically, FNU demonstrates persistence of disorganized fibrous tissue, woven bone, and cartilage at the nonunion site (Karnes et al., 2015).
2.3.2 Periprosthetic Osteolysis
Total joint replacement (TJR) is an effective surgical procedure to treat patients with end-stage arthritis. In 2011, approximately 1 million of TJRs were performed in the United States (AAOS, 2013). Wear particles and other orthopedic byproducts generated from the bearing surfaces of TJRs induce chronic inflammation and bone loss around the implant, leading to aseptic loosening, and revision surgery in nearly 10% of TJR patients (Purdue et al., 2007). Wear particles less than 10 μm are either phagocytized by macrophages, or enclosed by foreign body giant cells (FBGCs) (if >10 μm) (Cobelli, Scharf, Crisi, Hardin, & Santambrogio, 2011). Recognition of wear particles or DAMPs adhering to the particles via TLR2 and TLR4 induces the secretion of proinflammatory cytokines in the NF-κB-dependent pathway (Pearl et al., 2011). The proinflammatory cytokines including TNF-α and IL-1β further activate NF-κB in macrophages as a positive regulatory loop during the chronic inflammation, leading to OC activation, and osteolytic processes (Lin, Pajarinen, et al., 2016; Lin, Tamaki, et al., 2014). Inhibition of NF-κB activity suppresses PMMA and UHMWPE wear particle induced OC activation in vitro and in vivo, suggesting the therapeutic potential of targeting NF-κB pathway in the particle disease (Clohisy, Hirayama, Frazier, Han, & Abu-Amer, 2004; Lin, Pajarinen, et al., 2016).
Exposure of wear particles also impairs osteoblastic phenotypes and paracrine regulation functions in MSCs and OB-lineage cells. Reduced expression of collagen type 1, bone sialoprotein, and decreased bone mineralization was found in human and murine MSCs exposed to titanium or polyethylene wear particles (Chiu, Ma, Smith, & Goodman, 2009; Lin et al., 2015; Wang et al., 2002). Inhibition of NF-κB activity in the MSCs exposed to wear particles mitigated the reduced bone formation (Lin et al., 2015). The paracrine regulators including IL-8, GM-CSF (Haleem-Smith et al., 2012), M-CSF, and RANKL (Pioletti & Kottelat, 2004) are also upregulated in OB or MSC exposed to wear particles, which could further enhance inflammation and the osteolytic process.
2.3.3 Senile Osteoporosis
Aging is associated with chronic inflammation and increased reactive oxygen species. The concept of “inflamm-aging” has been suggested to accelerate the aging process (Franceschi et al., 2000). Aging-associated bone loss is marked by reduced bone formation ability (compared to increased OC activity in postmenopause osteoporosis) and is often referred to as “senile osteoporosis” (Dobbs, Buckwalter, & Saltzman, 1999). Senile osteoporosis is associated with increased risk of fracture in 44–65% of women and 25–42% of men during the lifetime (Nguyen, Ahlborg, Center, Eisman, & Nguyen, 2007).
Though NF-κB activation could be associated with inflamm-aging process (Salminen et al., 2008), direct evidence of a correlation with senile osteoporosis in humans remain unclear. In one study using a murine model, NF-κB activation (increased phosphorylation of RelA) has been reported in the trabecular bone in natural aging mice (Yu et al., 2014). Furthermore, increased NF-κB activity was found in the MSCs isolated from aged mice (15 months) compared with younger mice (Lin, Gibon, et al., 2016). Inhibition of the NF-κB pathway partially rescued the reduction of osteogenesis in aged MSCs. Increased RANKL and decreased OPG expression (thus leading to increased RANKL/OPG ratio and OC activation) was observed in aged MSCs. Further investigation is essential to clarify the correlation of NF-κB pathway in the process of senile osteoporosis.
3. INFLAMMATION AND NF-κB SIGNALING
NF-κB is one of the best characterized transcription factors that regulate inflammation and innate and adaptive immune reactions (Hayden & Ghosh, 2011; Lawrence, 2009). NF-κB signaling is activated by a variety of proinflammatory and danger signals as well as cell stress sensed by multiple receptors expressed on the cell membrane, endosomal compartment, and cytoplasm. Activation of NF-κB signaling leads to the production of various inflammatory cytokines, chemokines, adhesion molecules, transcription factors, and antimicrobial effector molecules that initiate and module the inflammatory reaction, and orchestrates the immediate host response against pathogens and tissue damage. NF-κB signaling is also involved in lymphoid organogenesis; activation and proliferation of CD4+ T cells; T cell polarization into various different effector T cell populations; and to the maturation of B cells. The role of NF-κB signaling in these adaptive immune functions has comprehensively been reviewed elsewhere (Hayden & Ghosh, 2011). In the context of bone, NF-κB signaling is directly involved in the differentiation and activation of bone resorbing OCs (Novack, 2011; Soysa & Alles, 2009). Chronic NF-κB activation has also been shown to impair both the differentiation of MSCs along the osteogenic pathway and OB-mediated bone formation (Lin, Tamaki, et al., 2014). Thus NF-κB signaling plays a role both in physiological bone remodeling as well as pathological bone loss occurring in such inflammatory conditions as rheumatoid arthritis and periimplant osteolysis of TJRs. Indeed, blocking the chronic NF-κB signaling in these and other inflammatory conditions limits inflammation and prevents bone loss (Lin, Tamaki, et al., 2014; Xu et al., 2009).
3.1 The NF-κB Protein Family
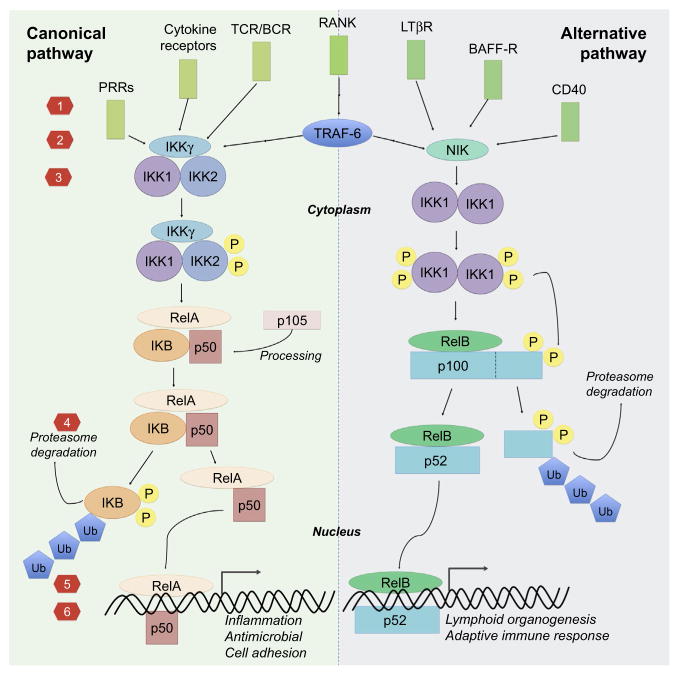
The NF-κB transcription factor family consists of five members p50 (NF-κ B1), p52 (NF-κB2), RelA (p65), RelB, and c-Rel (Huxford, Hoffmann, & Ghosh, 2011; Lawrence, 2009). All of the NF-κB subunits share a structurally conserved N-terminal sequence spanning 300 amino acid residues called the Rel homology domain (RHD) (Ghosh, May, & Kopp, 1998; Huxford et al., 2011). The RHD is responsible for DNA binding, dimerization, and nuclear translocation of the NF-κB subunits (Courtois & Gilmore, 2006; Huxford, Huang, Malek, & Ghosh, 1998). As described by Huxford, Hoff-man, and Ghosh, the RHD can be divided into three structural components—the N-terminal domain (NTD), dimerization domain (DD), and the nuclear localization sequence (NLS) polypeptide—all of which mediate the various activities of the RHD and subsequent NF-κB signaling (Huxford et al., 2011). At the C-terminal end of the RHD, the DD folds so that two antiparallel β-sheets form an immunoglobulin-like (Ig-like) structure; one of the sheets forms an interface for subunit dimer formation while the other mediates nonspecific DNA contacts (Chen & Ghosh, 1999). Similarly, the NTD contains an Ig-like fold that binds DNA both base-specifically and backbone nonspecifically. Subunits RelA, RelB, and c-Rel are produced as a mature protein while the p50 subunit is produced from an inactive precursor p105 and the p52 from precursor p100 via posttranslational processing in proteasome (Courtois & Gilmore, 2006; Gilmore, 2006; Lawrence, 2009). The production of p50 is constitutive while the production of p52 is regulated and induced upon the activation of the noncanonical NF-κB pathway (Fig. 1). In addition, the NF-κB p105 and p100 proteins are distinguished from Rel proteins by IκB-like inhibitory domains that play a role in the binding of inhibitory IκB proteins (Karin, Yamamoto, & Wang, 2004).
Fig. 1.
Canonical (left) and alternative (right) NF-κB pathway and the target strategies: (1) inhibition of cell-surface receptor binding, (2) inhibition of receptor adaptor proteins, (3) IKK inhibition, (4) blocking IκB degradation/upregulating IκB /inhibiting proteasome, (5) inhibit nuclear translocation, and (6) block DNA binding/transcriptional activation.
In resting state, NF-κB subunits reside in the cell cytoplasm non-covalently bound by a group of IκB proteins that maintain the NF-κB subunits in inactive form (Huxford et al., 2011; Whiteside & Israel, 1997). The mechanism underlying the inhibitory effects of IκB binding has been elucidated by determining the X-ray crystal structure of IκB α bound to p50:RelA dimers (Jacobs & Harrison, 1998). It is thought that the long-range electrostatic interactions between the C-terminal PEST region of IκB α and the NTD of RelA causes a conformational change that does not allow for RelA to bind DNA (Huxford et al., 1998; Jacobs & Harrison, 1998). Moreover, IκB α conceals the NLS peptide of p65, preventing its nuclear localization.
The degradation of these inhibiting proteins is regulated by a group of IκB kinase complexes IKK1 (IKKα), IKK2 (IKKβ), and IKKγ (NEMO) (Chen, 2005; Karin & Ben-Neriah, 2000). Once activated by upstream signaling cascades, IKKs phosphorylate the inhibitory IκB proteins leading to their ubiquitination by E3 ubiquitin-protein ligase followed by degradation in 26S proteasome. Once released the NF-κB subunits home to the nucleus as hetero- or homodimers and regulate the transcription of multitude of genes by binding to the gene promoter regions know as B sites; currently more than 500 NF-κB target genes have been recognized (Boston_University). The dimers bind to DNA via 10 flexible loops that extend from the Ig-like fold; this mechanism is unlike most transcription factors, which utilize alpha helices (Chen & Ghosh, 1999). Beyond this unique DNA-binding scheme, the NF-κB dimer arranges along the major groove for a full turn to create a “butterfly” structure.
Depending on the composition of the NF-κB dimer, the NF-κB can either induce or inhibit gene transcription; complexes containing RelA, RelB, and c-Rel function as transcription promoters while p50 and p52 lack the C-terminal transcription activation domains necessary to induce gene reading and can activate transcription only when paired with other NF-κB family members (Hayden & Ghosh, 2011); correspondingly p50 homodimers lacking activation domains function as transcription suppressors (Bohuslav et al., 1998). The signaling pathway leading to the degradation of IκB and the subsequent release of NF-κB subunits is known as the canonical (classical) NF-κB pathway, while the pathway culminating to the cleavage of p100 to active p52 is known as noncanonical (alternative) NF-κB pathway (Fig. 1).
3.2 Activators and Targets of Canonical Pathway
The canonical NF-κB signaling pathway is primarily involved in the sensing of danger due to tissue damage or infection, and is followed by rapid initiation and progression of an inflammatory reaction and antimicrobial functions (Huxford et al., 2011; Lawrence, 2009; Lin, Tamaki, et al., 2014). In the context of innate immunity, the pathway is activated by signals originating from two broad groups of receptors; the receptors for proinflammatory cytokines and the PRRs for various danger signal molecules (Hayden & Ghosh, 2014; Kawai & Akira, 2010). Downstream signals from these receptors convene to active a kinase complex formed by IKK1, IKK2, and IKKγ, with IKK2 playing the key role, leading to the release and nuclear translocation of mainly p50-RelA and p50-cRel dimers (Hayden & Ghosh, 2011). These factors promote the transcription of multiple proinflammatory cytokines, prostaglandins, chemokines, endothelial and leucocyte adhesion molecules as well as proteinases leading to recruitment and activation of further inflammatory cells, mainly neutrophils and macrophages. Antimicrobial effector molecules, such as defensing and reactive oxygen and nitrogen species, are also produced and the antigen presenting machinery induced for the subsequent activation of the adaptive immune system. In addition to transcription of proinflammatory signals, antiinflammatory cytokines such as IL-10 and IL-1rA as well as multiple inhibitors of NF-κB pathway, e.g., IκB proteins, are produced thus limiting the inflammatory reaction in a manner of an autocrine feedback loop (Lawrence, 2009).
Proinflammatory cytokines including TNF-α and IL-1 are among the best-known inducers and also target of the canonical NF-κB pathway (Hayden & Ghosh, 2014). These archetypal proinflammatory cytokines are abundantly produced during the inflammatory reaction and play a key role in multiple chronic inflammatory conditions. Binding of these cytokines to corresponding receptors TNF receptor 1 (TNFR1, widely expressed) and TNF receptor 2 (TNFR2, expressed on immune cells), and type I IL-1 receptor (IL-1R1) activates canonical NF-κB pathway signaling but also the MAP kinase/AP-1 pathway amplifying the inflammatory reaction.
The second set of receptors that activate the canonical NF-κB pathway are PRRs that sense danger signals originating from tissue damage and invading pathogens (Matzinger, 2002). The first recognized and the best-known PRR family is the TLRs (Kawai & Akira, 2010; Kumar, Kawai, & Akira, 2009). This set of receptors recognizes various evolutionally well-conserved molecular repeat structures expressed on various pathogens; the best-known examples include TLR2 ligand lipoteichoic acid (LTA) and TLR4 ligand lipopolysaccharide (LPS) both of which are fundamental structural components of Gram-positive or Gram-negative bacteria cell walls, respectively. TLRs also recognize conserved viral (e.g., single- and double-stranded RNA, recognized by TLR3, TLR7, and TLR8) and fungal (e.g., zymosan, recognized by TLR2) structures. In addition to these pathogen-derived molecules, or PAMPs, it has been suggested that several TLRs recognize endogenous ligands collectively known as alarmins (Bianchi, 2007; Kono & Rock, 2008). Other families of PPRs, including NLRs and RLRs, activate canonical NF-κB pathway and IRF3-mediated type 1 interferon production (Kawai & Akira, 2010, 2011). Unlike TLRs that are restricted to cell and endosomal membranes, NLRs and RLRs are located to the cell cytoplasm thus being optimally located to recognize viral structures and complementing the cells danger signal sensing machinery.
3.3 Activators and Targets of the Alternative Pathway
While the canonical NF-κB pathway is related to the rapid initiation and amplification of an inflammatory reaction, the best-known functions of the noncanonical pathway are related to lymphoid organogenesis and the activation and progression of adaptive immune response (Hayden & Ghosh, 2011; Lawrence, 2009). The activation of the pathway culminates in the induction of NF-κB-inducing kinase (NIK) and formation of IKK1 dimers. IKK1 phosphorylates p100 leading to its proteosomal processing into p52 and nuclear translocation of mainly p52/RelB dimers. The ligands that activate the noncanonical pathway include several TNF family members related to adaptive immune functions including lymphotoxin b, CD40 ligand, and B-cell activating factor (BAFF) but not TNF-α itself.
In the context of bone, the best-known function for the noncanonical NF-κB pathway is related to the bone resorption (Abu-Amer, 2013; Boyce, Yao, & Xing, 2010; Novack, 2011). The formation and function of bone resorbing OC are dependent on the receptor RANK expressed on circulating OC precursors (derived from monocytes) and its ligand, RANKL, produced by OBs, other MSCs, and activated T cells (Lacey et al., 1998; Suda et al., 1999). Both RANK and RANKL are necessary for OC formation. RANK signaling activates both the canonical and non-canonical NF-κB pathway via TRAF6 (Novack, 2011; Soysa & Alles, 2009). RANK signaling also activates the MAP kinase pathway with induction of the transcription factor c-Fos that is also necessary for OC formation (Grigoriadis et al., 1994). All of these pathways synergize to induce the expression of transcription factor NFATc1 that directly activates the transcription of OC-related genes and is considered as the master regulator of OC formation and sustained function (Kim & Kim, 2014; Takayanagi et al., 2002). The activity of RANKL is regulated on the one hand by a secreted decoy receptor, OPG that inhibits RANKL activity, and on the other hand by various inflammatory cytokines such as TNF and IL-1 and TLR ligands that enhance OC formation by synergizing with RANKL in activating the canonical NF-κB pathway (Osta, Benedetti, & Miossec, 2014; Simonet et al., 1997). Furthermore, the balance of RANKL and OPG production is regulated by these inflammatory signals typically increasing the RANKL/OPG ratio (Abu-Amer, 2013). Thus multiple mechanisms drive the increased OC formation and bone loss seen in the context of chronic inflammatory conditions.
4. NF-κB AND BONE REMODELING
4.1 Bone Remodeling
Bone remodeling is a necessary process to repair damaged bone and involves the resorption and formation of hard tissue. OCs, OBs, and osteocytes are the cells maintaining bone matrix homeostasis in bone. Immune cells, especially macrophages, are critical in the bone-remodeling process in response to damage and other inflammatory stimuli (Alexander et al., 2011; Cho et al., 2014; Guihard et al., 2012; Lin, Tamaki, et al., 2014). Under normal physiological conditions, osteocytes secrete sclerostin (van Bezooijen et al., 2004) and transforming growth factor β1 (TGF-β1) (Heino, Hentunen, & Vaananen, 2002) to suppress the activities of OB and OC, respectively. Osteocyte apoptosis caused by damage or inflammation is associated with a reduction of the suppressive paracrine regulators and thus, initiates bone remodeling at the damaged site. The basic multicellular unit (BMU) is a specialized structure formed in the bone-remodeling process. In an active BMU, OBs and OCs line the bone surface, covered by a canopy-like structure consisting of bone resident macrophages (known as osteomacs) (Chang et al., 2008), and acting in bone resorption and bone formation by a coupling reaction. The process is then terminated when the bone homeostasis is reestablished.
The crosstalk between immune cells and bone cells is tightly associated with mineral homeostasis and tissue repair in bone. However, inflammation-associated NF-κB activation is not limited to immune cells. The signals can be activated in OBs and OCs via direct exposure to the stimuli like PAMPs or DAMPs, or indirect regulation by the immune cells (Xu et al., 2009; Chang et al., 2013; Lin, Tamaki, et al., 2014; Lin et al., 2015). The fundamental roles of NF-κB activation in OC differentiation and activation are well defined (Boyle et al., 2003). The biological functions of NF-κB activation in OBs and MSCs are recently being clarified (Chang et al., 2013, 2009; Cho et al., 2010; Lin et al., 2015), although the detailed regulation of their bone-forming ability remains unclear. In this section, we summarize the current findings of NF-κB activation effects on bone-remodeling process (Fig. 2), including the studies using transgenic animal models or the stimulation by proinflammatory cytokines such as TNF-α and IL-1β. In addition, several reports have indicated that the TLR agonists can modulate the biological activities in OC (Itoh et al., 2003; Takami, Kim, Rho, & Choi, 2002) and OB (Hwa Cho, Bae, & Jung, 2006; Lombardo et al., 2009; Mo et al., 2008).
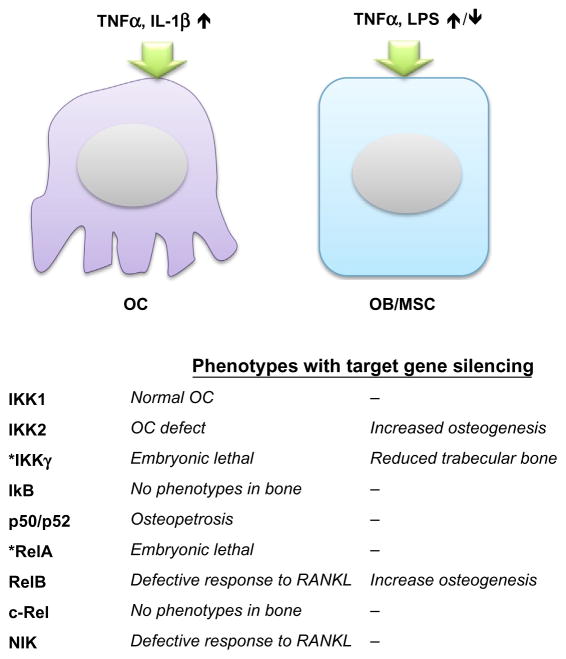
Fig. 2.
The roles of NF-κB pathway in OC and OB/MSC biological activity. The upstream NF-κB stimulators (TNFα, IL-1β) can enhance OC activity. The effect of NF-κB activation to OB/MSC is associated with timing, dose, duration, and the composition of the stimulators (TNFα, LPS, etc.). The table at the bottom summarized the specific role of NF-κB protein family reported in the studies using transgenic mice or pharmacological inhibitors. *IKKγ- and RelB-deficient mice are embryonic lethal, and their roles in OC have not been reported. The specific role of IKKγ in OB was identified using OB-specific transgenic mice model.
4.2 NF-κB in Osteoclasts
OCs are terminal differentiated myeloid lineage cells, which can be characterized by the unique multinuclear morphology and the expression of OCs-specific markers including tartrate-resistant acid phosphatase (TRAP), cathepsin K, calcitonin receptor, and β3 integrin (Boyle et al., 2003). Myeloid precursor cells differentiate into macrophages when exposed to macrophage colony-stimulating factor (M-CSF), or differentiate into OCs in the presence of M-CSF and RANKL.
RANKL can induce both classical and alternative NF-κB activation in OCs and their precursors (Novack, 2011). Classical activation of NF-κB requires IKK2 to phosphorylate and degrade IκB, and releases RelA/p50 or cRel/p50 heterodimer to translocate into the nucleus. Deletion of IKK2 (IKK2−/−) in transgenic mice caused defective osteoclastogenesis in OC precursors in response to RANKL, TNF-α, or IL-1β, which results in osteopetrosis (excessive bone formation) and resistance to inflammatory-associated bone loss in vivo (Ruocco et al., 2005). In contrast, mutation of IKK1 (IKK1AA) shows a defect of osteoclastogenesis in OC precursors in response to RANKL but not TNF-α or IL-1β in vitro. Notably, the defect of osteoclastogenesis was not observed in IKK1AA mice in vivo, which could be explained by compensatory effects from the paracrine regulation of OB. The role of IKKγ in skeletal development remains unclear due to the lethal phenotypes of severe liver degeneration in IKKγ-deficient mice (Rudolph et al., 2000). No phenotypes in bone have been reported in the transgenic mice lacking IκB or cRel expression (Gerondakis et al., 1996; Klement et al., 1996). Further studies are required to clarify their roles in skeletal development and bone remodeling.
Osteopetrosis and impaired osteoclastogenesis were reported in the transgenic mice with p50/p52 deletion, and the phenotypes were rescued by bone marrow cell transplantation (Franzoso et al., 1997; Iotsova et al., 1997). The transgenic mice with the single deletion in p50 or p52 showed no significant phenotypes in bone, suggesting the redundant roles of p50 and p52 to regulate OC functions and bone homeostasis. The RelA-deficient mice are embryonically lethal (Beg, Sha, Bronson, Ghosh, & Baltimore, 1995), while the mice with deficiency in RelA and TNFR1 live for 2–3 weeks (Rosenfeld, Prichard, Shiojiri, & Fausto, 2000). In the radiated wild-type mice transplanted with bone marrow cells from RelA/TNFR1-deficient mice, the OC numbers at the basal level or upon RANKL induction are significantly decreased (Vaira, Alhawagri, et al., 2008). RANKL-induced significant cellular apoptosis in RelA/TNFR1-deficient precursor cells in vitro through JNK/Bid/caspase 3 pathway, and blocking of proapoptotic Bid signaling protect the cells from RANKL-induced cell deaths and rescued the defect of osteoclastogenesis in p65-deficient cells. Taken together, the results suggested that RelA is essential for the anti-apoptotic signaling in OC in response to RANKL stimulation, but is not required for OC activation.
The alternative NF-κB pathway can be activated by RANKL but not the proinflammatory cytokines. In this process, NF-κB-inducing kinase (NIK) is stabilized in response to the stimulation and activates IKKα, which then process p100 into p52 to form RelB/p52 heterodimer (Sun, 2011). The nuclear translocation of RelB is specifically regulated by p100, and thus unique downstream signaling of NIK. Mild increased trabecular bone volume and normal OC numbers at the basal line are observed in both NIK-and RelB-deficient mice (Novack et al., 2003; Vaira, Johnson, et al., 2008). Nevertheless, administration of RANKL failed to induce osteoclastogenesis in the OC precursors with the defect in NIK or RelB in vitro (Novack, 2011). Overexpression of RelA cannot rescue defective osteoclastogenesis in RelB-deficient precursor cells induced by RANKL, suggested that RelB may be the key regulator of OC differentiation (Vaira, Johnson, et al., 2008).
The proinflammatory cytokines including TNF-α and IL-1β induce osteoclastogenesis via direct activation of OC precursor cells, or indirect induction of RANKL secretion in bone marrow stromal cells. Nevertheless, the dependence of RANKL/RANK signaling in the OC activation process is still in debate. Kobayashi et al. (2000) first reported that murine bone marrow myeloid cells differentiated into TRAP+ OCs in the presence of M-CSF and TNF-α. The bone-resorption ability in OCs and RANKL secretion in OBs stimulated by TNF-α was dependent on IL-1β (Kobayashi et al., 2000; Wei, Kitaura, Zhou, Ross, & Teitelbaum, 2005; Zwerina et al., 2007). Antibodies against TNF-α receptors but not RANKL inhibited this process, suggesting that the TNF-α-mediated OC activation is independent of RANKL/RANK signaling (Kobayashi et al., 2000). Lam et al. showed that TNF-α and M-CSF-mediated OC activation and required a permissive level of RANKL (Lam et al., 2000). Inhibition of RANKL by OPG at an earlier stage of OC differentiation (exposed to M-CSF alone) augmented TNF-α-mediated OC activation. Interestingly, bone marrow myeloid cells deficient in RANK can differentiate into OCs by TNF-α stimulation; this demonstrated the existence of RANKL/ RANK-independent pathway of OC activation (Kim et al., 2005).
The classical NF-κB activation can be stimulated by TNF-α, whereas the alternative NF-κB activation could be inhibited in OC precursors. TNF-α induced the accumulation of p100 in OC precursors, which inhibited the alternative NF- B activation (Yao, Xing, & Boyce, 2009). A recent study further demonstrated that the limitation of TNF-α-mediated OC activation was only observed in macrophage/OC precursors stimulated by M-CSF but not in combination with TNF-α (Zhao et al., 2015). Mechanistic studies showed that TNF-α-induced RelB expression and enhanced OC activation, but this process was self-limited by suppression of NFATc1.
4.3 NF-κB in MSCs/Osteoblasts
OBs are specialized bone-forming cells differentiated from MSCs, which have multilineage differentiation abilities including bone, cartilage, adipose tissue, etc. Runx2 is a master regulator of osteoblastogenesis which initiates the commitment of osteogenic differentiation into osteoprogenitors. The expression of Runx2, followed by osterix, induces alkaline phosphatase (ALP) and type I collagen secretion, and turn the cells into mature OBs located on the bone surface (Wu, Scadden, & Kronenberg, 2009). Mature OBs deposit the organic matrix for bone mineralization and, once surrounded by bone matrix, become osteocytes, which account for almost 95% of all bone cells. Osteoblastogenesis is guided by paracrine or endocrine factors including parathyroid hormone, bone morphogenic proteins (BMPs), Wnt signaling, and growth factors such as TGF-β1 (Novack, 2011). In addition, differentiated OBs secrete M-CSF, RANKL, and OPG to regulate OC activity in a coupling reaction during the bone-remodeling process.
The role of NF-κB in OC activation and differentiation has been well defined. However, the biological effects of NF-κB activation in OB differentiation and bone formation are still in debate. The first direct evidence for the suppressive role of canonical NF-κB signaling in osteogenesis was demonstrated by using transgenic mice expressing a dominant negative form of IKKγ in differentiated OBs as controlled by osteocalcin promoter. Inhibition of NF-κB activity in OBs increased trabecular bone mass and bone mineral density without affecting OC function in young (2–4 weeks old) mice. In addition, inhibition of NF-κB activity prevented ovariectomy-induced bone loss in adult mice by maintaining bone-forming ability in OBs. In a study using human, murine, and rat MSCs, inhibition of IKK2 by a small molecular inhibitor or gene deletion increased osteogenic ability at the basal level or in the presence of TNF-α or IL-17. Local administration of IKK2 inhibitor enhanced MSC-mediated bone repair in a murine calvarial bone defect model. This mechanistic study demonstrated that NF-κB activation induced the expression of Smurf1/2, the ubiquitin ligase controlling the degradation of β-catenin, and inhibits osteogenic differentiation (Chang et al., 2013). Expression of constitutive active IKK2 in OBs and cho-ndrocytes in transgenic mice controlled by Col2α1 promoter exhibits abnormal skeletal development with impeded bone formation and reduced bone mineral density. A heterozygous missense mutation on RelA in an osteopetrosis patient was revealed by trio-based whole exome sequencing, demonstrating the critical role of NF-κB signaling in human skeletal homeostasis.
In studies of noncanonical NF-κB signaling on bone formation, increased OB numbers and increased bone formation rate were found in the transgenic mice with NIK mutation. However, the effects of mutant NIK on osteogenesis had not been examined in MSCs and/or OBs. RelB-deficient mice developed age-related increased trabecular bone mass associated with increased bone formation. RelB-deficient MSCs had increased bone-forming ability in both in vitro studies and in the murine tibia defect model. These studies suggest that the noncanonical NF-κB signaling also plays a significant role in osteogenic differentiation.
Compared to the evidence in transgenic animal studies, the effects of NF-κB activation induced by extracellular stimulation in OB differentiation remain paradoxical (Osta et al., 2014). Bone mineral density and bone formation were elevated in the TNF-α or TNF-α receptor 1-deficient mice, whereas bone-resorption activity was not changed (Li et al., 2007). Direct injection of TNF-α into wild-type mice reduced osteogenic differentiation in MSCs, which was blocked in the ubiquitin ligase WWP1-deficient mice (Zhao et al., 2011). In vitro studies with primary bone marrow stromal cells or MC3T3 E1 (clone 14) cells demonstrated that 10 ng/ml TNF-α potently suppressed osteogenesis via suppressing Runx2 expression (Abbas, Zhang, Clohisy, & Abu-Amer, 2003; Gilbert, Rubin, & Nanes, 2005; Li et al., 2007). Interestingly, TNF-α-mediated inhibition of Runx2 is NF-κB dependent, whereas the suppression of osterix is depended on MEK1/ ERK1 signaling but independent of NF-κB (Lu, Gilbert, He, Rubin, & Nanes, 2006).
There is increasing evidence indicating an important induction role of TNF-α in osteogenic differentiation. Hess et al. demonstrated that treatment of 20 ng/ml TNF-α during osteogenic differentiation enhanced BMP2 expression, followed by Runx2 and osterix expression, and increased mineralization in human MSCs (Hess, Ushmorov, Fiedler, Brenner, & Wirth, 2009). Induction of constitutive active IKK2 by using retroviral vectors enhanced, whereas IκB impaired TNF-α-mediated osteogenesis induction. In studies of rat MSCs cultured in poly(ε-carprolactone) scaffold, the lower dose (0.1–5.0 ng/ml) inhibited, whereas the higher dose (50 ng/ml) of TNF-α enhanced mineralization in dexamethasone-pretreated cells (Mountziaris et al., 2013; Mountziaris, Tzouanas, & Mikos, 2010). The high dose of TNF-α mediating osteogenic induction was only observed with continuous (days 1–16) or early (days 1–4) treatments, but not with intermediate or later treated cells (Mountziaris et al., 2013). Early treatments of extracellular NF-κB stimulators including TNF-α, LPS, or peptidoglycan (the agonist for TLR2) enhanced osteogenesis in human adipose tissue-derived (Cho et al., 2010) or bone marrow-derived MSCs (Croes et al., 2015). Inhibition of NF-κB activity or silence of its downstream target TAZ impaired the induction of osteogenic differentiation, suggesting the direct regulation of NF-κB on TNF-α-mediated osteogenesis (Cho et al., 2010). In addition, Lu et al. demonstrated that human OBs and MSCs preconditioned with 1 ng/ml TNF-α for 1–3 days enhanced osteogenic differentiation via induction of BMP2 expression (Lu et al., 2013; Lu, Wang, Dunstan, & Zreiqat, 2012). Mechanistic studies have shown that inhibition of ERK1/2 or Wnt signaling impeded TNF-α-induced osteogenesis (Briolay et al., 2013; Lu et al., 2013); however, these studies did not address the role of NF-κB induced by TNF-α. In vivo studies have demonstrated that local injection of TNF-α at the early stage (24 h) augmented fracture healing by recruitment of muscle-derived stromal cells and infiltrated macrophages. Low and continuous levels of TNF-α were associated with proosteogenic effects, contributing to the formation of bone spurs (enthesis) by upregulating the activity of ALP (Ding et al., 2009; Lencel et al., 2011). However, the involvement of NF-κB signaling during the inflammation and healing processes remains unclear (Chan et al., 2015; Glass et al., 2011).
Taken together, although studies using transgenic animals demonstrated that NF-κB activation impairs normal skeletal development and bone-forming ability in OB-lineage cells, their effects on the osteogenic differentiation stimulated by extracellular signals are still in debate. The controversial finding in these reports could be due to the dose and the exposure times of NF-κB inducer, cell type used, and the conditions of osteogenic induction (DelaRosa & Lombardo, 2010; Osta et al., 2014).
5. PHARMACEUTICAL APPROACHES TO MODULATE NF-κB ACTIVITY
In this section, we will broadly discuss the structural aspects of NF-κB transcription factors, their interactions with select regulatory proteins, and how this knowledge can help in targeting NF-κB signaling for therapeutic purposes.
As aberrant NF-κB signaling is implicated in many disease processes, the development of NF-κB inhibitors has been widespread. To date, there are over 800 inhibitors that have been reported with, undoubtedly, many more to come (Gilmore & Garbati, 2011). There are step-wise approaches to inhibiting the NF-κB transduction pathway by: receptor inhibition, adaptor inhibition, IKK inhibition, IκB stabilization, cytoplasmic retention, and transcription factor inhibition (Gilmore & Garbati, 2011).
Since ligand binding to cell-surface receptors activates NF-κB signaling, inhibition of receptor binding can be used to block NF-κB activation. Most notably, anti-TNF-α antibodies such as etanercept and infliximab have been used to block activation of the canonical NF-κB pathway and have been used in several chronic inflammatory diseases (Gilmore & Garbati, 2011; Taylor & Feldmann, 2009). Similarly, denosumab, an anti-RANKL antibody, has been used to treat osteoporosis, and its role in preventing osteolytic lesions following total hip arthroplasty is in Phase 2 clinical trial (Clinicaltrials.gov, 2012). Once receptors are engaged, adaptor proteins such as TRAF are recruited to the cell membrane. Though these receptor or adaptor inhibitors are effective, their off-target effects on multiple signaling pathways and the compensatory effects from other NF-κB upstream regulators have made them less desirable as specific NF-κB inhibitors.
Most of the developments in NF-κB inhibitors have focused on targeting the IKK protein family since they are the central integrator of the NF-κB pathway without involvement of other cellular signaling. There are a variety of inhibitors that exert their effects via different mechanisms (Gilmore & Herscovitch, 2006): (1) The ATP analog that specifically interacts with IKK. For example, β-carboline, a natural ATP analog, specifically binds IKK2 to inhibit NF-κB signaling (Karin et al., 2004). Similarly, NSAIDs provide COX-independent antiinflammatory effects by competitively inhibiting the ATP-binding site of IKK2 (Karin et al., 2004). (2) Compounds bind to IKK proteins and mediate conformational changes. The synthetic BMS-345541 has been shown to inhibit IKK2 activity through allosteric effects (Karin et al., 2004). (3) Compounds bind to the Cys-179 residue on the activation loop on IKK protein and blocking the kinase activity. Several thiol-reactive compounds were shown to interact with IKK2 and inhibit the kinase activity, although the detailed mechanism remains unclear (Kwok, Koh, Ndubuisi, Elofsson, & Crews, 2001). In the canonical NF-κB pathway, NEMO and IKK1 are also comprised in the IKK complex. Therefore, dominant negative mutants or specific inhibitors to these proteins could also result in NF-κB signaling inhibition (Gilmore & Garbati, 2011; Scheidereit, 2006). For bone-related disease, the IKK inhibitor SAR113945 recently completed Phase 1 of clinical trials as a treatment for knee osteoarthritis (Clinicaltrials.gov, 2011). Notably, most of the current developed IKK inhibitors have targeted IKKβ and thus only inhibit the canonical NF-κB pathway. Inhibition of the noncanonical pathway via targeting IKK1 activity could be particularly important in osteolytic bone diseases, regarding the fact that IKK1 mutant in OC precursors showed defective osteoclastogenesis but normal TNF-α and IL1β signaling (Ruocco et al., 2005). As the IKK complex is not completely understood in itself, it is almost certain that more IKK inhibitors will be developed and/or discovered to match future understanding.
The next important step in the NF-κB signaling is the degradation of IκB. There are three main strategies for blocking IκB degradation: (1) promoting IκB synthesis, (2) blocking IκB ubiquitination, and (3) inhibition of the proteasome (Gilmore & Garbati, 2011). The most prominent proteasome inhibitor is bortezomib, which has shown efficacy in treating multiple myeloma as well as other hematologic and solid tumors (Gilmore & Garbati, 2011; Jagannath et al., 2010). Similarly, sulfasalazine, a medication used to treat inflammatory bowel disease, prevents NF-κB activation by blocking IκB degradation in response to TNFα and LPS (Karin et al., 2004; Wahl, Liptay, Adler, & Schmid, 1998).
Once IκB is degraded, the NF-κB dimer must translocate into the nucleus, so inhibitors of nuclear localization can block NF-κB signaling. As nuclear entry appears to be mediated by importin α, cell-permeable peptides with the NLS of p50 have been used to saturate importin α, and thus, block NF-κB dimer nuclear entry (Gilmore & Garbati, 2011; Letoha et al., 2005; Lin, Yao, Veach, Torgerson, & Hawiger, 1995; Torgerson, Colosia, Donahue, Lin, & Hawiger, 1998). However, these peptides were not specific to NF-κB and have not been used applied beyond the laboratory.
Finally, NF-κB signaling can be blocked at the level of DNA by directly blocking DNA binding or competitively inhibiting binding through the introduction of NF-κB decoy oligodeoxynucleotides (ODNs). For example, there are several compounds that target both IKK2 and NF-κB DNA binding such as parthenolide (Garcia-Pineres, Lindenmeyer, & Merfort, 2004; Gilmore & Garbati, 2011). However, these compounds likely affect other protein targets. Alternatively, NF-κB decoy oligonucleotides (ODN) specifically compete for binding of NF-κB dimers to their DNA targets, and could simultaneously inhibit both canonical and noncanonical NF-κB pathway. It has been shown that NF-κB decoy ODN significantly suppress cyto-kine and chemokine expression in macrophages, especially in the setting of periprosthetic osteolysis (Lin, Pajarinen, et al., 2016; Lin et al., 2015; Lin, Yao, et al., 2014). Moreover, there are several NF-κB decoy ODN-based therapies that have entered clinical trials for dermatitis and psoriasis (Gilmore & Garbati, 2011).
6. DRUG DELIVERY STRATEGIES IN BONE DISORDERS
6.1 Classification
Over 800 NF-κB inhibitors have been reported, and the number continues to increase (Gilmore & Garbati, 2011; Gilmore & Herscovitch, 2006). These inhibitors can be broadly divided into three categories including (1) proteins and peptides, (2) small molecules, and (3) nucleic acids. Proteins and peptides inhibitors include antibodies and growth factors and are highly specific with less off-target side effects (Craik, Fairlie, Liras, & Price, 2013). The common limitations in protein-based treatment are instability when applied in vivo, high dose requirement, high cost of manufacturing, undesirable immunogenic effects, contamination, and the limitations for oral administration. Small molecules are natural or synthetic nonpeptide molecules with small molecular size (<1000 Da) and have several advantages in clinical application. These molecules are characterized by greater tissue penetration and are less immunogenic due to their smaller size compared to the protein-based compounds (Lo, Ashe, Kan, & Laurencin, 2012). Other advantages include lower manufacturing costs and application for oral administration. However, these compounds could be less selective compared to the protein or nucleic acid-based drugs, raising the concern of nonspecific toxicity, limiting their clinical application (Balmayor, 2015; Torchilin, 2000). Nucleic acid-based molecules include such as oligonucleotides, small interfering RNAs (siRNAs), and microRNAs (miRNAs). These approaches are highly specific and directly silence protein translation via siRNA or miRNA, or inhibit gene transactivation via decoy ODN. In particular, modulating the transcriptional process such as NF-κB by using the decoy ODN is an emerging approach due to the complexity of the signal transduction pathway (Rad et al., 2015). Synthetic decoy ODN is a short fragment of DNA which has the consensus sequence of the binding site of the target transcription factor, thus decoy ODN has the ability to bind to free target transcription factor subsequently preventing these factors from binding to the specific promoter regions (Bielinska, Shivdasani, Zhang, & Nabel, 1990). Like protein-based therapy, nucleic acids are also limited by low cellular uptake and rapid degradation in biological fluids (De Stefano, 2011).
6.2 Drug Delivery to the Skeletal System
Local drug delivery is applicable to many of the bone disorders in a confined region including fractures and periprosthetic osteolysis. Though local delivery strategy has the advantage of reduced systemic adverse effects, systemic drug delivery is still desired for the long-term management during the disease process, and treatment for systemic bone diseases such as osteoporosis. Recent developments in bone cell-specific targeting vehicles allow gene modulation and transcriptional regulation in OC, OB, or MSC in skeletal system. Thus, the adverse effects of NF-κB therapy to nontargeted cell populations (i.e., lymphocytes) in the bone marrow can be reduced and advance its translational application. Here we summarize the current advanced techniques for local and systemic drug delivery in the skeletal system (Fig. 3).
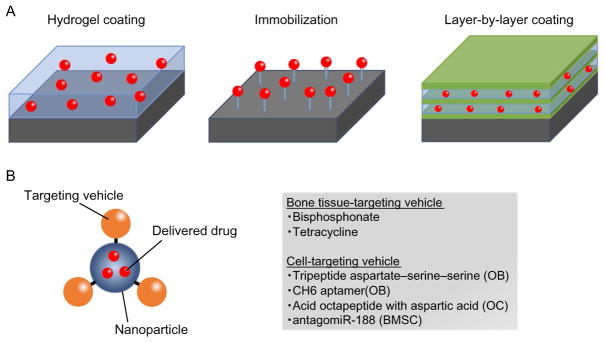
Fig. 3.
The strategies of drug delivery to the skeletal system. (A) Three surface coating procedures of the implants. (B) Systemic drug delivery conjugated with bone-targeting vehicle. OB, osteoblast; OC, osteoclast; BMSC, bone marrow mesenchymal stem cell.
6.2.1 Local Drug Delivery
Local drug delivery systems are designed to keep the localized and controlled release of biomolecules in a confined region. The advantages of local drug delivery include reduced systemic toxicity; avoidance of the risk of overdose; and upholding effective concentrations at target regions. The aims of drug delivery in orthopedics are to improve bone-healing processes, increase osseointegration, mitigate inflammation, and prevent infection. Implantable devices with controlled drug release are some of the most commonly used strategies for local drug delivery in the orthopedic field, and the techniques have also been applied to other diseases including cardiovascular diseases, cancer, periodontal diseases, and ophthalmologic diseases (Arruebo, Vilaboa, & Santamaria, 2010). Orthopedic implants are widely used for fracture fixation, TJR, spinal reconstruction, and other orthopedic applications. Several different strategies have been developed for surface coating of local drug release of orthopedic implants (Goodman, Yao, Keeney, & Yang, 2013; Raphel, Holodniy, Goodman, & Heilshorn, 2016), including the use of hydrogel, immobilization (Maia, Bidarra, Granja, & Barrias, 2013), and layer-by-layer coating (de Villiers, Otto, Strydom, & Lvov, 2011).
The layer-by-layer coating method is based on the alternating deposition of positively and negatively charged polyelectrolytes onto the implant surface. Charged biomolecules can be loaded onto the surface, and the amount of encapsulated biomolecules and release rate can be tuned by changing the concentration of the biomolecules, the number of layers, and the type of polyelectrolytes. Coating can be performed onto any shape and dimension of different material surfaces. Furthermore, due to simple process of layer-by-layer coating, various biomolecules have been applied for drug release from implants including proteins (Keeney et al., 2013; Min, Braatz, & Hammond, 2014), nucleic acids (Miyake et al., 2014; Tahara et al., 2011), and small molecules (Min et al., 2014). The limitations of layer-by-layer coating techniques include the fact that many layers are required to prevent rapid diffusion and burst release of biomolecules. Thus, long production times are needed, which may lead high batch-to-batch variability. Also, the influence of the layers on the mechanical stability after a press fit implantation into bone must be considered. Further studies are required to address such limitations.
Combination of drug delivery systems have been used to overcome the instability of target drugs such as ODN. De Rosa and La Rotonda reviewed the details of delivery systems including cationic liposomes, poly-ethylenimine (PEI), chitosan, and poly(lactide-co-glycolide) (PLGA) nanospheres which have the ability to increase the cellular uptake of ODN, prolong their stability, and localize the effect of ODN to target cells and tissues (De Rosa & La Rotonda, 2009). Furthermore, coating with chitosan showed a positive charge, which can penetrate easily through the negative charged cell membrane and enhance cellular uptake of ODN (Mao et al., 2001). Additionally, PLGA is thought to have the proton sponge mechanism to escape from the endosomal pathway (Panyam, Zhou, Prabha, Sahoo, & Labhasetwar, 2002). Taken together, combinations of multiple drug delivery systems could improve the drug release profile and keep the biological stability of target drugs in a confined region.
6.2.2 Systemic Delivery (Bone-Targeting Vehicle)
Early development of drug delivery to the skeletal system was focused on targeting the bone environment. The synthetic compounds including bis-phosphonates and tetracycline have high affinity for calcium ions and hydro-xyapatite (Pazianas & Abrahamsen, 2011; Russell, 2011). Therefore, the concept of bone-targeted delivery system is the use of biomolecules conjugated with bisphosphonates as ligands targeting bone mineral (Cole, Vargo-Gogola, & Roeder, 2016). Clinical applications investigating bisphosphonates as a targeting ligand include metabolic bone diseases such as osteoporosis (Bhandari, Newa, Chapman, & Doschak, 2012; Fujisaki et al., 1997; Morioka et al., 2010), bone infection (Houghton et al., 2008), rheumatoid arthritis (Hirabayashi et al., 2001), osteosarcoma, and cancer metastases to bone (Klenner et al., 1990; Reinholz et al., 2010; Torres Martin de Rosales, Finucane, Mather, & Blower, 2009). Intravenous injection or oral administration of bisphosphonate-conjugated vehicle delivered the desired compounds to bone due to their high affinity for calcium crystals (Widler, Jahnke, & Green, 2012). Also, bisphosphonates can regulate calcium homeostasis and inhibit OC activity, further enhancing the clinical application for several bone disorders. Conjugation of bisphosphonates with PLGA nanoparticles (Thamake, Raut, Gryczynski, Ranjan, & Vishwanatha, 2012) or N-(2-hydroxypropyl)methacrylamide (HPMA) (Miller et al., 2011) successfully delivered the small molecule compounds and siRNA to bone. However, recent reports suggested that long-term treatment of bisphosphonates-induced OB apoptosis (Orriss, Key, Colston, & Arnett, 2009), and can cause osteo-necrosis of the jaw (Favus, 2007). Although the direct link between bisphosphonates and jaw osteonecrosis remains unclear, preventive treatment for jaw osteonecrosis is important during bisphosphonates therapy. An alternative delivery strategy is to conjugate the therapeutic compounds with tetra-cycline, an antibiotic with high affinity to calcium ions and thus have bone-targeting ability (Neale et al., 2009). The development of tetracycline and its derivative to conjugate with drug carrying vehicles could be an efficient strategy to deliver agents to the skeletal system without induction of jaw osteonecrosis (Dang et al., 2016).
Recent development of drug delivery models for the skeletal system has advanced to cell-specific targeting vehicles to avoid the toxic effects on nontargeted cells. The bone-forming surface is characterized by low crystallized hydroxyapatite and amorphous calcium phosphate compared to the high crystallized hydroxyapatite in bone-resorption surface (Dang et al., 2016). The differences of chemical and physical properties enable the design of OC- or OB-targeting vehicles. For example, the tripeptite asparate–serine–serine (DSS) binds to bone formation sites preferably than bone-resorption site in vivo (Zhang et al., 2012). On the other hand, the acid octapeptide with aspartic acid has high affinity to hydroxyapatite and accumulated at bone-resorption surfaces (Wang et al., 2007). Conjunction of the bone formation or bone-resorption selective peptide with dio-leoyltrimethylammonium propane (DOTAP)-based cationic liposomes or HPMA nanoparticles successfully delivered siRNA, protein, or small molecule compounds to the desired region (Wang et al., 2007; Zhang et al., 2012).
A new developed technique, named as cell-SELEX (Gold, 1995), was able to screen and identify cell-specific molecules and target OB, OC, and even MSC, specifically (Dang et al., 2016). A random pool of 1013–1016 of ssDNA or ssRNA is used to screen for the cell-specific targeting molecule. CH6 is an OB-specific aptamer screened from cell-SELEX system (Liang et al., 2015). Conjugation of CH6 with lipid nanoparticles was able to deliver siRNA to OB at cellular level in vivo. Antagomir-188 is another example of MSC-specific aptamer screened from cell-SELEX system (Li et al., 2015), which can specifically regulate miRNA-188 in bone marrow MSCs in vivo. Taken together, the advanced techniques enable the NF-κB-targeting therapy in the skeletal system at the cellular level, reduce the potential toxic effects to the immune system, and advance the translational application in inflammatory bone disorders.
7. CONCLUSION
Despite direct evidence from transgenic animal studies demonstrating the essential roles of NF-κB in the activation of OC and the suppression of OB differentiation, bone-remodeling processes are sensitive to the timing, duration, dose, and composition of upstream NF-κB stimulators (Fig. 4). Acute inflammation or low dose inflammatory cytokines are indispensable and beneficial for bone defect healing processes. Therefore, temporal modulation or delayed inhibition could optimize the therapeutic effects of targeting NF-κB pathway in bone diseases associated with acute inflammation. Alternatively, overwhelming inflammatory signals (e.g., infection) or unresolved chronic inflammation (e.g., periprosthetic osteolysis) alters the balance of bone remodeling toward osteolytic processes, thus effective inhibition of NF-κB signaling is required to mitigate these inflammation-associated bone diseases.
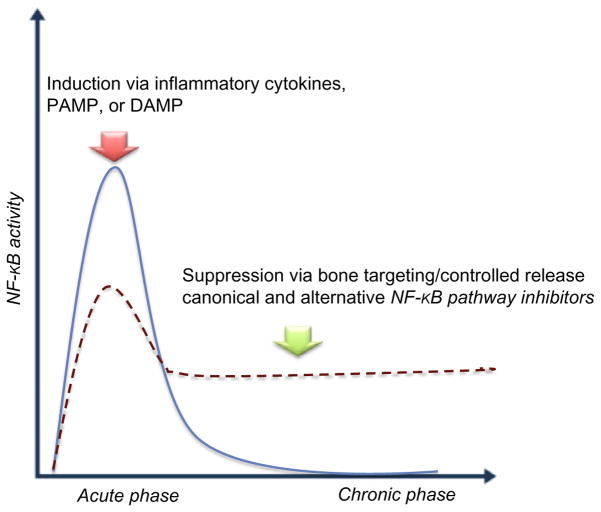
Fig. 4.
Temporal modulation of NF-κB activity in inflammatory-associated bone diseases. Blue line represents optimal condition of NF-κB signaling for bone-healing/bone-remodeling process. Red dash line represents the impaired inflammatory response at acute phase and/or persistent response at chronic phase. The arrows indicate the optimal bone regeneration conditions by either induction of NF-κB signaling at acute phase of inflammation (Red arrow), or suppression at chronic phase with unresolved inflammation (Green arrow).
There are many modalities to target the canonical or noncanonical NF-κB signaling cascades. In many cases, the strategy of NF-κB inhibition focuses on suppressing IKK2 kinase activity or maintaining IκB protein stability. These approaches have proved to be effective in blocking the canonical pathway; however, they cannot modulate noncanonical signaling and consequent osteoclastogenesis (Ruocco et al., 2005). Although the strategic use of NF-κB decoy ODN could simultaneously target both canonical and noncanonical signaling, the stability of the decoy ODN in vivo, or the potential toxic effects induced by chemically modified ODN, remain an obstacle to their translational application (Osako, Nakagami, & Morishita, 2012). Most likely, the best approach will be a combination of inhibitors that have specific activity, broad activity, and are synergistic, and provide a multistep inhibition of the inflammatory cascade. Enhanced understanding of situation-specific NF-κB activation and unique structural changes may also allow for the development of increasingly refined NF-κB target strategies.
The central roles of NF-κB as a master regulator of macrophages, OC, and OB make it possible to manipulate both the inflammation and bone-remodeling processes. Nevertheless, the critical functions of NF-κB in the immune system also raise concerns of toxicity with systemic treatment. Local delivery with controlled drug release systems is an efficient strategy for long-term manipulation of the NF-κB pathway with minimal side effects and can be applied to many bone diseases including fracture healing, per-iprosthetic osteolysis, osteoarthritis, etc. The discovery of bone-targeting drug delivery vehicles further advances the possibility to deliver siRNA, decoy ODN, or even small molecular inhibitors with minimal impacts on the patient’s immune function.
Taken together, the in vitro and in vivo evidence strongly suggest that NF-κB-targeting therapy has great potential to restore impaired bone-remodeling processes. The natural differences in pathogenesis between acute and chronic inflammation suggest that the optimal timing, dose, and targeting strategy to modulate NF-κB pathways are critical in order to achieve the desired therapeutic effects. Temporal modulation of NF-κB activity with advanced drug delivery strategies could efficiently mitigate osteolytic processes and enhance bone formation in the patients with inflammation-associated bone diseases.
References
- AAOS. 2013 http://www.aaos.org/Research/stats/patientstats.asp.
- Abbas S, Zhang YH, Clohisy JC, Abu-Amer Y. Tumor necrosis factor-alpha inhibits pre-osteoblast differentiation through its type-1 receptor. Cytokine. 2003;22(1–2):33–41. doi: 10.1016/s1043-4666(03)00106-6. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporosis International. 2013;24(9):2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of Bone and Mineral Research. 2011;26(7):1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- Arruebo M, Vilaboa N, Santamaria J. Drug delivery from internally implanted biomedical devices used in traumatology and in orthopedic surgery. Expert Opinion on Drug Delivery. 2010;7(5):589–603. doi: 10.1517/17425241003671544. [DOI] [PubMed] [Google Scholar]
- Balmayor ER. Targeted delivery as key for the success of small osteoinductive molecules. Advanced Drug Delivery Reviews. 2015;94:13–27. doi: 10.1016/j.addr.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bhandari KH, Newa M, Chapman J, Doschak MR. Synthesis, characterization and evaluation of bone targeting salmon calcitonin analogs in normal and osteoporotic rats. Journal of Controlled Release. 2012;158(1):44–52. doi: 10.1016/j.jconrel.2011.09.096. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. Journal of Leukocyte Biology. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bielinska A, Shivdasani RA, Zhang LQ, Nabel GJ. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990;250(4983):997–1000. doi: 10.1126/science.2237444. [DOI] [PubMed] [Google Scholar]
- Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, et al. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. The Journal of Clinical Investigation. 1998;102(9):1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston_University. http://www.bu.edu/nf-kb/gene-resources/target-genes/
- Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Annals of the New York Academy of Sciences. 2010;1192:367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Briolay A, Lencel P, Bessueille L, Caverzasio J, Buchet R, Magne D. Auto-crine stimulation of osteoblast activity by Wnt5a in response to TNF-alpha in human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2013;430(3):1072–1077. doi: 10.1016/j.bbrc.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. Journal of Experimental Medicine. 2008;205(11):2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JK, Glass GE, Ersek A, Freidin A, Williams GA, Gowers K, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Molecular Medicine. 2015;7(5):547–561. doi: 10.15252/emmm.201404487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. Journal of Immunology. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nature Medicine. 2009;15(6):682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nature Cell Biology. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: Structural views. Oncogene. 1999;18(49):6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: Sensing and reacting to damage. Nature Reviews. Immunology. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight poly-ethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. Journal of Biomedical Materials Research. Part A. 2009;89(1):242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, et al. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. Journal of Cellular Physiology. 2010;223(1):168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinicaltrials.gov. 2011 https://clinicaltrials.gov/ct2/show/NCT01113333?term=IKK+inhibitor&rank=1.
- Clinicaltrials.gov. 2012 https://clinicaltrials.gov/show/NCT01358669.
- Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. Journal of Orthopaedic Research. 2004;22(1):13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nature Reviews. Rheumatology. 2011;7(10):600–608. doi: 10.1038/nrrheum.2011.128. [DOI] [PubMed] [Google Scholar]
- Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Advanced Drug Delivery Reviews. 2016;99(Pt. A):12–27. doi: 10.1016/j.addr.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Cordova LA, Stresing V, Gobin B, Rosset P, Passuti N, Gouin F, et al. Orthopaedic implant failure: Aseptic implant loosening—The contribution and future challenges of mouse models in translational research. Clinical Science. 2014;127(5):277–293. doi: 10.1042/CS20130338. [DOI] [PubMed] [Google Scholar]
- Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: Implications for human disease. Oncogene. 2006;25(51):6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chemical Biology & Drug Design. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- Croes M, Oner FC, Kruyt MC, Blokhuis TJ, Bastian O, Dhert WJ, et al. Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PloS One. 2015;10(7):e0132781. doi: 10.1371/journal.pone.0132781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Liu J, Li F, Wang L, Li D, Guo B, et al. Targeted delivery systems for molecular therapy in skeletal disorders. International Journal of Molecular Sciences. 2016;17(3):428. doi: 10.3390/ijms17030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa G, La Rotonda MI. Nano and microtechnologies for the delivery of oligonucleotides with gene silencing properties. Molecules. 2009;14(8):2801–2823. doi: 10.3390/molecules14082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano D. Oligonucleotides decoy to NF-kappaB: Becoming a reality? Discovery Medicine. 2011;12(63):97–105. [PubMed] [Google Scholar]
- de Villiers MM, Otto DP, Strydom SJ, Lvov YM. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Advanced Drug Delivery Reviews. 2011;63(9):701–715. doi: 10.1016/j.addr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: Implications on therapeutic potential. Mediators of Inflammation. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, et al. TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sciences. 2009;84(15–16):499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Dobbs MB, Buckwalter J, Saltzman C. Osteoporosis: The increasing role of the orthopaedist. The Iowa Orthopaedic Journal. 1999;19:43–52. [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: Critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. Journal of Immunology. 2008;181(12):8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favus MJ. Diabetes and the risk of osteonecrosis of the jaw. The Journal of Clinical Endocrinology and Metabolism. 2007;92(3):817–818. doi: 10.1210/jc.2007-0098. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes & Development. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Tokunaga Y, Takahashi T, Kimura S, Shimojo F, Hata T. Osteotropic Drug Delivery System (ODDS) based on bisphosphonic prodrug. V. Biological disposition and targeting characteristics of osteotropic estradiol. Biological & Pharmaceutical Bulletin. 1997;20(11):1183–1187. doi: 10.1248/bpb.20.1183. [DOI] [PubMed] [Google Scholar]
- Garcia-Pineres AJ, Lindenmeyer MT, Merfort I. Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sciences. 2004;75(7):841–856. doi: 10.1016/j.lfs.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Gerondakis S, Strasser A, Metcalf D, Grigoriadis G, Scheerlinck JY, Grumont RJ. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. American Journal of Physiology—Endocrinology & Metabolism. 2005;288(5):E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Garbati MR. Inhibition of NF-kappaB signaling as a strategy in disease therapy. Current Topics in Microbiology and Immunology. 2011;349:245–263. doi: 10.1007/82_2010_105. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25(51):6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. The SELEX process: A surprising source of therapeutic and diagnostic compounds. The Harvey Lectures. 1995;91:47–57. [PubMed] [Google Scholar]
- Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34(13):3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/ macrophages depends on oncostatin M signaling. Stem Cells. 2012;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- Haleem-Smith H, Argintar E, Bush C, Hampton D, Postma WF, Chen FH, et al. Biological responses of human mesenchymal stem cells to titanium wear debris particles. Journal of Orthopaedic Research. 2012;30(6):853–863. doi: 10.1002/jor.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Research. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Seminars in Immunology. 2014;26(3):253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino TJ, Hentunen TA, Vaananen HK. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: Enhancement by estrogen. Journal of Cellular Biochemistry. 2002;85(1):185–197. doi: 10.1002/jcb.10109. [DOI] [PubMed] [Google Scholar]
- Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45(2):367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- Hirabayashi H, Takahashi T, Fujisaki J, Masunaga T, Sato S, Hiroi J, et al. Bone-specific delivery and sustained release of diclofenac, a non-steroidal anti-inflammatory drug, via bisphosphonic prodrug based on the Osteotropic Drug Delivery System (ODDS) Journal of Controlled Release. 2001;70(1–2):183–191. doi: 10.1016/s0168-3659(00)00355-2. [DOI] [PubMed] [Google Scholar]
- Houghton TJ, Tanaka KSE, Kang T, Dietrich E, Lafontaine Y, Delorme D, et al. Linking bisphosphonates to the free amino groups in fluoroquinolones: Preparation of osteotropic prodrugs for the prevention of osteomyelitis. Journal of Medicinal Chemistry. 2008;51(21):6955–6969. doi: 10.1021/jm801007z. [DOI] [PubMed] [Google Scholar]
- Huxford T, Hoffmann A, Ghosh G. Understanding the logic of IkappaB: NF-kappaB regulation in structural terms. Current Topics in Microbiology and Immunology. 2011;349:1–24. doi: 10.1007/82_2010_99. [DOI] [PubMed] [Google Scholar]
- Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95(6):759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- Hwa Cho H, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24(12):2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. The Journal of Clinical Investigation. 2009;119(2):305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nature Medicine. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, et al. Lipo-polysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. Journal of Immunology. 2003;170(7):3688–3695. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95(6):749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Kyle RA, Palumbo A, Siegel DS, Cunningham S, Berenson J. The current status and future of multiple myeloma in the clinic. Clinical Lymphoma, Myeloma & Leukemia. 2010;10(1):28–43. doi: 10.3816/CLML.2010.n.003. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annual Review of Immunology. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: A treasure trove for drug development. Nature Reviews. Drug Discovery. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Karnes JM, Daffner SD, Watkins CM. Multiple roles of tumor necrosis factor-alpha in fracture healing. Bone. 2015;78:87–93. doi: 10.1016/j.bone.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nature Immunology. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Keeney M, Waters H, Barcay K, Jiang X, Yao Z, Pajarinen J, et al. Mutant MCP-1 protein delivery from layer-by-layer coatings on orthopedic implants to modulate inflammatory response. Biomaterials. 2013;34(38):10287–10295. doi: 10.1016/j.biomaterials.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. Journal of Experimental Medicine. 2005;202(5):589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. Journal of Bone Metabolism. 2014;21(4):233–241. doi: 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, et al. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Molecular and Cellular Biology. 1996;16(5):2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenner T, Valenzuela-Paz P, Keppler BK, Angres G, Scherf HR, Wingen F, et al. Improvement of antitumor drug therapeutic index by toxicity reduction cisplatin-linked phosphonates in the treatment of the transplantable osteosarcoma in vitro and in vivo. Cancer Treatment Reviews. 1990;17(2):253–259. doi: 10.1016/0305-7372(90)90056-l. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. Journal of Experimental Medicine. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Reviews. Immunology. 2008;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chemistry & Biology. 2001;8(8):759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of Clinical Investigation. 2000;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencel P, Delplace S, Pilet P, Leterme D, Miellot F, Sourice S, et al. Cell-specific effects of TNF-alpha and IL-1beta on alkaline phosphatase: Implication for syndesmophyte formation and vascular calcification. Laboratory Investigation. 2011;91(10):1434–1442. doi: 10.1038/labinvest.2011.83. [DOI] [PubMed] [Google Scholar]
- Letoha T, Somlai C, Takacs T, Szabolcs A, Jarmay K, Rakonczay Z, Jr, et al. A nuclear import inhibitory peptide ameliorates the severity of cholecystokinin-induced acute pancreatitis. World Journal of Gastroenterology. 2005;11(7):990–999. doi: 10.3748/wjg.v11.i7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, et al. Micro-RNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. The Journal of Clinical Investigation. 2015;125(4):1509–1522. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. Journal of Bone and Mineral Research. 2007;22(5):646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- Liang C, Guo B, Wu H, Shao N, Li D, Liu J, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nature Medicine. 2015;21(3):288–294. doi: 10.1038/nm.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A, et al. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. Journal of Orthopaedic Research. 2016 doi: 10.1002/jor.23270. https://www.ncbi.nlm.nih.gov/pubmed/27105133, Epub ahead of print. [DOI] [PubMed]
- Lin TH, Pajarinen J, Sato T, Loi F, Fan C, Cordova LA, et al. NF-kappaB decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model. Acta Biomaterialia. 2016;41:273–281. doi: 10.1016/j.actbio.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, et al. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Engineering. Part A. 2015;21(5–6):875–883. doi: 10.1089/ten.tea.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomaterialia. 2014;10(1):1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Yao Z, Sato T, Keeney M, Li C, Pajarinen J, et al. Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: A preliminary report. Acta Biomaterialia. 2014;10(8):3747–3755. doi: 10.1016/j.actbio.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. The Journal of Biological Chemistry. 1995;270(24):14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Lo KWH, Ashe KM, Kan HM, Laurencin CT. The role of small molecules in musculoskeletal regeneration. Regenerative Medicine. 2012;7(4):535–549. doi: 10.2217/rme.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo E, DelaRosa O, Mancheno-Corvo P, Menta R, Ramirez C, Buscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Engineering. Part A. 2009;15(7):1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NF kappa B pathways. The Journal of Biological Chemistry. 2006;281(10):6297–6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY, Davies B, et al. Activation and promotion of adipose stem cells by tumour necrosis factor-alpha preconditioning for bone regeneration. Journal of Cellular Physiology. 2013;228(8):1737–1744. doi: 10.1002/jcp.24330. [DOI] [PubMed] [Google Scholar]
- Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells and Development. 2012;21(13):2420–2429. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- Maia FR, Bidarra SJ, Granja PL, Barrias CC. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomaterialia. 2013;9(11):8773–8789. doi: 10.1016/j.actbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of Pathology. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. Journal of Controlled Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson K, Chrysis D, Savendahl L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. Journal of Bone and Mineral Research. 2004;19(11):1805–1812. doi: 10.1359/JBMR.040805. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in Bioscience. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Miller K, Eldar-Boock A, Polyak D, Segal E, Benayoun L, Shaked Y, et al. Antiangiogenic antitumor activity of HPMA copolymer-paclitaxel-alendronate conjugate on breast cancer bone metastasis mouse model. Molecular Pharmaceutics. 2011;8(4):1052–1062. doi: 10.1021/mp200083n. [DOI] [PubMed] [Google Scholar]
- Min J, Braatz RD, Hammond PT. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials. 2014;35(8):2507–2517. doi: 10.1016/j.biomaterials.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Ihara S, Miyake T, Tsukada Y, Watanabe H, Matsuda H, et al. Prevention of neointimal formation after angioplasty using nuclear factor-kappaB decoy oligodeoxynucleotide-coated balloon catheter in rabbit model. Circulation. Cardiovascular Interventions. 2014;7(6):787–796. doi: 10.1161/CIRCINTERVENTIONS.114.001522. [DOI] [PubMed] [Google Scholar]
- Mo IF, Yip KH, Chan WK, Law HK, Lau YL, Chan GC. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biology. 2008;9:52. doi: 10.1186/1471-2121-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka M, Kamizono A, Takikawa H, Mori A, Ueno H, Kadowaki S-i, et al. Design, synthesis, and biological evaluation of novel estradiol–bisphosphonate conjugates as bone-specific estrogens. Bioorganic & Medicinal Chemistry. 2010;18(3):1143–1148. doi: 10.1016/j.bmc.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Mountziaris PM, Dennis Lehman E, Mountziaris I, Sing DC, Kasper FK, Mikos AG. Effect of temporally patterned TNF-alpha delivery on in vitro oste-ogenic differentiation of mesenchymal stem cells cultured on biodegradable polymer scaffolds. Journal of Biomaterials Science Polymer Edition. 2013;24(15):1794–1813. doi: 10.1080/09205063.2013.803455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31(7):1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale JR, Richter NB, Merten KE, Taylor KG, Singh S, Waite LC, et al. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorganic & Medicinal Chemistry Letters. 2009;19(3):680–683. doi: 10.1016/j.bmcl.2008.12.051. [DOI] [PubMed] [Google Scholar]
- Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. Journal of Bone and Mineral Research. 2007;22(6):781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- Novack DV. Role of NF-kappaB in the skeleton. Cell Research. 2011;21(1):169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. Journal of Experimental Medicine. 2003;198(5):771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Key ML, Colston KW, Arnett TR. Inhibition of osteoblast function in vitro by aminobisphosphonates. Journal of Cellular Biochemistry. 2009;106(1):109–118. doi: 10.1002/jcb.21983. [DOI] [PubMed] [Google Scholar]
- Osako MK, Nakagami H, Morishita R. Modification of decoy oligodeoxynucleotides to achieve the stability and therapeutic efficacy. Current Topics in Medicinal Chemistry. 2012;12(15):1603–1607. doi: 10.2174/156802612803531397. [DOI] [PubMed] [Google Scholar]
- Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-alpha on bone homeostasis. Frontiers in Immunology. 2014;5(48):48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteli M, Pountos I, Jones E, Giannoudis PV. Biological and molecular profile of fracture non-union tissue: Current insights. Journal of Cellular and Molecular Medicine. 2015;19(4):685–713. doi: 10.1111/jcmm.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB Journal. 2002;16(10):1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49(1):103–110. doi: 10.1016/j.bone.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, et al. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32(24):5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioletti DP, Kottelat A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials. 2004;25(27):5803–5808. doi: 10.1016/j.biomaterials.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clinical Orthopaedics and Related Research. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. Journal of Immunology. 2005;175(5):2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Rad SM, Langroudi L, Kouhkan F, Yazdani L, Koupaee AN, Asgharpour S, et al. Transcription factor decoy: A pre-transcriptional approach for gene down-regulation purpose in cancer. Tumour Biology. 2015;36(7):4871–4881. doi: 10.1007/s13277-015-3344-z. [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. American Journal of Pathology. 2014;184(12):3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Raphel J, Holodniy M, Goodman SB, Heilshorn SC. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–314. doi: 10.1016/j.biomaterials.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz MM, Zinnen SP, Dueck AC, Dingli D, Reinholz GG, Jonart LA, et al. A promising approach for treatment of tumor-induced bone diseases: Utilizing bisphosphonate derivatives of nucleoside antimetabolites. Bone. 2010;47(1):12–22. doi: 10.1016/j.bone.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. American Journal of Pathology. 2000;156(3):997–1007. doi: 10.1016/S0002-9440(10)64967-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/ IKKgamma-deficient mice. Genes & Development. 2000;14(7):854–862. [PMC free article] [PubMed] [Google Scholar]
- Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu LC, Cao Y, et al. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. Journal of Experimental Medicine. 2005;201(10):1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RGG. Bisphosphonates: The first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews. 2008;7(2):83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Scheidereit C. IkappaB kinase complexes: Gateways to NF-kappaB activation and transcription. Oncogene. 2006;25(51):6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nature Immunology. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Soysa NS, Alles N. NF-kappaB functions in osteoclasts. Biochemical and Biophysical Research Communications. 2009;378(1):1–5. doi: 10.1016/j.bbrc.2008.10.146. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrine Reviews. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Research. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara K, Samura S, Tsuji K, Yamamoto H, Tsukada Y, Bando Y, et al. Oral nuclear factor-kappaB decoy oligonucleotides delivery system with chitosan modified poly(D,L-lactide-co-glycolide) nanospheres for inflammatory bowel disease. Biomaterials. 2011;32(3):870–878. doi: 10.1016/j.biomaterials.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. Journal of Immunology. 2002;169(3):1516–1523. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Feldmann M. Anti-TNF biologic agents: Still the therapy of choice for rheumatoid arthritis. Nature Reviews. Rheumatology. 2009;5(10):578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials. 2012;33(29):7164–7173. doi: 10.1016/j.biomaterials.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of patho-physiological bone remodeling. Cytokine & Growth Factor Reviews. 2004;15(6):457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Drug targeting. European Journal of Pharmaceutical Sciences. 2000;11(Suppl 2):S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. Journal of Immunology. 1998;161(11):6084–6092. [PubMed] [Google Scholar]
- Torres Martin de Rosales R, Finucane C, Mather SJ, Blower PJ. Bifunctional bisphosphonate complexes for the diagnosis and therapy of bone metastases. Chemical Communications. 2009;32:4847–4849. doi: 10.1039/b908652h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. The Journal of Clinical Investigation. 2008;118(6):2088–2097. doi: 10.1172/JCI33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, et al. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(10):3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. Journal of Experimental Medicine. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. The Journal of Clinical Investigation. 1998;101(5):1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Miller SC, Shlyakhtenko LS, Portillo AM, Liu XM, Papangkorn K, et al. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjugate Chemistry. 2007;18(5):1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- Wang ML, Nesti LJ, Tuli R, Lazatin J, Danielson KG, Sharkey PF, et al. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. Journal of Orthopaedic Research. 2002;20(6):1175–1184. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. The Journal of Clinical Investigation. 2005;115(2):282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside ST, Israel A. I kappa B proteins: Structure, function and regulation. Seminars in Cancer Biology. 1997;8(2):75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- Widler L, Jahnke W, Green JR. The chemistry of bisphosphonates: From antiscaling agents to clinical therapeutics. Anti-Cancer Agents of Medicinal Chemistry. 2012;12(2):95–101. doi: 10.2174/187152012799014959. [DOI] [PubMed] [Google Scholar]
- Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. Journal of Bone and Mineral Research. 2009;24(5):759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, et al. NF-kappaB modulators in osteolytic bone diseases. Cytokine & Growth Factor Reviews. 2009;20(1):7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. The Journal of Clinical Investigation. 2009;119(10):3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Chang J, Liu Y, Li J, Kevork K, Al-Hezaimi K, et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nature Medicine. 2014;20(9):1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nature Medicine. 2012;18(2):307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Hou X, Yin X, Li Y, Duan R, Boyce BF, et al. TNF induction of NF-kappaB RelB enhances RANKL-induced osteoclastogenesis by promoting inflammatory macrophage differentiation but also limits it through suppression of NFATc1 expression. PloS One. 2015;10(8):e0135728. doi: 10.1371/journal.pone.0135728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Huang J, Zhang H, Wang Y, Matesic LE, Takahata M, et al. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells. 2011;29(10):1601–1610. doi: 10.1002/stem.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerina J, Redlich K, Polzer K, Joosten L, Kronke G, Distler J, et al. TNF-induced structural joint damage is mediated by IL-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11742–11747. doi: 10.1073/pnas.0610812104. [DOI] [PMC free article] [PubMed] [Google Scholar]