Abstract
Nucleic acids undergo naturally occurring chemical modifications. Over 100 different modifications have been described and every position in the purine and pyrimidine bases can be modified; often the sugar is also modified1. Despite recent progress, the mechanism for the biosynthesis of most modifications is not fully understood, owing, in part, to the difficulty associated with reconstituting enzyme activity in vitro. Whereas some modifications can be efficiently formed with purified components, others may require more intricate pathways2. A model for modification interdependence, in which one modification is a prerequisite for another, potentially explains a major hindrance in reconstituting enzymatic activity in vitro3. This model was prompted by the earlier discovery of tRNA cytosine-to-uridine editing in eukaryotes, a reaction that has not been recapitulated in vitro and the mechanism of which remains unknown. Here we show that cytosine 32 in the anticodon loop of Trypanosoma brucei tRNAThr is methylated to 3-methylcytosine (m3C) as a pre-requisite for C-to-U deamination. Formation of m3C in vitro requires the presence of both the T. brucei m3C methyltransferase TRM140 and the deaminase ADAT2/3. Once formed, m3C is deaminated to 3-methyluridine (m3U) by the same set of enzymes. ADAT2/3 is a highly mutagenic enzyme4, but we also show that when co-expressed with the methyltransferase its mutagenicity is kept in check. This helps to explain how T. brucei escapes ‘wholesale deamination’5 of its genome while harbouring both enzymes in the nucleus. This observation has implications for the control of another mutagenic deaminase, human AID, and provides a rationale for its regulation.
A deamination mechanism for C-to-U editing has been demonstrated for a subset of archaeal tRNAs, but the analogous enzyme is not found in eukaryotes6; the eukaryotic enzyme(s) have thus remained elusive. Downregulation of T. brucei tRNA adenosine to inosine deaminase (ADAT2/3) expression by RNAi leads to a decrease in both A-to-I editing at position 34 and C-to-U editing at position 32 of tRNAThrAGU, suggesting that it is involved in both editing events4. However, ADAT2/3 could not catalyse C-to-U in vitro7, but since position 32 of eukaryotic tRNAThr molecules is commonly modified to m3C (Extended Data Fig. 1)8, it raises the possibility that RNA editing may strictly target a methylated base, ultimately forming m3U (Fig. 1a). To test this, native T. brucei tRNAThr was purified9 and analysed by post-labelling/two-dimensional thin-layer chromatography (2D-TLC)10, revealing the presence of several modified nucleotides including m3C (Fig. 1b) and m3U, although lower levels of the latter were observed compared to m3C (Fig. 1b).
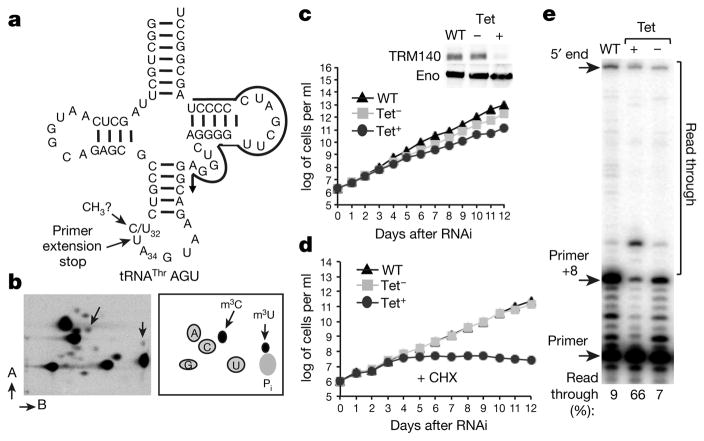
Figure 1. TRM140a modifies position 32 of tRNAThr.
a, A schematic of tRNAThrAGU. ‘CH3?’ marks the methylated site and the black arrow indicates the oligonucleotide used for primer extension. b, 2D-TLC of tRNAThrAGU from total T. brucei RNA, m3C and m3U are indicated by arrows. The A and B arrows refer to the two solvent systems used for 2D-TLC as described previously (ref. 4). c, Growth curves of wild-type (WT), uninduced (Tet−) and RNAi-induced (Tet+) cells and western blots with anti-TRM140a (TRM) and anti-enolase (ENO) antibodies (inset). Tet− and Tet+ refer to the absence or presence of tetracycline in the growth media, respectively. d, Growth curves as in b in the presence of cycloheximide (CHX). e, Primer extension with total RNA from wild-type, uninduced and RNAi-induced cells. ‘+8’ marks the stop indicative of m3C/m3U. The TLC and primer extension data are representative of at least three independent experiments. The growth curves are representative of five biological replicates with comparable results.
We identified two methyltransferase paralogues: Trm140a (927.10.1800) and Trm140b respectively11. RNAi of TRM140a (Fig. 1c) and TRM140b (data not shown) showed a slower growth rate when compared to an untreated control11. Primer extension analysis using an oligonucleotide primer specific for tRNAThrAGU (one of three iso-acceptors that undergoes C-to-U editing) and total RNA from each cell line generated a ‘strong’ stop one nucleotide short of the methylated position. This corresponds to a primer extended by 8 nucleotides (primer +8) indicative of m3C/m3U (Fig. 1a, e). The strong stop was significantly reduced in the RNAi sample when compared to either wild type or the untreated control (Fig. 1e). No changes were observed upon downregulation of the paralogous TRM140b (data not shown). The read-through observed with the wild-type and untreated samples was limited to <10%, while the level of read-through increased to 66% in the RNAi sample. This is consistent with the presence of a base-pair-blocking methylation such as m3C or m3U. Notably, only four other modifications are known to block reverse transcription (m1G, m2,2G, m1A and wybutosine derivatives); none has ever been found at position 32 of tRNA. RNAi experiments in the presence of a concentration of cycloheximide non-inhibitory for growth of wild-type cells (Fig. 1d, black triangles) showed that only downregulation of TRM140a by RNAi led to a reduced growth phenotype and eventually cell death (Fig. 1d, black circles), suggesting that this modification is important for protein synthesis.
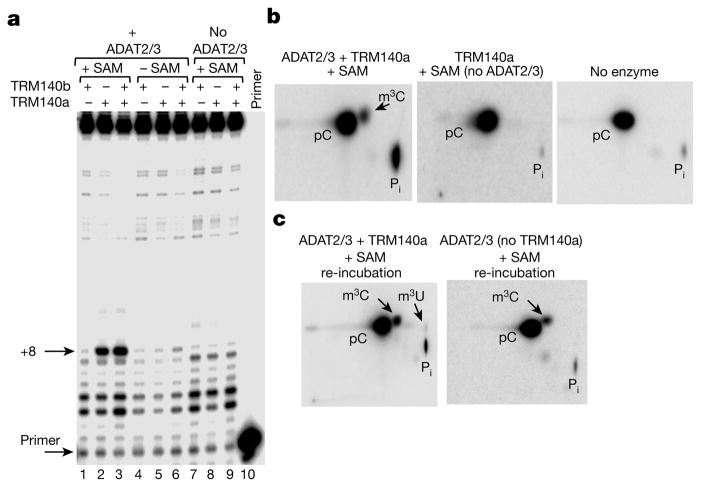
Failure to obtain in vitro methylation at position 32, with recombinant TRM140a led to testing for methylation in the presence of recombinant deaminase. We incubated in vitro transcribed tRNAThrAGU with TRM140a in the presence or absence of ADAT2/3 and the universal methyl donor S-adenosylmethionine (SAM), followed by primer extension analysis. A strong stop was observed (primer +8) but only when TRM140a, ADAT2/3 and SAM are present in the reaction (Fig. 2a, lanes 2 and 3). TRM140b alone was unable to generate a strong stop regardless of the presence or absence of the deaminase and its characterization was not pursued further (Fig. 2a, lanes 1 and 7). No strong stop was observed either in absence of SAM (Fig. 2a, lanes 4–6) or in the presence of SAM but with only one of the enzymes present (Fig. 2a, lanes 7–9). These results corroborate that TRM140a is the authentic tRNA m3C methyltransferase of T. brucei and demonstrate its strict dependence on the ADAT2/3 deaminase for activity.
Figure 2. TRM140a and ADAT2/3 are required for methylation and editing of tRNAThrAGU.
a, Primer extension as in Fig. 1a with a tRNAThr transcript. The presence of absence of recombinant enzymes and SAM is indicated. ‘Primer’ refers to a primer-alone reaction. b, A radioactive version of the transcript in a incubated with ADAT2/3, TRM140a and SAM (left panel), the methyltransferase and SAM but no deaminase, or no enzyme. pC, 5′ cytosine monophosphate; Pi, inorganic phosphate. c, Reincubation of m3C-containing tRNA with TRM140a and ADAT2/3 (left panel) and ADAT2/3 (no methyltransferase) (right panel); all reactions contain SAM. The data are representative of at least three independent experiments.
Similar experiments as above using a radioactive tRNAThr transcript and two-dimensional thin-layer chromatography (2D-TLC) analysis showed significant m3C formation (Fig. 2b). We still did not detect C-to-U or m3C-to-m3U formation, thus the two reactions might not occur simultaneously. To test this, we purified the radioactively labelled m3C-containing tRNAThr, which was then incubated with TRM140a, SAM and ADAT2/3; a new spot corresponding to m3U appeared in the 2D-TLC (Fig. 2c, left panel). Neither incubation of this substrate with SAM and ADAT2/3 alone, nor incubation with a tRNA transcribed with U at position 32 and any combination of the enzymes produced detectable m3U32 (not shown). Additionally, formation of m3U occurs at lower levels that m3C in vitro (Extended Data Fig. 4), which is reminiscent of the in vivo situation (Fig. 1).
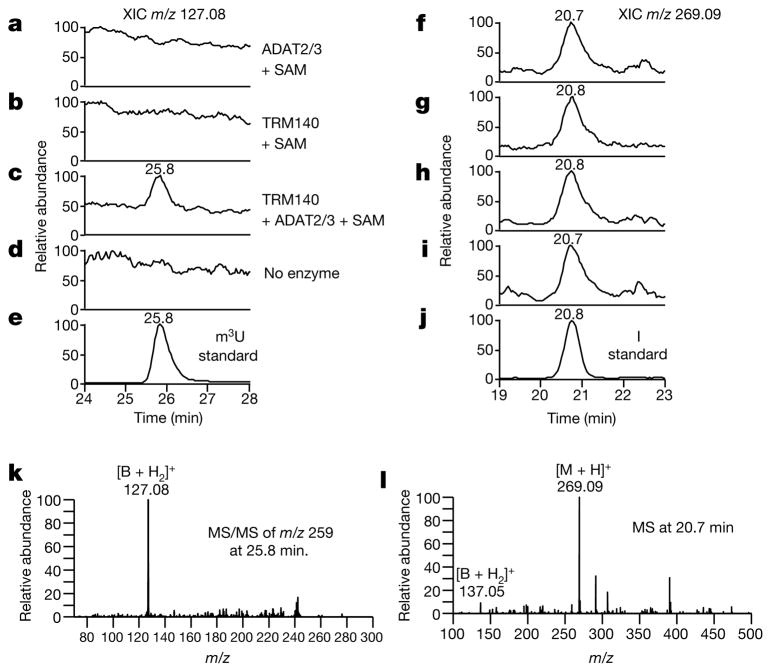
Unlabelled tRNA was also pre-incubated with TRM140a, SAM and ADAT2/3 to generate the m3C-containing substrate, which was purified and re-incubated with both enzymes. The resulting modified tRNA was then subjected to tandem liquid chromatography mass spectrometry (LC-MS/MS) after digestion to nucleosides12. A peak eluting at 25.8 min was observed by monitoring the MS/MS transition m/z 259→127, which corresponds to the elution time and mass spectral behaviour of a chemically synthesized m3U standard (Fig. 3c, e). This peak was not present when the m3C-containing tRNA transcript was incubated in the presence of SAM with either enzyme alone or with a ‘no enzyme’ control (Fig. 3a, b, d). Notably, all samples contained inosine (Fig. 3f–j) (m/z 269.09 at 20.8 min) resulting from deamination of position 34 by ADAT2/3, as previously described. However, inosine formation occurred independently of the methylation or editing status at position 32 as shown previously7. These data establish that formation of m3U is strictly dependent on prior base methylation of cytosine 32 while requiring the presence of both enzymes.
Figure 3. LC-MS/MS analysis confirms the in vitro formation of m3U.
a–e, tRNAThr transcript incubated with various enzymes analysed by LC-MS/MS. The extracted ion chromatogram (XIC) of m/z 127.08 ([B + H2]+ for m3U) of an MS/MS scan targeting m/z 259 ([M + H]+ ion for m3U) is shown. f–j, Same as in a–e showing inosine (XIC m/z 269) and the inosine standard eluting at 20.8 min. k, MS/MS spectra of m/z 259 at 25.8 min from c produced the expected product ion of m/z 127.08, also observed with the m3U standard. i, Mass spectrometry at 20.8 min showing the spectra for inosine with m/z 269.09 ([M + H]+) and m/z 137.05 ([B + H2]+) in all samples.
C-to-U editing of all three tRNAThr isoacceptors occurs in the nucleus of T. brucei (Extended Data Figs 2 and 3); the same must therefore be true of m3C-to-m3U editing. We purified nuclear and cytoplasmic RNA fractions from wild-type T. brucei as described previously13 and performed similar primer extension assays, which showed the presence of a strong stop one nucleotide short of position 32 for all tRNA isoacceptors in all sub-cellular fractions, indicating that methylation at position 32 occurs before export to the cytoplasm (Extended Data Figs 2c and 3). Western blots and immunofluorescence analysis with polyclonal antibodies against TRM140a showed that TRM140a also localizes to the nucleus of T. brucei (Extended Data Fig. 2a, b). Prior to this work we reported that although ADAT2/3 is mostly cytoplasmic, a portion of the enzyme is found in the nucleus4,7. Nuclear localization of ADAT2/3 can be explained by the mutual reliance upon both enzymes for activity.
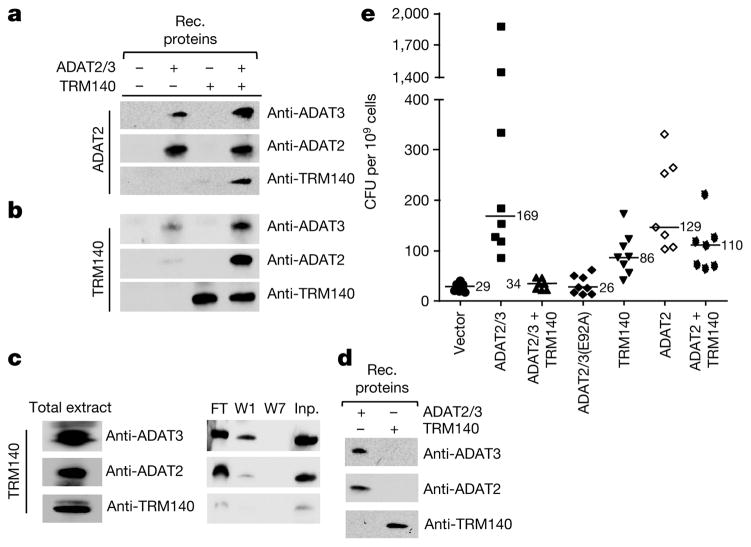
Enzyme interdependence may involve a direct interaction. To test this, immunoprecipitation experiments were performed with antibodies against recombinant ADAT2, ADAT3 and TRM140, which have negligible cross-reactivity (Fig. 4d). Co-immunoprecipitation experiments were performed by incubating recombinant ADAT2/3 with TRM140a, followed by precipitation with either anti-ADAT2 (Fig. 4a) or, reciprocally, with anti-TRM140a antibodies (Fig. 4b). In both cases, comparable amounts of the three proteins were immunoprecipitated. Similar experiments with total T. brucei extracts showed that anti-TRM140 antibodies also pull down equivalent amounts of ADAT2 and ADAT3 (Fig. 4c). These observations demonstrate a strong and specific interaction between the deaminase and methyltransferase in the nucleus of T. brucei.
Figure 4. Complex formation with TRM140a dampens the mutagenicity of ADAT2/3.
a, Immunoprecipitations with anti-ADAT2 antibodies and various protein combinations mixed before immunoprecipitation. The presence of each protein is then detected by western blots with antibodies against each protein (anti-ADAT3), (anti-ADAT2) or (anti-TRM140). Rec. refers to recombinant proteins purified from E. coli. b, Similar immunoprecipitations as in a but with anti-TRM140a antibodies. c, Total protein extracts from wild-type T. brucei immunoprecipitation as in b with anti-TRM140a antibodies, followed by western blot analysis as above. The flow-through (FT), washes 1 and 7 (W1 and W7) of the beads and the input (Inp.) are shown (right panels). d, Western blots with the recombinant proteins and the same antibodies as in c. All panels are representative of at least five independent experiments. e, Mutation frequencies for the rpoB gene in E. coli transformed with vector alone (vector) or plasmids expressing various combinations of enzymes as indicated, including a catalytically defective ADAT2/3 (ADAT2/3(E92A)). Each mark represents the frequency of mutation of an independent culture, shown as colony forming units (CFU) per 109 cells. Mean values for each condition are shown as horizontal lines and numbers within the graph. The source data (uncropped gels) for the western blots in panels a–d can be found in Supplementary Fig. 1.
ADAT2/3 efficiently deaminates DNA in vitro and proves highly mutagenic in a mutagenicity screen4. This raises the question of how this mutagenic enzyme is kept in check in the nucleus of T. brucei. In this regard ADAT2/3 is comparable to activation-induced cytidine deaminase (AID), which deaminates the immunoglobulin receptors while not affecting the rest of the genome14. It has been suggested that AID may associate with factors15 that modulate its mutagenicity. To explore if TRM140a prevents a potential mutagenic disaster created by ADAT2/3 nuclear localization, both enzymes were expressed separately or co-expressed in E. coli and scored for the appearance of rifampicin-resistant colonies4,14, which develop as a result of mutations in the rpoB gene. Expression of ADAT2/3 or ADAT2 alone led to accumulation of rifampicin-resistant colonies in E. coli (Fig. 4e). However, co-expression of TRM140a and ADAT2/3 reduced mutagenesis to background levels as compared to either expression of a deaminase catalytic mutant or an empty vector control (Fig. 4e). Co-expression of ADAT2 in absence of its partner (ADAT3) but presence of the methyltransferase still produced substantial mutagenesis (Fig. 4e), in agreement with the inability of ADAT2 alone to complement TRM140a methylation activity in vitro. We therefore suggest that methyltransferase– deaminase complex formation may have the added advantage of preventing undesirable mutagenic effects.
C-to-U editing of tRNA was first reported over 20 years ago16. Subsequent examples included the reassignment of a tRNAArg to tRNAAsp in marsupial mitochondria17,18 and creation of a tRNATrpUGA from tRNATrpCCA (ref. 19), essential for decoding in trypanosome mitochondria. Recently, several tRNAs in Arabidopsis thaliana were shown to undergo C-to-U editing at position 32 of the anticodon of tRNAThr (ref. 20) similar to T. brucei21. These processes share the lack of an in vitro assay for C-to-U editing—one of the standing challenges of the tRNA editing field in eukaryotes. At least in trypanosomes editing requires prior formation of m3C32 followed by formation of m3U32. Remarkably, both enzymes (ADAT2/3 and TRM140a) are required for both reactions. The existence of two intracellular pools of deaminase, the majority in the cytoplasm, but a significant portion in the nucleus of T. brucei, is then explained by the interdependence of both enzymes for activity. Notwithstanding the mutagenic activity of ADAT2/3, complex formation with the methyltransferase may also prevent genome alterations by an otherwise powerful mutator (ADAT2/3).
In mammalian cells, AID plays a critical role in antibody class diversification by specifically targeting the IgG receptor genes, while generally leaving the rest of the genome unblemished22. While the mechanism of this enzyme has been elucidated, the basis for its programmed specificity towards only a fraction of the genome is still unclear. The work presented here provides a rationale for controlling mutagenic enzymes through their interaction with other partners, as has been suggested previously. This, of course, leads to the question of how such substrate specificities evolved. Our data suggest that the answer may relate to the ability of certain protein–protein interactions to provide secondary functions based on extreme mutual dependability, as illustrated here by the interplay between TRM140a and ADAT2/3.
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
METHODS
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Plasmid constructs and recombinant protein expression and database searches
The two potential T. brucei paralogues of the m3C methyltransferase were identified by searching the trypanosome database (TriTryp) with the yeast m3C methyltransferase coding sequence. The resulting sequences were then used in the RNAi experiments and also for recombinant expression as described below. The coding regions of the ADAT2 and ADAT3 genes were cloned from T. brucei cDNA into the SalI/NotI and NdeI/XhoI sites of the pCDFduet-1 expression vector (Novagen) respectively. The final CDF-ADAT2/3 construct encodes T. brucei ADAT2 with an N-terminal hexahistidine tag and T. brucei ADAT3 with a C-terminal Flag tag. The TRM140a coding sequence was cloned into the pET100 expression vector placing a hexahistidine tag at its N terminus. These vectors were then individually transformed into BL21 DE3 PLys cells. A 10 ml overnight culture was used to inoculate 1 l of LB supplemented with the appropriate antibiotic (spectinomycin for the ADAT2/3 construct and ampicillin for the TRM140a construct). Cells were grown to an OD600 of 0.8 at 37 °C at which point the temperature was lowered to 22 °C and the cells were induced with 0.5 mM IPTG overnight. Cells were harvested, suspended in lysis buffer (200 mM KCl, 20 mM HEPES, pH 7.9) and lysed in an Emulsiflex C5 homogenizer. Lysates were clarified by centrifugation at 30,000 g for 20 min at 4 °C. The resulting supernatant was applied to a TALON Superflow affinity resin (BD Biosciences). Proteins were eluted using a step gradient of lysis buffer containing 15–250 mM imidazole. ADAT2/3 and TRM140a-containing fractions were pooled separately, dialysed against lysis buffer overnight, concentrated and further purified by gel filtration using a Superdex 200 column (GE Healthcare). Active complex fractions eluted with a peak at 212 kDa and 72 kDa for ADAT2/3 and TRM140a respectively.
Primer extension, deamination/methylation assays and mutation analysis
Primer extension assays were performed as described previously19, using radioactive oligonucleotide primers specific for the different tRNAThr isoacceptors from T. brucei. The presence of m3C or m3U blocks canonical base pairing during reverse transcription and generates a strong stop one nucleotide short of the methylated position. The appearance of such a strong stop was used as an indication of the presence of m3C/m3U. Per cent read-through during primer extension assays was calculated by quantifying the signal above the strong stop in the entire lane and divided by the signal from the entire lane above the primer (Fig. 1). This value was multiplied by 100.
Deamination assays were performed as described21. For the methylation assays tRNAThrAGU was generated by in vitro transcription with T7 RNA polymerase in a reaction mixture containing 50 mM Tris (pH 8.0), 1 mM spermidine, 30 mM MgCl2, 7.5 mM DTT, 4 mM ATP, 4 mM GTP, 4 mM UTP, and 0.1 mM CTP, 0.1 mg ml−1 DNA, and 50–100 mCi [α-32P-]CTP (3,000 Ci mmol−1, Perkin Elmer) for 3 h at 37 °C, followed by 8% polyacrylamide/7M urea gel electrophoresis, elution of tRNA, and suspension in TE (10 mM Tris-HCL, 0.1 mM EDTA, pH 8.0). The C-labelled transcript was incubated with equimolar amounts of recombinant TRM140a and/or ADAT2/3 (15 pmol final concentration) in the absence or presence of 0.5 mM S-adenosylmethionine (SAM). Reactions were incubated at 27 °C for 3 h. Following incubation the samples were extracted with Tris-equilibrated phenol (pH 8.0) precipitated in 0.3 M sodium acetate (pH 5.2) and 100% ethanol. The resulting pellets were digested to nucleotides with nuclease P1 and subjected to 2D liquid chromatography as described elsewhere4. Mutation frequencies for rpoB-mediated resistance to rifampicin were as described elsewhere, but using E. coli CJ236 (FΔ(HindIII)::cat (Tra+ Pil+ CamR)/ung-1 relA1 dut-1 thi-1 spoT1 mcrA) as host4. Expression of ADAT3 alone was not tested since this subunit is insoluble when expressed alone (data not shown).
Trypanosome cell lines and plasmid construction
Portions of the coding sequence of the Trm140a gene from T. brucei were cloned into the tetracycline-inducible RNAi vector p2T7-177. These plasmids were then linearized by Not I digestion (for genomic integration) and transfected into procyclic T. brucei 29–13 cells and clonal lines were obtained by limiting dilution. RNAi was induced after the addition of a final concentration of 1 μg ml−1 of tetracycline to SDM-79 growth medium. Cell counts were taken every 24 h using the Beckman Z2 Coulter counter over the course of 10 days post-induction. In these growth curves, the induced sample was compared to a non-induced control grown in the absence of tetracycline and a similar growth curve performed using wild-type cells. For the growth curves cumulative cells counts were used while accounting for culture dilutions. In the cycloheximide growth curve, a concentration of the drug was used that is non-inhibitory to wild-type cells. Targeted gene downregulation was confirmed by RT–PCR, as described elsewhere23.
Immunoprecipitation assays and western blots
For binding analysis of recombinant proteins, 10 μl of magnetic protein-G Dynabeads (Life Technologies, catalogue number 10007D) were washed with 100 μl of PBS (0.1 M sodium phosphate, pH 7.2, 0.15 M NaCl) then incubated with antigen-purified polyclonal antibody in 15 μl of PBS for 2 h on ice. Unbound antibody was removed from the beads by washing with 100 μl of PBS. Recombinant proteins at a final concentration of 500 nM were incubated with the antibody-bound beads for 40 min on ice. The beads were washed seven times with 100 μl of PBS containing 1.0% NP40, after which the binding components were eluted by boiling the beads for 15 min at 90 °C in SDS loading buffer and analysed by western blotting. For binding analysis of whole-cell extracts, 50 ml of protein-C-tagged ADAT3 procyclic T. brucei 29–13 cells at 7 × 106 cells ml−1 were pelleted at 3,000 r.p.m. for 20 min at 4 °C. The cells were washed twice in PBS then suspended in 400 μl of PBS with 1.0% NP40 and protease inhibitor cocktail (Sigma-Aldrich). The cell suspension was sonicated with a Sonifier 450 using a microprobe for three 30-s pulses with the settings at output 4.2, duty-cycle 25. The sample was spun for 10 min at 20,000 g to remove cellular debris. Total soluble cell extract was incubated with protein C antibody bound to beads (Roche, catalogue number 11815024001) and the rest of the immunoprecipitation experiment was carried out as described above. Reciprocal experiments with total extracts were also performed with anti-ADAT2; however, only traces of TRM140a were observed (not shown). This may be due to the fact that the overwhelming majority of ADAT2/3 resides in the cytoplasm, which according to our localization experiments is generally devoid of TRM140a.
The western blots were performed as previously described. In the blots in Fig. 1 the anti-enolase antibody was used as a loading control. All other antibodies were antigen-purified, using a recombinant protein affinity column as the antigen.
LC/MS analysis
Following the methylation assay with unlabelled transcripts, samples were extracted with Tris-saturated phenol (pH 8.0), and precipitated as above. For nucleoside analysis, the resulting tRNA was digested to nucleosides using nuclease P1 (Sigma-Aldrich) and Antarctic Phosphatase (New England Biolabs). The nucleosides were separated using a Hitachi D-7000 HPLC with a Hitachi L-7444 diode array detector at a flow rate of 0.3 ml min−1 at room temperature on a Supelcosil LC-18-S 250 × 2.1 mm column (Sigma-Aldrich). Nucleosides were separated using a combination of two buffers (buffer A: 5 mM ammonium acetate pH 5.3 and buffer B: acetonitrile:water, 40:60 (v/v)). These were used in a step-gradient which is a modified version of a previously described protocol (ref. 12): 0 min: 1% B, 5.8 min: 1% B, 9.2 min: 2% B, 10.9 min: 3% B, 12.7 min: 5% B, 32 min: 25% B, 38 min: 50% B, 43.5 min: 75% B, 45 min: 75% B, 50 min: 99% B, 55 min: 99% B, 60 min: 1% B. Column eluent was split into two fractions, with two-thirds being analysed by the UV detector and the remaining third was injected into an LTQ-XL linear ion trap mass spectrometer (Thermo Scientific). Mass spectra were recorded in the positive ion mode with a capillary temperature of 275 °C, spray voltage 4.0 kV and sheath gas, auxiliary gas and sweep gas of 45, 25 and 10 arbitrary units, respectively. Data-dependent MS/MS of the four most abundant ions were recorded throughout the LC/MS run using a collision energy of 35 arbitrary units.
Data availability
The data that support the findings of this study that are not included in this work are available from the corresponding author upon reasonable request.
Extended Data
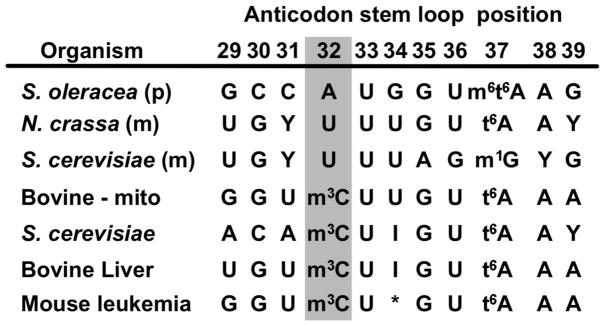
Extended Data Figure 1. Anticodon sequences for tRNAThr from various organisms.
This limited set represents the few tRNAThr molecules for which modifications have been mapped to specific nucleotide positions as deposited in tRNAdb8. Highlighted in grey is position 32 of the anticodon, showing that this position is often modified to 3-methylcytosine (m3C).
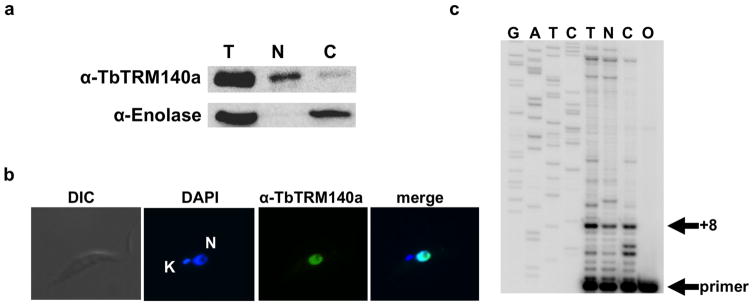
Extended Data Figure 2. TRM140a methyltransferase is a nuclear enzyme.
a, Western blot analysis of total (T), nuclear (N) and cytoplasmic (C) fractions isolated from T. brucei as previously described24 probed with polyclonal antibodies raised against recombinant TRM140a. The same membrane was probed with antibodies against enolase, serving as a cytosolic marker and also as a control fraction to show the degree of nuclear contamination in our cytoplasmic fraction. b, Immunofluorescence localization of TRM140a using the same antibodies as in top panel of a. The cells were also stained with DAPI to establish the position of the nucleus (N) and mitochondrial (K) genomes for reference. ‘Merge’ refers to the superposition of the two panels, which confirms the nuclear localization of this protein. c, Primer extension analysis of total RNA isolated from the same fractions as above, primer +8 marks the positions of the strong stop one nucleotide short of position 32 (the methylated position), indicating that m3C is already present in the nuclear tRNA before cytoplasmic export. G, A, T and C refer to an unrelated sequencing ladder used as a size marker. T, N and C are as in a and O represents a mock primer extension with oligonucleotide primer alone but in the absence of template.
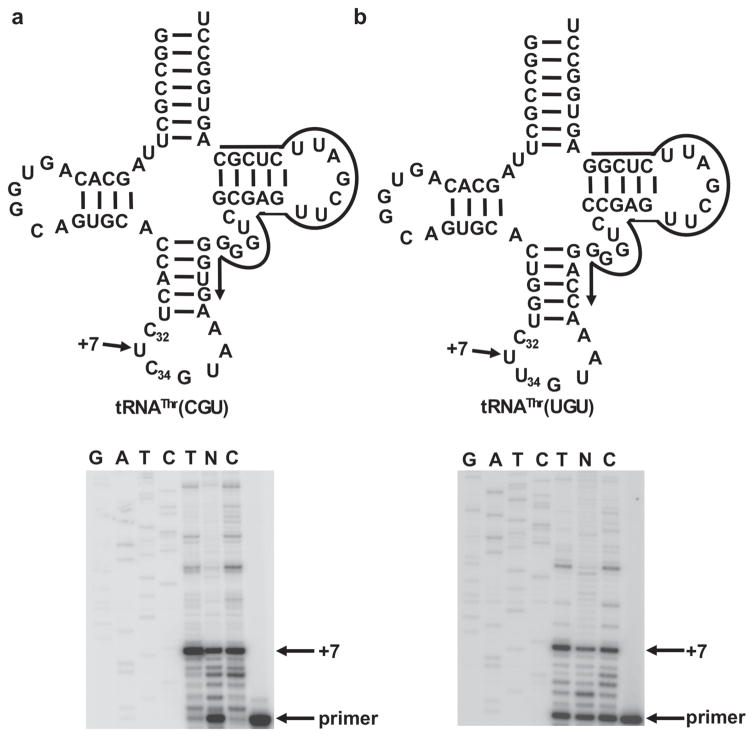
Extended Data Figure 3. Position 32 in the other tRNAThr isoacceptors is also methylated in the nucleus of T. brucei.
a, b, Similar reactions as in Extended Data Fig. 3c, but this time with oligonucleotide primers specific for tRNAThrCGU (a) and tRNAThrUGU (b), confirming that the strong stop in these tRNAs already appears in the nuclear fractions. This time, however, the expected product is at primer +7, as indicated.
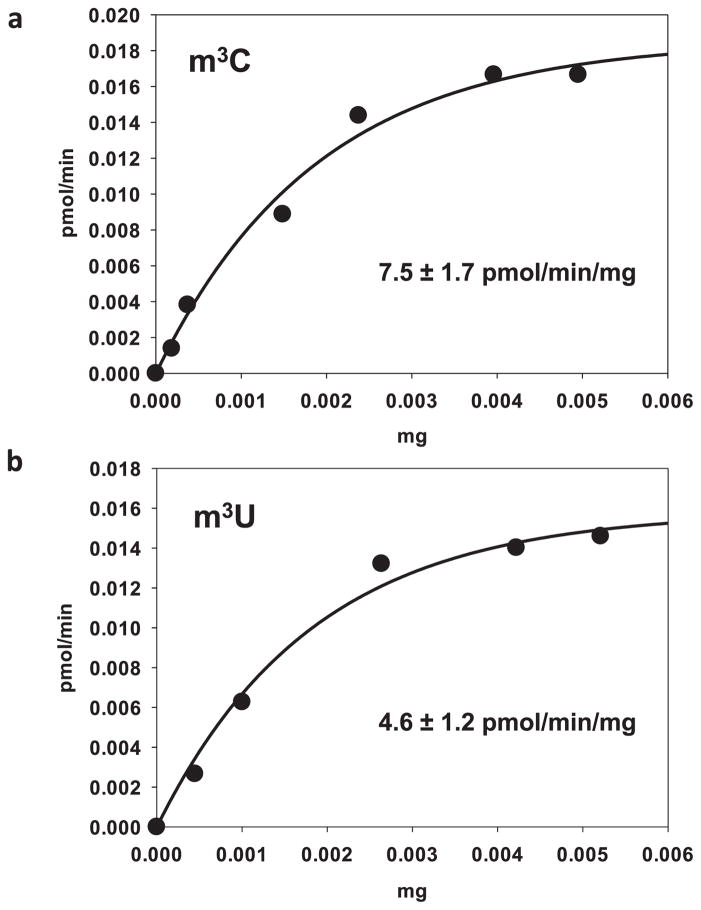
Extended Data Figure 4. Concentration curves for the formation of m3C and m3U.
a, Concentration curve with increasing amounts of TRM140, ADAT2/3 was kept at the same molar ratio as the methyltransferase. The specific activities of TRM140 are as indicated and expressed in pmol per min per mg. b, Similar experiment as in a but this time showing the m3U activity of ADAT2/3 in the presence of stoichiometric amounts of the methyltransferase.
Supplementary Material
Acknowledgments
The research was funded by NIH grants GM084065 to J.D.A. and GM058843 to P.A.L. and a Czech Science Foundation (15-21450Y) grant to Z.P. We thank V. Gopalan, J. Rinehart and D. Schoenberg for comments and suggestions on the manuscript.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions All authors contributed to the design of the experiments and data interpretation; experiments were performed by M.A.T. (Figs 1a, b, e, 2a–c), Z.P. (Fig. 1c–d), K.W.G (Fig. 3a–i), K.M.M. (Fig. 4a–d), I.M.C.F. (Fig. 4e). The manuscript was written by J.D.A. All authors discussed the results and commented on the manuscript.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
Reviewer Information Nature thanks J. Chaudhuri and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Machnicka MA, et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helm M, Alfonzo JD. Posttranscriptional RNA Modifications: playing metabolic games in a cell’s chemical Legoland. Chem Biol. 2014;21:174–185. doi: 10.1016/j.chembiol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crain PF, et al. Modification of the universally unmodified uridine-33 in a mitochondria-imported edited tRNA and the role of the anticodon arm structure on editing efficiency. RNA. 2002;8:752–761. doi: 10.1017/s1355838202022045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio MA, et al. An adenosine-to-inosine tRNA-editing enzyme that can perform C-to-U deamination of DNA. Proc Natl Acad Sci USA. 2007;104:7821–7826. doi: 10.1073/pnas.0702394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CW. In: Modification and Editing of RNA. Grosjean, Benne, editors. ASM Press; 1998. pp. 363–375. [Google Scholar]
- 6.Randau L, et al. A cytidine deaminase edits C to U in transfer RNAs in Archaea. Science. 2009;324:657–659. doi: 10.1126/science.1170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaston KW, et al. C to U editing at position 32 of the anticodon loop precedes tRNA 5′ leader removal in trypanosomatids. Nucleic Acids Res. 2007;35:6740–6749. doi: 10.1093/nar/gkm745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jühling F, et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spears JL, Gaston KW, Alfonzo JD. Analysis of tRNA editing in native and synthetic substrates. Methods Mol Biol. 2011;718:209–226. doi: 10.1007/978-1-61779-018-8_13. [DOI] [PubMed] [Google Scholar]
- 10.Randerath K, Chia LS, Gupta RC, Randerath E. Structural analysis of nonradioactive RNA by postlabeling: the primary structure of baker’s yeast tRNA Leu/CUA. Biochem Biophys Res Commun. 1975;63:157–163. doi: 10.1016/s0006-291x(75)80024-6. [DOI] [PubMed] [Google Scholar]
- 11.Fleming IM, et al. A tRNA methyltransferase paralog is important for ribosome stability and cell division in Trypanosoma brucei. Sci Rep. 2016;6:21438. doi: 10.1038/srep21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R, Cao X, Yu N, Limbach PA. Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods. 2016;107:73–78. doi: 10.1016/j.ymeth.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapushoc ST, Alfonzo JD, Simpson L. Differential localization of nuclear-encoded tRNAs between the cytosol and mitochondrion in Leishmania tarentolae. RNA. 2002;8:57–68. doi: 10.1017/s1355838202012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 15.Petersen-Mahrt S. DNA deamination in immunity. Immunol Rev. 2005;203:80–97. doi: 10.1111/j.0105-2896.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 16.Lonergan KM, Gray MW. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993;259:812–816. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- 17.Börner GV, Mörl M, Janke A, Pääbo S. RNA editing changes the identity of a mitochondrial tRNA in marsupials. EMBO J. 1996;15:5949–5957. [PMC free article] [PubMed] [Google Scholar]
- 18.Mörl M, Dörner M, Pääbo S. C to U editing and modifications during the maturation of the mitochondrial tRNAAsp in marsupials. Nucleic Acids Res. 1995;23:3380–3384. doi: 10.1093/nar/23.17.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfonzo JD, Blanc V, Estévez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNATrp allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J. 1999;18:7056–7062. doi: 10.1093/emboj/18.24.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Karcher D, Bock R. Identification of enzymes for adenosine-to-inosine editing and discovery of cytidine-to-uridine editing in nucleus-encoded transfer RNAs of Arabidopsis. Plant Physiol. 2014;166:1985–1997. doi: 10.1104/pp.114.250498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio MA, Ragone FL, Gaston KW, Ibba M, Alfonzo JD. C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei. J Biol Chem. 2006;281:115–120. doi: 10.1074/jbc.M510136200. [DOI] [PubMed] [Google Scholar]
- 22.Teng G, Papavasiliou FN. Immunoglobulin somatic hypermutation. Annu Rev Genet. 2007;41:107–120. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- 23.Sample PJ, et al. A common tRNA modification at an unusual location: the discovery of wyosine biosynthesis in mitochondria. Nucleic Acids Res. 2015;43:4262–4273. doi: 10.1093/nar/gkv286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapushoc ST, Alfonzo JD, Rubio MA, Simpson L. End processing precedes mitochondrial importation and editing of tRNAs in Leishmania tarentolae. J Biol Chem. 2000;275:37907–37914. doi: 10.1074/jbc.M007838200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study that are not included in this work are available from the corresponding author upon reasonable request.