Abstract
Serum concentrations of PBDEs were measured using gas chromatography-tandem mass spectrometry in 80 children aged 15-71 months. Demographic and behavioral data were collected on parental questionnaires; a research nurse recorded anthropometric measures and insurance status. For a subset of children (n=17), PBDEs were measured in house dust and child handwipes sampled during a home visit. In linear and Tobit regression, log-transformed PBDE congeners were modeled as a function of child characteristics, including neighborhood-level socioeconomic indicators. BDE congeners 47, 99, and 100 were highly correlated and summed for analysis; BDE-153 was examined individually. PBDE serum concentrations were associated with socioeconomic factors; for example, a $20,000 increase in median household income in a child's ZIP code was associated with a 34% decrease (95%CI=14-49%) in BDE-153 and a 26% decrease (95%CI=6-42%) in ΣBDE-47,-99,-100. Lower body-mass index (BMI) z-score and household smoking were strong predictors of higher BDE-153 levels. Among children who participated in a home visit, serum PBDE was positively correlated with handwipe PBDE (Spearman r ΣBDE-47,-99,-100 = 0.48, p=0.09), but not dust PBDE. Results indicate socioeconomic factors and BMI are strong predictors of serum PBDE levels among young children. PBDEs measured on handwipes are more predictive of serum PBDE levels than vacuum-collected dust.
Introduction
Polybrominated diphenyl ethers (PBDEs) are chemical flame retardants added to a wide range of consumer products including furniture foam, plastics, electronics, and textiles. They have been phased out of production and import in the United States (pentaPBDE and octaPBDE formulations as of 2004 and decaPBDE as of 2013).1 However, their persistence, lack of chemical bonding to the products containing them, and the long useful lives of such products lead to their continued presence in house dust, the major route of human exposure.2 Serum levels of these compounds are an order of magnitude higher in the United States and Canada compared to other countries3,4, likely due to historically strict flammability standards.5 There is concern about the impact of these chemicals on human health due to their structural similarity to thyroid hormones (e.g., thyroxine)6 and evidence of developmental neurotoxicity in human and animal studies.7
An analysis of 2420 pooled serum sampled collected from children across the age spectrum in Australia showed PBDE concentrations peak in 2-5 year old children, with average levels among 2-3 year-olds being five times as high as adults over age 30.8 This observation was consistent with a smaller study of mothers and their 1.5-4 year old children in the United States.9 The high serum levels among young children are likely due to higher dust ingestion rates in early life due to hand-to-mouth behaviors.10 Exposures in this age group are of particular concern due to the importance of thyroid function to neurodevelopment in early life.11 More recently, among 300 Texas children 0-13 years, serum concentrations were highest in the 4-6 year age group prompting the authors to call for additional studies to characterize young children's exposure to PBDEs and to identify factors that contribute to these high exposures.12
We conducted a study to investigate predictors of PBDE serum levels among children age 15-71 months (1-<6 years), ages corresponding with peak PBDE serum levels and a life stage of intensive neurodevelopment and growth. We included a socioeconomically and racially diverse study population to investigate possible disparities in exposure specifically in this age group. In a subgroup of our study population we also investigated relationships between PBDE serum levels and vacuum-collected house dust samples and dust collected on child handwipes.
Materials and Methods
Study population
To avoid the discomfort and anxiety of a blood draw among young children, we enrolled children who would be under general anesthesia while undergoing one or more of the following routine procedures: myringotomy (ear tube surgery), adenoidectomy, or tonsillectomy. Children were healthy at the time of surgery. Enrollment took place from June 2011 through February 2012, and informed consent was obtained from a parent on the day of surgery. Study protocols were approved by the Institutional Review Board at Emory University. Eligible children were 15 months to 71 months old (<6 years), born in the United States, without any current illness or underlying health condition, and not taking current medications known to affect endocrine function.
After consenting, parents completed a short questionnaire, which included information about the child's age, race and ethnicity (non-Hispanic white; non-Hispanic black; other: Asian, Pacific Islander, Multiracial, and any Hispanic ethnicity), breastfeeding history and duration, medications, parental occupations, smokers in the household, and residential address. A research nurse recorded the child's height and weight and the insurance provider listed as the payer for the child's surgery. The child's residential ZIP code was linked to ZCTAs (ZIP code tabulation areas) from the American Community Survey for the 5 year average for 2007-201113 to obtain the percent of households living in poverty and the median household income in the ZIP code of the child's residence.
Body mass index (BMI) was calculated for each child from height and weight and converted to an age- and sex-specific z-score for analysis because the distribution of BMI varies dramatically by age in early life. We used the U.S. national standard from the Centers for Disease Control and Prevention14 and the available CDC SAS program15 to calculate BMI z-scores for children who were at least 24 months. Because the CDC reference does not provide BMI z-scores below 24 months of age, we used the World Health Organization BMI distributions16 to impute the BMI z-score for children under age 24 months (n=10) based on the difference between the CDC and WHO z-scores at 24 months.
Serum PBDE Quantification
After the surgical procedure(s) and while the child was still under general anesthesia, the study nurse drew at most 15 mL of venous blood in two serum separator Vacutainer® tubes (average =10 mL). The blood samples were transported next door to the Rollins School of Public Health on the day of collection and were manually inverted, allowed to clot for 60 minutes, and then centrifuged (Thermo Scientific®; IEC Medispin) for 30 minutes at 3000 rpm to separate the serum from the whole blood sample. The serum was then aliquoted into two freezer-safe storage vials, one for PBDE analysis and one for thyroid hormone analysis. The serum samples were stored at -20°C.
Prepared serum samples were analyzed for seven PBDE congeners (BDE-47,-85,-99,-100,-153,-154, and -209) using gas chromatography-tandem mass spectrometry (GS-MS/MS) or GC-MS (only for BDE-209) at the Laboratory for Exposure Assessment and Method Development in Environmental Research (LEADER) at Emory University's Rollins School of Public Health (Agilent Technologies; 7000 GC/MS Triple Quad). The serum PBDE extraction method was developed based on three previous methods reported in the literature and further refined to optimize extraction recovery and analytic precision.17-19 For analysis of the targeted congeners, a 1-mL aliquot of each serum sample was spiked with a known concentration of isotopically labeled PBDEs, deproteinated with the addition of formic acid, and thoroughly mixed. Each sample was then extracted twice with 5 mL hexane and passed through a self-packed silica/acidified-silica cartridge to remove residual fats. Breakthrough was collected and cartridges were then eluted with 10 mL of a 1:19 dichloromethane:hexane solution and collected. This extractant (breakthrough plus eluate) was dried and reconstituted in 50 μL of toluene prior to instrumental analysis.
Extracted samples were analyzed using GC-MS/MS with electron impact ionization in the multiple reaction monitoring mode with one quantification and one confirmation parent-to-product ion pair monitored for each analyte, except for PBDE-209 which was analyzed using the selected ion monitoring mode (SIM). Quantification was performed using isotope dilution calibration covering concentration ranges of 5, 10, 20, 50, 100, 200, 500 ng/mL for BDE-209 and 0.2, 0.5, 1, 2.5, 5, 10, 25, 50 ng/mL for all other congeners. In each analytical run, a laboratory background sample (non-fortified pooled human serum), a solvent-based calibration curve, and two quality control (QC) samples [pooled human serum fortified at two levels (40 ng/mL and 200 ng/mL, for PBDE-209; 1 ng/mL and 25 ng/mL, for all other congeners)] were prepared and analyzed concurrently with unknown samples.
For each congener, values (shown in Table 1) greater than the amount measured in blanks or the lowest standard and a signal-to-noise ratio of greater than 3 were considered above the method limit of detection (LOD). Using data from QC samples, method accuracy was within 100±20% at both fortified levels for most compounds with the exception of BDE-100 at the lower fortified concentration (78%) and method precision was less than 8% as determined by relative standard deviation. For a peak to be identified as one of our target analytes, it had to co-elute with its isotopically labeled internal standard (differentiated by mass), have ions within 20% of the theoretical ion ratio for naturally occurring isotopes of bromine, and have the correct quantification and confirmation.
Table 1. Distribution of PBDE congener concentrations by volume (ng/mL) and on the lipid basis (ng/g lipid) in serum samples of 80 young children recruited from Children's Healthcare of Atlanta [Geometric means calculated for congeners with > 50% detection; for observations below the LOD, the LOD/√2 is included in calculation of mean].
| Congener | LOD ng/mL |
LOD ng/g |
<LOD N (%) |
Mean (GM) ng/mL(ng/g) |
Minimum ng/mL(ng/g) |
25th %ile ng/mL(ng/g) |
Median ng/mL(ng/g) |
75th %ile ng/mL(ng/g) |
Maximum ng/mL(ng/g) |
|---|---|---|---|---|---|---|---|---|---|
| BDE-47 | 0.002 | 0.3-1.0 | 0 | 0.148 (36.2) | 0.024 (4.4) | 0.091 (22.9) | 0.142 (38.9) | 0.260 (59.5) | 2.470 (625.9) |
| BDE-85 | 0.005 | 0.6-2.5 | 71 (89) | — | <LOD | <LOD | <LOD | <LOD | 0.063 (16.0) |
| BDE-99 | 0.002 | 0.3-1.0 | 0 | 0.039 (9.7) | 0.008 (1.4) | 0.021 (5.8) | 0.038 (9.6) | 0.074 (16.8) | 0.744 (188.7) |
| BDE-100 | 0.002 | 0.3-1.0 | 13 (16) | 0.018 (4.3) | <LOD | 0.009 (2.2) | 0.023 (6.1) | 0.044 (11.2) | 0.484 (122.6) |
| BDE-153 | 0.016 | 2.0-7.9 | 29 (36) | 0.028 (6.8) | <LOD | <LOD | 0.028 (5.9) | 0.052 (11.9) | 0.303 (76.8) |
| BDE-154 | 0.007 | 0.9-3.5 | 68 (85) | — | <LOD | <LOD | <LOD | <LOD | 0.088 (22.3) |
| BDE-209 | 0.100 | 12.7-50.0 | 80 (100) | — | <LOD | <LOD | <LOD | <LOD | <LOD |
| Sum BDE3 | — | — | — | 0.214 (52.2) | 0.036 (6.5) | 0.127 (31.6) | 0.194 (53.9) | 0.394 (89.7) | 3.698 (937.1) |
| Lipids (mg/dL) | |||||||||
| Cholesterol | 102.8 | 10.2 | 77.7 | 101.1 | 127.9 | 262.2 | |||
| Triglycerides | 127.8 | 37.2 | 100.8 | 127.4 | 140.4 | 275.3 | |||
| Total lipids (2.27×Cholesterol)+Triglycerides+62.320 | 423.4 | 200.0 | 353.4 | 403.8 | 478.1 | 788.0 | |||
Total lipid analysis
Total serum triglyceride content was measured using the BioVision Triglyceride Quantification Assay Kit (BioVision Research Products; Mountain View, CA), and total cholesterol content was measured using the Cayman Cholesterol Assay Kit (Cayman Chemical Company; Ann Arbor, MI) according to manufacturer instructions. Total lipids were calculated using conventional methods based on these individual lipid components.20
Dust and Handwipe Collection
A subset of the participants were contacted after their surgical appointment to participate in a home visit for collection of house dust from the child's primary residence. Parents were contacted by phone and offered a $25 gift card for participating. Collection of child handwipes was not included in the original study protocol, but was added soon after the study began, in response to emerging findings.21,22
Prior to the home visit, participants were asked to refrain from vacuuming so there would be enough dust volume to collect. Parents identified the room in which the child spent the most time awake and this room was sampled. Using a solvent-cleaned high volume small surface sampler (HVS3) vacuum (CS3 Inc.; Bend, OR) and solvent-cleaned Teflon® catch bottle, a 1 m2 area of floor was sampled according to a standard operating procedure; if sufficient volume was not obtained, a second 1m3 area was sampled. Samples were transported to the LEADER laboratory the same day they were collected and were processed within 7 days of sampling. Dust samples were sieved twice to a particle size of less than 250 μm and stored at room temperature in a solvent-cleaned amber jar until analysis.
Handwipes were collected from the child at the same home visit, and prior to the visit parents were asked to prevent their children from washing their hands. The child's hands were wiped up to the wrist with a sterile gauze pad that had been soaked in isopropyl alcohol, one pad per hand.21,22 The two gauze wipes were combined in a solvent-cleaned brown amber jar and stored at room temperature until analysis.
Dust and Handwipe PBDE Quantification
Dust sample preparation was based on a modification of Król et al.23 PBDEs were extracted from dust using accelerated solvent extraction (ASE) and a clean-up method to remove contaminants prior to analysis. Dust samples (50 mg mixed with purified diatomaceous earth) were placed into the ASE cells and then spiked with a known amount of isotopically labelled internal standards. The samples were extracted in duplicate using the ASE 350 (Dionex; Sunnyvale, CA) system at 1500 psi using 2 static extraction cycles of 10% dichloromethane in hexane. The extractant was dried and reconstituted in 50 μL of toluene prior to instrumental analysis.
Handwipes were extracted using a method modified from Stapleton et al.24 Handwipes were spiked with internal standard and sonicated in 30 mL of dichloromethane a total of 3 times. Extracts were combined, dried, reconstituted with 2 mL hexane, and cleaned using pre-conditioned 500 mg/3 mL SupelClean ENVIFlorisil SPE cartridges (Sigma-Aldrich, St. Louis, MO). The extract was dried and reconstituted in 50 μL toluene prior to instrumental analysis.
All dust and handwipe samples were analyzed for seven congeners (BDE-47,-85,-99,-100,-153,-154, and -209) using gas chromatography/mass selective detection (GC/MSD) in house (Agilent Technologies; 5975 GC/MSD System). The GC/MSD was operated using electron impact ionization and in SIM mode. Blanks and QC samples were included with each run. The QC solutions were made in hexane at 100 ng/mL and 300 ng/mL, respectively, for BDE-47,-85,-99,-100,-153, and -154. For BDE-209 only one QC solution at 150 ng/mL was used. 100 uL of these solutions was used to prepare the QC samples for each analytical run.
For quantification, a solvent-based calibration curve was included. For BDE-47,-85,-99,-100,-153, and -154, the concentrations were 0.1, 0.25, 0.5, 1.25, 2.5, 12.5, 35, and 37.5 ng (per handwipe or gram dust). For PBDE-209, the concentrations were 5, 10, 20, 50, 100, 200, 500 ng (per handwipe or gram dust). Method accuracy was within 100±20%. Method precision was less than 15%, as determined by relative standard deviation. The LODs derived from the standard calibration curves are expressed in ng/wipe for handwipes and ng/gram for dust samples in Table 1. PBDE concentrations were blank subtracted and duplicates were averaged.
Statistical Analysis
Based on the detection frequencies and covariation of the congeners measured in our study as well as previous work showing differences in the predictors of serum BDE-47, -99, -100 versus serum BDE-153,22,25 we separately assessed predictors of BDE-153 and predictors of the sum of the three congeners BDE-47, -99, -100 (ΣBDE3); where BDE-100 was less than the LOD, the LOD/√2 was substituted for calculation of the sum. We assessed predictors of serum PBDE concentrations using linear regression to model serum concentrations of ΣBDE3, and Tobit regression to model serum concentrations of BDE-153 to accommodate the left-censored nature of BDE-153 serum values,26 for which 36% of samples were below the limit of detection.26,27 Standard errors from Tobit models incorporate the uncertainty due to the LOD.27 Serum concentrations were natural log-transformed due to their skewed distribution, and modeled on the volume basis (ng/mL) adjusting for lipid concentrations as a covariate; lipids were also natural log-transformed to correspond to the serum PBDE dependent variable. For the home visit subset, Spearman correlation coefficients were calculated between (a) serum PBDE concentrations (ng/mL) and house dust PBDE concentrations (ng/g) and (b) serum PBDE concentrations (ng/mL) and handwipe PBDE concentrations (ng/wipe). Sensitivity analyses included using generalized estimating equations to account for the two sets of siblings; analyzing serum BDE-153 levels in linear regression with observations below the LOD imputed as the LOD divided by √2; and modeling serum PBDE concentrations on the lipid basis (ng/g) instead of adjusting for lipids as a covariate. All analyses were conducted in SAS Version 9.4 (SAS, Cary, NC).
Results
Of 95 parents approached to have their children participate in the study, 89 (94%) initially consented to participate in the study, including two pairs of siblings. Nine children were excluded because: child was too dehydrated to draw sufficient blood (n=5), failure to return survey (n=2), outside age eligibility (n=1), and an unresolvable quality control issue with PBDE analysis in the serum sample (n=1). Characteristics of the analyzed study population are shown in Table 2 along with the geometric mean (GM) serum concentration of BDE-47 and BDE-153 for each group (arithmetic means shown for lipids). The most common race of study participants was black (41%) and 64% were enrolled in Medicaid. More than half of the study population was 2 or 3 years old (54%). Geometric mean BDE-47 and BDE-153 serum levels were highest among children living in ZIP codes with high poverty or low median household income, children with low BMI, children living in a household with active smokers (particularly for BDE-153) and black children.
Table 2. Description of Study Population (n=80).
| Characteristic | N (%) | BDE-47 ng/g lipid GM (GSD) |
BDE-153 ng/g lipid GM (GSD) |
|---|---|---|---|
| Sex | |||
| Female | 35 (44) | 46.6 (2.5) | 7.7 (2.4) |
| Male | 45 (56) | 29.8 (2.3) | 6.2 (2.3) |
| Age | |||
| 15 mo. - < 2 years | 11 (14) | 56.0 (3.3) | 7.0 (2.8) |
| 2 - < 3 years | 23 (29) | 31.5 (2.2) | 6.6 (1.8) |
| 3 - < 4 years | 20 (25) | 42.0 (2.1) | 6.5 (2.4) |
| 4 - < 5 years | 13 (16) | 27.4 (2.1) | 5.9 (2.3) |
| 5 - < 6 years | 13 (16) | 33.8 (2.6) | 8.5 (2.9) |
| Breastfeeding | |||
| 0 | 33 (41) | 42.6 (2.7) | 6.4 (2.1) |
| < 6 months | 21 (26) | 30.7 (1.9) | 6.9 (2.3) |
| ≥ 6 months | 26 (33) | 33.8 (2.4) | 7.2 (2.6) |
| Race/ethnicity | |||
| non-Hispanic black | 33 (41) | 43.2 (2.2) | 8.2 (2.1) |
| non-Hispanic white | 31 (39) | 34.2 (2.9) | 6.5 (2.6) |
| Othera | 16 (20) | 28.2 (2.2) | 5.1 (2.2) |
| BMI z-score | |||
| < 0 | 27 (34) | 42.7 (2.4) | 10.0 (2.6) |
| 0 - <1 | 24 (30) | 31.7 (3.0) | 5.9 (2.3) |
| 1 - <2 | 20 (25) | 35.6 (2.0) | 5.7 (2.0) |
| 2+ | 9 (11) | 32.8 (1.7) | 4.7 (1.4) |
| Insurance | |||
| private | 29 (36) | 35.7 (2.4) | 5.6 (2.0) |
| public (Medicaid) | 51 (64) | 36.5 (2.4) | 7.6 (2.5) |
| Smoker in household | |||
| yes | 14 (17) | 38.2 (2.8) | 11.0 (3.0) |
| no | 56 (69) | 33.3 (2.4) | 6.1 (2.1) |
| missing | 10 (13) | 53.9 (2.0) | 6.5 (1.9) |
| Median Income in Residential Zip Codeb |
|||
| < $44,100 | 27 (34) | 45.7 (1.9) | 9.9 (2.2) |
| $44,100-$57,000 | 26 (33) | 38.4 (2.5) | 6.1 (2.3) |
| > $57,000 | 27 (34) | 27.1 (2.6) | 5.1 (2.2) |
| Percent poverty in Residential Zip Codeb |
|||
| <12.4% | 27 (34) | 28.9 (2.7) | 5.2 (2.1) |
| 12.4%-19.0% | 29 (36) | 37.3 (2.3) | 6.5 (2.4) |
| >19.0% | 24 (30) | 45.0 (2.2) | 9.6 (2.3) |
Table 1 shows the distribution of PBDE concentrations by congeners and for the sum of congeners BDE-47, BDE-99 and BDE-100 (ΣBDE3), which were the most frequently detected (BDE-47 and BDE-99 detected in 100%). BDE-209 was not detected above the LOD in any of the serum samples; however, the LOD was much higher for BDE-209 than other congeners. The distribution of PBDE serum levels was right-skewed with children with the highest serum PBDE concentrations two orders of magnitude higher than the children with the lowest exposures. Table S1 in the Supporting Information shows the correlations between the commonly detected congeners. BDE-47, -99 and -100 were highly correlated with each other (r>0.7), and were also positively correlated with BDE-153 to a lesser degree(r=0.4-0.6).
There were 32 participant households who met the eligibility criteria for the dust collection arm of the study (residence within 30 miles of Emory University, provided a contact phone number on the survey, and had English speaking ability). Of these, 10 could not be contacted, 7 were contacted and declined participation, and 15 successfully completed the house dust collection protocol. Two houses included siblings, resulting in 17 children with available house dust samples, and 13 with handwipes. Median time between blood draw and dust sampling was 33 days, and no children had changed residences between blood draw and house dust sampling.
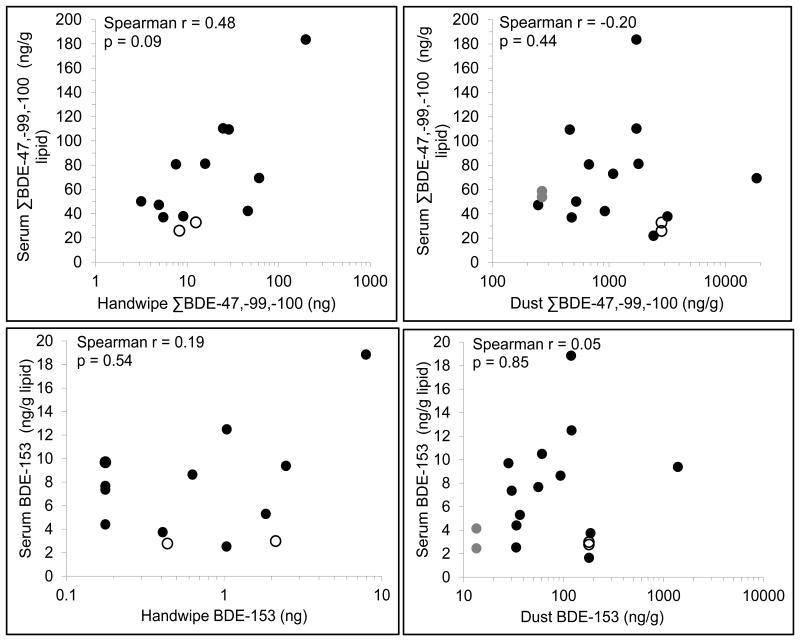
The detection frequencies and distribution of PBDE concentrations collected in house dust (in ng/g dust) and from child handwipes (in ng/wipe) are shown in Table 3. In contrast to the serum samples, BDE-209 was detected in all of the dust and hand wipe samples, and had the largest geometric mean measured on hand wipes; in dust samples, concentrations of BDE-47, -99, and -100 showed the highest levels per gram dust. Like serum levels, PBDE concentrations in dust and on hand wipes were highly right-skewed. Figure 1 shows dust and hand wipe concentrations plotted against serum levels for the 17 children with dust samples and the 13 children with handwipes. The strongest correlation observed was between serum and handwipe concentrations for ΣBDE-47, -99, and -100 (r=0.48).
Table 3. PBDE concentrations in dust (ng/g) sampled from 15 households and on handwipes (ng/wipe) sampled from 13 children during a home visit.
| Congener | House Dust Sample (n=15), ng/g | Handwipes (n=13), ng/wipe | ||||||
|---|---|---|---|---|---|---|---|---|
| LOD | N(%)<LOD | GM | Range | LOD | N(%)<LOD | GM | Range | |
| BDE-47 | 0.1 | 0 | 404 | 95 – 6,012 | 0.1 | 0 | 7.1 | 1.4 – 84.3 |
| BDE-85 | 0.1 | 0 | 50 | 8 - 841 | 0.1 | 2 (15%) | 0.4 | <0.1 – 6.7 |
| BDE-99 | 0.1 | 0 | 648 | 127 - 10,702 | 0.1 | 0 | 7.2 | 1.5 – 95.2 |
| BDE-100 | 0.1 | 0 | 118 | 24 – 1,863 | 0.1 | 0 | 1.4 | 0.2 – 18.7 |
| BDE-153 | 0.25 | 0 | 76 | 13 – 1,393 | 0.25 | 4 (31%) | 0.7 | <0.25 – 7.9 |
| BDE-154 | 0.25 | 0 | 54 | 10 - 869 | 0.25 | 5 (47%) | 0.5 | <0.25 – 6.4 |
| BDE-209 | 1.0 | 0 | 80 | 29 - 202 | 1.0 | 0 | 7.5 | 3.0 - 21.3 |
| Sum BDE3 | - | - | 1,173 | 246 – 18,577 | - | - | 15.8 | 3.1 – 198.2 |
Figure 1.
Correlation between serum and dust PBDE concentrations (17 children) and serum and
handwipe PBDE concentrations (13 children) for ΣBDE -47, -99, -100 and BDE-153.
[Siblings, indicated by symbols  and
○, have identical house dust PBDE concentrations but unique handwipe
concentrations and serum concentrations]
and
○, have identical house dust PBDE concentrations but unique handwipe
concentrations and serum concentrations]
Table 4 shows results from the linear and Tobit regression models of ΣBDE3 and BDE-153, respectively. Crude results are from separate models including one variable at a time, adjusting for lipids; adjusted results are from a model including lipids as well as the other child characteristics shown in the adjusted column (race/ethnicity, sex, age, BMI, breastfeeding duration (linear term), and ZIP code median income). Only the most predictive (judged by p-values) of the three correlated socioeconomic status indicators (i.e. median income in zip code, percent poverty in zip code, and insurance type) was included in the adjusted model. Because serum PBDE levels are natural log-transformed, β-coefficients from the models are exponentiated to show the multiplicative change in serum PBDE for a change in the independent variable of interest. Poverty and median income in the child's residential ZIP code were strong predictors of ΣBDE3. A $20,000 increase in median household income (approximate inter-quartile range) predicted a 26% decrease (95%CI=6-42%) in ΣBDE3 and a 34% decrease (95%CI=14-49%) in serum BDE-153. Enrollment in Medicaid predicted higher BDE-153 levels (53% increase relative to private insurance in unadjusted models, 95% CI= -7, 152%). Females had 55% higher concentrations (95%CI=8-122%) of ΣBDE3. For both ΣBDE3 and BDE-153, in lipid-only adjusted models, non-Hispanic African Americans had an estimated 33-35% higher serum concentration than non-Hispanic white children (p>0.05). However, there was no longer suggestion of this racial disparity after neighborhood socioeconomic factors were controlled.
Table 4. Crude and adjusted exponentiated β-coefficients and 95% confidence intervals from linear regression models of ΣBDE-47, -99, -100 and Tobit regression models of BDE-153 among 80 children [all results are adjusted for lipids].
| Crude Exponentiated β-coefficient (95%CI) |
p-value | Adjusted Exponentiated β-coefficient (95% CI) |
p-value | |
|---|---|---|---|---|
| ΣBDE-47, -99, -100 (linear regression) | ||||
|
| ||||
| Race/ethnicity | ||||
| Non-Hispanic white | ref | 0.121 | ref | 0.561 |
| Non-Hispanic black | 1.33 (0.88, 2.01) | 1.00 (0.64, 1.56) | ||
| Other | 0.80 (0.48, 1.33) | 0.78 (0.48, 1.27) | ||
| Sex | ||||
| Female | 1.54 (1.07, 2.24) | 0.022 | 1.55 (1.08, 2.22) | 0.019 |
| Age (per 12 month increase) | 0.92 (0.80, 1.07) | 0.283 | 0.96 (0.84, 1.10) | 0.591 |
| Reported household smokingc | 1.10 (0.66, 1.85) | 0.709 | 0.97 (0.60, 1.54) | 0.883 |
| BMI (per unit increase in z-score) | 0.95 (0.83, 1.09) | 0.487 | 0.97 (0.85, 1.10) | 0.589 |
| Breastfeeding (per month duration) | 0.99 (0.96, 1.03) | 0.769 | 0.99 (0.95, 1.02) | 0.464 |
| Breastfeeding (categorical) | ||||
| None | ref | 0.432 | ||
| 1-5 months | 0.75 (0.47, 1.20) | |||
| 6+ months | 0.80 (0.51, 1.25) | |||
| Insurance type | ||||
| Private | ref | 0.778 | ||
| Medicaid | 1.06 (0.71, 1.57) | |||
| Median income in ZIP code (per $20,000 increase) |
0.72 (0.57, 0.90) | 0.003 | 0.74 (0.58, 0.94) | 0.015 |
| Percent poverty in ZIP code (per 10% increase) |
1.31 (1.07, 1.62) | 0.010 | ||
|
| ||||
| BDE-153 (Tobit regression) | ||||
|
| ||||
| Race/ethnicityb | ||||
| Non-Hispanic white | ref | 0.137 | ref | 0.672 |
| Non-Hispanic black | 1.35 (0.80, 2.28) | 1.02 (0.64, 1.62) | ||
| Otherb | 0.70 (0.36, 1.37) | 0.81 (0.48, 1.37) | ||
| Sex | ||||
| Female | 1.31 (0.81, 2.11) | 0.272 | 1.11 (0.76, 1.62) | 0.591 |
| Age (per 12 month increase) | 1.03 (0.86, 1.24) | 0.704 | 1.04 (0.90, 1.21) | 0.555 |
| Reported household smoking c | 1.98 (1.06, 3.70) | 0.032 | 2.07 (1.28, 3.37) | 0.003 |
| BMI (per unit increase in z-score) | 0.77 (0.65, 0.91) | 0.003 | 0.77 (0.67, 0.88) | <0.001 |
| Breastfeeding (per month duration) | 1.02 (0.98, 1.07) | 0.309 | 1.03 (1.00, 1.07) | 0.086 |
| Breastfeeding (categorical) | ||||
| None | ref | 0.843 | ||
| 1-5 months | 1.12 (0.61, 2.05) | |||
| 6+ months | 1.18 (0.67, 2.08) | |||
| Insurance type | ||||
| Private | ref | 0.092 | ||
| Medicaid | 1.53 (0.93, 2.52) | |||
| Median income in ZIP code (per $20,000 increase) |
0.63 (0.47, 0.84) | 0.002 | 0.66 (0.51, 0.86) | 0.002 |
| Percent poverty in ZIP code (per 10% increase) |
1.33 (1.02, 1.72) | 0.033 | ||
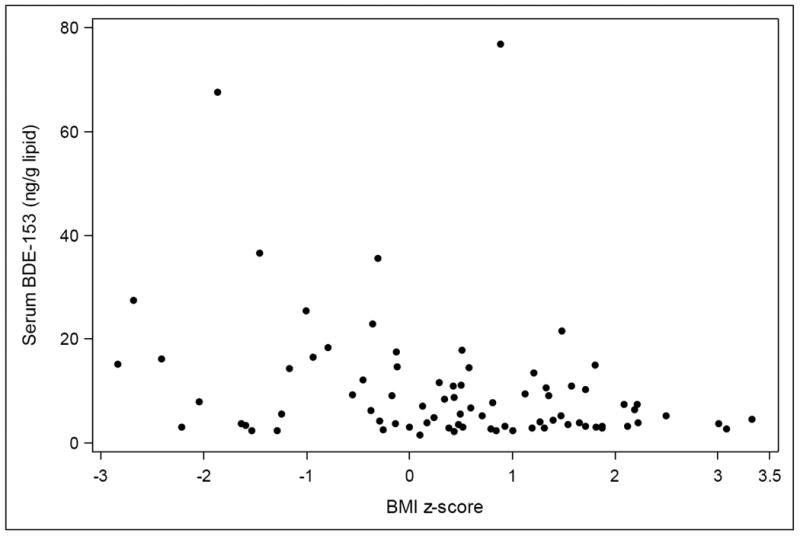
In contrast to results for ΣBDE3, reported household smoking was associated with a doubling of BDE-153 serum concentrations (95% CI=28-237%), and a one unit increase in BMI z-score was associated with a 23% decrease in concentrations (95% CI=12-33%, p<0.001). This inverse relationship between serum BDE-153 and BMI z-score is shown in Figure 2. In adjusted BDE-153 models there was little evidence that females had higher BDE-153 serum concentrations, but there was suggestion that longer breastfeeding duration predicted higher BDE-153 levels, with a 3% increase per reported month of duration (95% CI= 0-7%, p=0.086). Sensitivity analyses for serum BDE-153 imputing LOD/√2 for observations below the LOD in linear regression analyses yielded identical conclusions (Supporting Information, Table S2), and in our sensitivity analysis excluding children less than 24 months of age, BMI z-score remained a strong predictor of lower BDE-153 concentrations (p<0.001). This BMI result was also robust to exclusion of outlier BMI z-scores (<-2.5 or >2.5). Modeling PBDEs on the lipid basis (instead of controlling for them in the model), and using generalized estimating equations to account for correlation between siblings (or excluding one of each sibling pair) had no impact on the results (data not shown).
Figure 2. Serum BDE-153 concentrations by body mass index (BMI) z-score among 80 children age 1-5 years.
Discussion
In this study we assessed predictors of PBDE serum levels among 1-5 year-old children in the United States, a population with some of the highest PBDE serum levels in the world due to extensive production and use of PBDEs in consumer products in the U.S. and hand-to mouth behaviors in the first years of life. In this socioeconomically diverse study population enrolled between 2011-2012, we observed higher PBDE serum levels among children living in neighborhoods with more poverty and lower median incomes and an inverse relationship between BMI and serum levels of BDE-153, a congener with one of the longer estimated half-lives.28 Serum levels of BDE-153 were also positively associated with household smoking, and suggestively associated with breastfeeding duration. Results of our handwipe and dust substudy suggested stronger correlations between serum levels and handwipe concentrations compared to serum and vacuum-collected house dust concentrations.
Two previous studies investigated predictors of PBDE serum levels specifically among toddlers and preschool children in the United States: one among children with autism, developmental delays, and general population controls (n=94) aged 24 - 60 months in California,29 and another among children aged 12-36 months recruited through pediatric clinics in North Carolina (n=77).22 There was evidence in both studies that maternal and/or paternal college education predicted lower PBDE levels, although in the North Carolina population higher maternal education was associated with higher BDE-153 serum levels. However, in their study, breastfeeding duration was a strong predictor of BDE-153 and BDE-153 associations with maternal education and breastfeeding duration were not independent in multivariate models. Our results showing neighborhood environments of higher poverty and lower income predict higher PBDE serum levels adds evidence of socioeconomic disparities in PBDE exposure among young children. Our results suggesting enrollment in Medicaid predicts higher BDE-153 levels is also consistent with this pattern. Higher BDE-153 serum levels among children living in households with active smokers has not been previously reported to our knowledge. It is possible that household smoking is acting as a proxy of unmeasured individual-level socioeconomic factors not captured by insurance type. Alternatively smoking behaviors in the mother (e.g., hand-to-mouth behaviors) may directly affect PBDE levels in the child. In light of a previous report indicating PBDE levels in breast milk are higher among smokers30, our finding warrants further research on maternal smoking and infant and child PBDE exposure.
Our results showing significantly higher ΣBDE3 among females contradict results from North Carolina, where male sex predicted higher ΣBDE3, tempering conclusions about sex as a predictor of PBDE serum levels among young children. In our study there was also suggestion of a positive association between breastfeeding duration and BDE-153, but not as strong as in the North Carolina population.22 We note that extended breastfeeding was less common in our population with only 7.5% reporting breastfeeding for more than 12 months as compared with 34% in the North Carolina study population, and the oldest children in the North Carolina study were only 36 months, with less time on average elapsed since breastfeeding. Because of these population differences we might expect breastfeeding to contribute relatively less to measured serum BDE levels in our study population.
Differences in the predictors of serum ΣBDE3 and BDE-153 may be explained by differences in pharmacokinetics between congeners. BDE-153 exhibits greater fat deposition in rats31 and considerably longer estimated half-lives in humans than other congeners,28 although precise estimates of half-lives of these compounds in human tissues remain a research need. The higher fat deposition of BDE-153 could explain the strong inverse association with BMI if more adipose tissue leads to more sequestration of these lipophilic compounds from the blood supply. The longer half-life could also explain the suggestive relationship between breastfeeding and BDE-153 but not ΣBDE3 as the time elapsed since breastfeeding for most of our study population and shorter half-lives of BDE-47, -99 and -100 may preclude our ability to detect associations with breastfeeding for these congeners. If the blood samples were obtained at an age when the child was breastfeeding, it is possible that a relationship with these congeners would be observed. By the same logic, the stronger correlation between serum levels and handwipe levels of ΣBDE3 compared to BDE-153 could be due to the greater influence of past exposures on current serum levels for BDE-153, with serum ΣBDE3 more influenced by recent exposures that are captured on a current handwipe. Based on the estimated half-lives, BDE-153 should be a better measure of lifetime cumulative exposures in this age group.
Our finding of a strong inverse association between BMI and serum levels of BDE-153 highlights the lack of equivalence between PBDE body burden and PBDE serum levels and the complexity of pharmacokinetic processes governing serum concentrations of lipophilic compounds in early childhood, a life stage of rapid growth and profound changes in body fat distribution.14 Inverse associations between BMI and PBDE serum levels have been observed in previous cross-sectional studies of 7 year-old Latino children in California24,32 and 6-8 year-old girls in Ohio and California.33 In both of these study populations, associations were most evident for BDE-153 specifically. Our study provides new evidence of this relationship at the ages when serum levels of these compounds are greatest8,12 and BMI is rapidly changing. Our results can also be viewed as consistent with evidence in adults that recent weight gain reduces serum levels34, as all weight gain among these young children would have been relatively recent. It is possible that sequestration of these compounds into adipose tissue may render children with higher BMI less vulnerable to the effects of PBDEs.35 However, we note that PBDEs stored in adipose tissue may have other impacts, and lead to mobilization of these compounds into circulation and bioavailability at later life stages.35
Although we were only able to collect house dust samples and handwipes for a subset of our participants (n=17 and 13 respectively), our results are generally consistent with those of Stapleton et al., which showed higher correlations between serum and handwipe PBDEs than serum and vacuum-collected housedust PBDEs among toddlers. The estimated correlation (r) between serum ΣBDE3 and hand wipe ΣBDE3 of 0.48 is similar to the correlation of 0.59 estimated by Stapleton et al. among 77 toddlers22. It is challenging to obtain individual serum samples on young children for research purposes, and collection of handwipes offer a promising noninvasive approach to characterize personal exposure. The observed relationship between BMI and serum levels of BDE-153 also highlights that measured serum levels may not be a gold standard measure of ingested (or absorbed/inhaled) PBDEs. A perfect correlation between ingested PBDEs and serum levels would not be expected because serum levels are a product of both ingestion of PBDEs and pharmacokinetic processing (e.g., metabolism, elimination, storage) that vary across individuals. Because external dose, serum concentrations, and body burden (i.e., the total sum of PBDEs in all body tissue, including adipose) are different conceptual targets for defining exposure in an epidemiologic study, the exposure measure of interest will vary depending on the study's hypothesis; for some study questions, handwipe PBDEs may be preferable to serum levels since they are unaffected by BMI and other individual characteristics.
It is noteworthy that all of the children in this study were born after the US phase-out in 2004 of the pentaBDE and octaBDE commercial formulations, the source of the congeners studied here except for BDE-209.36 Despite this, children in our cohort had a geometric mean of 36.2 ng/g lipid for BDE-47, almost double the geometric mean of 20.5 ng/g lipid among the over-12 year U.S. population measured in 2003-2004 before the phase-out.3 Geometric mean BDE-47 levels in our study (2011-2012) were comparable to similar-aged children in Texas in 2009,12 but lower than California children in 2003-2005 (age 2-5 years)29 and 2008-2009 (age <8 years)37 and higher than North Carolina toddlers (age 1-3 years) in 2009-2011.22 With two-thirds of our cohort enrolled in Medicaid, we speculate that our study population is on average of lower socioeconomic status than most previous studies. BDE-209, the major congener of decaPBDE, was not detected in any serum samples, which may reflect its much shorter estimated half-life of 15 days,38 but may also reflect the high limit of detection for BDE-209 in our analysis. While BDE-209 was detected in all the dust and handwipe samples, unlike previous studies21,22 it was not the dominant congener in dust. Consistent with previous studies in children and adults in the United States, the distribution of PBDE serum levels in our study was skewed with certain individuals exhibiting serum levels orders of magnitude higher than others.12 Anecdotally, the child in our study with the highest serum levels of PBDEs (626 ng/g lipid of BDE-47) had parents who reported being unemployed and a contact phone that was disconnected so a home visit could not be scheduled.
The strong relationship between socioeconomic environment and PBDE exposure also has implications for concern about confounding by SES in epidemiologic studies of PBDEs and neurodevelopment. Isolating the impact of PBDEs on global measures of neurological functioning such child behavior and intelligence is challenging because of concerns about residual confounding by socioeconomic factors that are difficult to quantify and adequately control in statistical models. Given that PBDEs have been phased out of production in new consumer products, but remain in older consumer products that tend to have long useful lives (e.g., furniture), socioeconomic disparities in PBDE exposures are likely to increase over time. Identification of specific mechanisms of effect on neurodevelopment in young children would complement emerging studies showing relationships between PBDE exposures and IQ, reading ability, and behavior,39-41 and may be less vulnerable to residual confounding by socioeconomic factors than these more downstream measures of neurological function known to be impacted by poverty. For example, we recently reported associations between serum PBDEs and thyroid hormones, one possible mechanism of impacts on child neurodevelopment, in this same population.42 While SES is strongly related to PBDE serum levels in our population, controlling for SES factors had no impact on estimated associations with thyroid parameters. Finally, we note that other flame retardant compounds, such as 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)-3,4,5,6-tetrabromophthalate (BEH-TEBP), two components of the Firemaster 550 mixture, were not measured in our study and may show different patterns of association with child characteristics.43
The paucity of studies in this age group is likely due to the challenge in obtaining blood samples; we enrolled children undergoing myringotomy and related surgeries, which allowed us to obtain voluntary blood samples with a high participation rate (94%) in a socioeconomically diverse population. It is possible that the predictors of PBDE serum levels observed in this sample are not generalizable beyond the population of children receiving these surgical procedures. However, even if these results are only relevant to children receiving such surgeries this represents a large group; approximately 667,000 children under age 15 had myringotomy with tube insertion in the United States in 2006.44 We have no a priori reason to expect that the conditions leading to these surgeries or the surgeries themselves would affect serum concentrations of persistent organic pollutants.
In summary, we observed evidence of association between PBDE serum concentrations and markers of SES, BMI and household smoking among 1-5 year-old children. Our results also add to the literature suggesting an association between BDE-153 and history of breastfeeding, and indicating that handwipes are more positively correlated with serum PBDE levels than housedust concentrations. These results have implications for confounding by socioeconomic factors in epidemiologic studies and highlight the complex pharmacokinetics of these lipophilic environmental contaminants in early life, a period of profound growth.
Supplementary Material
Acknowledgments
The authors thank P. Barry Ryan and Priya D'Souza for their contributions in the laboratory. Funding provided by National Institute of Environmental Health Sciences grant R21ES019697.
Footnotes
The authors declare no competing financial interest.
Supporting Information. Partial Spearman correlation coefficients between BDE congener concentrations. Exponentiated coefficients and 95% confidence intervals from linear regression models of BDE-153. This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.United States Environmental Protection Agency. [Last accessed July 18, 2016];2012 https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenylethers-pbdes-significant-new-use.
- 2.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Exposure Sci Environ Epidemiol. 2008;18(1):2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 3.Sjödin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003-2004. Environ Sci Technol. 2008;42(4):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114(11):1770–1775. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards. Environ Sci Technol. 2008;42(21):8158–64. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 6.Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119(2):168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linares V, Bellés M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89(3):335–56. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- 8.Toms LM, Sjödin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2-5 years of age) than in infants and adults. Environ Health Perspect. 2009;117(9):1461–5. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol. 2010;1(13):44. 5256–62. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 10.United States Environmental Protection Agency. Exposure Factors Handbook 2011 Edition (Final) U.S. Environmental Protection Agency; Washington, DC: 2011. EPA/600/R-09/052F. [Google Scholar]
- 11.Braverman LE, Cooper D. Werner & Ingbar's the Thyroid: A Fundamental and Clinical Text. Lippincott Williams & Wilkins; Philadelphia, PA: 2012. [Google Scholar]
- 12.Sjödin A, Schecter A, Jones R, Wong LY, Colacino JA, Malik-Bass N, Zhang Y, Anderson S, McClure C, Turner W, Calafat AM. Polybrominated diphenyl ethers, 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153), and p,p'-dichlorodiphenyldichloroethylene (p,p'-DDE) concentrations in sera collected in 2009 from Texas children. Environ Sci Technol. 2014;48(14):8196–202. doi: 10.1021/es5016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Community Survey. 2012 http://www2.census.gov/programs-surveys/acs/summary_file/2011/data/5_year_by_state/
- 14.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat. 2000;11(246):2002. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [Accessed 7/8/16]; http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 16.World Health Organization. Child growth standards: WHO Anthro (version 3.2.2, January 2011) and macros. (n.d.) Retrieved Aug 7, 2014, from http://www.who.int/childgrowth/software/en/
- 17.Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J Anal Toxicol. 2000;24:696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- 18.Sandau CD, Sjodin A, Davis MD, Barr JR, Maggio VL, Waterman AL, Preston KE, Preau JL, Jr, Barr DB, Needham LL, Patterson DG., Jr Comprehensive solid-phase extraction method for persistent organic pollutants. Validation and application to the analysis of persistent chlorinated pesticides. Anal Chem. 2003;75:71–77. doi: 10.1021/ac026121u. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Rhind SM. Optimized determination of polybrominated diphenyl ethers and polychlorinated biphenyls in sheep serum by solid-phase extraction-gas chromatography-mass spectrometry. Talanta. 2011;84:487–493. doi: 10.1016/j.talanta.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989 Jul-Aug;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 21.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, WebsterF T. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ Health Perspect. 2011;119(9):1247–52. doi: 10.1289/ehp.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Król S, Zabiegała B, Namieśnik J. Determination of polybrominated diphenyl ethers in house dust using standard addition method and gas chromatography with electron capture and mass spectrometric detection. J Chromatogr A. 2012 Aug 3;1249:201–14. doi: 10.1016/j.chroma.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Stapleton HM, Kelly SM, Allen JG, Mcclean MD, Webster TF. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environ Sci Technol. 2008 May 1;42(9):3329–34. doi: 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- 25.Bradman A, Castorina R, Sjödin A, Fenster L, Jones RS, Harley KG, Chevrier J, Holland NT, Eskenazi B. Factors associated with serum polybrominated diphenyl ether (PBDE) levels among school-age children in the CHAMACOS cohort. Environ Sci Technol. 2012 Jul 3;46(13):7373–81. doi: 10.1021/es3003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 27.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht A, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- 29.Rose M, Bennett DH, Bergman A, Fängström B, Pessah IN, Hertz-Picciotto I. PBDEs in 2-5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol. 2010;44(7):2648–53. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ Res. 2003 Oct;93(2):186–94. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 31.Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci. 2006;94(1):28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- 32.Erkin-Cakmak A, Harley KG, Chevrier J, Bradman A, Kogut K, Huen K, Eskenazi B. In Utero and Childhood Polybrominated Diphenyl Ether Exposures and Body Mass at Age 7 Years: The CHAMACOS Study. Environ Health Perspect. 2015;123(6):636–42. doi: 10.1289/ehp.1408417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Windham GC, Pinney SM, Sjodin A, Lum R, Jones RS, Needham LL, Biro FM, Hiatt RA, Kushi LH. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ Res. 2010;110(3):251–7. doi: 10.1016/j.envres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, Vainio H, Weiderpass E. Organochlorines in Swedish women: determinants of serum concentrations. Environ Health Perspect. 2003;111(3):349–55. doi: 10.1289/ehp.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121(2):162–9. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaGuardia MJ, Hale R, Harvey E. Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-,and Deca-PBDE Technical Flame-retardant Mixtures. Environ Sci Technol. 2006;40:6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 37.Wu XM, Bennett DH, Moran RE, Sjödin A, Jones RS, Tancredi DJ, Tulve NS, Clifton MS, Colón M, Weathers W, Hertz-Picciotto I. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environmental Health. 2015;14(1):23. doi: 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K. Apparent Half-Lives of Hepta- to Decabrominated Diphenyl Ethers in Human Serum as Determined in Occupationally Exposed Workers. Environ Health Perspect. 2006;114(2):176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Yolton K, Webster GM, Sjödin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect. 2016 doi: 10.1289/EHP478. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowell WJ, Lederman SA, Sjödin A, Jones R, Wang S, Perera FP, Wang R, Rauh VA, Herbstman JB. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3-7 years. Neurotoxicol Teratol. 2015;52(Pt B):143–50. doi: 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, Dietrich KN, Lanphear BP. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ Health Perspect. 2014;122(8):856–62. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson MH, Barr DB, Marcus M, Muir AB, Lyles RH, Howards PP, Pardo L, Darrow LA. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environ Res. 2016;149:222–30. doi: 10.1016/j.envres.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;(24):46. 13056–66. doi: 10.1021/es303879n. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen KA, Hall MJ, Golosinskiy A. National health statistics reports. Hyattsville, MD: National Center for Health Statistics; 2009. Ambulatory Surgery in the United States, 2006. no 11. Revised. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.