Abstract
Objective: This review evaluates costs and benefits associated with acquiring, implementing, and operating clinical decision support systems (CDSSs) to prevent cardiovascular disease (CVD).
Materials and Methods: Methods developed for the Community Guide were used to review CDSS literature covering the period from January 1976 to October 2015. Twenty-one studies were identified for inclusion.
Results: It was difficult to draw a meaningful estimate for the cost of acquiring and operating CDSSs to prevent CVD from the available studies (n = 12) due to considerable heterogeneity. Several studies (n = 11) indicated that health care costs were averted by using CDSSs but many were partial assessments that did not consider all components of health care. Four cost-benefit studies reached conflicting conclusions about the net benefit of CDSSs based on incomplete assessments of costs and benefits. Three cost-utility studies indicated inconsistent conclusions regarding cost-effectiveness based on a conservative $50,000 threshold.
Discussion: Intervention costs were not negligible, but specific estimates were not derived because of the heterogeneity of implementation and reporting metrics. Expected economic benefits from averted health care cost could not be determined with confidence because many studies did not fully account for all components of health care.
Conclusion: We were unable to conclude whether CDSSs for CVD prevention is either cost-beneficial or cost-effective. Several evidence gaps are identified, most prominently a lack of information about major drivers of cost and benefit, a lack of standard metrics for the cost of CDSSs, and not allowing for useful life of a CDSS that generally extends beyond one accounting period.
Keywords: Decision support systems, clinical; economics; economics, medical
INTRODUCTION
The 2016 statistical update from the American Heart Association estimated that the annual cost of cardiovascular disease (CVD) and stroke in 2011–2012 was $193.1 billion in medical care and about $123 billion in lost productivity from premature death.1 A substantial part of this burden is avoidable by preventing and controlling major risk factors for CVD, including hypertension, hypercholesterolemia, and diabetes.1 Clinical decision support systems (CDSSs) are computer-based tools used to assist health providers in preventing and controlling these risk factors. The Community Preventive Services Task Force recently recommended CDSSs for CVD prevention2 based on evidence from a systematic review conducted for the Community Guide.3 The evidence showed that CDSSs improved screening for CVD risk factors and improved practices for CVD-related preventive care services, clinical tests, and treatments. The objective of the present study is to assess the economic value of CDSS interventions for CVD prevention based on a systematic review of the literature.
METHODS AND MATERIALS
A full description of general methods for Community Guide systematic economic reviews is available online.4 Briefly, Community Guide systematic economic reviews have the multiple objectives of providing evidence-based estimates of what it costs to implement an intervention, calculating the expected benefits from averted health care costs and worksite productivity losses due to reduced morbidity and mortality, and making a judgment of economic value based on estimated cost-benefit or cost-effectiveness. Estimates of economic outcomes vary considerably across evaluation studies. Therefore, Community Guide economic review methods attempt to account for the major elements that drive intervention costs and benefits identified a priori based on information gained from published literature and subject matter experts.
Following the general methods, a systematic review team was constituted to evaluate CDSSs for CVD prevention, including subject matter experts on CVD and CDSSs from various agencies, organizations, and academic institutions, together with expert systematic reviewers from the Community Guide branch at CDC. The team worked under the oversight of the Task Force.
CDSSs for CVD prevention were defined as computer-based information systems designed to assist health care providers in primary, secondary, and tertiary care settings implement guidelines and evidence-based practices by providing: (1) tailored reminders to conduct or schedule preventive and screening services; (2) assessments of patients’ risk of developing CVD based on their medical history; and (3) alerts when CVD-related physiologic indicators are not at goal. The CDSS interface with the provider occurs at the point of care, based on individual patient data, and assists providers with recommendations for screening, preventive care, and treatment of patients who have risk factors for CVD such as obesity, inactivity, smoking, hypertension, hyperlipidemia, or diabetes. Improved clinician action should mitigate the risk factors and ultimately lead to reduced morbidity and mortality from CVD, thereby also improving economic outcomes. The complete definition and analytic framework are available in the published review of effectiveness.3
Search strategy and inclusion criteria
The studies for this systematic economic review were drawn from the results of 3 searches:
- The primary source was the set of studies referenced in a broad systematic review published in 2012 (Bright et al.5) and the accompanying report to the Agency for Healthcare Research and Quality.6
- Period: January 1975 to January 2011
- Databases: PubMed, CINAHL, PsycINFO, PubMed NLM, and Web of Science
- Scope: CDSS in all health topics
- The Bright et al. search was updated to cover more recent studies.
- Period: January 2011 to October 2015
- Databases: PubMed, CINAHL, PsycINFO, PubMed NLM, and Web of Science
- Scope: CDSS in CVD prevention
- A search was conducted within economics-related databases using the strategy implemented in Bright et al.
- Period: January 1970 to October 2015
- Databases: JSTOR, EconLit, Centre for Reviews and Dissemination
- Scope: CDSS in CVD prevention.
The 3 searches used terms to identify evaluation studies of CDSSs to prevent CVD across health care settings and ages. The studies referenced in the Bright et al. review were further screened to identify those related to CVD prevention, because that review considered CDSS use under all health topics. Details, including the complete list of search terms for the domains of CDSS intervention, target population, CVD prevention, the databases searched, and an update are available online,7 as are similar details for the economics-focused search.8
Studies were included in this review if they:
met the intervention definition,
were in English,
were implemented in a high-income economy,9 and
reported one or more of: the cost of intervention, change in health care cost, change in productivity, other economic benefit, cost-benefit, or cost-effectiveness.
CDSS interventions are generally implemented in clinics, hospitals, and other health care settings, and the cost of implementation is borne by the same organizations. However, benefits can accrue to patients, their employers, or insurers. This review took both a health system and societal perspective when assessing cost and benefit, so that reported estimates are meaningful from both the public and commercial perspective of these implementers and funders.
Intervention cost
The cost to develop a CDSS is the cost of compiling evidence-based narrative guidelines and programming the guidelines and decisions into code to produce prompts for provider action. Resources are needed to then implement the system throughout the practice and for all providers. The day-to-day use and maintenance of the CDSS require staff time and other resources, and are categorized under operating cost. In summary, the components of capital cost are development and implementation and the components of operating cost are maintenance and operation. Annualized intervention cost was estimated by distributing the one-time cost of development and implementation equally over the assumed 5-year life of the system and adding this annual amortized cost, discounted at 3%, to the annual cost of operation. Details regarding the conceptualization and measurement of intervention cost is presented in Supplementary Appendix 1.
A CDSS can be embedded within an electronic health record (EHR) system containing patient demographics and all health-related data, or it can be built on a registry that contains information only for patients with a specific disease or condition. The cost of collecting and populating the database with patient information, whether for an EHR or a registry, is not considered part of the CDSS intervention cost.
A CDSS can also change the economic efficiency of the care process by altering either the resources needed to care for a patient or the number of patients cared for with the same level of resource use. The benefits of this efficiency accrue to the health care facility implementing the CDSS and would be observed in operation cost per patient or per patient visit.
Intervention economic benefits
Effective CDSS interventions reduce CVD risk factors, such as systolic blood pressure. The reduction in risk factors, in turn, reduces morbidity and mortality and increases the quantity and quality of years lived, measured as quality-adjusted life years (QALYs) saved.
The impact of CDSS on health care cost is the difference in the cost of health care products and services used by the intervention and control groups or the pre to post change where there is no control group. Implementation of a CDSS can increase or decrease health care utilization by the patient, owing to either adherence to guidelines for care (increased cost) or improved health (decreased cost). The sum of these 2 changes is observed in changes in the component cost of outpatient, inpatient, and emergency room (ER) visits, medications, and labs. A full accounting of health care cost would include all 5 of these components; this review considers them to be of equal weight and an estimate that includes at least 3 of the 5 to be reasonably complete.
Reduced illness and increased years of life lived contribute to fewer illness-related absences from work, better performance when present, and a longer period of productivity. Hence, effective CDSS implementations that prevent CVD are expected to increase worksite productivity.
Reduced morbidity and mortality also increase the quantity and quality of life years lived. This is captured in outcomes such as QALYs saved or disability-adjusted life years averted.
Summary economic outcomes
Cost-benefit analysis compares economic benefit to intervention cost, where both benefit and cost are monetized and expressed in dollar terms; an intervention is cost-beneficial when economic benefit exceeds intervention cost.
Net cost (intervention cost plus health care cost) per QALY gained produces cost-utility, which is a type of cost-effectiveness assessment. An intervention is cost-effective when net cost per QALY gained is <$50,000. A threshold is applied because it is necessary to determine cost-effectiveness10 and $50,000 is chosen for the threshold because it is a conservative estimate and the one most widely used in the literature.11
Measurements, metrics, and reviewer decisions
Intervention cost can differ for practices of different sizes, because the scale of CDSS implementation ranges from small clinics to large health centers. The review team decided that intervention cost should be characterized by the size of practices where the CDSS is implemented. However, this review did not find any studies that fully characterized the association between cost of CDSS implementation and size of practice, whether based on number of physicians or patients in the practice. Therefore, we used the following categories of practice size (based on number of physicians) reported in the 2012 survey of practices by the American Medical Association12 to classify practices: small, 1–4 physicians (40% of respondents); medium, 5–24 physicians (35% of respondents); large, ≥25 physicians (25% of respondents). For studies that reported only the number of patients, we estimated the number of physicians based on an average patient panel and workload of US primary care physicians.13 All other cost and economic benefit estimates from included studies were standardized to a per-patient per-year basis when possible.
All monetary values were converted to 2015 US dollars. The Consumer Price Index from the Bureau of Labor Statistics14 was used to adjust for inflation. Purchasing Power Parity indices from the World Bank were used to convert from foreign currencies to US dollars.15 Results are summarized using medians and interquartile intervals.
RESULTS
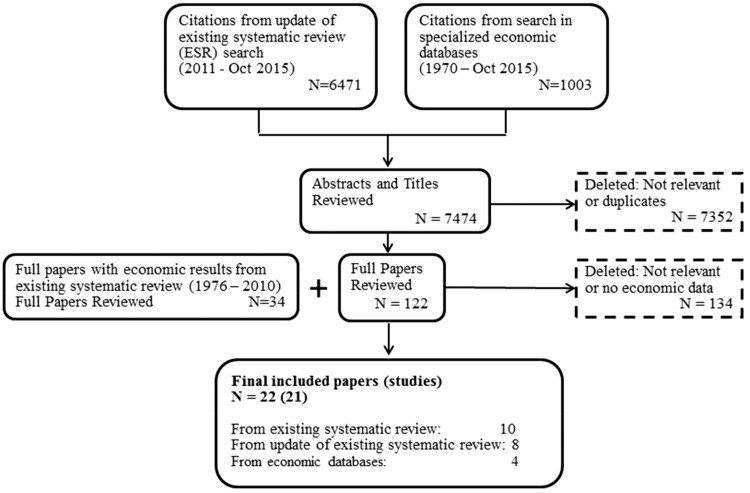
Twenty-one studies from the 7508 papers screened met the inclusion criteria (Figure 1). Seventeen studies16–28 reported on EHR-based CDSSs, 1 study29 piloted a CDSS residing on a stand-alone desktop, 130 was based on a diabetes registry, and 2 studies31–33 covered both EHR-based and registry-based CDSSs (2 papers32,33 reported on the same research and are counted as 1 study for this review). Table 1 provides an overview of additional characteristics of the included 21 studies. Most of the studies were based in the United States (67%) and implemented in clinics (94%), based on trials with a control group (63%) or models based on trials (25%). The studies covered CDSSs implemented alone (36%), or in combination with patient reminders/education (18%) or with team-based care (23%), where primary care providers and patients worked together with other providers to improve care and self-management support for patients. Most studies focused on diabetes as a risk factor (47%), followed by other CVD risks. More than 90% of the studies were published since 2000. Details of the included studies are available online.34
Figure 1.
Search process
Table 1.
Characteristics of included studies and proportion of studies with each characteristic
| Characteristic | No. of studies(% of all reviewed studies) |
|---|---|
| Country | |
| United States | 1416,20,22–24,26–28,30–33,35–37 |
| (67%) | |
| Non-United States | 717–19,21,25,29,38 |
| (33%) | |
| Setting | |
| Clinic | 1816–25,27–33,35,37 |
| (86%) | |
| Hospital | 326,36,38 |
| (14%) | |
| Study type | |
| Trial | 1516,17,19,20,23,24,26–30,35–38 |
| (71%) | |
| Model | 132,33 |
| (5%) | |
| Trial + Model | 418,21,22,25 |
| (19%) | |
| Survey | 131 |
| (5%) | |
| CDSS risk factor focus | |
| Diabetes | 1018,22,25,28,30–33,36–38 |
| (47%) | |
| Hypertension | 124 |
| (5%) | |
| Hyperlipidemia | 217,19 |
| (10%) | |
| Hypertension and hyperlipidemia | 121 |
| (5%) | |
| Including multiple CVD risk factors | 716,20,23,26,27,29,35 |
| (33%) | |
| Control group | |
| Pre and post with no control group | 517,27,36–38 |
| (24%) | |
| With control group | 1416,18–26,28–30,35 |
| (67%) | |
| Survey, model with no control group | 231–33 |
| (10%) | |
| Intervention added to CDSS | |
| Team-based care | 518,24,28,36,38 |
| (23%) | |
| Provider incentives | 222,35 |
| (9%) | |
| Provider audit and feedback | 121 |
| (5%) | |
| Patient phone reminders or report to patient | 420,23,31–33 |
| (18%) | |
| Quality improvement | 235,37 |
| (9%) | |
| None | 816,17,19,25–27,29,30 |
| (36%) | |
| Publication period | |
| 1990s | 220,26 |
| (9%) | |
| 2000s | 916,17,19,21,24,28,30–33 |
| (43%) | |
| 2010s | 1018,22,23,25,27,29,35–38 |
| (48%) |
CDSS, clinical decision support system; CVD, cardiovascular disease
Intervention cost
Twelve studies reported estimates of intervention cost, which are presented in Table 2 for registry- and EHR-based CDSS implementations. The estimates are characterized further by size of the health care practice and whether studies included cost of development and implementation, ongoing cost of operations, or both. Nine18,21–23,25,27,30–33 of the 12 studies reported the intervention cost of CDSS implementation specifically (Table 2). Of the remaining 3 studies, 2 that provided the cost of intervention did not include the cost of CDSS, with 135 reporting the cost of adding provider incentives to the CDSS implementation and the other36 providing only the labor cost of the staff involved in team-based care. The intervention cost from another study29 was computed by the review team from the incremental cost per unit reduction in low-density lipoprotein-c reported in the study, and is therefore assumed to include change in health care cost of outpatient visits and medications.
Table 2.
Intervention cost with components and characteristics of CDSSs
| Study | Sizea | Development and Implementation Cost Included | Ongoing Cost Included | Reasonably Complete Estimates | Annual Costb (5-year life) |
|
|---|---|---|---|---|---|---|
| Per Patient | Per Practice | |||||
| Registry-based | ||||||
| Adler-Milstein 0731 | Small | Y | Y | N | $69 | $9511 |
| Adler-Milstein 0731 | Medium | Y | Y | Y | $23 | $20,649 |
| Adler-Milstein 0731 | Large | Y | Y | Y | $14 | $75,964 |
| Blanchfield 0630 | Medium | Y | Y | N | $106 | $132,438 |
| Bu 07a,b32,33 | Small | Y | Y | Y | $55 | NR |
| EHR-based | ||||||
| Adler-Milstein 0731 | Mediumc | Y | Y | Y | $56 | $49,808 |
| Bardach 1335 | Small | N | N | N | $9 | $18,650d |
| Bu 07a,b32,33 | Small | Y | Y | Y | $170 | NR |
| Cleveringa 1018 | Small | Y | Y | Y | $73 | $4794 |
| Fretheim 0621 | Small | Y | N | N | NR | $346 |
| Gilmer 1222 | Medium | Y | Y | Y | $43 | $20,595 |
| Khan 1023 | Small | Subscription fee | Subscription fee | Y | $63 | $7318 |
| Munoz 1236 | Large | N | N | N | $61 | $386,750e |
| O’Reilly 1225 | Small | Y | N | N | $27 | $3739 |
| Shih 1127 | Small | Y | N | N | $4 | $7053 |
| Zamora 1329 | Small | NR | NR | N | $225f | NR |
CDSS, clinical decision support system; EHR, electronic health record; Y, Yes; N, No; NR, not reported
aSize of practice based on number of providers: small, 1–4 physicians; medium, 5–24 physicians; large, ≥25 physicians.
bCapital cost amortized over 5 years at a 3% discount rate.
cStudy assumes the implementation is perfectly scalable.
dCost of provider incentives only.
eAnnual budget reported to include cost of team-based care staff.
fBased on reported incremental cost per milligram/deciliter reduction in low-density lipoprotein-c.
All 3 studies that assessed the cost of CDSSs implemented in diabetes registries included both the one-time capital cost and ongoing operations cost. Based on a survey of users and vendors, 131 of the studies estimated that the annual cost per practice for a registry-based CDSS was about $9,500 for small, $20,600 for medium, and $76,000 for large. The corresponding estimates for cost per patient per year were $69, $23, and $14, respectively. A US study32,33 that modeled the cost of scaling up a registry-based CDSS nationwide estimated the annual per patient costs at $55, which corresponds closely with the survey-based estimate for a small practice. On the other hand, another US study30 estimated the annual cost of a medium-size CDSS at $106 per patient ($132,400 per practice) based on data collected during a controlled trial.
Eight studies reported the intervention cost of EHR-embedded CDSS, of which 518,22,23,31–33 included both the cost of development and implementation and the ongoing cost of operation, and 321,25,27 included only the one-time cost of development and implementation. Among the 5 studies that provided reasonably complete assessments of intervention cost, the mean annual costs were $102 per patient18,23,32,33 and $6056 per practice18,23 for small practices, and $49 per patient and $35,201 per practice22,31 for medium-sized practices.
Change in health care cost
Change in health care cost attributable to the intervention was reported in 15 studies: 1316–25,28,37,38 EHR-based implementations, 130 registry-based, and 132,33 reporting both types (Table 3). Interventions in addition to the CDSS were present in several studies; therefore, the effect on health care cost and other outcomes cannot be attributed to the CDSS alone. This is especially the case where intensive interventions were added, as for 4 CDSS interventions that included team-based care,18,24,28,38 followed by those that added less-intensive interventions, such as quality improvement,37 provider incentives,22 provider audit and feedback,21 and patient reminders.20,32,33
Table 3.
Health care cost: components and estimates
| Type of CDSS Study | Additional Intervention | Time Horizon | Components |
Reasonably Complete Estimate | Change in Health Care Cost Per Patient Per Yeara | ||||
|---|---|---|---|---|---|---|---|---|---|
| Outpatient | Inpatient | ER | Drugs | Labs | |||||
| Registry-based | |||||||||
| Blanchfield 0630 | None | 1 year | ✓ | ✓ | ✓ | Y | +$6 | ||
| Bu 07ab32,33 | Patient reminders | 10 years | ✓ | ✓ | N | −$127 | |||
| EHR-based | |||||||||
| Apkon 0516 | None | 2 months | ✓ | ✓ | ✓ | Y | +$355 | ||
| Bassa 0517 | None | 1 year | ✓ | ✓ | ✓ | Y | −$133 | ||
| Bu 07ab32,33 | None | 10 years | ✓ | ✓ | N | −$94 | |||
| Cleveringa 1018 | TBC | 10 years | ✓ | ✓ | ✓ | Y | All patients: +$148; patients with CVD: +$98 | ||
| Cobos 0519 | None | 1 year | ✓ | ✓ | ✓ | Y | −$107 | ||
| Frame 9420 | Patient reminders | 2 years | ✓ | N | $0 | ||||
| Fretheim 06b21 | Provider audit and feedback | 1 year | ✓ | N | −$10 | ||||
| Gilmer 1222 | Provider incentives | 40 years | ✓ | ✓ | ✓ | Y | −$46 | ||
| Herring 1338 | TBC | 9 months | ✓ | N | −$539 | ||||
| Khan 1023 | None | 32 months | ✓ | ✓ | N | −$236 | |||
| Murray 0424 | TBC | 1 year | ✓ | ✓ | ✓ | Y | −$2986 | ||
| O’Reilly 1225 | None | 40 years | ✓ | ✓ | ✓ | Y | −$23 | ||
| Oxendine 1437 | Quality improvement | 1 year | ✓ | ✓ | N | +$6532 during intervention | |||
| −$1960 1 year post | |||||||||
| Smith 0828 | TBC | 1 year | ✓ | ✓ | N | Outpatient was | |||
| −$349. Total with inpatient was | |||||||||
| −$2800 (authors noted that inpatient cost increase was primarily due to elective surgeries for musculoskeletal pain). | |||||||||
CDSS, clinical decision support system; ER, emergency room; TBC, team-based care; Y, Yes, N, No.
aHealth care cost for intervention versus control group or pre to post change where there is no control group.
Based on 8 studies16–19,22,24,25,30 that included at least 3 of 5 components of health care cost, the median change in health care cost per patient per year was −$35 (interquartile interval [IQI]: −$127 to $75). However, the estimated change in health care cost cannot be attributed to CDSSs alone for 218,24 interventions that included team-based care. With these studies removed, the remaining 6 studies produced a median change in health care cost per patient per year of −$35 (IQI: −$114 to $93).
Worksite productivity
No studies assessed the economic benefit of worksite productivity improvements for patients whose health improved through use of a CDSS.
Cost-benefit and cost-effectiveness
Economic benefit was compared to intervention cost in 221,23 studies of EHR-based CDSSs and 132,33 study of both EHR- and registry-based CDSSs (Table 4). The same time horizons were used for benefits and cost in these studies. All 3 studies included fewer than 3 components of health care cost and did not estimate productivity effects; they were therefore incomplete assessments of economic benefit. One study21 that reported a benefit-to-cost ratio of 2.03:1 considered only the averted cost of medication and did not include ongoing operating cost in its estimate for intervention cost. The second study23 provided a reasonably complete assessment of intervention cost but only considered the cost of inpatient stays and ER visits, estimating benefit-to-cost at 3.8:1. The third study32,33 estimated the benefit of averted inpatient stays and outpatient visits to a reasonably complete assessment of intervention cost at 2.3:1 for a registry-based CDSS but 0.55:1 for an EHR-based CDSS. The latter unfavorable ratio arose primarily because the only high-quality trial of an EHR-based CDSS for diabetes management at the time showed an increase in systolic blood pressure for the intervention group, which translated to increased CVD risk and CVD events in the economic modeling. In summary, the results from cost-benefit studies are incomplete assessments and indicate mixed conclusions on whether economic benefits exceed the cost of CDSS interventions to prevent CVD.
Table 4.
Cost-benefit and cost per QALY saved estimates
| Study Type of CDSS | Benefit-to-Cost Ratio |
|---|---|
|
|
|
3.8:1 |
| 0.55:1 | |
| 2.3:1 | |
| Study Type of CDSS | Cost per QALY saved |
|
|
|
$16,500 |
|
$143,000 |
CDSS, clinical decision support system; CVD, cardiovascular disease; EHR, electronic health record; QALY, quality-adjusted life year.
Three studies provided estimates of cost per QALY saved (Table 4), where the same time horizons were used for net cost and adjusted life years lived outcomes: 2 reported that the interventions were cost-effective at $49,000(18) and $16,50022 and 1 reported that it was not cost-effective at $143,000.25 Estimates for health care cost from all 3 studies were reasonably complete, but 1 study25 did not include annual operating cost in the intervention cost. Further, the 2 studies with cost per QALY that saved <$50,000 included interventions in addition to CDSS, namely team-based care18 and provider incentives.22 The demonstration of cost-effectiveness from these 2 studies is for the combination intervention and cannot be attributed to CDSS alone.
DISCUSSION
A recent symposium concluded39 that methodologies are yet to be developed that can rigorously evaluate the economic value of health information technologies at the population and national levels even though economic value is evident from individual observations of their success at the local and organizational levels. The symposium noted the difficulty in transitioning from judgments of economic value at the level of specific implementations to a judgment about the aggregate of the implementations: costs and benefits have to be summed over implementations with different organizational contexts, technologies, functions, outcomes, scales, and scope. This systematic economic review of one type of health information technology, namely CDSS, encountered similar difficulties among others in synthesizing the economic evidence from various implementation instances.
The cost and economic benefits of CDSS implementations from included studies were poorly reported, and many studies did not adhere to sound evaluation or accounting practices. Only a few studies provided a complete accounting of cost to develop, implement, maintain, and operate a CDSS. More complete economic evaluations are necessary to obtain reliable estimates for intervention cost across types and sizes of health care settings. Reported economic benefits of a CDSS are often determined or guided by the implementation’s disease or risk-factor focus (eg, hypertension, CVD, diabetes, depression), functionality (eg, provider prompts, management of orders, disease management), or the implementer’s objective (eg, containing cost with cheaper drugs or averting hospital readmissions). However, despite the heterogeneity in the research literature around the focus, functionality, and objective of CDSS implementations, certain guidelines for the evaluation of economic costs and benefits can still be described.
Starting with cost, important features of a CDSS from an evaluation perspective are its useful life, which generally spans multiple years, and the substantial one-time cost of development and implementation. In addition to the one-time cost, maintaining and operating the CDSS requires technical and medical staff time. The present review estimated intervention cost as the sum of these 2 components, based on recommended accounting practices and tax rules. Details and references are provided in Supplementary Appendix 1. Because the cost of a CDSS is generally borne by the practice and can differ by practice size, it is useful to communicate the intervention cost in both per-practice and per-patient terms. Most included evaluation studies did one or the other. An additional advantage of intervention cost measured in per-patient terms is its immediate comparability to health care cost and health effects that are generally reported in per-patient terms.
Moving to the benefits side of the evaluation, only those benefits that likely resulted from changes in provider clinical decisions and any consequent change in patient behavior and health should be ascribed to the CDSS implementation. The societal perspective is recommended for economic evaluations to account for all health care, regardless of who pays for the various components. It is important to know what impact the CDSS has on total health care utilization; following the guidelines prompted by the CDSS can increase outpatient visits and/or medications within primary care but can avert very costly inpatient stays and ER visits in tertiary care. This is not to say a partial perspective is without merit. A primary care study evaluating a CDSS to treat hypertension could report the intervention cost per unit reduction in blood pressure ($/mmHg), a useful statistic for clinic managers.
Evaluations are more complex when one or more additional interventions occur along with the CDSS. Both the intervention cost and economic benefit (including QALY saved) must be ascribable to the CDSS when statements are made about the cost-benefit or cost-effectiveness of CDSS implementations. It is often feasible to estimate the cost of the CDSS and additional intervention(s) separately, but obtaining separate estimates for benefits from the component intervention(s) is likely to be difficult, whether analytically or by study design. For example, in an intervention where team-based care was implemented along with the CDSS, a cost-benefit analysis that included only the cost of CDSS in intervention cost and the combined effect of both team-based care and the CDSS in the benefits would be incomplete. In the absence of complete data, statements about the economic value of the CDSS would have to include appropriate caveats.
Many studies included in this review focused on a single CVD risk factor, such as diabetes, high cholesterol, or high blood pressure. Outcomes for lifetime cost per QALY saved or cost-benefit ratios were often modeled based on measured improvements in a single or a few risk, such as blood pressure, blood glucose, or cholesterol. However, a CDSS for CVD prevention and control would, in practice, be implemented with a multiplicity of functionalities, including simple provider and patient reminders for screening and testing, patient risk assessments, and medication and lifestyle counseling recommendations across the range of risk factors and indicators for CVD (and diabetes).
The results presented in the current review are consistent with those found in the broader review of evidence for CDSS implementations for all diseases and conditions.5,6 That review found that CDSSs/Knowledge Management Systems reduced health care cost and produced cost-savings, but it reached mixed conclusions about cost-effectiveness. Similar to the current review, that review also called for evaluations to standardize the metrics for efficiency and cost.
CONCLUSION
An overall conclusion cannot be reached about the cost-effectiveness or cost-benefit of CDSSs for CVD prevention and control. The evidence on cost and benefit is limited by many estimates that do not account for major components, and mixed evidence when the estimates are reasonably complete. Further, the reported cost and benefit in many studies included the effect of interventions in addition to the CDSS.
The quality of economic evidence for CDSSs can improve with more evaluations that acknowledge its capital good features and account for both development and operating cost over its span of useful life.
Supplementary Material
ACKNOWLEDGMENTS
This authors acknowledge the Division for Heart Disease and Stroke Prevention, Centers for Disease Control (CDC), for support and subject matter expertise, particularly the late David B. Callahan, MD. We thank members of our coordination team in the Community Guide Branch at CDC and from other areas of CDC, and our external partners: Kimberly J. Rask, MD, PhD, Emory University, Atlanta; Daniel T. Lackland, DrPH, Medical University of South Carolina, Charleston; and Lynne T. Braun, PhD, ANP, FAAN, FACAA, Rush College of Nursing, Chicago. The authors acknowledge Randy W. Elder, PhD, Kate W. Harris, BA, and Onnalee Gomez, MS, from the Community Guide Branch at CDC for their assistance throughout the review, and John Tibbs, BA, MBA, Office of Noncommunicable Diseases, Injury, and Environmental Health, CDC, for assistance with accounting concepts for capital expenditures. The authors also thank Gillian Sanders, PhD, Duke Evidence-Based Practice Center, and Amy Kendrick, RN, MSN, MS-CRM, Duke Clinical Research Institute, Durham, North Carolina, for the references of additional economic studies identified but not included in the Agency for Healthcare Research and Quality review.
The work of Gibril Njie and Krista Proia was supported with stipends from the Oak Ridge Institute for Science and Education.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
FUNDING
The work of Gibril J. Njie and Krista K. Proia were supported with stipends from the Oak Ridge Institute for Science and Education.
COMPETING INTERESTS
The authors have no competing interests to declare.
CONTRIBUTORS
All authors contributed substantially to the conception and design of the work. VJ, ABT, SKC, GJN, KKP, and DPH drafted the manuscript, and all authors contributed to revising it critically for important intellectual content. VJ and SKC contributed to acquisition, analysis, and interpretation of data. All authors have approved the manuscript for publication and agree to be accountable for all aspects of the work.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS et al. Executive summary: heart disease and stroke statistics 2016 update—a report from the American Heart Association. Circulation. 2016;133(4):447–54. [DOI] [PubMed] [Google Scholar]
- 2. Community Preventive Services Task Force. Clinical decision support systems recommended to prevent cardiovascular disease. Am. J. Prev. Med. 2015;49(5):796–99. [DOI] [PubMed] [Google Scholar]
- 3. Njie GJ, Proia KK, Thota AB et al. Clinical decision support systems and prevention: a Community Guide cardiovascular disease systematic review. Am. J. Prev. Med. 2015;49(5):784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guide to Community Preventive Services. Economic Reviews. 2014. Available at: https://www.thecommunityguide.org/about/economic-reviews. Accessed September 14, 2016. [Google Scholar]
- 5. Bright TJ, Wong A, Dhurjati R et al. Effect of clinical decision-support systems: a systematic review. Ann. Intern. Med. 2012;157(1):29–43. [DOI] [PubMed] [Google Scholar]
- 6. Lobach D, Sanders GD, Bright TJ et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. AHRQ Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PMC free article] [PubMed] [Google Scholar]
- 7. Guide to Community Preventive Services. Cardiovascular Disease Prevention and Control: Clinical Decision-Support Systems (CDSS): Search Strategy. 2013. Available at: https://www.thecommunityguide.org/findings/cardiovascular-disease-clinical-decision-support-systems-cdss. Accessed September 14, 2016. [Google Scholar]
- 8. Guide to Community Preventive Services. Cardiovascular Disease Prevention and Control: Clinical Decision-Support Systems (CDSS): Search Strategy – Economic Review. 2014. Available at:https://www.thecommunityguide.org/findings/cardiovascular-disease-clinical-decision-support-systems-cdss. Accessed September 14, 2016. [Google Scholar]
- 9. World Bank Country and Lending Groups. 2010. http://data.worldbank.org/about/country-and-lending-groups. Accessed May 21, 2015.
- 10. Eichler H-G, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28. [DOI] [PubMed] [Google Scholar]
- 11. Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–78. [DOI] [PubMed] [Google Scholar]
- 12. Kane CK, Emmons DW. New Data on Physician Practice Arrangements: Private Practice Remains Strong Despite Shifts Toward Hospital Employment. 2013. Available at: http://www.nmms.org/sites/default/files/images/2013_9_23_ama_survey_prp-physician-practice-arrangements.pdf. Accessed May 12, 2015. [Google Scholar]
- 13. Østbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann. Fam. Med. 2005;3(3):209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Available at: http://www.bls.gov/cpi/cpid1512.pdf. Accessed February 8, 2016.
- 15. World Bank. Purchasing power parities. Available at: http://data.worldbank.org/indicator/PA.NUS.PRVT.PP. Accessed May 21, 2015.
- 16. Apkon M, Mattera JA, Lin Z et al. A randomized outpatient trial of a decision-support information technology tool. Arch. Intern. Med. 2005;165(20):2388–94. [DOI] [PubMed] [Google Scholar]
- 17. Bassa A, Del Val M, Cobos A et al. Impact of a clinical decision support system on the management of patients with hypercholesterolemia in the primary healthcare setting. Dis. Manage. Health Outcomes. 2005;13(1):65–72. [Google Scholar]
- 18. Cleveringa FG, Welsing PM, van den Donk M et al. Cost-effectiveness of the diabetes care protocol, a multifaceted computerized decision support diabetes management intervention that reduces cardiovascular risk. Diabetes Care. 2010;33(2):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cobos A, Vilaseca J, Asenjo C et al. Cost effectiveness of a clinical decision support system based on the recommendations of the European Society of Cardiology and other societies for the management of hypercholesterolemia: Report of a cluster-randomized trial. Dis. Manage. Health Outcomes. 2005;13(6):421–32. [Google Scholar]
- 20. Frame PS, Zimmer JG, Werth PL, Hall WJ, Eberly SW. Computer-based vs manual health maintenance tracking. A controlled trial. Arch. Fam. Med. 1994;3(7):581–88. [DOI] [PubMed] [Google Scholar]
- 21. Fretheim A, Aaserud M, Oxman AD. Rational prescribing in primary care (RaPP): economic evaluation of an intervention to improve professional practice. PLoS Med. 2006;3(6):e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilmer TP, O'Connor PJ, Sperl-Hillen JM et al. Cost-effectiveness of an electronic medical record based clinical decision support system. Health Serv. Res. 2012;47(6):2137–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan S, Maclean CD, Littenberg B. The effect of the Vermont Diabetes Information System on inpatient and emergency room use: results from a randomized trial. Health Outcomes Res. Med. 2010;1(1):e61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray MD, Harris LE, Overhage JM et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: results of a randomized controlled trial. Pharmacotherapy. 2004;24(3):324–37. [DOI] [PubMed] [Google Scholar]
- 25. O'Reilly D, Holbrook A, Blackhouse G, Troyan S, Goeree R. Cost-effectiveness of a shared computerized decision support system for diabetes linked to electronic medical records. J. Am. Med. Inform. Assoc. 2012;19(3):341–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of "corollary orders" to prevent errors of omission. J. Am. Med. Inform. Assoc. 1997;4(5):364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shih SC, McCullough CM, Wang JJ, Singer J, Parsons AS. Health information systems in small practices. Improving the delivery of clinical preventive services. Am. J. Prev. Med. 2011;41(6):603–09. [DOI] [PubMed] [Google Scholar]
- 28. Smith SA, Shah ND, Bryant SC et al. Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clin. Proc. 2008;83(7):747–57. [DOI] [PubMed] [Google Scholar]
- 29. Zamora A, de Bobadilla FF, Carrion C et al. Pilot study to validate a computer-based clinical decision support system for dyslipidemia treatment (HTE-DLP). Atherosclerosis. 2013;231(2):401–04. [DOI] [PubMed] [Google Scholar]
- 30. Blanchfield BB, Grant RW, Estey GA, Chueh HC, Gazelle GS, Meigs JB. Cost of an informatics-based diabetes management program. Int. J. Technol. Assess. Health Care. 2006;22(2):249–54. [DOI] [PubMed] [Google Scholar]
- 31. Adler-Milstein J, Bu D, Pan E et al. The cost of information technology-enabled diabetes management. Dis. Manag. 2007;10(3):115–28. [DOI] [PubMed] [Google Scholar]
- 32. Bu D, Pan E, Johnston D et al. The value of information technology-enabled diabetes management. Center for Information Technology Leadership, Healthcare Information and Management System Society, Charleston, MA: 2007. Available at: http://www.partners.org/cird/pdfs/CITL_ITDM_Report.pdf. Accessed May 9, 2013. [Google Scholar]
- 33. Bu D, Pan E, Walker J et al. Benefits of information technology-enabled diabetes management. Diabetes Care. 2007;30(5):1137–42. [DOI] [PubMed] [Google Scholar]
- 34. Guide to Community Preventive Services. Cardiovascular Disease Prevention and Control: Clinical Decision-Support Systems (CDSS). Summary Evidence Table: Economic Review. Available at: https://www.thecommunityguide.org/sites/default/files/assets/SET-CDSS-econ-2013.pdf. Accessed September 14, 2016. [Google Scholar]
- 35. Bardach NS, Wang JJ, De Leon SF et al. Effect of pay-for-performance incentives on quality of care in small practices with electronic health records: a randomized trial. JAMA. 2013;310(10):1051–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munoz M, Pronovost P, Dintzis J et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt. Comm. J. Qual. Patient. Safety/Jt. Comm. Resources. 2012;38(5):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oxendine V, Meyer A, Reid PV, Adams A, Sabol V. Evaluating diabetes outcomes and costs within an ambulatory setting: a strategic approach utilizing a clinical decision support system. Clin. Diabetes. 2014;32(3):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herring R, Russell-Jones D, Pengilley C et al. Management of raised glucose, a clinical decision tool to reduce length of stay of patients with hyperglycaemia. Diabet. Med. 2013;30(1):81–87. [DOI] [PubMed] [Google Scholar]
- 39. Payne TH, Bates DW, Berner ES et al. Healthcare information technology and economics. J. Am. Med. Inform. Assoc. 2013;20(2):212–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.