Abstract
Memory deficits have a significant impact on the quality of life of patients with epilepsy and currently no effective treatments exist to mitigate this comorbidity. While these cognitive comorbidities can be associated with varying degrees of hippocampal cell death and hippocampal sclerosis, more subtle changes in hippocampal physiology independent of cell loss may underlie memory dysfunction in many epilepsy patients. Accordingly, animal models of epilepsy or epileptic processes exhibiting memory deficits in the absence of cell loss could facilitate novel therapy discovery. Mouse corneal kindling is a cost-effective and non-invasive model of focal to bilateral tonic-clonic seizures that may exhibit memory deficits in the absence of cell loss. Therefore, we tested the hypothesis that corneal kindled C57BL/6 mice exhibit spatial pattern processing and memory deficits in a task reliant on DG function and that these impairments would be concurrent with physiological remodeling of the DG as opposed to overt neuron loss. Following corneal kindling, C57BL/6 mice exhibited deficits in a DG-associated spatial memory test – the metric task. Compatible with this finding, we also discovered saturated, and subsequently impaired, LTP of excitatory synaptic transmission at the perforant path to DGC synapse. This saturation of LTP was consistent with evidence suggesting that perforant path to DGC synapses in kindled mice had previously experienced LTP-like changes to their synaptic weights: increased postsynaptic depolarizations in response to equivalent presynaptic input and significantly larger amplitude AMPA receptor mediated spontaneous EPSCs. Additionally, there was evidence for kindling-induced changes in the intrinsic excitability of DGCs: reduced threshold to population spikes under extracellular recording conditions and significantly increased membrane resistances observed in DGCs. Importantly, quantitative immunohistochemical analysis revealed hippocampal astrogliosis in the absence of overt neuron loss. These changes in spatial pattern processing and memory deficits in corneal kindled mice represent a novel model of seizure-induced cognitive dysfunction associated with pathophysiological remodeling of excitatory synaptic transmission and granule cell excitability in the absence of overt cell loss.
Keywords: Seizures, corneal kindling, hippocampus, dentate gyrus, hyperexcitability, synaptic plasticity, cognitive deficits, spatial pattern processing, intrinsic plasticity
Introduction
Epilepsy is a neurological disorder that affects one in 26 individuals worldwide (Hesdorffer et al., 2011). Besides spontaneous recurrent seizures, patients with epilepsy frequently experience behavioral, emotional, and cognitive comorbidities (Jensen, 2011). For example, approximately one in four individuals with temporal lobe epilepsy exhibit cognitive impairment during neuropsychological evaluation, most notably on memory tests (Hermann et al., 2006). These cognitive comorbidities are increasingly recognized as an equal (or even more disabling) part of epilepsy; cognitive dysfunction does not universally disappear once seizures are well controlled which leads to a poor quality of life for patients and their families, even in the absence of seizures (Jacobs et al., 2009). Furthermore, there are currently no treatments for memory deficits experienced by patients with epilepsy (Giovagnoli and Avanzini, 2000; Hrabok et al., 2013). Accordingly, the NINDS Epilepsy Research Benchmarks recognizes this as a significant challenge and calls for increased efforts aimed at establishing animal models that will in turn aid in the development of optimal treatments for cognitive comorbidities in epilepsy (Kelley et al., 2009; Long et al., 2016). The discovery of treatments for this constellation of debilitating cognitive symptoms would be greatly facilitated by such animal models and an improved understanding of the underlying pathophysiology contributing to these symptoms.
When considering the pathophysiology of memory dysfunction in epilepsy, neuron death and atrophy of the hippocampus may be a reasonable underlying cause in many cases. Indeed, patients with temporal lobe epilepsy (TLE) exhibit varying degrees of cell death and hippocampal sclerosis (HS) (see review (de Lanerolle et al., 2012)), and similar neuronal damage can be recapitulated in a number of animal models (Chauvière et al., 2009; Covolan and Mello, 2000; Friedman et al., 1994; Gröticke et al., 2008; Rao et al., 2006). However, not all epilepsy patients with memory impairment have severe hippocampal damage (Aikiä et al., 1995; Giovagnoli and Avanzini, 1999; Lah et al., 2014; Loiseau et al., 1983; Martins et al., 2015), and hippocampal cell loss with or without HS is not always associated with memory impairment (L. H. Castro et al., 2013; C. S. M. Schmidt et al., 2015). Moreover, associations between hippocampal volume loss and performance on memory tests are highly variable (Äikiä et al., 2001). These observations suggest, at least in some epilepsy patients (e.g. cryptogenic TLE), more subtle changes in hippocampal physiology may underlie memory dysfunction, and this patient population in particular might be expected to benefit most from pharmacological therapies aimed at restoring normal memory. Thus, a better understanding of these physiological changes is needed, and animal models of epileptic processes that exhibit memory dysfunction without overt cell loss or HS would be particularly useful.
Rodent kindling models that employ direct electrical stimulation of limbic structures may represent an ideal model of human complex partial seizures (focal aware or impaired awareness seizures, (Fisher et al., 2017)) with comorbid memory dysfunction. However, the underlying pathological changes leading to memory dysfunction in these models can vary considerably depending on the age of the animal, the location of stimulation, and the extent of kindling (Hannesson and Corcoran, 2000; Morimoto et al., 2004). While some kindling studies employing direct electrical stimulation methods report minimal neuronal loss (Brandt et al., 2004; Haas et al., 2001; Singh et al., 2013; Tooyama et al., 2002), others report striking neuronal loss, sometimes resembling hippocampal sclerosis, after more extensive kindling protocols (Cavazos et al., 1994; Cavazos and Sutula, 1990; Chen et al., 2010; Sutula, 1990). Furthermore, all of these models suffer from the time- and labor-intensive nature of invasive electrode implantation surgeries and post-operative care. However, the corneal kindling model of seizures in mice, which has been validated as a rapid screening model of partial limbic seizures that secondarily generalize in humans (focal to bilateral tonic-clonic seizures, (Fisher et al., 2017)), has become a useful tool in the discovery of novel anti-seizure drugs, in part, because of its non-invasive, cost-effective and relatively easy methodology (Matagne and Klitgaard, 1998; Potschka and Löscher, 1999; Rowley and White, 2010).
While the pharmacological responsiveness of corneal kindled seizures in CF1 mice has been thoroughly examined and shown to both correlate well with that of the hippocampal kindled rat and be consistent with human focal limbic seizures (Rowley and White, 2010), their cognitive comorbidities and underlying pathophysiology are only now beginning to be examined. Recently, corneal kindled CF1 mice were shown to be cognitively indistinguishable from control mice in a behavioral paradigm that relies on both hippocampal and extra-hippocampal areas – the novel object/place recognition task (Barker-Haliski et al., 2016). Additionally, Loewen et al., (2016) recently reported that corneal kindled CF1 mice do not exhibit neuronal loss within the hippocampus but do exhibit significant astrogliosis in both area CA1 and the dentate gyrus (DG). These reactive astrocytes indicate that corneal kindled seizures may induce pathological changes in important hippocampal areas such as the DG; the full nature of these changes, and whether they are beneficial or detrimental, remains to be determined. Taken together, these data suggest that corneal kindled mice lack overt hippocampal neuron loss and, despite showing no cognitive dysfunction in a task that relies on extra-hippocampal function, may yet still exhibit cognitive dysfunction in other tests of learning and memory that rely heavily on hippocampal function. Furthermore, it is unknown whether corneal kindling in other strains, particularly C57BL/6 mice, similarly spare hippocampal neurons.
In the present study, we tested the hypothesis that corneal kindled C57BL/6 mice exhibit spatial pattern processing deficits in a task reliant on DG function and that these impairments are concurrent with physiological remodeling of the DG as opposed to overt neuron loss. To accomplish this, we evaluated neurodegeneration in the hippocampus of corneal kindled C57BL/6 mice as well as their performance in a DG-mediated spatial pattern processing test that relies on the rodents’ natural tendencies to explore changes in the distance between two objects – the “metric task” (Ennaceur, 2010; Goodrich-Hunsaker et al., 2008; Lee et al., 2005). Next, we examined physiological changes in the DG of corneal kindled mice that may contribute to memory impairment. We used in-vitro extracellular and whole-cell patch-clamp electrophysiology to evaluate changes in synaptic transmission, granule cell excitability, short- and long-term synaptic plasticity (paired pulse depression, post-tetanic potentiation, and long-term potentiation), and saturation of LTP in the DG. Our results demonstrate that corneal kindled C57BL/6 mice, in the absence of overt cell loss, exhibit DG-associated cognitive deficits associated with pathophysiological remodeling of excitatory synaptic transmission and granule cell excitability that is consistent with saturation of LTP at the perforant path – dentate granule cell synapse.
Methods
Animals
Fifty, five- to six-week-old male C57BL/6 mice (15–20 g, Charles River, Raleigh, NC, U.S.A.) were used in this study. The first cohort (n = 20) of mice was used for the metric task, immunofluorescence, and synaptic plasticity experiments. The second cohort (n = 20) was used for basal synaptic transmission and patch-clamp electrophysiology experiments. The third cohort (n = 10) was used for LTP saturation experiments. All experiments were conducted between three days and 2 weeks after kindling. All mice were group housed in a light- and temperature-controlled (12 h on/12 h off) environment and permitted access to food and water ad libitum throughout the study. All experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Utah Institutional Animal Care and Use Committee. All efforts were made to minimize the number and suffering of animals used.
Corneal Kindling
C57Bl/6 mice were corneal kindled using a protocol adapted from CF1 mice (Rowley and White, 2010). Briefly, a 0.9% saline solution containing 0.5% tetracaine hydrochloride was applied to each eye to provide local anesthesia and electrical conductivity. Mice were stimulated twice daily (4 h apart) with corneal stimulation of 1.5 mA (60 Hz) for 3 s. Mice were considered fully kindled when five consecutive stage five seizures were achieved according to a modified Racine scale (Racine, 1972): 0 = no reaction or immobility; 1 = jaw clonus; 2 = myoclonic twitches in the forelimbs and head nodding; 3=clonic convulsions in the forelimbs; 4 = clonic convulsions in the forelimbs with rearing and falling; and 5 = generalized clonic convulsions associated with loss of balance. Mice were stimulated daily until 5 d before being evaluated for memory deficits and stimulated once three days before beginning electrophysiology experiments to confirm persistence of the fully-kindled state. Additionally, control mice were also brought into the kindling facility and handled but not stimulated.
Metric Task
Habituation for the metric task began three days after ceasing daily kindling stimulation. At the start of each day, one cohort of mice (n = 10 kindled, n = 10 control, 20–25 g) was acclimated to the testing room for 1 h prior to behavioral testing. Mice were then individually habituated to a square plexiglass arena (40 L × 40 W × 60 H cm) for two consecutive days by freely exploring the testing arena for 10 min. Animals were then placed into an empty holding cage for 5 min, and returned to the arena for an additional 5 min of exploration. During habituation, the arena contained two objects in separate locations that were not used during testing and were not moved during habituation. Distinct visual spatial cues were always located 15 cm from each side of the arena. On day 3, testing was conducted in 3 phases (see Fig. 2 panel A for schematic): during phase 1 (acquisition), mice were individually allowed to explore the arena that contained two dissimilar objects placed 30 cm apart for 15 min. In Phase 2 (delay), the animal was removed from the arena and placed in a holding cage for 5 min, while the arena underwent a minimal cleaning protocol to wipe up any feces and exchange the objects for a duplicate pre-cleaned pair, with the objects now placed 8 cm apart. During phase 3 (testing), the mouse was placed back in the arena for an additional 5 min. Before each trial, the arena and four objects (a duplicate pair of dissimilar objects) were pre-cleaned with a 4% HDQ solution.
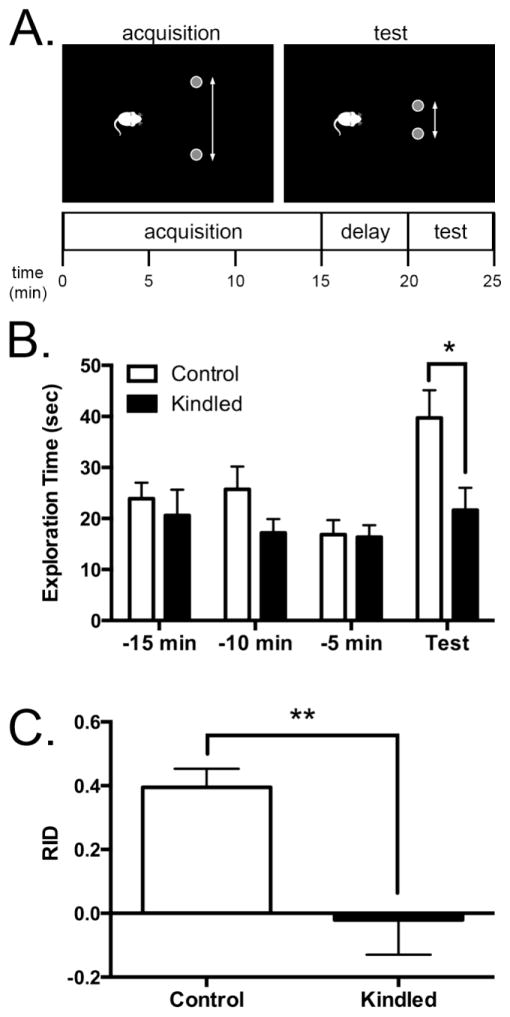
Figure 2.
Corneal kindled mice exhibit DG-dependent memory impairment during the metric task. A, Schematic illustrating the metric task. B, Total object exploration time averaged by phase. There were no significant differences between the control and kindled mice until the test phase, when kindled mice spent significantly less time exploring the objects compared to control mice (p < 0.05, t-test, n = 10 per treatment group). C, Averaged recognition index (RID) in kindled mice was significantly less than the average RID in control mice (**p < 0.01, t-test,).
During testing, animals were tracked using EthoVision software (Noldus, Leesburg, VA, U.S.A.). An observer who was blinded to the experimental group manually scored object exploration. Exploration was defined as investigative behavior, where the mouse was orienting its head towards the object when within 2 cm of the object, or touching or smelling the object with its nose, whiskers, or forepaws. A mouse that was climbing on the objects, or in close proximity without actively attending to the objects, was not counted as exploration. Throughout phases 1 and 3, total object exploration time, regardless of which object was being explored, was recorded in 300 second epochs and averaged by experimental group. Performance was also expressed as a recognition index (RID):
RIDs were calculated for each mouse and averaged by treatment group.
Immunofluorescence
All immunohistochemistry was performed as reported by Loewen et al., (2016). Briefly, four mice (20–25g) per treatment group were anesthetized and transcardially perfused with 0.1M phosphate-buffer solution (PBS) followed by 4% paraformaldehyde (PFA). Brains were removed, post-fixed in 4% PFA at 4°C for 7 hours, transferred to a 15/30% sucrose gradient for cryoprotection, and frozen on dry ice. Next, brains were coronally cut on a freezing stage microtome (Leica Microsystems, Buffalo Grove, IL, U.S.A) into 25 μm-thick sections through dorsal hippocampus and stored in 0.1M PBS at 4°C. Sections immediately caudal to the septum (3 sections/animal for each stain; 4 animals/treatment group) from corneal kindled and control animals were slide mounted and batch processed. First, sections were washed three times with 0.1 M PBS and blocked with 0.25% bovine serum albumin and 0.2% Triton X-100 in PBS for one hour. Sections were then incubated with NeuN 555 MAB377A5 (1:500, EMD Millipore LLC, Billerica, MD, U.S.A.) and GFAP C9205 (1:1000, Sigma-Aldrich, St. Louis, MO, U.S.A.) for two hours. All slides were then counterstained with DAPI D9542 (1:100, Sigma-Aldrich), rinsed in 0.1M PBS and coversliped with ProLong® Gold antifade reagent (Thermo Fisher Scientific, Grand Island, NY, U.S.A.).
Imaging and Analysis
Images were captured with a Nikon A1 confocal microscope (Nikon Instruments, Melville, NY, U.S.A.) using 20x/NA 1.0 air and 60x/NA 1.0 oil immersion objectives and analyzed utilizing NIS Software. Briefly, the left dorsal DG, hilus, and CA1 were identified using a 100 W halogen lamp and a filter set for DAPI to avoid selection bias. Once the region was selected, laser-scanning mode was used to collect images in the z-axis. A minimum of 12 z-stack optical images (1 μm thick) were imaged for each stain from triplicate sections.
Raw grey scale 16-bit images from the channel of each cell marker (NeuN and GFAP) were utilized to perform quantification analysis. To remove bias in the analysis, an automated macro created using ImageJ software (National Institutes of Health) was utilized (Loewen et al., 2016). The total field area stained by each cell marker was measured for each optical section through the stained tissue section. These values were then averaged for each tissue section and averaged by treatment group and brain region.
Hippocampal Brain Slice Preparation
Acute hippocampal brain slices were prepared daily as described previously (West et al., 2014) from either a kindled or control mouse beginning no fewer than 3 d-post kindling. Briefly, mice (20–25 g) were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and brains were rapidly removed and submerged in ice-cold (4°C) oxygenated sucrose-based artificial cerebral spinal fluid (ACSF) bubbled with 95% O2/5% CO2. Sucrose-based ACSF contained the following (in mM): Sucrose (200.0), KCl (3.0), Na2PO4 (1.4), MgSO4 (3.0), NaHCO3 (26.0), glucose (10.0), and CaCl2 (0.5). The pH and osmolarity of the sucrose-based ACSF were adjusted to 7.30–7.35 and 290–300 mOsm, respectively, before each experiment. Next, coronal brain slices (350μm) containing dorsal hippocampus were cut using a vibrating microtome (VT1000S, Leica Microsystems Inc.). Slices were then transferred to oxygenated standard ACSF and incubated for 2 h at room temperature prior to recording. Standard ACSF was made from the same recipe as sucrose-based ACSF, only sucrose was replaced with NaCl (126.0 mM), the MgSO4 concentration lowered to 1.0 mM, and the CaCl2 concentration raised to 2.5 mM.
Brain Slice Electrophysiology - Extracellular
Extracellular field excitatory post-synaptic potentials (fEPSPs) were recorded by transferring eight coronal brain slices containing dorsal hippocampus from either hemisphere into the integrated brain slice chambers (IBSCs) of the Scientifica Slicemaster (Scientifica, Ukfield, East Sussex, U.K.), a high throughput semi-automatic brain slice recording system, which conducts separate but simultaneous recordings (Stopps et al., 2004). All slices were perfused with regular ACSF (2 mL/min) at 30°C. For LTP experiments, ACSF contained 10 μM picrotoxin (PTX). Twisted Nichrome-Formvar stimulating electrodes were placed in the middle-third of the molecular layer in the upper blade of the DG to stimulate the medial perforant path; recording electrodes were placed in the middle-third of the molecular layer approximately 400 – 600 μm away from the stimulating electrode. Input/output (I/O) relationships were measured by incrementally stimulating for 100 μs in 0.5 V steps until a population spike was observed or 20 V maximum stimulus intensity was reached. Data were acquired using pClamp 10 interfaced to a Digidata 1550A data acquisition board (Molecular Devices, Sunnyvale, CA, U.S.A.) at a sampling rate of 10 kHz, low-pass filtered at 1 kHz, and high-pass filtered at 3 Hz. The magnitude of the fEPSP was determined by measuring the 20–80% slope of the rising phase and expressed as an average for each stimulation strength by treatment group.
Long-term potentiation of excitatory synaptic transmission
After obtaining fEPSPs and conducting I/O curves, baseline stimulation strength was set to 50% and slices received one stimulation pulse every 30 s. Once stable baselines were obtained for 30 min (maximum allowable fEPSP amplitude drift ± 20%), LTP was induced via theta-burst stimulation (TBS) (pulse duration = 200 μs; intra-train frequency = 100 Hz; inter-train frequency = 5 Hz; 4 trains of 4 pulses = 1 burst; 4 bursts with an inter-burst interval of 20 s). LTP induction was then followed by 60 min of single stimulation pulses every 30 s. To assess saturation of LTP, an additional 4 LTP induction stimuli (TBS), starting at 60 minutes after the initial TBS and occurring every 15 minutes, were delivered. Single stimulation pulses were then delivered for an additional 30 minutes and the potentiation of fEPSP slope (at 135 minutes) was directly compared to the potentiation achieved by a single TBS (at 60 minutes). All fEPSPs were normalized to the average slope of the last 5 fEPSPs prior to the first TBS.
Short-term plasticity of excitatory synaptic transmission
To explore changes in presynaptic short-term plasticity, the baseline stimulation strength was set to 50% of the range between the minimum and maximum detectable fEPSP (as indicated by the appearance of a population spike during I/O curves) and a paired-pulse protocol was conducted where an initial stimulus was given, followed by a second pulse with an inter-pulse interval ranging from 25–300 ms, in sequentially increasing 25 ms intervals; each paired-pulse was separated by a 30 s interval. Paired-pulse ratio (PPR) was quantified as a percentage by dividing the slope of the second pulse by the slope of the first and expressed as an average for each interval by treatment group.
Brain Slice Electrophysiology - Voltage-clamp
To evaluate the effects of kindling on excitatory and inhibitory synaptic transmission onto DG granule cells (DGCs), the whole cell patch clamp technique was used for voltage clamp recordings. An individual brain slice from dorsal hippocampus was transferred to a perfusion chamber (RC-27L, Warner Instruments, Hamden, CT, U.S.A.), held between two nylon nets, and perfused with oxygenated ACSF (2 mL/min) at room temperature. Patch electrodes (2.5–3.5 MΩ) were filled with the following internal solution (in mM): Cs Methanesulfonate (CH3CsO3S, 140.0), HEPES (10.0), EGTA (1.0), CaCl2 (0.5), Glucose (10.0), ATP (2.0), GTP (0.5), and QX-314 (5.0)–300 mOsm, pH=7.30 (CsOH). Cells were “blindly” patched by forming tight seals (4–8 GΩ) with cells located in the GC layer of the DG. All recordings were performed using a Multiclamp 700 B amplifier and the pClamp 10 (Clampex) software package interfaced to a Digidata 1440 A digitizer (Molecular Devices); recordings were digitized at 10 kHz and filtered at 1 kHz. For recording spontaneous excitatory and inhibitory post-synaptic currents (sEPSCs & sIPSCs), cells were held at −70 mV and 0 mV, respectively. Access and membrane resistance, temperature, and holding current were monitored in 30 s increments; changes in these parameters >20% rendered a cell excluded from analysis. The amplitude, interevent interval (IEI), length, and time constants for sEPSCs and sIPSCs were analyzed using Mini Analysis Program v6.0.1 (Synaptosoft, Decatur, GA, U.S.A.) with the amplitude threshold for events set to 10 pA. All measurements were obtained by averaging amplitude, IEI, length or time constant from a 30 s recording epoch for each cell after recording for 15 min at either −70 mV or 0 mV. Measurements from each neuron were then averaged by experimental group.
Statistical Analysis
All numerical values were expressed as the mean ± SEM, and all error bars on graphs represent SEM, except for the immunofluorescence data represented by box and whisker plots. Metric task, immunofluorescence, and patch-clamp data were compared using a two-tailed Student’s t-test. LTP and PPR data were compared using a repeated measure two-way ANOVA, and I/O curves were compared using repeated measure two-way ANOVA or linear regression. Brain slice physiology replicates are represented by # slices/# animals. All statistical analyses were preformed using GraphPad Prism V5.0c (GraphPad Software, San Diego, CA); *, **, or *** indicate a p-value of <0.05, <0.01, and <0.001, respectively.
Results
Corneal kindling in C57BL/6 mice
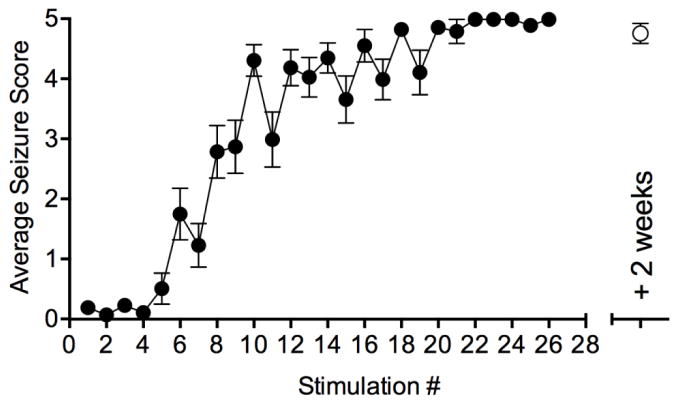
To model the development of seizures in C57BL/6 mice, we corneal kindled three cohorts of mice (n = 25) by stimulating twice daily until each mouse had achieved at least five consecutive stage five seizures (Racine, 1972; Rowley and White, 2010). Our kindling paradigm illustrates that C57BL/6 male mice were fully kindled by stimulation #26 on day 13 (Fig. 1A). We also stimulated two of these cohorts of mice (n = 15) two weeks later to ensure they were still kindled 3 d prior to beginning electrophysiology experiments. Despite using a stimulus strength (1.5 mA) half of that used for corneal kindled CF1 mice (30 mA), C57BL/6 mice developed fully kindled stage 5 seizures in response to a similar number of stimulations as seen in CF1 mice (Rowley and White, 2010).
Figure 1.
Corneal kindling curve in mice. Male C57BL/6 mice (closed circles, n = 25, 15–20 g) were stimulated twice daily and their average seizures scores determined according to the Racine scale. Mice took approximately 26 stimulations (13 days) to become fully kindled. Mice were still fully kindled after 2 weeks without stimulation (open circles, n=15).
Corneal kindled mice exhibit DG-dependent spatial memory deficits
Three days following cessation of kindling stimuli, corneal kindled and control mice (n=10 per group) were habituated and tested for spatial memory impairments in the metric task (Goodrich-Hunsaker et al., 2008; Lee et al., 2005) (Fig. 2A). Corneal kindled mice spent significantly less time exploring the objects in the spatially-novel test configuration compared to non-stimulated control mice (Fig 2B; control: 39.7 ± 5.4 sec, kindled: 21.6 ± 4.4 sec, p < 0.05, t test). Additionally, corneal kindled mice had a significantly lower average RID compared to non-kindled mice (Fig 2C; control RID: 0.40 ± 0.06, kindled RID: −0.02 ± 0.11, p < 0.01, t test). We also quantified distance traveled and found no significant differences between corneal kindled and control mice (p > 0.05, t-test). These results indicate that corneal kindled mice exhibited impaired spatial pattern processing when performing a behavioral test that relies on proper DG function.
Corneal kindled mice exhibit astrogliosis in the absence of overt hippocampal cell loss
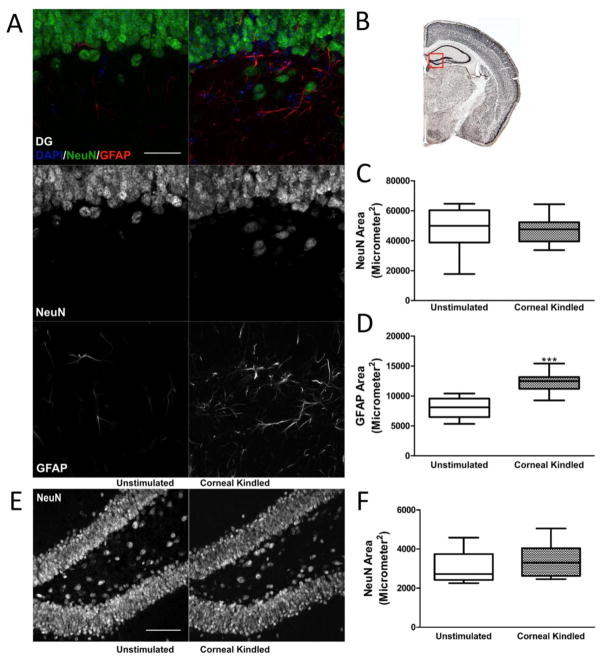
To determine whether corneal kindled mice exhibit cell loss or astrogliosis in the DG and CA1, we performed immunofluorescence staining for both neuronal nuclear antigen (NeuN), a neuron specific protein (Kim et al., 2009), and glial fibrillary acidic protein (GFAP), a marker for astrocyte activation (Anderson et al., 2014), on sections from both corneal kindled and non-kindled control mice (n=12 sections from four mice/group). Quantification of NeuN reactivity in the DG granule cell (DGC) layer revealed there was no significant difference between kindled and control animals (Fig 3A–C), whereas GFAP immunoreactivity was significantly increased (Fig 3D, GFAP area, control: 8050 ± 485.7 μm2; kindled: 12271 ± 515.8 μm2, p < 0.001, t test). Additional quantification of NeuN reactivity in the hilus revealed no difference between kindled and control animals (Fig 3E, F).
Figure 3.
Corneal kindled mice exhibit increased activation of astrocytes in the DG in the absence of overt neuronal cell loss. A, Representative images of NeuN and GFAP staining from the DG. Scale bar: 50 μm. B, The region of interest that was quantified. C, Quantification of NeuN staining in the DG revealed no significant differences (n = 12 sections from 4 mice per group), however, D, quantification of GFAP staining revealed increased expression in kindled mice (p < 0.001, t test). E, Representative images of NeuN staining used to quantify staining in the hilar region of the DG. Scale bar: 100 μm. F, Quantification of NeuN staining in the hilus revealed no significant differences between kindled and control animals.
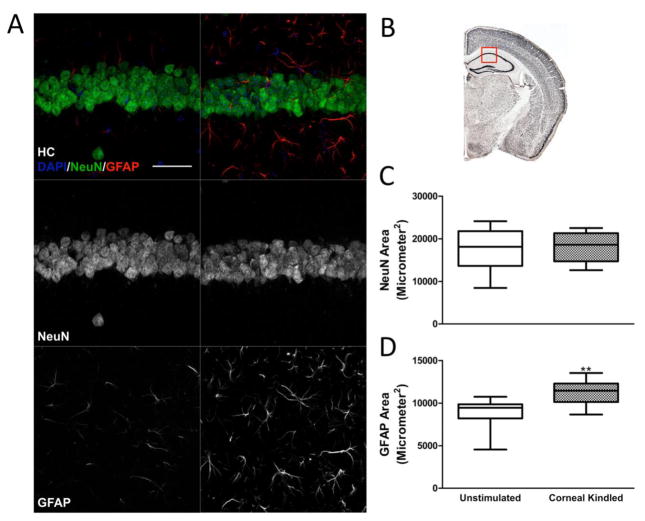
We also quantified NeuN and GFAP expression in hippocampal area CA1. Quantification of NeuN reactivity revealed there was no significant difference between kindled and control animals (Fig 4A–C), whereas GFAP immunoreactivity was significantly increased (Fig 4D, GFAP area, control: 8945 ± 497.7 μm2; kindled: 11296 ± 387.8 μm2, p < 0.01, t test). Together, these results suggest that corneal-kindled C57BL/6 mice exhibit increased astrocyte activation in the DG and CA1 in the absence of overt neuron loss, consistent with results found in corneal kindled CF1 mice (Loewen et al., 2016).
Figure 4.
Corneal kindled mice exhibit increased astrocyte activation in hippocampal area CA1 in the absence of overt neuronal cell loss. A, Representative images of NeuN and GFAP staining in CA1 (Scale bar: 50 μm) and B, the region of interest that was quantified. C, Quantification of NeuN staining in CA1 revealed no significant differences (n = 12 sections from 4 mice per group), however, D, quantification of GFAP staining revealed increased expression in kindled mice (p < 0.01, t test).
Long-term potentiation is attenuated in the dentate gyrus of corneal kindled mice
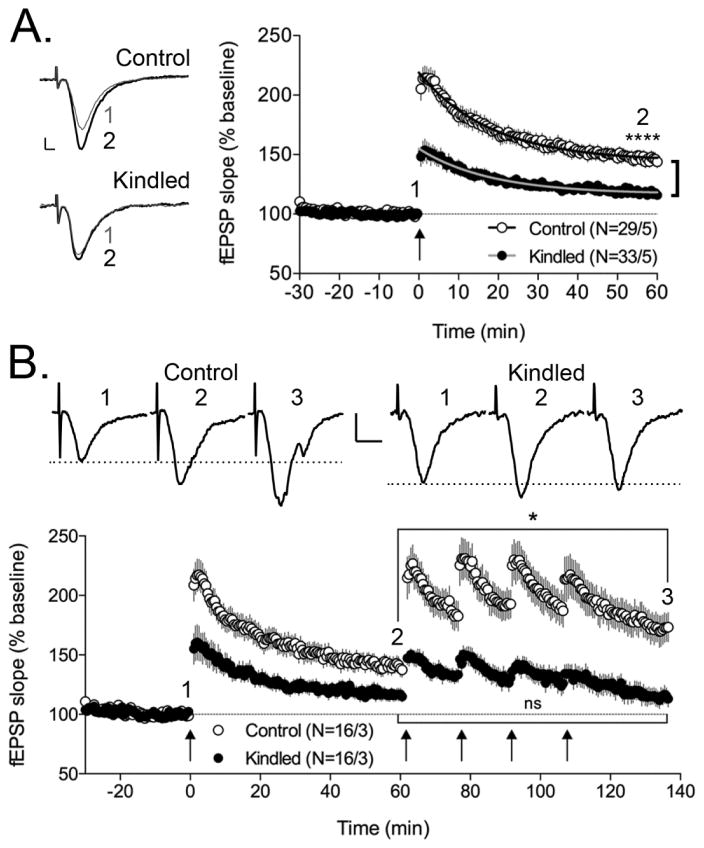
To evaluate long-term synaptic plasticity in the DG of corneal kindled mice, we examined TBS-induced LTP at the medial perforant path to DGC synapse in acute hippocampal brain slices from kindled and control mice. This LTP induction stimulus also induces an intermediate form of plasticity known as post-tetanic potentiation (PTP). PTP decay time constants (tau) were evaluated in brain slices from both control and kindled mice and were not significantly different (Control: tau = 18.4 min, 95% CI 15.5 – 22.5 min; Kindled: tau = 17.2 min, 95% CI 13.8 – 22.7 min). However, kindled mice exhibited significantly reduced LTP compared to control mice 30–60 min following TBS (Fig 5A: Control: 146.1 ± 5.4%, n=29/5; kindled: 117.6 ± 3.5%, n = 33/5 animals; F(1,60) = 23.89, p < 0.0001, two-way repeated-measures ANOVA). These results demonstrate that corneal kindled mice have impaired long-term synaptic plasticity at excitatory synaptic inputs onto DGCs.
Figure 5.
Brain slices from corneal kindled mice exhibit attenuated LTP at the perforant path – dentate granule cell synapse due to saturation. A, Representative fEPSPs from 30 s prior to (1, gray) and 60 min post-TBS (2, black) in control and kindled mice are illustrated. Scale bars 0.25 mV, 2 ms. are applicable to both control and kindled traces. fEPSP slope from control (open circles, n = 29/5) and kindled animals (closed circles, n = 33/5) conducted in the presence of 10 μM PTX is plotted as a function of time. fEPSP slopes are normalized to the average of the last 4 fEPSPs before TBS. TBS occurred at time = 0 min (indicated by an arrow). Single exponential decay curves fit to the data, used to evaluate the decay time constants for PTP, are represented by solid black (control) and gray (kindled) lines. Kindled mice exhibited significantly attenuated LTP between 30 and 60 min post-TBS (****p < 0.0001, two-way ANOVA). B, Saturation of LTP in brain slices from kindled mice is indicated by the lack of further potentiation in response to multiple TBS. Representative fEPSPs from 30 s prior to TBS (1), 60 min after the 1st TBS (2), and 135 min after the 1st TBS (3), in control and kindled mice are illustrated. Scale bars 0.25 mV, 5 ms. are applicable to both control and kindled traces. The dotted line illustrates the amplitude of the baseline fEPSPs (1). Again, fEPSP slope from control (open circles, n = 16/3) and kindled animals (closed circles, n = 16/3) conducted in the presence of 10 μM PTX is plotted as a function of time. fEPSP slopes are normalized to the average of the last 4 fEPSPs before the first TBS at time 0 min. Four additional TBS are delivered at 60 min, 75 min, 90 min, and 105 min (indicated by arrows). Control slices show significant further potentiation of fEPSPs (*, p=0.0306, unpaired t test) while slices from kindled mice do not (ns = not significant, p=0.8433, unpaired t test).
The attenuated LTP observed in kindled mice may be the result of saturation of synaptic plasticity; kindling stimuli may induce LTP-like plasticity at these, as well as other, synapses and produce a “ceiling-effect” where additional potentiation of synaptic strength is not possible. Indeed, this saturation effect in response to both experimental and pathological conditions has been observed by others (E. I. Moser et al., 1998; E. I. Moser and M. B. Moser, 1999; Schubert et al., 2005; Weng et al., 2011). To test this hypothesis, four additional LTP induction stimuli (TBS), spaced in 15 minute increments, were delivered starting 60 minutes after the first TBS (Figure 5B). As can be observed in brain slices from control mice, these additional stimuli do induce a significant further potentiation of synaptic strength (Fig 5B: Control at 60 min: 140.2 ± 7.4%, Control at 135 min: 172.6 ± 12.2%, n=16/3, p=0.0306, unpaired t test). However, in brain slices from kindled mice, these additional stimuli do not induce a significant further potentiation of synaptic strength (Fig 5B: Kindled at 60 min: 116.7 ± 3.9%, Kindled at 135 min: 115.1 ± 7.1%, n=16/3, p=0.8433, unpaired t test). These results indicate that LTP is largely saturated in corneal kindled mice such that further potentiation via experimental stimuli (TBS) is constrained.
Basal synaptic transmission and granule cell excitability are strengthened in the dentate gyrus of corneal kindled mice
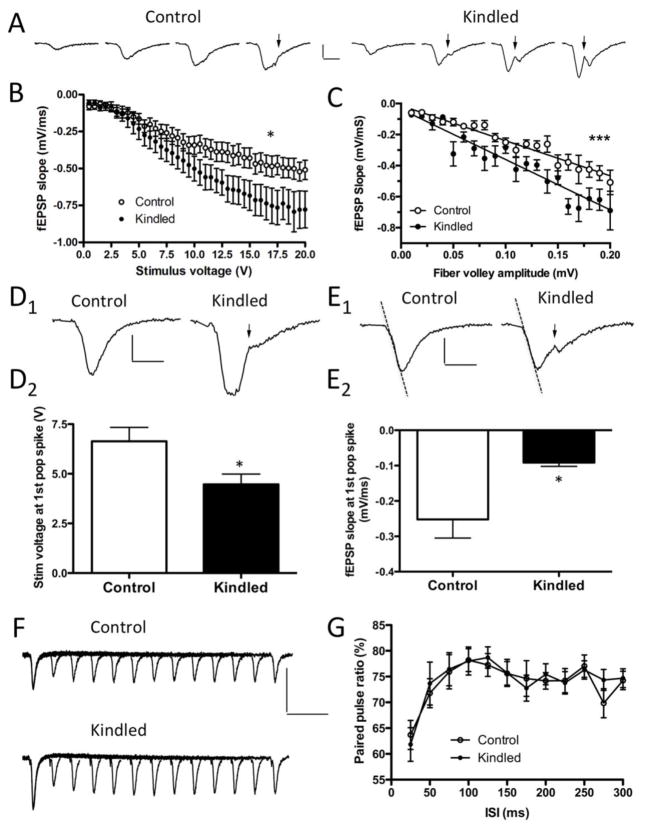
To further explore potential alterations in synaptic transmission in this model of focal limbic seizures, we examined basal synaptic transmission at the medial perforant path to DGC synapse in acute hippocampal brain slices prepared from kindled and control animals (n = 14/2 per group). fEPSPs obtained during acquisition of input/output (I/O) relationship curves revealed an increased post-synaptic response at equivalent stimulation strengths in kindled mice (Fig. 6B; stimulation strengths 10 – 20 V, F(1, 25) = 4.309, p = 0.0483, two-way repeated measures ANOVA). To further explore this relationship and rule out potential confounds associated with slice to slice stimulation variability, we plotted fEPSP response as a function of presynaptic input determined by fiber volley amplitudes. DGCs from kindled mice had a greater postsynaptic response for an equivalent presynaptic input over a range of increasing stimulation strengths (Fig 6C; F(1, 751) = 12.3206, p = 0.0005, linear regression). Moreover, DGCs from kindled mice required a lower stimulation strength (Fig. 6D; average stimulation strength at first population spike, control: 6.63 ± 0.70 V, kindled: 4.46 ± 0.05 V, p = 0.0206, t test) and a smaller post-synaptic depolarization (Fig. 6E; average slope of fEPSP at first population spike, control: −0.25 ±0.05 mV/ms, kindled: −0.09 ±0.01 mV/ms, p = 0.0077, t test) to achieve a population spike. Additionally, quantification of PPRs showed no differences in short-term synaptic plasticity at the medial perforant path to DGC synapse (Fig. 6F, 6G). These results demonstrate that while the presynaptic perforant path input to the DG is unaltered, DGCs from kindled animals exhibit a larger post-synaptic depolarization in response to equivalent presynaptic input. This result is consistent with the LTP saturation results (Fig. 5B) that suggest kindling induces a broad LTP-like potentiation of synaptic strength. Additionally, the results in Figure 6 indicate that DG synapses in kindled mice require less presynaptic input and less postsynaptic depolarization to induce a population spike. Taken together, these results suggest that DGCs from kindled mice have pathologically increased synaptic strength and altered post-synaptic electroresponsive membrane properties consistent with a hyperexcitable/seizure-susceptible state.
Figure 6.
Input/output curves along the perforant path indicate that DGCs are hyperexcitable. A, Representative fEPSPs from Input/output (I/O) relationships in both control and kindled animals. From left to right, each fEPSP is a response from a 5, 10, 15 or 20 V stimulus. Population spikes are indicated by a solid arrow. B, I/O curves by experimental group. fEPSP slopes are shown as a function of stimulus strength. Kindled mice exhibited an increased postsynaptic response compared to control animals (* p = 0.0483, two-way repeated measures ANOVA, n = 14/2 per treatment group). C, I/O curves as a function of fiber volley amplitudes. Kindled mice exhibited an increased postsynaptic response compared to control animals (*** p = 0.0005, linear regression; n = 14/2 per group). D, DGCs of kindled mice develop a population spike in response to lower stimulus strengths. D1, Representative traces from a 5.5 V stimulus. Note the population spike (solid arrow) is only present in the representative trace from the kindled animal. D2, Average stimulus voltage when a population spike was first detected during I/O relationships was lower in kindled animals (p = 0.0206, t test). E, Action potentials in DGCs of kindled mice are triggered by comparatively smaller post-synaptic depolarizations as indicated by the slope of the fEPSP when the first population spike was identified. E1 Representative fEPSPs. The dashed lines illustrate two fEPSPs with the same slope. Note that the population spike (solid arrow) is only present in the representative trace from the kindled animal. E2, Average slope of the fEPSP when a population spike was first detected during I/O relationships was lower in kindled animals (p = 0.0077, t test). F, Representative traces of fEPSPs evoked by paired-pulse stimulations of varying interstimulus intervals (ISI), and G, paired-pulse ratios (PPRs) plotted as a function of the (ISI). PPRs did not differ between kindled and control animals. Scale bars for fEPSPs: 0.5 mV, 5 ms; PPR: 0.5 mV, 50 ms.
Dentate gyrus granule cells exhibited increased membrane resistances and amplitudes of sEPSCs
Extracellular recordings of LTP and I/O relationships presented above, which represent population responses, indicate a kindling-induced saturation of LTP-like synaptic plasticity in the DG. Furthermore, analysis of pair-pulse short-term synaptic plasticity (Fig 6F, 6G) suggest that kindling does not alter release probability at the perforant path axon terminals. Taken together, these data suggest that the kindling-induced plasticity resembles classical NMDA-receptor-dependent LTP (as opposed to compound-LTP) where the potentiation of synaptic strength results almost exclusively from increased insertion of post-synaptic AMPA receptors (Blundon and Zakharenko, 2008). To test this hypothesis, we recorded the amplitudes and frequencies of spontaneous EPSCs and IPSCs in DGCs from control and kindled mice.
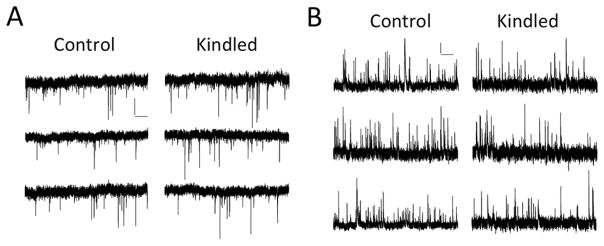
To record sEPSCs in acute hippocampal brain slices and minimize the contributions of GABAergic IPSCs, we held the membrane potential of DGCs at −70 mV. DGCs from kindled mice (n = 12 cells from 5 animals) had significantly increased membrane resistances compared to non-kindled control animals (n=14 cells from 5 animals) (Fig. 7A,B, representative traces; Table 1, control: 312.8 ± 21.5 MΩ, kindled: 431.2 ± 39.4 MΩ, p < 0.05, t test). While there were no differences in IEI (the reciprocal of frequency), the amplitudes of sEPSCs in kindled mice were significantly increased compared to control mice (Table 1; control: 15.7 ± 0.6 pA, kindled: 17.9 ± 0.7 pA, p < 0.05, t test). We also observed no difference in the decay rate or half-width of sEPSCs (Table 1). After recording sEPSCs from DGCs at −70mV, we held the cells at 0 mV to minimize the contributions of glutamatergic EPSCs and recorded sIPSCs. We found no difference in IEI, amplitude, decay rate, or half-width of sIPSCs (Table 1). These data are consistent with an increased insertion of post-synaptic AMPA receptors in DGCs from kindled mice without changes in the release of glutamate from presynaptic terminals or any obvious changes in inhibitory synaptic transmission. Furthermore, the increased amplitudes of post-synaptic AMPA-receptor mediated EPSCs without any changes in frequency of spontaneous release of glutamate in corneal kindled mice is consistent with the hypothesis that kindling induces a non-specific LTP of synaptic weights at the perforant path – DGC synapse that prevents further potentiation of specific synapses in response to salient spatial sensory stimuli.
Figure 7.
DGCs from kindled mice receive sEPSCs with increased amplitudes but did not exhibit any changes in spontaneous inhibitory synaptic transmission. A, Representative sEPSCs and B, sIPSCs from control and kindled mice when DGCs are held at −70 mV or 0 mV, respectively. Scale bars: 10 pA, 1 sec.
Table 1.
Results from voltage-clamp experiments showing the effects of corneal kindling on excitatory and inhibitory postsynaptic currents in dentate granule cells.
| Rm (MΩ) | IEI (msec) | Amp (pA) | Decay (msec) | Half-width (msec) | ||
|---|---|---|---|---|---|---|
| Excitatory (−70mV) | Control | 312.8 ± 21.5 | 902.5 ± 21.5 | 15.7 ± 0.6 | 6.5 ± 0.7 | 6.4 ± 0.7 |
| Kindled | 431.2 ± 39.4* | 1119.0 ± 166.3 | 17.9 ± 0.7* | 6.4 ± 0.7 | 6.0 ± 0.6 | |
| Inhibitory (0 mV) | Control | 127.0 ± 20.2 | 201.1 ± 23.5 | 22.0 ± 1.4 | 11.8 ± 0.5 | 10.1 ± 0.5 |
| Kindled | 153.9 ± 19.8 | 191.8 ± 28.0 | 22.7 ± 0.9 | 11.7 ± 0.7 | 10.4 ± 0.7 |
Measurements are shown as mean ± SEM. Rm = membrane resistance; IEI = interevent interval; Amp = amplitude (*p < 0.05, t-test, n = 12/5 kindled animals, and n = 14/5 control animals).
Discussion
The present study is the first to identify a distinct cognitive dysfunction, and a number of associated physiological changes likely to contribute to this aberrant behavior, in the corneal kindling mouse model of focal seizures. Specifically, this study demonstrates that corneal kindled mice exhibit spatial pattern processing deficits in a behavioral task that is known to rely heavily on the DG (Goodrich-Hunsaker et al., 2008; 2005; Hunsaker et al., 2008). In support of this finding, we also discovered impaired LTP of excitatory synaptic transmission at the perforant path to DGC synapse. Unlike the results obtained from control mice, the impaired plasticity in brain slices from kindled mice was also incapable of further potentiation in response to multiple LTP induction stimuli. This saturation of LTP was consistent with evidence suggesting that perforant path to DGC synapses in kindled mice had previously experienced LTP-like changes to their synaptic weights: increased postsynaptic depolarizations in response to equivalent presynaptic input over a range of stimulation strengths and significantly larger amplitude AMPA receptor mediated spontaneous EPSCs. Additionally, there was evidence for kindling-induced changes in the intrinsic excitability of DGCs: reduced threshold to population spikes under extracellular recording conditions and significantly increased membrane resistances observed in DGCs. We did not observe any differences in the frequency of excitatory or inhibitory spontaneous events in kindled animals, and PPRs of fEPSPs were also unchanged, suggesting that kindling-induced alterations to synaptic transmission in the DG are largely postsynaptic in DGCs only. Finally, these behavioral and physiological anomalies were observed in the absence of overt neuronal loss in the hippocampus as a whole. Thus, spatial pattern processing and memory deficits in corneal kindled mice represents a novel model of seizure-induced cognitive dysfunction associated with pathophysiological remodeling of excitatory synaptic transmission and granule cell excitability in the absence of overt cell loss.
Long-term potentiation (LTP) is a form of activity dependent plasticity that can be experimentally observed at synapses both in vivo and in vitro. Because this plasticity is generally agreed to be necessary for information storage, LTP has gained acceptance as a widely-used model of learning and memory (Cooke and Bliss, 2006; Neves et al., 2008). Implicit in this model is the expectation that the memory engram is composed of selectively potentiated synapses specific to the learned experience. Alternatively, a non-selective and broad saturation of synaptic strength, such that there is minimal availability of non-potentiated synapses to strengthen in response to salient experiences, would be expected to impair learning and memory. Indeed, this has been experimentally observed by using repeated tetanic stimulation of afferent pathways prior to training in a number of behavioral learning and memory tasks (C. A. Castro et al., 1989; E. I. Moser et al., 1998; E. I. Moser and M. B. Moser, 1999; Otnaess et al., 1999). Furthermore, saturation of LTP has also been observed in a model of Rett syndrome (a genetic postnatal neurological disorder associated with a high incidence of seizures and cognitive dysfunction, (Weng et al., 2011)) and in the rat amygdala-kindling model (Schubert et al., 2005). As with many other kindling models, amygdala kindling is often associated with learning and memory deficits (Hannesson and Corcoran, 2000). In this report, we demonstrate that a DG-dependent behavior is impaired by corneal kindling and that this impairment is correlated with saturated, and subsequently attenuated, LTP in the DG. Importantly, our observation of impaired LTP at the perforant path-DGC synapse parallels findings in tissue from humans with epilepsy (Beck et al., 2000). Furthermore, it has been shown that LTP in the DG is necessary for the performance of pattern separation tasks in mice (McHugh et al., 2007). Therefore, while we cannot rule other pathophysiological effects such as spontaneous interictal excitability (Holmes, 2013; Shatskikh et al., 2006) or altered synaptic weights at other synapses in the hippocampus and beyond, the saturation of LTP that we observed in the DG of corneal kindled mice is likely to substantially contribute to their spatial pattern learning and memory deficits.
Numerous pathological changes may have contributed to the saturated, and subsequently attenuated, LTP in the DG of corneal kindled mice. For instance, the development of seizures in response to initially subconvulsive stimulations during kindling occurs via synaptic and cellular mechanisms similar to those of LTP (Albensi et al., 2007). Therefore, corneal kindling may have saturated the capability for further synaptic potentiation at the perforant-DGC synapse via typical postsynaptic changes known to commonly occur as a result of NMDA receptor-dependent LTP (Huang et al., 1992; Meador, 2007). For instance, trafficking of AMPA receptors to the postsynaptic membrane has long been appreciated as a prominent mechanism underlying LTP at excitatory synapses (Herring and Nicoll, 2016; Kessels and Malinow, 2009; Kneussel and Hausrat, 2016; Malenka, 2003; Malinow, 2003). Although not universally true, especially in some kindling models where AMPA receptor expression tends to not change or can even show decreased expression patterns, increased expression of AMPA receptors is also found in the hippocampus of a number of animal models of epilepsy and human TLE tissues (Behr et al., 2000; Cincotta et al., 1991; Ekonomou et al., 2001; Gitaí et al., 2010; Hosford et al., 1991; Lopes et al., 2013; Mathern et al., 1997; 1998; Schroeder et al., 1998). The data presented in this report suggests that corneal kindling of C57Bl/6 mice leads to an increased functional expression of AMPA receptors at the perforant path – DGC synapse; specifically, the strengthening of basal synaptic transmission and increased sEPSC amplitudes documented in Figures 6–7 and Table 1 are consistent with the increased expression, insertion, and/or phosphorylation of AMPA receptors on DGCs. This increased functional expression of AMPA receptors is likely the result of the broad LTP-like plasticity induced by kindling stimulations. Further, the associated cognitive dysfunction observed suggests that these plastic increases in AMPA receptor expression are likely to have occurred at the majority of perforant path – DGC synapses since weaker tetanic stimulations that do not saturate synaptic plasticity at nearly all synapses in the tetanized terminal field do not impair hippocampal-dependent spatial learning (E. I. Moser and M. B. Moser, 1999). The resulting increase in synaptic strength would be expected to prevent further increases in synaptic strength through a ceiling effect and create a hyperexcitable substrate that facilitates the expression of seizures.
Synaptic plasticity is not the only type of experience dependent plasticity that is known to occur in the CNS; another common form of learning and memory related plasticity is known as intrinsic plasticity: the ability of neurons to alter their intrinsic excitability in response to experience (Daoudal and Debanne, 2003; Sehgal et al., 2013). Additionally, intrinsic plasticity can mediate adaptive or maladaptive changes in CNS function in response to disease and injury (Beck and Yaari, 2008). Indeed, plasticity of excitability has been observed in hippocampal tissues from epilepsy patients and various animal models of epilepsy (Camp, 2012; Graef and Godwin, 2010; Jung et al., 2007; Stegen et al., 2012). In accordance with these previous studies, we present data in this report consistent with maladaptive plasticity of intrinsic excitability in DGCs from corneal kindled mice. Specifically, population spike threshold analysis (Figure 6D and 6E) revealed that population spikes in DGCs from corneal kindled mice occur in response to significantly lower stimulation strengths and smaller fEPSPs. While the observation that lower stimulation strengths result in population spikes may simply be the result of a larger postsynaptic AMPA receptor response (as previous discussed), altered synaptic plasticity alone is insufficient to explain the altered population spike threshold data presented in Figure 6E; under the conditions of this analysis, synaptic strength is normalized and population spikes were observed in response to significantly less synaptic input in corneal kindled mice DGCs. Instead, these results are consistent with the significantly increased membrane resistances measured in DGCs from corneal kindled mice.
Although the specific ion channel alterations responsible for changes in DGC resistances remain to be discovered, it is clear from the data presented here that maladaptive changes in intrinsic excitability occur in DGCs from corneal kindled mice. These types of maladaptive plastic changes in ion channels have been referred to as “acquired channelopathies” (Wolfart and Laker, 2015). In addition to the saturation of LTP described above, DGC hyperexcitability in corneal kindled mice could have contributed to impaired performance of pattern separation during the metric task. The DG is the anatomical entranceway into the hippocampus and is critical for pattern separation, defined as the ability to discriminate similar synaptic inputs and transform them into dissimilar outputs (Amaral et al., 2007; B. Schmidt et al., 2012). DGCs accomplish this function, in part, by maintaining a hyperpolarized resting membrane potential and low firing probability – and thus, gate information flow into the hippocampus (Coulter and Carlson, 2007; Hsu, 2007). Moreover, pathological increases in DG excitability have been associated with impaired performance during pattern separation tasks in rodents (Jinde et al., 2012; Park et al., 2015) and humans alike (Bakker et al., 2012); attenuating aberrantly increased DG excitability restored pattern separation ability in these studies. These findings support the idea that DGC hyperexcitability in corneal kindled mice could contribute to the impairment of spatial pattern processing during the metric task reported here.
In addition to the physiological changes directly examined in this report and discussed above, other kindling-induced changes in hippocampal function may have contributed to the cognitive dysfunction observed in these mice. For instance, we cannot rule out the possibility that changes in adult hippocampal neurogenesis may have contributed to DG-mediated memory deficits observed in corneal kindled C57Bl/6 mice as attenuating aberrantly increased hippocampal neurogenesis has been shown to prevent cognitive impairment in animal models of epilepsy (Botterill et al., 2015; Fournier et al., 2013; Jessberger et al., 2007). That being said, no changes in neurogenesis were observed in the DG of corneal kindled CF1 mice (Loewen et al., 2016). Additionally, we cannot rule out the possibility that increased activation of astrocytes in the DG, observed in the form of increased GFAP immunoreactivity in this study, may have also contributed to DGC hyperexcitability and cognitive dysfunction; activation of astrocytes has been shown to be sufficient to alter glutamate uptake by astrocytes and cause seizures in mice (Robel et al., 2015; Takahashi et al., 2010). However, data reported here does support the exclusion of changes in presynaptic release probability at both glutamatergic and GABAergic synapses onto DGCs as consequences of corneal kindling. This assertion is supported by a lack of significant alterations in short-term synaptic plasticity (paired-pulse depression of excitatory synaptic transmission over a range of interpulse intervals) and spontaneous EPSC and IPSC frequencies. Additional evidence supporting a lack of corneal kindling-induced changes in inhibitory synaptic transmission are data suggesting no changes in IPSC amplitudes or overt cell loss in the hilus.
The effects of corneal kindling on DG-mediated cognitive function and associated changes in synaptic and intrinsic plasticity described in this report add to the growing body of literature describing the varying effects of multiple models of acquired epilepsy on neuronal hyperexcitability and cognitive dysfunction. It is noteworthy that these models vary substantially in a number of important ways. Studies that employ chemoconvulsants or direct electrical stimulation to produce status epilepticus and subsequent spontaneous seizures in rodents report increased excitability in the hippocampus that is thought to facilitate seizure activity and contribute to memory dysfunction but is often attributed to gross anatomical changes such as neuronal loss – primarily the loss of inhibitory interneurons that synapse onto DGCs, or the loss of excitatory mossy cells within the hilus of the DG that drive inhibition, as well as aberrant neuronal sprouting and reorganization (Chauvière et al., 2009; Cossart et al., 2001; Harvey and Sloviter, 2005; Jinde et al., 2012; Sloviter et al., 2003; 2006; Sun et al., 2007). In contrast, electrical kindling models also exhibit hippocampal-dependent memory impairment and neuronal hyperexcitability, but usually in the absence of overt hippocampal cell loss (see review (Hannesson and Corcoran, 2000)), which suggests more subtle changes in hippocampal physiology underlie these deficits (although there are exceptions to this rule: see (Cavazos et al., 1994; Cavazos and Sutula, 1990; Sutula, 1990)). Our results parallel the findings in electrical kindling models that report neuronal hyperexcitability, impaired long-term synaptic plasticity, and hippocampal-dependent spatial memory deficits in the absence of overt hippocampal damage (Brandt et al., 2004; Haas et al., 2001; Hannesson et al., 2001; Leung and Shen, 2006; Morimoto et al., 2004; Singh et al., 2013; Tooyama et al., 2002). However, it is noteworthy that these other electrical kindling models are constrained by the time- and labor-intensive nature of invasive implantation surgeries that cause a proinflammatory response in the brain. Our results were found in a non-invasive and cost-effective corneal kindling model of focal seizures that is not constrained by severe hippocampal damage or laborious implantation surgeries. Accordingly, this model is suitable for the screening and development of new therapies for epilepsy-related memory deficits. This point is significant since developing treatments for memory impairments in epilepsy may be hampered in animal models that exhibit overt hippocampal damage. Indeed, a growing body of literature suggests cognitive dysfunction in epilepsy may result from pathophysiology independent of cell death or the seizures themselves (Bender et al., 2013; Holmes, 2016). Thus, subtle changes in hippocampal physiology may underlie memory dysfunction in many epilepsy patients, and this patient population in particular might be expected to benefit most from pharmacological therapies aimed at restoring normal cognitive function.
Finally, the path forward regarding the development of mechanistic, or even symptomatic, pharmacological treatments for cognitive dysfunction in epilepsy is obscured by the double-edged sword of exacerbating cognitive dysfunction with anticonvulsants or lowering seizure thresholds with existing cognition enhancing compounds. Specifically, one potential source of cognitive impairment in TLE patients whose seizures are well controlled by medication is side effects from the medications themselves (Brooks-Kayal et al., 2013; West et al., 2014). However, there is proof-of-concept clinical data suggesting that it is possible to achieve both a procognitive and simultaneous anticonvulsant effect in patients with Alzheimer’s disease with comorbid seizures; in this study, Keppra (levetiracetam) significantly improved cognitive function and simultaneously reduced seizure burden (Cumbo and Ligori, 2010). On the other hand, symptomatically addressing cognitive dysfunction associated with epilepsy by prescribing cognition-enhancing compounds such as the acetylcholinesterase inhibitors Aricept (Donepezil) and Exelon (Rivastigmine) is not recommended since both of these drugs were listed in the top 10 drugs most frequently associated with convulsive Adverse Drug Reactions (ADRs) according to the WHO ADR database (Kumlien and Lundberg, 2010). Furthermore, Donepezil has been directly tested for its ability to improve memory in epilepsy patients; in one trial, it had no effect on cognition or seizures presumably due to an inadequate dose (Hamberger et al., 2007), while in another trial epilepsy patients on Donepezil tended to have increased seizure frequency and two patients had increased tonic–clonic seizures (Fisher et al., 2001). These mixed and/or negative clinical results further highlight the need for new models of epilepsy with comorbid cognitive dysfunction that can be readily used in unbiased drug screening efforts. Additionally, these models may be used to better understand the underlying pathophysiology associated with cognitive dysfunction in epilepsy with the intention of using this data to direct the development of targeted treatments. The data presented here supports the use of the corneal kindling model of focal seizures in C57Bl/6 mice in advancing these goals and providing a much-needed platform upon which promising new therapies for seizures and cognitive comorbidities will be identified.
Highlights.
Corneal kindled mice exhibit dentate gyrus-associated spatial memory impairment.
This spatial memory impairment occurs in the absence of overt neuron loss.
Saturated and impaired LTP in the dentate gyrus correlates with memory impairment.
Additional intrinsic plasticity changes are found in granule cells of kindled mice.
These mice may be used to screen drugs to treat cognition impairment in epilepsy.
Acknowledgments
We would like to thank the Anticonvulsant Drug Development Program at the University of Utah for scientific, technical, and logistical support. In particular, we’d like to thank Mr. Isaac Henson for assistance with the production of corneal kindled mice, and both Mr. Jerry Saunders and Ms. Peggy Billingsley for assistance with all aspects of acute brain slice electrophysiology experiments. Finally, we’d like to thank Dr. Raymond Kesner and Ms. Genevieve Smith for their helpful suggestions regarding the metric task.
Funding: This work was supported by contract #HHSN271201100029C (HSW), contract #HHSN271201600048C (KSW), and summer student fellowships from The Waterford School, Sandy, UT 84093-2902, U.S.A. and Juan Diego Catholic High School, Draper, UT, 84020-9035, U.S.A.
Abbreviations
- ACSF
artificial cerebral spinal fluid
- DG
dentate gyrus
- DGC
dentate granule cell
- fEPSP
field excitatory post-synaptic potential
- GFAP
glial fibrillary acidic protein
- HS
hippocampal sclerosis
- IEI
interevent interval
- LTP
long-term potentiation
- NeuN
neuronal nuclear antigen
- PBS
phosphate-buffer saline
- PFA
paraformaldehyde
- pp
perforant path
- PPR
paired-pulse ratio
- PTP
post-tetanic potentiation
- PTX
picrotoxin
- RID
recognition index
- sEPSC
spontaneous excitatory post-synaptic current
- sIPSC
spontaneous inhibitory post-synaptic currents
- TBS
theta-burst stimulation
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikiä M, Kälviäinen R, Riekkinen PJ. Verbal learning and memory in newly diagnosed partial epilepsy. Epilepsy Res. 1995;22:157–164. doi: 10.1016/0920-1211(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Äikiä M, Salmenperä T, Partanen K, Kälviäinen R. Verbal Memory in Newly Diagnosed Patients and Patients with Chronic Left Temporal Lobe Epilepsy. Epilepsy Behav. 2001;2:20–27. doi: 10.1006/ebeh.2000.0140. [DOI] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Haliski ML, Vanegas F, Mau MJ, Underwood TK, White HS. Acute cognitive impact of antiseizure drugs in naive rodents and corneal-kindled mice. Epilepsia. 2016;57:1386–1397. doi: 10.1111/epi.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Goussakov IV, Lie A, Helmstaedter C, Elger CE. Synaptic plasticity in the human dentate gyrus. J Neurosci. 2000;20:7080–7086. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Behr J, Heinemann U, Mody I. Glutamate receptor activation in the kindled dentate gyrus. Epilepsia. 2000;41(Suppl 6):S100–3. doi: 10.1111/j.1528-1157.2000.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Bender AC, Natola H, Ndong C, Holmes GL, Scott RC, Lenck-Santini PP. Focal Scn1a knockdown induces cognitive impairment without seizures. Neurobiology of Disease. 2013;54:297–307. doi: 10.1016/j.nbd.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon JA, Zakharenko SS. Dissecting the components of long-term potentiation. The Neuroscientist. 2008;14:598–608. doi: 10.1177/1073858408320643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botterill JJ, Brymer KJ, Caruncho HJ, Kalynchuk LE. Aberrant hippocampal neurogenesis after limbic kindling: Relationship to BDNF and hippocampal-dependent memory. Epilepsy Behav. 2015;47:83–92. doi: 10.1016/j.yebeh.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Brandt C, Ebert U, Löscher W. Epilepsy induced by extended amygdala-kindling in rats: lack of clear association between development of spontaneous seizures and neuronal damage. Epilepsy Res. 2004;62:135–156. doi: 10.1016/j.eplepsyres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, Kanner AM, O’Brien TJ, Whittemore VH, Winawer MR, Patel M, Scharfman HE. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54(Suppl 4):44–60. doi: 10.1111/epi.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp AJ. Intrinsic neuronal excitability: a role in homeostasis and disease. Front Neurol. 2012;3:50. doi: 10.3389/fneur.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro CA, Silbert LH, McNaughton BL, Barnes CA. Recovery of spatial learning deficits after decay of electrically induced synaptic enhancement in the hippocampus. Nature. 1989;342:545–548. doi: 10.1038/342545a0. [DOI] [PubMed] [Google Scholar]
- Castro LH, Silva LCAM, Adda CC, Banaskiwitz NHC, Xavier AB, Jorge CL, Valerio RM, Nitrini R. Low prevalence but high specificity of material-specific memory impairment in epilepsy associated with hippocampal sclerosis. Epilepsia. 2013;54:1735–1742. doi: 10.1111/epi.12343. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- Chauvière L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. Journal of Neuroscience. 2009;29:5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wu J, Hua D, Shu K, Wang JZ, Li L, Lei T. The c-Jun N-terminal kinase inhibitor SP600125 is neuroprotective in amygdala kindled rats. Brain Res. 2010;1357:104–114. doi: 10.1016/j.brainres.2010.07.082. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Young NA, Beart PM. Unilateral up-regulation of glutamate receptors in limbic regions of amygdaloid-kindled rats. Exp Brain Res. 1991;85:650–658. doi: 10.1007/BF00231751. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 2010;17:461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Lee TS, Spencer DD. Histopathology of Human Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, DelgadoEscueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. New York: Oxford University Press; 2012. p. 1199. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Smith AL, Angelatou F. Changes in AMPA receptor binding and subunit messenger RNA expression in hippocampus and cortex in the pentylenetetrazole-induced “kindling” model of epilepsy. Brain Res Mol Brain Res. 2001;95:27–35. doi: 10.1016/s0169-328x(01)00230-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Bortz JJ, Blum DE, Duncan B, Burke H. A pilot study of donepezil for memory problems in epilepsy. Epilepsy Behav. 2001;2:330–334. doi: 10.1006/ebeh.2001.0221. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Cross JH, D’Souza C, French JA, Haut SR, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, Scheffer IE, Schulze-Bonhage A, Somerville E, Sperling M, Yacubian EM, Zuberi SM. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–542. doi: 10.1111/epi.13671. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Botterill JJ, Marks WN, Guskjolen AJ, Kalynchuk LE. Impaired recruitment of seizure-generated neurons into functional memory networks of the adult dentate gyrus following long-term amygdala kindling. Exp Neurol. 2013;244:96–104. doi: 10.1016/j.expneurol.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Pellegrini-Giampietro DE, Sperber EF, Bennett MV, Moshé SL, Zukin RS. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci. 1994;14:2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Avanzini G. Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurol Scand. 2000;101:295–300. doi: 10.1034/j.1600-0404.2000.90257a.x. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Avanzini G. Learning and memory impairment in patients with temporal lobe epilepsy: relation to the presence, type, and location of brain lesion. Epilepsia. 1999;40:904–911. doi: 10.1111/j.1528-1157.1999.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Gitaí DLG, Martinelli HN, Valente V, Pereira MGAG, Oliveira JAC, Elias CF, Bittencourt JC, Leite JP, Costa-Neto CM, Garcia-Cairasco N, Paçó-Larson ML. Increased expression of GluR2-flip in the hippocampus of the Wistar audiogenic rat strain after acute and kindled seizures. Hippocampus. 2010;20:125–133. doi: 10.1002/hipo.20590. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioral Neuroscience. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Graef JD, Godwin DW. Intrinsic plasticity in acquired epilepsy: too much of a good thing? Neuroscientist. 2010;16:487–495. doi: 10.1177/1073858409358776. [DOI] [PubMed] [Google Scholar]
- Gröticke I, Hoffmann K, Löscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol. 2008;213:71–83. doi: 10.1016/j.expneurol.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshé SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Palmese CA, Scarmeas N, Weintraub D, Choi H, Hirsch LJ. A randomized, double-blind, placebo-controlled trial of donepezil to improve memory in epilepsy. Epilepsia. 2007;48:1283–1291. doi: 10.1111/j.1528-1167.2007.01114.x. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Corcoran ME. The mnemonic effects of kindling. Neurosci Biobehav Rev. 2000;24:725–751. doi: 10.1016/s0149-7634(00)00033-6. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland J, Pollock M, Mohapel P, Wallace AE, Corcoran ME. Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J Neurosci. 2001;21:4443–4450. doi: 10.1523/JNEUROSCI.21-12-04443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol. 2016;78:351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Benn EKT, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology. 2011;76:23–27. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL. Effect of Seizures on the Developing Brain and Cognition. Semin Pediatr Neurol. 2016;23:120–126. doi: 10.1016/j.spen.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL. EEG abnormalities as a biomarker for cognitive comorbidities in pharmacoresistant epilepsy. Epilepsia. 2013;54(Suppl 2):60–62. doi: 10.1111/epi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford DA, Crain BJ, Cao Z, Bonhaus DW, Friedman AH, Okazaki MM, Nadler JV, McNamara JO. Increased AMPA-sensitive quisqualate receptor binding and reduced NMDA receptor binding in epileptic human hippocampus. J Neurosci. 1991;11:428–434. doi: 10.1523/JNEUROSCI.11-02-00428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabok M, Sherman EMS, Bello-Espinosa L, Hader W. Memory and health-related quality of life in severe pediatric epilepsy. Pediatrics. 2013;131:e525–32. doi: 10.1542/peds.2012-1428. [DOI] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Jacobs MP, Leblanc GG, Brooks-Kayal A, Jensen FE, Lowenstein DH, Noebels JL, Spencer DD, Swann JW. Curing epilepsy: progress and future directions. Epilepsy Behav. 2009;14:438–445. doi: 10.1016/j.yebeh.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD, Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. Journal of Neuroscience. 2007;27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde S, Zsiros V, Jiang Z, Nakao K, Pickel J, Kohno K, Belforte JE, Nakazawa K. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012;76:1189–1200. doi: 10.1016/j.neuron.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. Journal of Neuroscience. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MS, Jacobs MP, Lowenstein DH NINDS Epilepsy Benchmark Stewards. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50:579–582. doi: 10.1111/j.1528-1167.2008.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Hausrat TJ. Postsynaptic Neurotransmitter Receptor Reserve Pools for Synaptic Potentiation. Trends Neurosci. 2016;39:170–182. doi: 10.1016/j.tins.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19:69–73. doi: 10.1016/j.seizure.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Lah S, Mohamed A, Thayer Z, Miller L, Diamond K. Accelerated long-term forgetting of verbal information in unilateral temporal lobe epilepsy: Is it related to structural hippocampal abnormalities and/or incomplete learning? J Clin Exp Neuropsychol. 2014;36:158–169. doi: 10.1080/13803395.2013.874405. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behavioral Neuroscience. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B. Hippocampal CA1 kindling but not long-term potentiation disrupts spatial memory performance. Learn Mem. 2006;13:18–26. doi: 10.1101/lm.66106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, Wilcox KS. Neuronal Injury, Gliosis, and Glial Proliferation in Two Models of Temporal Lobe Epilepsy. Journal of Neuropathology and Experimental Neurology. 2016;75:366–378. doi: 10.1093/jnen/nlw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau P, Strube E, Broustet D, Battellochi S, Gomeni C, Morselli PL. Learning impairment in epileptic patients. Epilepsia. 1983;24:183–192. doi: 10.1111/j.1528-1157.1983.tb04878.x. [DOI] [PubMed] [Google Scholar]
- Long C, Fureman B, Dingledine R. 2014 Epilepsy Benchmarks: Progress and Opportunities. Epilepsy Curr. 2016;16:179–181. doi: 10.5698/1535-7511-16.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MW, Soares FMS, de Mello N, Nunes JC, Cajado AG, de Brito D, de Cordova FM, da Cunha RMS, Walz R, Leal RB. Time-dependent modulation of AMPA receptor phosphorylation and mRNA expression of NMDA receptors and glial glutamate transporters in the rat hippocampus and cerebral cortex in a pilocarpine model of epilepsy. Exp Brain Res. 2013;226:153–163. doi: 10.1007/s00221-013-3421-8. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond, B, Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Guillery-Girard B, Clochon P, Bulteau C, Hertz-Pannier L, Chiron C, Eustache F, Jambaqué I. Associative episodic memory and recollective processes in childhood temporal lobe epilepsy. Epilepsy Behav. 2015;44:86–89. doi: 10.1016/j.yebeh.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Bertram EH, Babb TL, Pretorius JK, Kuhlman PA, Spradlin S, Mendoza D. In contrast to kindled seizures, the frequency of spontaneous epilepsy in the limbic status model correlates with greater aberrant fascia dentata excitatory and inhibitory axon sprouting, and increased staining for N-methyl-D-aspartate, AMPA and GABA(A) receptors. Neuroscience. 1997;77:1003–1019. doi: 10.1016/s0306-4522(96)00516-7. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Leite JP, Kornblum HI, Mendoza D, Lozada A, Bertram EH. Hippocampal AMPA and NMDA mRNA levels and subunit immunoreactivity in human temporal lobe epilepsy patients and a rodent model of chronic mesial limbic epilepsy. Epilepsy Res. 1998;32:154–171. doi: 10.1016/s0920-1211(98)00048-5. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Meador KJ. The basic science of memory as it applies to epilepsy. Epilepsia. 2007;48(Suppl 9):23–25. doi: 10.1111/j.1528-1167.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. Is learning blocked by saturation of synaptic weights in the hippocampus? Neurosci Biobehav Rev. 1999;23:661–672. doi: 10.1016/s0149-7634(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Otnaess MK, Brun VH, Moser MB, Moser EI. Pretraining prevents spatial learning impairment after saturation of hippocampal long-term potentiation. Journal of Neuroscience. 1999;19:RC49. doi: 10.1523/JNEUROSCI.19-24-j0007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA. Experience-Dependent Regulation of Dentate Gyrus Excitability by Adult-Born Granule Cells. Journal of Neuroscience. 2015;35:11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H, Löscher W. Corneal kindling in mice: behavioral and pharmacological differences to conventional kindling. Epilepsy Res. 1999;37:109–120. doi: 10.1016/s0920-1211(99)00062-5. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, Sutor B, Sontheimer H. Reactive astrogliosis causes the development of spontaneous seizures. Journal of Neuroscience. 2015;35:3330–3345. doi: 10.1523/JNEUROSCI.1574-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy Res. 2010;92:163–169. doi: 10.1016/j.eplepsyres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2012;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Schmidt CSM, Lassonde M, Gagnon L, Sauerwein CH, Carmant L, Major P, Paquette N, Lepore F, Gallagher A. Neuropsychological functioning in children with temporal lobe epilepsy and hippocampal atrophy without mesial temporal sclerosis: a distinct clinical entity? Epilepsy Behav. 2015;44:17–22. doi: 10.1016/j.yebeh.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Becker A, Hoellt V. Sensitivity and density of glutamate receptor subtypes in the hippocampal formation are altered in pentylenetetrazole-kindled rats. Exp Brain Res. 1998;120:527–530. doi: 10.1007/s002210050427. [DOI] [PubMed] [Google Scholar]
- Schubert M, Siegmund H, Pape HC, Albrecht D. Kindling-induced changes in plasticity of the rat amygdala and hippocampus. Learn Mem. 2005;12:520–526. doi: 10.1101/lm.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR. Learning to learn - intrinsic plasticity as a metaplasticity mechanism for memory formation. NEUROBIOLOGY OF LEARNING AND MEMORY. 2013;105:186–199. doi: 10.1016/j.nlm.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatskikh TN, Raghavendra M, Zhao Q, Cui Z, Holmes GL. Electrical induction of spikes in the hippocampus impairs recognition capacity and spatial memory in rats. Epilepsy & Behavior. 2006;9:549–556. doi: 10.1016/j.yebeh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Singh SP, He X, McNamara JO, Danzer SC. Morphological changes among hippocampal dentate granule cells exposed to early kindling-epileptogenesis. Hippocampus. 2013;23:1309–1320. doi: 10.1002/hipo.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen M, Kirchheim F, Hanuschkin A, Staszewski O, Veh RW, Wolfart J. Adaptive intrinsic plasticity in human dentate gyrus granule cells during temporal lobe epilepsy. Cereb Cortex. 2012;22:2087–2101. doi: 10.1093/cercor/bhr294. [DOI] [PubMed] [Google Scholar]
- Stopps M, Allen N, Barrett R, Choudhury HI, Jarolimek W, Johnson M, Kuenzi FM, Maubach KA, Nagano N, Seabrook GR. Design and application of a novel brain slice system that permits independent electrophysiological recordings from multiple slices. J Neurosci Methods. 2004;132:137–148. doi: 10.1016/j.jneumeth.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP. Experimental models of temporal lobe epilepsy: new insights from the study of kindling and synaptic reorganization. Epilepsia. 1990;31(Suppl 3):S45–54. doi: 10.1111/j.1528-1157.1990.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Takahashi DK, Vargas JR, Wilcox KS. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiology of Disease. 2010;40:573–585. doi: 10.1016/j.nbd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooyama I, Bellier JP, Park M, Minnasch P, Uemura S, Hisano T, Iwami M, Aimi Y, Yasuhara O, Kimura H. Morphologic study of neuronal death, glial activation, and progenitor cell division in the hippocampus of rat models of epilepsy. Epilepsia. 2002;43(Suppl 9):39–43. doi: 10.1046/j.1528-1157.43.s.9.10.x. [DOI] [PubMed] [Google Scholar]
- Weng SM, Mcleod F, Bailey MES, Cobb SR. Synaptic plasticity deficits in an experimental model of rett syndrome: long-term potentiation saturation and its pharmacological reversal. Neuroscience. 2011;180:314–321. doi: 10.1016/j.neuroscience.2011.01.061. [DOI] [PubMed] [Google Scholar]
- West PJ, Saunders GW, Remigio GJ, Wilcox KS, White HS. Antiseizure drugs differentially modulate θ-burst induced long-term potentiation in C57BL/6 mice. Epilepsia. 2014;55:214–223. doi: 10.1111/epi.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Laker D. Homeostasis or channelopathy? Acquired cell type-specific ion channel changes in temporal lobe epilepsy and their antiepileptic potential. Front Physiol. 2015;6:168. doi: 10.3389/fphys.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]