Abstract
Exposure to drugs early in life has complex and long-lasting implications for brain structure and function. This review summarizes work to date on the immediate and long-term effects of prenatal exposure to cocaine. In utero cocaine exposure produces disruptions in brain monoamines, particularly dopamine, during sensitive periods of brain development, and leads to permanent changes in specific brain circuits, molecules, and behavior. Here, we integrate clinical studies and significance with mechanistic preclinical studies, to define our current knowledge base and identify gaps for future investigation.
Keywords: brain, in utero, prenatal, psychostimulant, dopamine
Introduction
Cocaine abuse and addiction continue to be major public health concerns in the United States and across the world. According to the 2014 National Survey on Drug Use and Health, 1.5 million Americans age 12 or older (or ∼0.6% of the population) self-reported current cocaine use. Equally concerning is cocaine use by pregnant women. Among pregnant women aged 15 to 44, 5.4% were current illicit drug users (SAMHSA, 2014). Furthermore, nearly 28% of women become aware of pregnancy on average 2.5 months post conception (Ayoola et al., 2009), increasing the risk for fetal drug exposure. In fact, it has been estimated that over 7.5 million U.S. children have been prenatally exposed to cocaine (Chae and Covington, 2009).
The cocaine epidemic emerged in United States in the 1980s and early 1990s. Epidemiological studies conducted during this time indicated that up to 27% of women used cocaine during pregnancy (Frank et al., 1988; Chasnoff 1989; Gomby and Shiono, 1991), and initial reports suggested potentially dramatic adverse effects for the newborn child at birth (Chasnoff et al., 1985; MacGregor et al., 1987). This led to fears in the general media that an entire generation of “crack babies” may have been “lost.” Unfortunately, many of these fears were exaggerated and, subsequently, public notions swung in the opposite direction, suggesting that prenatal cocaine exposure (PCE) may produce no or only subtle effects on intellectual abilities. However, debate and discussion have prevailed regarding the consequences of PCE on brain and behavioral development and, perhaps not surprisingly, the truth is actually somewhere in the middle. In fact, more recent studies report that PCE produces persisting deficits in higher cognitive functions, such as attention, executive function, and language (Sheinkopf et al., 2009; Ackerman et al., 2010; Bandstra et al., 2011; Carmody et al., 2011; Liu et al., 2013; Akyuz et al., 2014; Allen et al., 2014).
One of the issues that fuels the debate is the inherent variability in findings from studies in human subjects. For example, studies that rely on self-reporting of illicit drug use are subject to an underreporting bias, especially regarding drug use during pregnancy (Frank et al., 1988; Gomby and Shiono, 1991; Ostrea et al., 1992). For instance, in one study, only 25% of mothers who had abused cocaine during pregnancy admitted to using it (Ostrea et al., 1992). In addition, multi-drug use, including alcohol, marijuana, and nicotine, is common among individuals who use cocaine and presents another confounding variable. Poor nutrition, drug dose, route of administration, timing, frequency, and duration of exposure also add further complexities.
As PCE continues to be a significant public health concern, whether it can affect early brain development and to what extent remains a significant question from scientific, public health, and public policy perspectives. As a result, scientific attention has focused on animal models of PCE. Studies using animal models permit rigorous and hypothesis-driven exploration of the consequences of PCE on the development of the brain and behavior, and have the potential to help settle this debate. However, the vast majority of the studies using animal models have focused on behaviors and molecular mechanisms associated with drug reinforcement, sensitization, or reward (Simansky and Kachelries, 1996; Glatt et al., 2000; Paule et al., 2000; Rocha et al., 2002; Crozatier et al., 2003; Malanga and Kosofsky, 2003; Stanwood and Levitt, 2003; Malanga et al., 2007; Malanga et al., 2008; Malanga, 2009), rather than on the types of cognitive functions analyzed in human subjects. In other words, direct extrapolation of the data from studies on animal models to human subjects tends to pose its own challenges.
Given these circumstances, our goal here is to critically evaluate findings from human and animal model studies with a predisposition toward identifying common themes and directions on the short- and long-term consequences of PCE on brain structure and function.
Human Studies
Cocaine use during pregnancy affects two generations simultaneously—the mother and her child. Pregnant women who use cocaine have an increased risk for premature rupture of the membranes and hemorrhage, as well as spontaneous abortion or fetal death (Oro and Dixon, 1987; Townsend et al., 1988; Handler et al., 1991; Kistin et al., 1996). The consequences that PCE imposes on child include increased risk for prematurity, intrauterine growth retardation, respiratory distress, seizure,; decreased head circumference, low birth weight and reduced length, malformations of the genitourinary track, bowel and cerebral infarctions/malformations, and an increased risk for sudden infant death syndrome (Chasnoff et al., 1985; Bingol et al., 1987; Oro and Dixon, 1987; Ryan et al., 1987; Braude et al., 1987; Chasnoff et al., 1987; Chasnoff et al., 1988; Riley et al., 1988; Doberczak et al., 1988; Chasnoff and Griffith, 1989; Frank et al., 1990; Dominguez et al., 1991; Handler et al., 1991; Lester et al., 1991; Bada et al., 2002; Bada et al., 2005; Bauer et al., 2005).
Behavioral Assessments
PCE is associated with lower scores in the Apgar evaluation (Ryan et al., 1987; Chasnoff et al., 1989b) as well as in the Neonatal Behavioral Assessment Scale (NBAS) (Chasnoff et al., 1985; Doberczak et al., 1988; Chasnoff et al., 1989a; Schneider and Chasnoff, 1992; Delaney-Black et al., 1996; Bauer et al., 2005). There appears to be a correlation between the timing of gestational cocaine exposure and the infant's behavioral profile. For example, cocaine use during the first and second trimesters increased the number of abnormal reflexes, whereas cocaine use during the second and third trimester reduced motor maturity and muscle tone (Richardson et al., 1996b). Unfortunately, many early studies using the NBAS involved the concomitant maternal use of other drugs, such as opioids, marijuana, alcohol, and tobacco. Thus, singling out the role of PCE in these outcomes has been difficult.
PCE produces deficits in language development and emotional reactivity, as well as impairments in arousal and attention (Davis et al., 1992; Chasnoff, 1992; Chiriboga et al., 1993; Azuma and Chasnoff, 1993; Griffith et al., 1994; Mayes et al., 1995; Mayes et al., 1996; Jacobson et al., 1996; Delaney-Black et al., 1996; Richardson et al., 1996a; Bada et al., 2007; Lewis et al., 2007; Bandstra et al., 2011; Carmody et al., 2011; Lewis et al., 2011) (see Fig. 1). Many behavioral and neurophysiological findings have suggested that PCE disrupts arousal regulation. Because the regulation of arousal serves as a gating mechanism for orientation and attention, it has important implications for information processing, learning, and memory. In fact, deficits in arousal appear early and persist well into adolescence and adulthood. For instance, cocaine-exposed 3-month old infants demonstrated deficits in reactivity to novel stimuli. These infants were more likely to exhibit crying and negative affect on novel stimulus presentations, as well as deficits in information processing and habituation, suggesting deficits in arousal and attention regulation (Mayes et al., 1995; Mayes et al., 1996; Mayes et al., 1998). Children prenatally exposed to cocaine also have decreased inhibition and deficits in their ability to sustain attention, which demonstrate abnormal cognitive processing and executive function (Richardson et al., 1996a; Espy et al., 1999; Leech et al., 1999; Linares et al., 2006). Specifically, when persistence was measured in a Stanford-Binet Intelligence scale (persistence is not always measured and may account for variability in the overall findings among different standardized tests), 3-year old toddlers prenatally exposed to cocaine demonstrated poor task persistence and increased irritability and distractibility, compared to control subjects (Azuma and Chasnoff, 1993). Even at older ages (6, 9, and 11 years), deficits in attention and inhibitory control were present following PCE. Once again, this effect on attention and arousal has been shown to be more robust with higher levels of PCE (Carmody et al., 2011).
Figure 1. Summary of neurobehavioral findings in children exposed to cocaine prenatally.
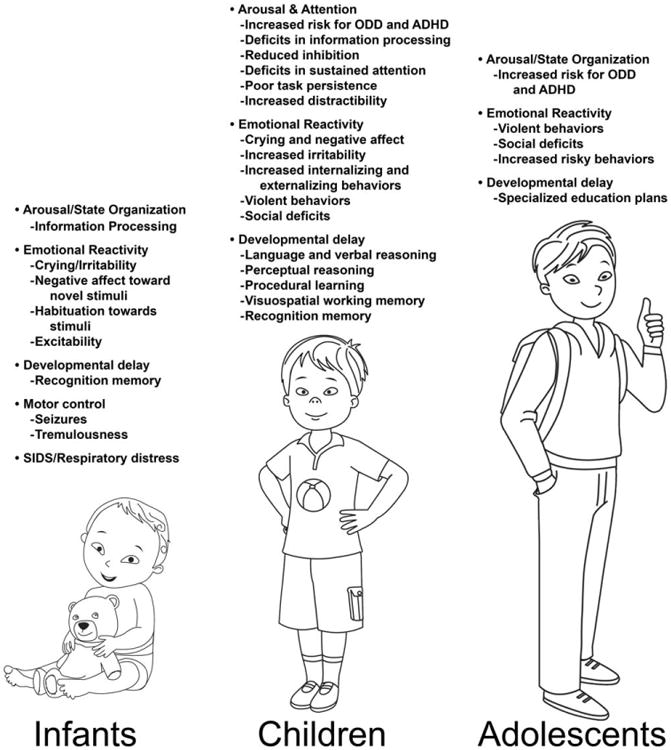
Other related cognitive deficits following PCE include visual attention deficits and difficulties in sustained attention (Heffelfinger et al., 2002) (Fig. 1). In addition, children exposed prenatally to cocaine have also shown impairments in procedural learning, and demonstrated reduced visuospatial working memory. Specifically, the cocaine-exposed group demonstrated slower responses, made more errors, and showed less consolidation in learning compared to non-exposed children, signifying a possible deficit in procedural learning and spatial working memory (Schroder et al., 2004; Mayes et al., 2007). These cognitive deficits seem to appear early on such that some studies have shown impaired recognition memory in infants following PCE (Struthers and Hansen, 1992).
Specific language and cognitive deficits have also been reliably detected (Fig. 1). These include language delays and impairments in verbal reasoning in preschool-aged children (Angelilli et al., 1994; Griffith et al., 1994; Bandstra et al., 2004; Lewis et al., 2004; Morrow et al., 2004). Using the Wechsler Intelligence Scale for Children–Fourth Edition to assess math, reading, and written language ability, preschool children prenatally exposed to cocaine were found to have lower perceptual reasoning IQ associated with language deficits. Once again greater effects were seen in those children that were exposed to higher amounts of cocaine (compared to a lower cocaine-exposed group and control subjects) (Singer et al., 2008). Other language deficits in infants and toddlers include failure to vocalize other than crying, delayed acquisition of words, limited use of newly learned words, failure to call the primary caregiver “mama” or “dada” until after 15 months of age, and long periods of silence (Davis et al., 1992). Longitudinal studies examining language delays include deficits in receptive, expressive, and total language domains that are persistent throughout childhood and last, at least, into adolescence (Singer et al., 2001; Morrow et al., 2003b; Bandstra et al., 2004; Lewis et al., 2007; Lewis et al., 2011; Bandstra et al., 2011).
Prenatally cocaine-exposed children have also been found to have increased risk for behavioral problems and impaired social development, compared to non-exposed children (Yolton and Bolig, 1994; Bland-Stewart et al., 1998; Richardson, 1998; Delaney-Black et al., 1998; Linares et al., 2006; Bada et al., 2007) (Fig. 1). Specifically, temperament differences have been found at both 1 and 3 years of age, with the onset of behavioral problems starting at 3 years following PCE (Richardson, 1998). Similarly, in another clinical study of preschool- and elementary school-aged children (3-, 5-, and 7-year old), children with PCE demonstrated increased externalizing behaviors (e.g., aggressive or defiant behaviors), as well as increased internalizing behaviors (e.g., social withdrawal, nervousness or irritability, and fearfulness) at all three ages, even after controlling for other factors such as environment. Once again, higher levels of cocaine exposure in utero were associated with greater risk for these behaviors (Bada et al., 2007). In addition, these children had delayed conceptual development. Emotional and social deficits, including blunted and dulled emotions, less secure attachment, limited play skills, less social play behavior, passive and withdrawn personalities, and rapid mood swings were also found in children prenatally exposed to cocaine (Rodning et al., 1989; Gittler and McPherson, 1990; Grace, 1993).
Brain Anatomy
Abnormal brain development likely underlies the PCE-induced behavioral deficits described above. For instance, decreased head circumference and microcephaly are commonly found in infants prenatally exposed to cocaine, particularly high levels of cocaine (Bingol et al., 1987; Ryan et al., 1987; Zuckerman et al., 1989; Chasnoff et al., 1989a; Fulroth et al., 1989; Frank et al., 1990; Gillogley et al., 1990; Little and Snell, 1991; Nulman et al., 2001; Bada et al., 2002; Bauer et al., 2005; Akyuz et al., 2014). Additionally, studies that find a decrease in head circumference also typically find behavioral and cognitive deficits. In fact, head circumference is thought to be a good predictor of neurobehavioral deficits in those children prenatally exposed to cocaine.
Brain imaging studies have also been used to examine structural and volumetric changes in the brain following PCE. Specifically, magnetic resonance imaging (MRI) has revealed smaller cortical and subcortical structures, including a smaller caudate (Rao et al., 2007; Rivkin et al., 2008) [although see also (Avants et al., 2007)], corpus callosum (Dow-Edwards et al., 2006), and pallidum (Liu et al., 2013), following PCE. In contrast, PCE appears to be associated with a larger amygdala (Rao et al., 2007). Akyuz et al. (2014) and Liu et al. (2013) used MRI to examine changes in brain structure following PCE at two separate time points (8–10-year old children and 13–15-year adolescents). Initial studies of 8–10-year olds revealed a trend toward a reduction in overall brain volume in those prenatally exposed to cocaine. The specific structures in which the volume was significantly reduced included cortical gray matter, the thalamus, and the putamen. The size reductions in the thalamus and putamen were inversely correlated with the amount of cocaine exposure in utero. In addition, MRI scans using a smaller cohort of 13–15-year olds determined that the reductions in the volume of these structures persisted at least into the teenage years (Akyuz et al., 2014). Diffusion tensor imaging has shown that PCE is associated with increased diffusion in the frontal brain regions, including the medial and lateral white matter tracts, suggesting immature development (Warner et al., 2006) and a reduction in the mean cortical gray matter and total parenchymal volumes (Rivkin et al., 2008). In comparison, arterial spin-labeling perfusion fMRI has shown that adolescents prenatally exposed to cocaine exhibit a global reduction in resting cerebral blood flow and have a relative increased cerebral blood flow in the frontal and limbic regions, suggesting a compensatory mechanism (Rao et al., 2007).
Seizures
PCE is also associated with increased risk of seizures in newborns and changes in brain wave activity (Doberczak et al., 1988; Shih et al., 1988; Chasnoff et al., 1989a; Kramer et al., 1990; Carzoli et al., 1991; Scher et al., 2000; Lester et al., 2002). In one study, 100% of the infants prenatally exposed to cocaine experienced seizures within 36 hr of delivery; 50% of these infants had repetitive seizures during their neonatal hospitalization stay, but did not have reoccurrences during a follow-up period of 4 to 12 months. The other 50%, however, continued to experience neonatal seizures after their initial month of life and a smaller subset continued to experience seizures even after 6 months of life, suggesting long-term neurodevelopmental effects of early cocaine exposure (Kramer et al., 1990). In a separate study by Doberczak et al. (1988), which focused on EEG patterns, 90% of the infants that were prenatally exposed to cocaine displayed deficits in neurophysiological behavior, such as increased CNS irritability, and over 50% had abnormal EEGs. These effects, however, were transient such that by week 2, only 20% of infants displayed abnormal EEGs, and by 3 to 12 months of age, only 1 infant out of 10 displayed abnormal EEG patterns. Lastly, brain stem auditory responses were shown to have prolonged inter-peak latencies and prolonged absolute latencies, indicating that the myelination of these neurons may be impaired or retarded (Shih et al., 1988; Lester et al., 2002). Collectively, these results suggest that abnormal brain function appears as early as infancy following PCE.
Functional Imaging Studies
Functional MRI studies of school age children (8–9-year old) showed that PCE was associated with greater activation of the right inferior frontal cortex and caudate following a response inhibition task, whereas non-PCE children showed greater activation in temporal and occipital regions (Sheinkopf et al., 2009). These results suggest that PCE likely affects the development of brain regions involved in attention and response inhibition. To assess the location and degree of neural activation underlying working memory, teenagers approximately 15-year old were subjected to an executive function task. Teens prenatally exposed to cocaine demonstrated performances and fMRI activation patterns during this task that were comparable to those of control subjects, indicating no differences between these groups (Hurt et al., 2008). These seeming discrepancies may be due to high versus low levels of cocaine exposure, as well as other confounding variables, and therefore necessitate future studies to further understand the functional long-term effects of PCE.
Animal Models
Behavioral Consequences
A large body of literature suggests that PCE leads to a number of deficits in simple, unlearned behaviors, such as surface righting, cliff avoidance, orientation, state control, negative geotaxis, motor development, and tremors (Sobrian et al., 1990; Kunko et al., 1993; Sobrian et al., 1995; Lidow, 2003; He et al., 2004) (see Table 1: Behavioral & Cognitive Consequences for examples), whereas, others have found no effects of PCE on reflexes (Spear et al., 1989a; Morris et al., 1996a; Paule et al., 1996) or state (Zmitrovich et al., 1992). However, one behavior that is consistently found in animal models of PCE is important for pup survival: ultrasonic vocalizations (USV). As with humans, neonates emit calls that indicate to the dam a change in environment or arousal state, for instance. These cries are critical in the mother-infant relationship. Studies have shown that neonates exposed gestationally to cocaine display altered vocalization patterns. In rodents and chickens, PCE can result in decreases in the number of vocalizations (Hahn et al., 2000; Cox et al., 2012; Lippard et al., 2015), while others have shown increases in USVs relative to controls; although this effect normalized soon after in rodents (Kabir et al., 2014; Zeskind et al., 2014). As in humans, others have shown that USV emitted from PCE-exposed rodents also differ from their control peers in the acoustic structure, harmonics, duration, and pitch of the calls (Hahn et al., 2000; Cox et al., 2012; Zeskind et al., 2014).
Table 1. Behavioral and Cognitive Consequences.
| Measuring variable | Species | Control groups | Dose & route of PCE | Testing age | Outcomes | References |
|---|---|---|---|---|---|---|
| Unlearned behaviors | Rhesus Monkey | Cocaine absent fruit given to pregnant dams | 10 mg/kg 2×daily Oral (fruit) | P1–28 | Deficits in orientation, state control, and motor maturity; impaired attention and increased tremulousness | He et al., 2004 |
| Motor | Rat | Saline injected pregnant dams | 60 mg/kg 1×daily,SC | P60–90 | Reduced threshold for flurothyl and kainic acid induced seizures | Baraban and Schwartzkroin, 1997 |
| Social communication | Mouse Rat Mouse Rat Rat |
Non-injected, saline injected, and PF-saline injected pregnant dams | 20 mg/kg 1×daily,SC 15 mg/kg 2×daily,SC 20 mg/kg 2×daily,SC 15 mg/kg 2×daily,SC 15 mg/kg 2×daily,SC |
P2, 3, 4 P1, 14, 21 P25,35,45 P5 P1 |
Altered number and rate of USVs; changes in the pitch, amplitude, duration, and harmonics under basal and stress-induced conditions and social interaction paradigms. |
Hahn et al., 2000 Cox et al., 2012 Kabir et al., 2014 Zeskind et al., 2014 Lippard et al., 2015 |
| Attention & cognition | Mouse | Saline, Phent, and PF-injected pregnant dams | 10, 20, 40 mg/kg 2×daily,SC | P9 P50, 100 |
Reduction in first order Pavlovian conditioning |
Kosofsky and Wilkins, 1998 Wilkins et al., 1998b |
| Attention & cognition | Rabbit | Saline injected pregnant dams | 20 mg/kg 1×daily,SC 3 mg/kg 2×daily,IV |
P27–29 P90–122 |
Working memory deficits |
Sobrian et al., 1995 Thompson et al., 2005 |
| Attention | Rat | Saline injected pregnant dams | 0.5, 1,3 mg/kg 1×daily (E8-E14) and 2×daily (E15-E21), IV | P220 | Impaired attention |
Gendle et al., 2003 Gendle et al., 2004b |
| Attention | Rabbit | Saline injected pregnant dams | 4 mg/kg 2×daily | P90 | Deficits in discrimination learning and attentional processing | Romano and Harvey, 1998 |
| Cognition | Rat | Saline injected pregnant dams | 40 mg/kg 1×daily,SC | P7 | Deficits in discrimination learning of an odor/milk association | Spear et al., 1989a, 1989b |
| Cognition | Rat | Saline injected pregnant dams | 3 mg/kg 2×daily,IV 30 mg/kg 1×daily,IP |
P42–50 P102–107 P64–68 |
Deficits in non-spatial working memory |
Morrow et al, 2002a, 2002b Salas-Ramirez et al., 2010 |
| Cognition | Rat Mouse |
Saline injected Pregnant Dams | 20 mg/kg 1×daily,IP 15 mg/kg 1×daily,SC 20 mg/kg 2×daily,SC 30 mg/kg 1×daily,IP |
P25 P27–31 P120 P69–73 |
Spatial memory deficits |
Bashkatova et al., 2005 Cutler et al., 1996 Lu et al., 2012 Salas-Ramirez et al., 2010 |
| Attention & cognition | Mouse | PF and ad-lib pregnant dams w/o cocaine inhalation | 10-50 mg of cocaine granules sublimated within an inhalation chamber | P30+ | Deficits in sustained visuo-spatial attention and spatial working memory | He et al., 2006b |
| Cognition | Mouse | Saline injected Pregnant Dams | 20 mg/kg 2×daily,SC | P60–P90 | Changes in the recall of fear extinction | Kabir et al., 2013 |
| Social behavior | Mouse | Saline injected PF and non-PF pregnant dams | 40 mg/kg 1×daily,SC 20 mg/kg 2×daily,SC |
P28–36 P24, 35 |
Altered social interactions and pinning behavior |
Wood et al., 1994 Wood et al., 1995 Kabir et al., 2014 |
| Social behavior & aggression | Rat | Non-treated and PF-saline injected pregnant dams | 40 mg/kg 1×daily,SC | P16, 29, 60–70 | Decreased ability to compete successfully for a nipple and increased aggression towards conspecifics | Johns et al., 1998 |
| Reward | Rat Mouse |
PF and non-PF saline injected pregnant dams | 20 mg/kg 2×daily,SC | ∼P150 | Increased cocaine seeking self-administration |
Keller et al., 1996b Rocha et al., 2002 |
| Reward | Rat Rabbit |
Non-treated and PF-saline injected pregnant dams | 40 mg/kg 1×daily,SC 3 mg/kg 2×daily, IV |
P110–150 P54–60 |
Behavioral tolerance and reduced sensitivity to psychostimulants (cocaine and AMPH) |
Heyser et al., 1994 Stanwood and Levitt, 2003 |
Attentional and cognitive deficits are also commonly found in animal models of PCE. Neonatal rhesus monkeys have been shown to have attentional deficits (He et al., 2004). PCE also results in long-lasting cognitive and attentional deficits in adult rodents and rabbits (Kosofsky and Wilkins, 1998; Wilkins et al., 1998b; Gendle et al., 2004a; Gendle et al., 2004b; Thompson et al., 2005), with specific emphasis on deficits in visual or visuospatial attention (Gendle et al., 2003; He et al., 2006b), similar to those human studies previously mentioned (Heffelfinger et al., 1997; Heffelfinger et al., 2002); however, see (Morrow et al., 2002b). While the underlying mechanism for these deficiencies is not clear, alterations in attentional processing following PCE have been found in adult rodents (Mactutus, 1999; Bayer et al., 2000; Brunzell et al., 2002; Morgan et al., 2002) and rabbits (Romano and Harvey, 1998). Some data suggest that these defects arise from PCE-induced changes in the dopamine D1 receptor signaling (Bayer et al., 2000).
Such attentional deficits may contribute to deleterious cognitive effects following PCE. While some researchers have found little to no evidence of cognitive deficits (Morris et al., 1996b; Paule et al., 1996; Galler and Tonkiss, 1998; Markowski et al., 2000), the majority of findings suggest the opposite. These deficits are apparent at a very early age, as prenatally cocaine-exposed neonatal rats were significantly impaired in the acquisition and retention of milk-odor associations relative to controls (Spear et al., 1989b), and demonstrated an initial inability to develop associations in a test of Pavlovian conditioning (Kosofsky and Wilkins, 1998). They also exhibited increased latencies to acquire conditioning to an aversive stimulus (Goodwin et al., 1992). In contrast, primates (∼6 months of age) do not exhibit any overt learning and memory deficits (Paule et al., 1996). In adults, a number of studies have found significant alterations in egocentric learning (Vorhees et al., 1995), as well as working memory—both spatial and non-spatial—following PCE (Sobrian et al., 1995; Cutler et al., 1996; He et al., 2006b; Morrow et al., 2002b; Bashkatova et al., 2005; Thompson et al., 2005; Salas-Ramirez et al., 2010; Lu et al., 2012); although these effects are potentially sex- and/or dose-dependent (Levin and Seidler, 1993; Vorhees et al., 1995). Others have noted deficits in visual discrimination tasks (Romano and Harvey, 1996, 1998; Chelonis et al., 2003), passive and active avoidance (Church and Overbeck, 1990), fear extinction recall (Kabir et al., 2013), as well as Pavlovian conditioning tests (Kosofsky, 1998; Wilkins et al., 1998b), among the different animal models of PCE.
As with humans, animals exposed prenatally to cocaine exhibit alterations in social behaviors. Similar to the PCE-induced cognitive deficits, these effects appear early in the postnatal period. For instance, PCE-exposed pups do not effectively compete with unexposed pups for time at the nipple, demonstrating deficits in social competition (Wood et al., 1994). Exposed rodents also exhibit less play behavior (e.g., pinning) and altered social interaction early on (Wood et al., 1994; Wood et al., 1995; Kabir et al., 2014). These deficits in social behavior seem to persist such that adults generally demonstrate deficits in social interaction (Johns and Noonan, 1995; Overstreet et al., 2000; Estelles et al., 2006b; Williams and Johns, 2014) and, most predominantly, increases in aggressive behaviors (Goodwin et al., 1992; Wood and Spear, 1998; Estelles et al., 2005). Maternal care is also an important factor in proper infant development, and dams administered cocaine demonstrate aberrant maternal behaviors, which can exacerbate the deleterious effects of PCE [for review, see (Williams and Johns, 2014)]. Moreover, female offspring exposed to cocaine in utero exhibit aberrant maternal behaviors themselves, including elevated aggression and disrupted pup retrieval time (Hess et al., 2002; McMurray et al., 2008). While it has been suggested that oxytocin plays a role in the long-term effects of PCE on social behavior (Williams and Johns, 2014) and that environmental enrichment ameliorates these outcomes (Neugebauer et al., 2004), additional research is needed to understand the underlying mechanisms involving PCE's role in social behaviors.
PCE-exposed animals also demonstrate distinct impairments in motor functions (Table 1). PCE invokes alterations in basal locomotor activity and rearing (Hutchings et al., 1989; Johns et al., 1992b; Peris et al., 1992; Vathy et al., 1993; Peeke et al., 1994; Laferriere et al., 1995; Vorhees et al., 1995; Tonkiss et al., 1996; Romano and Harvey, 1998; Johns et al., 1998; Schrott et al., 1998; Sithisarn et al., 2011); however see (Fung et al., 1989; Estelles et al., 2006b). Moreover, ambulatory and stereotypic effects were exacerbated following acute administration of dopaminergic ligands (e.g., cocaine, amphetamine, quinpirole, methylphenidate) (Meyer et al., 1992; Peris et al., 1992; Kunko et al., 1993; Sobrian et al., 1995; Kunko et al., 1996; Simansky and Kachelries, 1996; Stewart et al., 1998; Glatt et al., 2000; Tilakaratne et al., 2001; Torres-Reveron and Dow-Edwards, 2006; Estelles et al., 2006a; Lu et al., 2009; Sasaki et al., 2014); however, see (Benson et al., 1996). Changes in response to such challenges indicate that PCE affects dopaminergic circuitry and/or the corticostriatal motor pathway during development (Jones et al., 2000; Stanwood et al., 2001a; Tilakaratne et al., 2001; Crandall et al., 2004; McCarthy et al., 2011; McCarthy and Bhide, 2012; Sasaki et al., 2014), thus resulting in long-term dysfunction to these and related circuits. Interestingly, administering low concentration, intermittent doses of psychostimulants results in an intensified motor response in PCE-exposed animals. Termed behavioral sensitization, this phenomenon is one indicator of “drug wanting,” or the potential for addiction to a substance (Steketee and Kalivas, 2011). In addition, a number of studies indicate that prenatal exposure to cocaine exacerbates the stereotypic responses but not necessarily the locomotor effects, during behavioral sensitization (Byrnes et al., 1993; Melnick and Dow-Edwards, 2001; Crozatier et al., 2003; Guerriero et al., 2005). PCE can also decrease the rewarding or reinforcing properties of psychostimulants and other drugs of abuse (Heyser et al., 1992a; Heyser et al., 1992b; Heyser et al., 1994; Hecht et al., 1998; Gulley et al., 1999; Stanwood and Levitt, 2003; Estelles et al., 2006a; Malanga et al., 2007). However, others have shown that animals exposed to PCE are more sensitive to the rewarding properties of drugs. For instance, studies on PCE-exposed rodents indicate increased impulsivity (Sobrian et al., 2003; Hamilton et al., 2011), which can portend the potential for addiction, whereas in other studies PCE rodents exhibit augmented sensitivity to cocaine reward in conditioned place preference (Dow-Edwards et al., 2014), brain stimulation-reward (Lin and Kellogg, 1996; Malanga et al., 2008), and self-administration (Keller et al., 1996b; Rocha et al., 2002). Overall, these results suggest that gestational cocaine exposure affects the vulnerability of offspring to administer drugs of abuse later in life.
Finally, the effects of PCE are not limited to these behavioral domains. Animals developmentally exposed to cocaine demonstrate increased depression-like behaviors (Bilitzke and Church, 1992; Sobrian et al., 2003), elevated anxiety-like behavior (Johns et al., 1992a; Johns et al., 1992b; Johns et al., 1998; Salas-Ramirez et al., 2010; Sithisarn et al., 2011), abnormal sexual behavior (Raum et al., 1990; Cutler et al., 1996; Vathy and Marson, 1998), atypical responses in acoustic startle-prepulse inhibition (Sobrian et al., 1990; Bilitzke and Church, 1992; Overstreet et al., 2000), altered reactivity to stressors (Spear et al., 1989b; Campbell et al., 2000; Huber et al., 2001; Gendle et al., 2004a; Malanga et al., 2007), respiratory dysfunction (Moss et al., 1995; Lipton et al., 1996), catalepsy or seizures (Meyer et al., 1994; Baraban and Schwartzkroin, 1997), and arousal or state dysregulation (Moss et al., 1995; Strother et al., 1998; Gendle et al., 2004b), to name a few. Given the overlapping outcomes of preclinical PCE studies with those same effects in humans, using animal models to delve deeper into the biological mechanisms and underpinnings of PCE will potentially improve the outcomes for exposed humans.
Brain Anatomy
A primate model of PCE reported reduced head circumference (Ronnekleiv et al., 1998) and lasting effects on the overall brain and cortical volume, the total number of neocortical neurons, and the cell density, similar to the findings from human studies (Lidow, 1995; Lidow and Song, 2001b). In contrast, in rodent models, although some studies have also reported a reduction in brain volume and cortical thickness in the embryonic, newborn, and adult brain (Gressens et al., 1992b; He et al., 2006b; Lee et al., 2011), most rodent studies have failed to find significant effects on measures, such as brain and cortical structure and volume, as well as biparietal thickness of the cortex and thickness of cortical layers following PCE (Wang et al., 1995c; Jones et al., 1996; Johnson et al., 2002; Morrow et al., 2002a; Crandall et al., 2004; McCarthy and Bhide, 2012), suggesting a possible species-specific effect. In addition, no significant changes have been found for sub-cortical structures, such as the caudate, putamen, or amygdala (Hamilton et al., 2010). Interestingly, significant effects were found in some of these animal models between the saline pair-fed controls and the ad-lib controls, indicating the possible effect that nutrition may play in brain and cortical development (Kosofsky et al., 1994; Wilkins et al., 1998a).
In addition to potential deficits in brain volume and/or cortical thickness, body growth retardation has also been reported both in utero and postnatally by some preclinical studies. For instance, although these results are variable, some have demonstrated in utero growth retardation (Henderson and McMillen, 1990; Church and Rauch, 1992; Gressens et al., 1992a; Akbari et al., 1994) and decreased weight in newborns (Kosofsky et al., 1994); these effects appear to be dependent on the dose and duration of cocaine exposure. In contrast, others have suggested that no effect exists or only exists in comparison against saline controls and not saline pair-fed controls (Heyser et al., 1990; Wiggins and Ruiz, 1990a; Wiggins and Ruiz, 1990b; Kunko et al., 1993; Wilkins et al., 1998a). Taken together, there is relevant evidence from preclinical studies to support the aforementioned clinical studies, and, as with the clinical studies, the confounding factors add to discrepancies and variability in these important findings.
Neurotransmitter Mechanisms
Studies using animal models have significant advantages over human studies because animal studies permit detailed experimental analysis of neurotransmitter signaling mechanisms at the level of the neurotransmitter itself, as well as its transporters, receptors and second messenger systems. As cocaine binds to monoamine transporters in the fetal brain, PCE research has focused on dopamine (DA) serotonin (5-HT) and norepinephrine (NET) signaling mechanisms (see Table 2: Cellular and Molecular Findings). In one study using a guinea pig model of PCE, Lidow et al. (1999) described a decrease in the D1R and D2R expression in the dorsal forebrain within a few days of initial exposure (E25, with treatment beginning on E20); however, these expression levels significantly increased later in embryonic development (E40–E50) (Lidow et al., 1999). Similarly, β-adrenergic receptor expression in the embryonic brain was also initially decreased following the onset of cocaine exposure, but then was found to be significantly increased later throughout embryonic development (Lidow et al., 1999). In fact, this increase in β-adrenergic receptor expression seems to persist throughout adolescence and into adulthood in regions of the forebrain, such as the striatum and frontal cortex (Henderson et al., 1991). In addition, 5-HT1A and 5-HT2 receptor expression is also significantly decreased during early embryonic development, but then appears to normalize by late embryonic development (Lidow et al., 1999). In the adolescent brain, no changes in the 5-HT fiber density have been found following PCE (Wang et al., 1996b). Overall, 5-HT and its metabolite 5-HIAA appear to be decreased at P60, but then levels normalize by P180, indicating possibly transient effects of cocaine on 5-HT content (Henderson and McMillen, 1993). A variety of other models have seen substantial changes in the 5-HT system that persist postnatally (Snyder-Keller and Keller, 1993; Akbari et al., 1994; Meyer et al., 1996; Schrott and Sparber, 2001; Bolanos et al., 2000; Cabrera-Vera et al., 2000; Bolanos et al., 2002; Johns et al., 2002; Yan, 2002; Chen et al., 2004; Williams et al., 2011).
Table 2. Cellular and Molecular Findings.
| Measuring variable | Species | Control groups | Dose, route | Testing age | Outcomes | References |
|---|---|---|---|---|---|---|
| Monoamine expression | Guinea Pig | Saline injected pregnant dams | 20 mg/kg 2× daily, SC | E25, 30, 35, 40, 50 | Transient initial downregulation of DA, 5HT, and NE receptor expression followed by upregula-tion of DA and NE receptor expression. | Lidow et al., 1999 |
| 5HT system | Rat | Saline injected pregnant dams | 15 mg/kg 2×daily, SC | P30, 60, 180 | 5-HT its metabolite 5-HIAA reduced at P60 but normalized by P180 | Henderson and McMillen, 1993 |
| DA system | Rabbit | Saline injected pregnant dams | 2–4 mg/kg 2×daily, IV | E22, E25, P1, P10, P20, P50, P100 E15 |
Reduction in D1R coupling to GPCR in frontal and cingulate cortices due to improper D1R trafficking to the cell membrane. |
Wang et al., 1995a, 1995b, 1995c Friedman et al., 1996 Friedman and Wang 1998 Jones et al., 2000 Zhen et al., 2001 Harvey, 2004 Stanwood and Levitt, 2007 |
| DA system | Mouse | Saline injected pregnant dams | 20 mg/kg 2×daily, SC | E15 | Reduction in DAT activity, attenuation of D1R function and upregulation of D2R function in the dorsal and basal forebrain | Kubrusly and Bhide, 2010 |
| DA system | Rhesus Monkey Mouse |
Saline injected pregnant dams | 3 mg/kg, 4×daily, IM 3 mg/kg, 4×daily, IM 20 mg/kg 2×daily, SC |
E60 E70 E15 |
Transient changes in D1R and D2R expression in cortical and subcortical regions of the embryonic brain |
Choi and Ronnekleiv, 1996 Fang et al., 1997 Kubrusly et al., 2010 |
| DA system | Rabbit Rat |
Saline injected pregnant dams | 4 mg/kg 2×daily, IV 10 mg/kg 2×daily, SC |
P10, 50, 100 P40–190 |
Brain region specific alterations in DA release on stimulation by either K+ in vitro or by in vivo cocaine injection |
Wang et al., 1995b Keller et al., 1996a |
| Cortical lamination (inclusive of all neuronal types) | Mouse, Rhesus Monkey | Saline injected pregnant dams and cocaine absent fruit | 10, 20 mg/kg 2×daily, IP 20 mg/kg 2×daily, SC 10 mg/kg 2×daily, oral |
E15, E17, PI, P3, P60 P60, 3 yrs |
Lack of discernable lamination in specific regions of the frontal cortex. |
Gressens et al., 1992a, 1992b Kosofsky et al., 1994 Lidow, 1995 Lidow et al., 2001 |
| GABAergic system | Mouse Rat |
Saline injected pregnant dams | 10 and 20mg/kg 2×daily, SC 20mg/kg 2×daily, SC 20 mg/kg 2×daily, IP |
E13,15,17 E15,17,19 |
Decreased tangential migration of GABA neurons and reduced GABA neuron numbers in the dorsal forebrain |
Crandall et al., 2004 McCarthy et al., 2011 Lee et al., 2011 |
| GABAergic system | Mouse | Saline injected pregnant dams | 20 mg/kg 2×daily, SC | P60 | Reduced numerical density of PV + GABA neurons and decreased GABA-to-projection neuron ratio in the mPFC | McCarthy et al., 2012a, 2012b |
| GABAergic system | Rat | Saline injected pregnant dams | 3 mg/kg 2×daily, IV | P45 | Decreased number of spindle-shaped PV-ir neurons, PV-ir dendrites, and candles impinging on oyramidal neurons in the mPFC |
Morrow et al., 2003a Morrow et al., 2005 |
| Glutamatergic system | Rat | Saline injected pregnant dams | 20 mg/kg 2×daily, IP | E15, E17, E19 | Altered radial migration of pyramidal cells in the dorsal forebrain | Lee et al., 2011 |
| Glutamatergic system | Mouse Rabbit |
PF and non-PF saline injected and non-injected pregnant dams | 20 mg/kg 2×daily, SC 2–4 mg/kg 2×daily, IV |
P60 P1, 3, 5, 7, 10, 14, 20, 58 |
Reduced dendritic bundling and wavy appearance in frontal and anterior cingulate cortical pyramidal neurons |
Kosofsky et al., 1994 Jones et al., 1996 Murphy et al., 1997 Stanwood et al., 2001a, 2001b |
| Excitation and Inhibition | Rat | Saline injected pregnant dams | 15 mg/kg, IP3mg/kg, IV | P16, P42 P22–24 |
Reduced GABA receptor surface expression and decreased inhibition resulting in long-term potentiation of excitatory synapses in the mPFC |
Lu et al., 2009 Huang et al., 2011 |
| Glial cells | Mouse Rhesus Monkey |
Saline injected pregnant dams and cocaine absent fruit | 10, 20 mg/kg 2×daily, IP 10 mg/kg 2×daily, oral |
E17 P60 3 yrs |
Transient reduction in GFAP expression |
Gressens et al., 1992b Lidow, 1995 Lidow et al., 2001 |
| BDNF | Rat Mouse |
Saline injected pregnant dams | 30 mg/kg 1×daily, SC 20 mg/kg 2×daily, SC |
E13, 15, 17, P7, P30, P60 | Transient decrease in basal fore-brain BDNF protein expression at E15. Normalized constitutive expression in postnatal mPFC and striatum |
Yan et al., 2004 McCarthy et al., 2011 Kabir et al., 2013 Kabir et al., 2014 McCarthy et al., 2014 |
| Epigenetic modifications | Mouse | Saline injected pregnant dams | 20 mg/kg 2×daily, SC | P30, P60 | Increased Ach3k9,14 and decreased MeCP2 binding at BDNF exons in the mPFC | Kabir et al., 2013, 2014 |
| Proliferation & differentiation | Mouse | Saline injected pregnant dams | 20 mg/kg 2×daily, SC | E18 | Increased β-catenin protein levels in the dorsal forebrain | Novikova et al., 2005b |
| Proliferation | Rhesus Monkey | cocaine absent fruit | 10 mg/kg 2×daily, Oral (fruit) | E73, 113 | Periodic fluctuations in cell production within the fetal cortical proliferative zones at 1.5 and 10 hr following 3-HT injections. No change at E113 | Lidow and Song, 2001a, 2001b |
| Proliferation | Human primary neurons and A2B5+ progenitor cells | Control medium | 1, 10, 100 μM | 20 week old fetal cells and A2B5 + progenitor cells | Down regulated cyclin A2 induced reduction in proliferation | Lee et al., 2008 |
| Apoptosis and cell surviva | Mouse Rhesus Monkey Mouse Human |
Control medium cocaine absent fruit Saline injectedPregnant Dams |
100–500 μM 10 mg/kg 2×daily, Oral (fruit) 20 mg/kg 2×daily, SC 100 μM |
E15 E65 E18 E140 |
Increased cell death within cortical regions and within specific cell populations. Upregulation of the expression of apoptosis and cell survival genes |
Nassogne et al., 1995 Nassogne et al., 1997 He et al., 1999 Novikova et al., 2005a, 2005b Lee et al., 2009 |
There have been extensive studies on the role of dopamine in PCE. PCE produces increases in extracellular DA levels, resulting in sustained activation of the dopamine receptors (DARs), and subsequent impairment of receptor function. Specifically, PCE attenuates D1 receptor (D1R) activity by uncoupling the D1R from its Gs protein partners (Wang et al., 1995a; Friedman et al., 1996; Friedman and Wang, 1998; Jones et al., 2000; Zhen et al., 2001; Harvey, 2004; Kubrusly and Bhide, 2010). This effect persists throughout adolescence and into adulthood. The reduction in D1R-Gs coupling results from D1R internalization, due to improper D1R trafficking to the cell membrane (Stanwood and Levitt, 2007). These alterations in DA signaling could have profound and lasting effects on the developing brain. Specifically, D1R loss of function and PCE both produce morphological changes in the apical dendrites of pyramidal neurons, disruptions in their laminar positioning, changes in neurite outgrowth, and altered GABA neuron migration in the cerebral cortex (Bhide, 2009; Frederick and Stanwood, 2009; Thompson et al., 2009).
PCE also alters DAR binding site densities, protein expression, and mRNA expression (Choi and Ronnekleiv, 1996; Fang et al., 1997; Kubrusly and Bhide, 2010). Decreased D1R expression in the dorsal forebrain was observed in our mouse model on embryonic day 15; however, these effects were transient such that expression returned to levels similar to those in saline-exposed controls by adulthood (Kubrusly and Bhide, 2010). In the postnatal brain, studies have found decreased D1R expression and increases in the D2R mRNA expression in the striatum (Leslie et al., 1994), whereas another study did not find such changes in D1R expression in the striatum but rather only increased D2R expression (Scalzo et al., 1990).
Dopamine transporter (DAT) expression and function were also altered in the embryonic and adult brain following PCE (Leslie et al., 1994; Fang and Ronnekleiv, 1999; Kubrusly and Bhide, 2010). In addition, a transient reduction in the density of neurons containing tyrosine hydroxylase, the biosynthetic enzyme of DA and norepinephrine (NE), in the substantia nigra and ventral tegmental area were reported in the prenatally cocaine exposed embryos (Ronnekleiv and Naylor, 1995). In contrast, no changes in the overall DA content were found in the adolescent and adult forebrain areas (Wang et al., 1995b); however, on stimulation with K+, DA release was decreased in the frontal cortex and cingulate cortex in slice cultures from prenatally cocaine exposed embryos (Wang et al., 1995b). Moreover, this effect seems to be region specific, as no effect was found in the striatum following K+ stimulation (Wang et al., 1995b). In a separate study, subtle increases in DA release were found in the nucleus accumbens (NAc) following a tail pinch, whereas a robust increase in DA release was found following a cocaine injection in the NAc of young rats prenatally exposed to cocaine (Keller et al., 1996a). Overall, these changes in DA signaling in specific brain regions provide valuable clues regarding the potential mechanisms that may underlie many of the behavioral and cognitive deficits typically seen following PCE.
Neuronal Migration
PCE also alters tangential and radial migration of neurons in the developing rodent brain (Crandall et al., 2004; Lee et al., 2011; McCarthy et al., 2011) (Table 2). Specifically, rodent studies have shown that following PCE, fewer GABA neurons were present in the intermediate zone of the developing cortex during early and mid corticogenesis (Lee et al., 2011; McCarthy et al., 2011). These effects were transient, however, because by E17, a period of late corticogenesis, the deficits were no longer apparent (McCarthy et al., 2011). Interestingly, PCE led to a significant reduction in the lateral to medial gradient of GABA numerical densities at E17, with fewer GABAergic neurons in the medial prefrontal cortex (mPFC), compared to the dorsal PFC. Thus, PCE prevented the flattening of the lateral-to-medial gradient in the distribution of GABAergic neurons within the dorsal forebrain (McCarthy et al., 2011).
Once GABAergic neurons reach the dorsal forebrain via tangential migration, they begin to migrate radially until they settle in their final laminar positions within the dorsal forebrain. Whether PCE specifically affects the ability of GABAergic neurons to migrate radially on their arrival in the dorsal forebrain is not known; however, decreases in the numerical densities of GABA neurons within certain laminae of the cerebral cortex may suggest deficits in their radial migration. PCE alters the radial migration of the excitatory glutamatergic pyramidal cells in the embryonic brain (Lee et al., 2011; McCarthy et al., 2011). Following PCE, there is an increase in the number of post-mitotic cortical projection neurons present in the ventricular zone/subventricular zone (VZ/SVZ), indicating an inability of these cells to migrate away from the proliferative zones of the VZ and SVZ and into the post-mitotic zones (Lee et al., 2011; McCarthy et al., 2011).
Although the specific molecular mechanisms associated with PCE-mediated delay in neuronal migration remain unclear, the delay in tangential migration could be due to a combination of factors, including cocaine's direct effects on DA signaling and function. In fact, we have shown that the tangential migration of GABA neurons is influenced by DAR activation (Crandall et al., 2007). D1R activation promotes tangential neuronal migration, whereas D2R activation inhibits it. The second mechanism by which cocaine may be exerting its effects involves an increase in Nkx2-1 expression in the basal forebrain. Nkx2-1 is a homeobox transcription factor that mediates tangential migration of GABA neurons from the basal to the dorsal forebrain, and neurons with higher Nkx2-1 expression are more likely to remain in the basal forebrain and not migrate out via tangential migration. We found higher Nkx2-1 expression in the basal forebrain during early brain development (E13 and E15), thereby providing an alternative mechanism for this delayed tangential migration (McCarthy et al., 2011). Thirdly, 5-HT has been shown to directly influence GABA interneuron migration via the 5-HT6 receptor (Riccio et al., 2009). Due to cocaine's effects on the 5-HT system, this could potentially be another mechanism by which PCE is exerting its effects on the developing brain.
Lastly, cocaine's effects on neuronal migration may be a result of impaired brain-derived neurotrophic factor (BDNF) expression (see also Table 2). BDNF is a neurotrophin involved in neuronal migration, cell survival, growth, differentiation, neurite outgrowth, and synaptogenesis (Huang and Reichardt, 2003; Binder and Scharfman, 2004). BDNF is highly expressed in the fetal brain, particularly in the basal and dorsal forebrain (Meyer et al., 1996; McCarthy et al., 2011). BDNF protein expression is reduced in the basal forebrain of PCE embryos during a gestational period of peak tangential neuronal migration (McCarthy et al., 2011), and application of exogenous BDNF to basal forebrain explants from PCE embryos rescued the migration defects, implicating BDNF as a molecular mediator of cocaine effects on tangential migration (McCarthy et al., 2011; McCarthy et al., 2014). (See below for additional discussion of possible roles for BDNF).
These effects on neuronal migration even persist throughout adulthood (Gressens et al., 1992b; Kosofsky et al., 1994; Lidow, 1995; Lidow et al., 2001; Lidow and Song, 2001b; McCarthy et al., 2012b). Specifically, in a non-human primate PCE study, newly generated cortical neurons labeled with [3H]-thymidine during the fetal period (E65) did not reach their final position within the cortical plate of the visual cortex in both adolescent and adult rhesus monkeys. Specifically, in PCE monkeys, neurons born at this time were spread across cortical layers IV, V, VI, and the white matter rather than being restricted to layers IV and V, resulting in a neocortex lacking discernible lamination (Lidow, 1995; Lidow and Song, 2001b). Mouse models of PCE have also reported a disruption in the final laminar position of these labeled neurons, ultimately resulting in the blurring of the laminar borders within the dorsolateral PFC (Gressens et al., 1992b; Kosofsky et al., 1994). Furthermore, these neuronal migration defects are not limited to the cortical regions: PCE has also been shown to disrupt the laminar position of pyramidal cells in the hippocampal CA1 cell layer (Baraban et al., 1999). Despite several different animal models suggesting long-term deficits in laminar positioning following PCE, other studies that used a lower prenatal cocaine dose failed to show similar results in regions of the mPFC and the anterior cingulate cortex (ACC) (Jones et al., 1996; Wang et al., 1996a; Morrow et al., 2005). In conclusion, it appears that deficits in migration following PCE appear to leave persisting deficits in the adult brain, and may contribute to deficits in cognitive function in exposed individuals.
Cytoarchitecture: Cell Densities, Differentiation and Synaptogenesis
GABA neurons
Long-term repercussions of PCE have been found in the GABA and glutamate neurotransmitter systems. This should not be surprising, given the effects of PCE on the migration and laminar positioning of GABA and glutamate neurons during brain development. One study in particular found a reduction in the number of GABA neurons in the mPFC (McCarthy and Bhide, 2012). The reduction was more pronounced in the upper mPFC (layers II-III), and could be partially attributed to selective reduction in the numbers of parvalbumin (PV)-containing GABA neurons. PCE also produces a decrease in the GABA-to-projection neuron ratio in the mPFC (McCarthy and Bhide, 2012). In contrast, several studies have shown no detectable differences in the total number or numerical densities of GABA neurons in other brain regions, such as the PFC, piriform cortex, ACC, and entorhinal cortex (Wang et al., 1995c; Wang et al., 1996a; Stanwood et al., 2001a; Stanwood et al., 2001b; Morrow et al., 2003a,), which may be partially attributed to a different cocaine dose and animal model. Interestingly, Morrow et al. also examined the types of PV-containing GABA neurons in the mPFC based on their shape and found a decrease in the number of spindle shaped PV-immunoreactive (PV-ir) neurons in PCE rats, indicating that differences in cell numbers and densities may be particular to certain subtypes of cells (Morrow et al., 2005). Conversely, other studies have demonstrated increased expression of PV-ir in the dendrites of interneurons in the PFC, ACC, and piriform cortices (Wang et al., 1995c; Wang et al., 1996a; Murphy et al., 1997; Stanwood et al., 2001a; Stanwood et al., 2001b). These effects were even seen at low doses of cocaine exposure (2–4 mg/kg) and were specific for periods of peak corticogenesis. Moreover, these effects are not limited solely to PV-ir cells. Others have shown a reduction in the GABAergic axo-axonic structures in the mPFC called candles, which are the axon terminals of GABAergic cells that synapse on the axons of excitatory pyramidal neurons, and are important for the inhibitory control of excitatory neurons (Morrow et al., 2003a). Overall, these deficits suggest alterations in neocortical connectivity that can contribute to the aforementioned behavioral and cognitive deficits following PCE.
Glutamatergic neurons
PCE has also been shown to produce long-lasting anatomical alterations in the glutamatergic system. In fact, one study found an increase in the numerical density of glutamatergic neurons (McCarthy and Bhide, 2012). Several studies in a rabbit model of PCE have also shown that PCE produces alterations in the morphology and distribution of glutamatergic pyramidal neurons (Jones et al., 1996; Jones et al., 2000; Stanwood et al., 2001a; Stanwood et al., 2001b; Ismail and Bedi, 2007), likely through a dopaminergic mechanism (Stanwood et al., 2005; Stanwood and Levitt, 2007). There is also an attenuation of AMPAR-mediated long-term depression within the mPFC following PCE, likely due to changes in the phosphorylation and activity of trafficking molecules important for AMPAR synaptic targeting (Bakshi et al., 2009). Furthermore, PCE has been shown by others to lead to decreased inhibition and increased excitation in the mPFC, by elevating neuronal excitability and activity-induced long-term potentiation (LTP) (Lu et al., 2009; Huang et al., 2011). In addition, increased excitability of hippocampal pyramidal neurons via a reduction in action potential threshold has also been found (Baraban and Schwartzkroin, 1997), possibly due to a reduction in the surface expression of GABAA receptor subunits, α1, β2, and β3 (Lu et al., 2009). Because LTP can result from a reduction in the GABAA receptor-mediated inhibition of mPFC pyramidal neurons, PCE-induced excitability of these neurons is attributed to the reduction in GABAergic inhibition. Lastly, cocaine-exposed rats showed increased Fos expression in the mPFC after handling, compared to saline controls, but decreased footshock-induced Fos (Morrow et al., 2002a); also suggesting a disruption in the excitatory/inhibitory balance in the mPFC, which may contribute to the underlying behavioral and cognitive deficits following PCE.
Although PCE-induced deficits in neuronal migration may underlie the persistent alterations in GABA and glutamatergic neuron distribution and density, this is not the only plausible mechanism. Developmental processes, such as neurogenesis, proliferation, cell death, and differentiation may themselves mediate such changes; particularly as the cocaine exposure is ongoing during these critical windows of development (see below).
PCE also produces changes in cortical excitatory neuronal morphology. Normally, the apical dendrites of glutamatergic projection neurons ascend to the pial surface in long and straight bundles. In contrast, PCE has been shown to produce thinner and longer “wavy” apical dendrites in the ACC, PFC, and entorhinal cortex (Kosofsky et al., 1994; Jones et al., 1996; Murphy et al., 1997; Stanwood et al., 2001b). Interestingly, no changes were found in the somatosensory cortex, a DA-poor brain region, indicating that cocaine's effect on DA likely plays a role during dendritogenesis. In addition, PCE increased the number of excitatory synaptic inputs, also indicating cocaine's potential effects on regional excitability. This may represent another mechanism (in addition to decreased inhibition) by which PCE enhances the activation of frontal pyramidal neurons (Morrow et al., 2007). Interestingly, the onset of corticogenesis and cocaine's effects on modified neurite outgrowth is paralleled by the uncoupling of the D1R and its G protein signaling, as previously described (Stanwood and Levitt, 2007).
A number of other mechanisms have been proposed to explain the effects of PCE on cellular function. One such example is via the Wnt and cadherin signaling pathways. Cadherins are a family of transmembrane proteins that are involved in cell-cell adhesion and their activity is mediated in a Ca2+-dependent manner. It has been demonstrated that these molecules are important for cell motility and migration, specifically elongation, branching, and synapse formation of neuronal processes (Ranscht, 2000; Togashi et al., 2002; Yu and Malenka, 2003; Poskanzer et al., 2003;), roles that are facilitated via a β-catenin-α-catenin bridge (Ranscht, 2000; Logan and Nusse, 2004). In fact, Wnt-/β-catenin-induced gene transcription is known to be involved in the regulation of cell proliferation and neuron-specific differentiation of progenitor cells. Interestingly, following PCE, there was an increase in β-catenin protein levels throughout the frontal cortex of E18 mouse embryos (Novikova et al., 2005b). In addition, the transcription of various genes involved in the Wnt and cadherin signaling pathways was also affected in these embryos, indicating that cocaine's effects on the Wnt-cadherin system likely plays an important role in mediating cocaine's effects on the cytoarchitecture and the subsequent behavioral and cognitive deficits.
Glial cells
PCE has been shown to affect not only neuronal cell densities but also glial cells. In fact, multiple studies have shown a reduction in the density of radial glial fibers in the embryonic brain and in the expression of glial fibrillary acidic protein (GFAP) in the postnatal brain following PCE (Gressens et al., 1992b; Lidow, 1995). Specifically, the mouse model demonstrated these findings in the E17 embryonic brain (Gressens et al., 1992b), whereas, the primate model demonstrated reduced GFAP labeling in the upper cortical layers in 2-month old rhesus monkeys (Lidow, 1995). However, in the same primate model, by 3 years of age, no differences in the neocortical GFAP labeling could be detected, indicating a delay in gliogenesis following PCE (Lidow et al., 2001). In fact, defasciculation of radial glial bundles was demonstrated in a mouse model of PCE (Kosofsky et al., 1994) and may be the reason for undetectable differences in those studies that examine glial fiber densities in the postnatal brain (Jones et al., 1996).
Neurogenesis
PCE has been shown to alter neurogenesis in the developing brain. Many studies indicate that PCE produces significant effects on the proliferation of cells in the dorsal cerebral wall (Lee et al., 2008; Lidow and Song, 2001a; Lee et al., 2011), whereas a mouse model of PCE found no such differences (Crandall et al., 2004; McCarthy et al., 2011). In one study, using a non-human primate animal model, PCE was shown to produce opposing effects on neurogenesis in the embryonic neocortex during cocaine exposure—decreased neurogenesis shortly after cocaine exposure and increased neurogenesis 10 hr later in the short term and increased neurogenesis later (Lidow and Song, 2001a). Similar observations were made in neonatal rats (Anderson-Brown et al., 1990). Together, these findings indicate periodic fluctuations in cell proliferation within the fetal brain. This is likely due to the fact that cocaine has previously been shown to lead to the accumulation of dividing cells at the G1/S transition, such that when cocaine levels decline, cells are then able to enter S phase and continue their progression within the cell cycle (Di Francesco et al., 1990). Subsequently, Lidow and Song (2001a, 2001b) examined the long-term effects of PCE on neurogenesis and found no such differences. Therefore, the initial suppression of cell proliferation produced by cocaine administration in this study leads to a compensatory burst of proliferative activity which consequently normalizes the effects that PCE has on neurogenesis over time.
These results are difficult to interpret, however, without further analysis of cell cycle parameters and cell output rates. In one PCE study that did examine the cell output using Ki67-BrdU double-labeling to count the number of cells that became post-mitotic over a 24-hr period, no significant differences were found (McCarthy et al., 2011). Interestingly, there was an increase in the number of postmitotic cells in the VZ and SVZ of the cerebral wall, compared to the intermediate zone and cortical plate, indicating a possible delay in the migration of these cells away from the proliferative zones (McCarthy et al., 2011). Overall, differences among the experimental techniques and animal models used in these studies may account for diverse results. These include using different thymidine analogs ([3H]thymidine versus BrdU), differences in the timing of the thymidine analog injection (1.5 vs. 10 vs. 24 hr), differences in the gestational timing between animal models, the route of administration (oral administration versus subcutaneous injection), different doses used for each (10 mg/kg twice daily versus 20 mg/kg twice daily), along with differences in the pharmacodynamic profile of cocaine for each of these animal models.
A possible mechanism for these changes in the cell cycle was elucidated using cell cultures with neural progenitor AF5 cells. This study found that 24 hr of cocaine treatment reduced the total number of cells within the culture and also reduced BrdU incorporation (Lee et al., 2008). Specifically, cocaine exposure resulted in a dose-dependent increase in the number of cells in G1 phase of the cell cycle, whereas fewer cells were found in S phase, indicating, similar to the aforementioned findings, that cocaine had suppressed the G1-to-S phase transition. Subsequently, microarray, qRT-PCR, and western blot analysis all revealed a decrease in the expression of cyclin A2, a cell cycle-related gene following PCE. This effect of cocaine on cyclin A2 expression was also confirmed using human fetal cortical cells exposed to cocaine in vitro and tissue from the neocortex of rat embryos receiving PCE during early (E13–E14) and middle (E15–E16) periods of neurogenesis. In this same study, cocaine exposure during early and late neurogenesis also reduced the percent of BrdU-Ki67 double labeling in the VZ, but not the SVZ (Lee et al., 2008), also indicating changes in the cell cycle. It should be noted that although changes in transcription factors or cell cycle regulatory molecules may occur following cocaine exposure, these effects may be transient.
Cell death
Once again, contrasting findings are reported regarding the effects of PCE on apoptosis in the brain (Nassogne et al., 1995; Nassogne et al., 1997; He et al., 1999; Novikova et al., 2005a; Lee et al., 2009; McCarthy et al., 2011). In a primate model that examined the effects of PCE on cell death during early corticogenesis (E50–E65), TUNEL labeling of DNA fragmentation (indicative of apoptosis) was increased in all neocortical regions examined, including the proliferative zones, the intermediate zone, and the cortical plate (including the marginal zone) (He et al., 1999). This study did not, however, distinguish between neurons and glial cells. When others did differentiate between neuronal and glial cell types, apoptosis was found to be specific to certain cell types. Using an in vitro culture system, embryonic cortical cells were exposed to cocaine for either 48 or 96 hr at various concentrations of cocaine (100–500 μM) (Nassogne et al., 1995; Nassogne et al., 1997). Both studies found that by examining the cell numbers, morphological features, and TUNEL labeling of DNA fragmentation, increasing concentrations of cocaine and increased length of exposure each promoted apoptosis to greater extents. These studies also found that cocaine differentially affects neurons and not glial cells, as measured by MAP2 and GFAP immunostaining, respectively. In contrast, others have shown that PCE in the mouse embryo does not, in fact, induce detectable apoptosis in regions of the mPFC, ganglionic eminence, and striatum (McCarthy et al., 2011).
Previous reports have suggested that cocaine exposure regulates expression of various genes associated with cell death and cell survival in different cell populations of the neocortex (Novikova et al., 2005a; Lee et al., 2009). Specifically, using microarray and qRT-PCR analysis, one particular study demonstrated that PCE in the mouse upregulated 35 pro-apoptotic and 8 anti-apoptotic genes and downregulated 4 pro-apoptotic and 6 anti-apoptotic genes (Novikova et al., 2005a). This study did not, however, distinguish between neuronal and glial cell types. Another study that did differentiate between the two found no changes in the expression of genes related to cell death or cell survival in neurons; whereas gene expression changes were found in glial cells (Lee et al., 2009). Specifically, the pro-apototic genes IL-1β and BAX were upregulated in microglia whereas the anti-apoptotic genes 14-3-3ε and HVEM were upregulated in astrocytes following 24 hr of cocaine exposure in a culture containing human fetal cortical cells at 20 weeks of gestation (Lee et al., 2009). Collectively, these findings suggest that cocaine affects multiple apoptosis-regulating pathways, and indicate that different cell types that comprise the developing neocortex respond to cocaine differently. Taken together, differences among cell types may partially contribute to the variability seen across the PCE literature. In addition, the cocaine dose, route of administration, animal model, pharmacodynamics profile of cocaine in each of these models, as well as genetic and nutritional factors likely play a large role.
BDNF
BDNF expression has been intimately associated with the mesolimbic dopamine pathway (McGinty et al., 2010; McCarthy et al., 2012a) and repeated exposure to cocaine leads to increased BDNF expression in the mesolimbic pathway (for review see (McGinty et al., 2010; McCarthy et al., 2012a; Li and Wolf, 2015). It is found within the dopaminergic neurons of the VTA, glutamatergic neurons of the PFC, and GABAergic neurons of the NAc. As changes in BDNF expression have been shown in the embryonic brain following PCE and both the GABA and glutamatergic systems are altered by PCE, changes in BDNF expression may also occur in the adult brain, and potentially underlie the cognitive deficits typically seen in children prenatally exposed to cocaine (Yan et al., 2004; McCarthy et al., 2011; Tropea et al., 2011; Kabir et al., 2014). A role for BDNF has been found in rat pups that were prenatally exposed to cocaine, such that lower total BDNF protein content under basal conditions in the hippocampus was found, whereas, no differences were found in the frontal cortex or striatum (Yan et al., 2004) (Table 2). Interestingly, depolarization of neurons in the hippocampus, cortex, and striatum slices resulted in blunted BDNF expression in PCE pups compared to saline-treated controls (Yan et al., 2004), indicating activity-dependent changes in BDNF signaling. Other studies that examined BDNF expression under basal conditions in adolescent and adult mice found no change in BDNF protein expression in multiple brain regions at either age (Tropea et al., 2011, Kabir et al 2013, 2014). At the gene level, there was a transient increase in mRNA expression of exon IV in the mPFC of adolescents whereas no changes were found in adult mice (Kabir et al., 2013; Kabir et al., 2014). In addition, the mRNA and protein expression of egr1, a downstream signaling molecule of BDNF, was also increased in the adolescent mice following PCE (Kabir et al., 2013). These alterations in BDNF protein and mRNA expression were accompanied by increased acetylation of histone 3 lysine residues 9 and 14 acetylation (acH3K9,14) of exon IV in adolescent mice and a possible decrease in methyl-CpG-binding protein 2 (MeCP2) of exons I and IV of adolescent and adult mice, both of which are thought to be associated with an open histone configuration and an increase in gene transcription (Kabir et al., 2013; Kabir et al., 2014) (Table 2). The same group examined BDNF expression, with regard to fear extinction behavior in prenatally cocaine exposed mice, and found decreased BDNF protein expression in the mPFC of adult mice, compared to saline controls during fear extinction tasks (Kabir et al., 2013). Subsequently, BDNF microinfusion into the mPFC rescued these behavioral deficits in fear extinction, a cognitive behavior that is heavily dependent on mPFC BDNF levels (Peters et al., 2010; Kabir et al., 2013). Similarly, this group also completed a study that used a single nucleotide polymorphism commonly found in the BDNF gene (of which 20–30% of Caucasians carry), and found changes in BDNF expression in the hippocampus, a region important for learning and memory, as well as deficits in the fear extinction task following PCE (Kabir et al., 2012). Collectively, changes in BDNF expression and signaling seem to persist throughout adolescence and into adulthood, and are associated with behavioral deficits associated with learning and extinction memory. Thus, these long-lasting molecular and epigenetic changes may be playing a crucial role in BDNF-mediated cognitive outcomes.
Concluding Remarks
Our aim in this review was to provide evidence suggesting behavioral and cognitive deficits following PCE and to provide insights into the underlying cellular and molecular mechanisms. PCE has been shown to have serious consequences for the mother and the child. In addition, children prenatally exposed to cocaine exhibit long-term deficits in arousal and attention, emotional reactivity, and reward systems. Recent functional imaging studies reveal subtle, but significant, behavioral and cognitive effects. However, a detailed analysis of executive function, decision-making, emotional dysregulation, and propensity for risky behaviors have yet to be elucidated. Findings from longitudinal studies in young adults that were prenatally exposed are now becoming available and reveal persistent behavioral and cognitive deficits following PCE. Specifically, brain regions with DA-rich innervation, such as the PFC, ACC, or striatum, which are involved in attention and arousal, are thought to be impaired in PCE children, and yet these circuits do not fully mature until the third decade of life. Therefore, these longitudinal studies are crucial to fully understand cocaine's effects.
Importantly, preclinical animal models of PCE have provided substantial mechanistic evidence, suggesting that PCE does indeed alter brain developmental trajectories leading to long-term behavioral and cognitive deficits. These animal models allow us to control for confounding factors, such as maternal multidrug use, genetic variability, and postnatal environment, which are unavoidable limitations of human studies. In addition, animal models can tease apart the effects of cocaine on brain, behavioral, and cognitive development, based on factors such as gestation timing and the level of cocaine exposure. Recent and ongoing animal models studies have also allowed us to explore epigenetic changes and transgenerational effects of PCE through its effects on germ cells.
Taken together, both clinical and preclinical studies continue to provide valuable insight into the effects of PCE on early brain development, as well as its long-term behavioral and cognitive implications.
Acknowledgments
Supported by grant from R01MH086629, R21DA35588 (to G.D.S.), a NARSAD Independent Investigator award, and the FSU College of Medicine and The Jim and Betty Ann Rodger's Chair Fund (to P.G.B.).
References
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100 beta and induced microcephaly: reversal by postnatal 5-HT1A receptor agonist. Neurosci Lett. 1994;170:141–144. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Akyuz N, Kekatpure MV, Liu J, et al. Structural brain imaging in children and adolescents following prenatal cocaine exposure: preliminary longitudinal findings. Dev Neurosci. 2014;36:316–328. doi: 10.1159/000362685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JW, Bennett DS, Carmody DP, et al. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicol Teratol. 2014;41:65–70. doi: 10.1016/j.ntt.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Brown T, Slotkin TA, Seidler FJ. Cocaine acutely inhibits DNA synthesis in developing rat brain regions: evidence for direct actions. Brain Res. 1990;537:197–202. doi: 10.1016/0006-8993(90)90358-i. [DOI] [PubMed] [Google Scholar]
- Angelilli ML, Fischer H, Delaney-Black V, et al. History of in utero cocaine exposure in language-delayed children. Clin Pediatr. 1994;33:514–516. doi: 10.1177/000992289403300901. [DOI] [PubMed] [Google Scholar]
- Avants BB, Hurt H, Giannetta JM, et al. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ayoola AB, Stommel M, Nettleman MD. Late recognition of pregnancy as a predictor of adverse birth outcomes. Am J Obstet Gynecol. 2009;201:156.e151–156.e156. doi: 10.1016/j.ajog.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, et al. Gestational cocaine exposure and intrauterine growth: maternal lifestyle study. Obstet Gynecol. 2002;100:916–924. doi: 10.1016/s0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, et al. Low birth weight and pre-term births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- Bakshi K, Gennaro S, Chan CY, et al. Prenatal cocaine reduces AMPA receptor synaptic expression through hyperphosphorylation of the synaptic anchoring protein GRIP. J Neurosci. 2009;29:6308–6319. doi: 10.1523/JNEUROSCI.5485-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Vogel AL, Morrow CE, et al. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Accornero VH, et al. Estimated effects of in utero cocaine exposure on language development through early adolescence. Neurotoxicol Teratol. 2011;33:25–35. doi: 10.1016/j.ntt.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Schwartzkroin PA. Effects of prenatal cocaine exposure on the developing hippocampus: intrinsic and synaptic physiology. J Neurophysiol. 1997;77:126–136. doi: 10.1152/jn.1997.77.1.126. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Wenzel HJ, Castro PA, Schwartzkroin PA. Hippocampal dysplasia in rats exposed to cocaine in utero. Brain Res Dev Brain Res. 1999;117:213–217. doi: 10.1016/s0165-3806(99)00106-6. [DOI] [PubMed] [Google Scholar]
- Bashkatova V, Meunier J, Maurice T, Vanin A. Memory impairments and oxidative stress in the hippocampus of in-utero cocaine-exposed rats. Neuroreport. 2005;16:1217–1221. doi: 10.1097/00001756-200508010-00017. [DOI] [PubMed] [Google Scholar]
- Bauer CR, Langer JC, Shankaran S, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- Bayer LE, Brown A, Mactutus CF, et al. Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J Neurosci. 2000;20:8902–8908. doi: 10.1523/JNEUROSCI.20-23-08902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson KA, Ali SF, Wilson MC. The effects of prenatal cocaine exposure on dopaminergic challenge and receptor binding in Wistar rats. Ann N Y Acad Sci. 1996;801:289–300. doi: 10.1111/j.1749-6632.1996.tb17449.x. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Bilitzke PJ, Church MW. Prenatal cocaine and alcohol exposures affect rat behavior in a stress test (the Porsolt swim test) Neurotoxicol Teratol. 1992;14:359–364. doi: 10.1016/0892-0362(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors (Chur, Switzerland) 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol N, Fuchs M, Diaz V, et al. Teratogenicity of cocaine in humans [published erratum appears in. J Pediatr 1987 Mar; 110(3):350] J Pediatr. 1987;110:93–96. doi: 10.1016/s0022-3476(87)80297-4. [DOI] [PubMed] [Google Scholar]
- Bland-Stewart LM, Seymour HN, Beeghly M, Frank DA. Semantic development of African-American children prenatally exposed to cocaine. Semin Speech Lang. 1998;19:167–186. doi: 10.1055/s-2008-1064043. quiz 186–167. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Trksak GH, Glatt SJ, Jackson D. Prenatal cocaine exposure increases serotonergic inhibition of electrically evoked acetylcholine release from rat striatal slices at adulthood. Synapse. 2000;36:1–11. doi: 10.1002/(SICI)1098-2396(200004)36:1<1::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Trksak GH, Cohen OS, Jackson D. Differential serotonergic inhibition of in vitro striatal [3H]acetylcholine release in prenatally cocaine-exposed male and female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1339–1348. doi: 10.1016/s0278-5846(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Braude MC, Szeto HH, Kuhn CM, et al. Perinatal effects of drugs of abuse. Fed Proc. 1987;46:2446–2453. [PubMed] [Google Scholar]
- Brunzell DH, Ayres JJ, Meyer JS. Effects of prenatal cocaine exposure on latent inhibition in 1-year-old female rats. Pharmacol Biochem Behav. 2002;72:795–802. doi: 10.1016/s0091-3057(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Pritchard GA, Koff JM, Miller LG. Prenatal cocaine exposure: decreased sensitization to cocaine and decreased striatal dopamine transporter binding in offspring. Neuropharmacology. 1993;32:721–723. doi: 10.1016/0028-3908(93)90087-j. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Garcia F, Pinto W, Battaglia G. Neurochemical changes in brain serotonin neurons in immature and adult offspring prenatally exposed to cocaine. Brain Res. 2000;870:1–9. doi: 10.1016/s0006-8993(00)02382-9. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Bliven TD, Silveri MM, et al. Effects of prenatal cocaine on behavioral adaptation to chronic stress in adult rats. Neurotoxicol Teratol. 2000;22:845–850. doi: 10.1016/s0892-0362(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol Teratol. 2011;33:61–68. doi: 10.1016/j.ntt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carzoli RP, Murphy SP, Hammer-Knisely J, Houy J. Evaluation of auditory brain-stem response in full-term infants of cocaine-abusing mothers. Am J Dis Child. 1991;145:1013–1016. doi: 10.1001/archpedi.1991.02160090065024. [DOI] [PubMed] [Google Scholar]
- Chae SM, Covington CY. Biobehavioral outcomes in adolescents and young adults prenatally exposed to cocaine: evidence from animal models. Biol Res Nurs. 2009;10:318–330. doi: 10.1177/1099800408330395. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ. Drug use and women: establishing a standard of care. Ann N Y Acad Sci. 1989;562:208–210. doi: 10.1111/j.1749-6632.1989.tb21019.x. (1) [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ. Cocaine, pregnancy, and the growing child. Curr Prob Pediatr. 1992;22:302–321. doi: 10.1016/0045-9380(92)90027-v. discussion 322. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR. Cocaine: clinical studies of pregnancy and the newborn. Ann N Y Acad Sci. 1989;562:260–266. doi: 10.1111/j.1749-6632.1989.tb21024.x. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Burns WJ, Schnoll SH, Burns KA. Cocaine use in pregnancy. N Engl J Med. 1985;313:666–669. doi: 10.1056/NEJM198509123131105. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Burns KA, Burns WJ. Cocaine use in pregnancy: perinatal morbidity and mortality. Neurotoxicol Teratol. 1987;9:291–293. doi: 10.1016/0892-0362(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Chisum GM, Kaplan WE. Maternal cocaine use and genitourinary tract malformations. Teratology. 1988;37:201–204. doi: 10.1002/tera.1420370304. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, MacGregor S, et al. Temporal patterns of cocaine use in pregnancy: perinatal outcome. JAMA. 1989a;261:1741–1744. [PubMed] [Google Scholar]
- Chasnoff IJ, Hunt CE, Kletter R, Kaplan D. Prenatal cocaine exposure is associated with respiratory pattern abnormalities [see comments] Am J Dis Child. 1989b;143:583–587. doi: 10.1001/archpedi.1989.02150170085028. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol. 2003;25:437–446. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Chen Z, Waimey K, Van de Kar LD, et al. Prenatal cocaine exposure potentiates paroxetine-induced desensitization of 5-HT2A receptor function in adult male rat offspring. Neuropharmacology. 2004;46:942–953. doi: 10.1016/j.neuropharm.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Bateman DA, Brust JCM, Allen Hauser W. Neurologic findings in neonates with intrauterine cocaine exposure. Pediatr Neurol. 1993;9:115–119. doi: 10.1016/0887-8994(93)90045-e. [DOI] [PubMed] [Google Scholar]
- Choi WS, Ronnekleiv OK. Effects of in utero cocaine exposure on the expression of mRNAS encoding the dopamine transporter and the D1, D2 and D5 dopamine receptor subtypes in fetal rhesus monkey. Brain Res Dev Brain Res. 1996;96:249–260. doi: 10.1016/0165-3806(96)00123-x. [DOI] [PubMed] [Google Scholar]
- Church MW, Overbeck GW. Prenatal cocaine exposure in the Long-Evans rat: II. Dose-dependent effects on offspring behavior. Neurotoxicol Teratol. 1990;12:335–343. doi: 10.1016/0892-0362(90)90052-e. [DOI] [PubMed] [Google Scholar]
- Church MW, Rauch HC. Prenatal cocaine exposure in the laboratory mouse: effects on maternal water consumption and offspring outcome. Neurotoxicol Teratol. 1992;14:313–319. doi: 10.1016/0892-0362(92)90037-b. [DOI] [PubMed] [Google Scholar]
- Cox ET, Hodge CW, Sheikh MJ, et al. Delayed developmental changes in neonatal vocalizations correlates with variations in ventral medial hypothalamus and central amygdala development in the rodent infant: effects of prenatal cocaine. Behav Brain Res. 2012;235:166–175. doi: 10.1016/j.bbr.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, Hackett HE, Tobet SA, et al. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, et al. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier C, Guerriero RM, Mathieu F, et al. Altered cocaine-induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain Res Dev Brain Res. 2003;147:97–105. doi: 10.1016/j.devbrainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol. 1996;18:635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- Davis E, Fennoy I, Laraque D, et al. Autism and developmental abnormalities in children with perinatal cocaine exposure. J Natl Med Assoc. 1992;84:315–319. [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ostrea E, Jr, et al. Prenatal cocaine and neonatal outcome: evaluation of dose-response relationship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, et al. Prenatal cocaine exposure and child behavior. Pediatrics. 1998;102:945–950. doi: 10.1542/peds.102.4.945. [DOI] [PubMed] [Google Scholar]
- Di Francesco P, Pica F, Favalli C, et al. Inhibition of rat fibroblast cell proliferation at specific cell cycle stages by cocaine. Cell Biol Int Rep. 1990;14:549–558. doi: 10.1016/0309-1651(90)91182-4. [DOI] [PubMed] [Google Scholar]
- Doberczak TM, Shanzer S, Senie RT, Kandall SR. Neonatal neurologic and electroencephalographic effects of intrauterine cocaine exposure. J Pediatr. 1988;113:354–358. doi: 10.1016/s0022-3476(88)80283-x. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Aguirre Vila-Coro A, Slopis JM, Bohan TP. Brain and ocular abnormalities in infants with in utero exposure to cocaine and other street drugs. Am J Dis Child. 1991;145:688–695. doi: 10.1001/archpedi.1991.02160060106030. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards DL, Benveniste H, Behnke M, et al. Neuroimaging of prenatal drug exposure. Neurotoxicol Teratol. 2006;28:386–402. doi: 10.1016/j.ntt.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, Iijima M, Stephenson S, et al. The effects of prenatal cocaine, post-weaning housing and sex on conditioned place preference in adolescent rats. Psychopharmacology. 2014;231:1543–1555. doi: 10.1007/s00213-013-3418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Kaufmann PM, Glisky ML. Neuropsychologic function in toddlers exposed to cocaine in utero: a preliminary study. Dev Neuropsychol. 1999;15:447–460. [Google Scholar]
- Estelles J, Rodriguez-Arias M, Maldonado C, et al. Prenatal cocaine exposure alters spontaneous and cocaine-induced motor and social behaviors. Neurotoxicol Teratol. 2005;27:449–457. doi: 10.1016/j.ntt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Estelles J, Rodriguez-Arias M, Maldonado C, et al. Gestational exposure to cocaine alters cocaine reward. Behav Pharmacol. 2006a;17:509–515. doi: 10.1097/00008877-200609000-00017. [DOI] [PubMed] [Google Scholar]
- Estelles J, Rodriguez-Arias M, Maldonado C, et al. Prenatal cocaine alters later responses to morphine in adult male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006b;30:1073–1082. doi: 10.1016/j.pnpbp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fang Y, Ronnekleiv OK. Cocaine upregulates the dopamine transporter in fetal rhesus monkey brain. J Neurosci. 1999;19:8966–8978. doi: 10.1523/JNEUROSCI.19-20-08966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Janowsky A, Ronnekleiv OK. Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1- and D2-like receptor binding densities. Brain Res Dev Brain Res. 1997;104:163–174. doi: 10.1016/s0165-3806(97)00151-x. [DOI] [PubMed] [Google Scholar]
- Frank DA, Zuckerman BS, Amaro H, et al. Cocaine use during pregnancy: prevalence and correlates. Pediatrics. 1988;82:888–895. [PubMed] [Google Scholar]
- Frank DA, Bauchner H, Parker S, et al. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. J Pediatr. 1990;117:622–626. doi: 10.1016/s0022-3476(05)80702-4. [DOI] [PubMed] [Google Scholar]
- Frederick AL, Stanwood GD. Drugs, biogenic amine targets and the developing brain. Dev Neurosci. 2009;31:7–22. doi: 10.1159/000207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, Wang HY. Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system. Ann N Y Acad Sci. 1998;846:238–247. doi: 10.1111/j.1749-6632.1998.tb09741.x. [DOI] [PubMed] [Google Scholar]
- Friedman E, Yadin E, Wang HY. Effect of prenatal cocaine on dopamine receptor-G protein coupling in mesocortical regions of the rabbit brain. Neuroscience. 1996;70:739–747. doi: 10.1016/s0306-4522(96)83011-9. [DOI] [PubMed] [Google Scholar]
- Fulroth R, Phillips B, Durand DJ. PErinatal outcome of infants exposed to cocaine and/or heroin in utero. Am J Dis Child. 1989;143:905–910. doi: 10.1001/archpedi.1989.02150200057018. [DOI] [PubMed] [Google Scholar]
- Fung YK, Reed JA, Lau YS. Prenatal cocaine exposure fails to modify neurobehavioral responses and the striatal dopaminergic system in newborn rats. Gen Pharmacol. 1989;20:689–693. doi: 10.1016/0306-3623(89)90108-0. [DOI] [PubMed] [Google Scholar]
- Galler JR, Tonkiss J. The effects of prenatal protein malnutrition and cocaine on the development of the rat. Ann N Y Acad Sci. 1998;846:29–39. [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, et al. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, et al. Prenatal cocaine exposure does not alter working memory in adult rats. Neurotoxicol Teratol. 2004a;26:319–329. doi: 10.1016/j.ntt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gendle MH, White TL, Strawderman M, et al. Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav Neurosci. 2004b;118:290–297. doi: 10.1037/0735-7044.118.2.290. [DOI] [PubMed] [Google Scholar]
- Gillogley KM, Evans AT, Hansen RL, et al. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. Am J Obstet Gynecol. 1990;163:1535–1542. doi: 10.1016/0002-9378(90)90621-d. [DOI] [PubMed] [Google Scholar]
- Gittler J, McPherson M. Prenatal substance abuse. Child Today. 1990;19:3–7. [PubMed] [Google Scholar]
- Glatt SJ, Bolanos CA, Trksak GH, et al. Prenatal cocaine exposure alters behavioral and neurochemical sensitization to amphetamine in adult rats. Neuropharmacology. 2000;39:599–610. doi: 10.1016/s0028-3908(99)00181-1. [DOI] [PubMed] [Google Scholar]
- Gomby DS, Shiono PH. Estimating the number of substance-exposed infants. Future Child. 1991;1:17–25. [Google Scholar]
- Goodwin GA, Heyser CJ, Moody CA, et al. A fostering study of the effects of prenatal cocaine exposure: II. Offspring behavioral measures. Neurotoxicol Teratol. 1992;14:423–432. doi: 10.1016/0892-0362(92)90053-d. [DOI] [PubMed] [Google Scholar]
- Grace C. The southern association on children under Six: September 1, 1991-August 31, 1992. Dimens Early Child. 1993;21:29–30. [Google Scholar]
- Gressens P, Gofflot F, Van Maele-Fabry G, et al. Early neurogenesis and teratogenesis in whole mouse embryo cultures. Histochemical, immunocytological and ultrastructural study of the premigratory neuronal-glial units in normal mouse embryo and in mouse embryos influenced by cocaine and retinoic acid. J Neuropathol Exp Neurol. 1992a;51:206–219. doi: 10.1097/00005072-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Gressens P, Kosofsky BE, Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992b;140:113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- Griffith DR, Azuma SD, Chasnoff IJ. Three-year outcome of children exposed prenatally to drugs. J Am Acad Child Adolesc Psychiatry. 1994;33:20–27. doi: 10.1097/00004583-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Guerriero RM, Rajadhyaksha A, Crozatier C, et al. Augmented constitutive CREB expression in the nucleus accumbens and striatum may contribute to the altered behavioral response to cocaine of adult mice exposed to cocaine in utero. Dev Neurosci. 2005;27:235–248. doi: 10.1159/000085997. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Billman SP, Gilliam DM, George FR. Operant-self-administration of ethanol in mice prenatally exposed to cocaine. J Addict Dis. 1999;18:77–89. doi: 10.1300/J069v18n03_08. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Benno RH, Schanz N, Phadia E. The effects of prenatal cocaine exposure and genotype on the ultrasonic calls of infant mice. Pharmacol Biochem Behav. 2000;67:729–738. doi: 10.1016/s0091-3057(00)00418-4. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2010;210:481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Nader MA. Behavioral characterization of adult male and female rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2011;213:799–808. doi: 10.1007/s00213-010-2038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler A, Kistin N, Davis F, Ferré C. Cocaine use during pregnancy: perinatal outcomes. Am J Epidemiol. 1991;133:818–825. doi: 10.1093/oxfordjournals.aje.a115961. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- He N, Song ZM, Lidow MS. Cocaine induces cell death within the primate fetal cerebral wall. Neuropathol Appl Neurobiol. 1999;25:504–512. doi: 10.1046/j.1365-2990.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- He N, Bai J, Champoux M, et al. Neurobehavioral deficits in neonatal rhesus monkeys exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:13–21. doi: 10.1016/j.ntt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006a;28:198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Inhalational model of cocaine exposure in mice: neuroteratological effects. Neurotoxicol Teratol. 2006b;28:181–197. doi: 10.1016/j.ntt.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Alterations in the reinforcing efficacy of cocaine in adult rats following prenatal exposure to cocaine. Behav Neurosci. 1998;112:410–418. doi: 10.1037//0735-7044.112.2.410. [DOI] [PubMed] [Google Scholar]
- Heffelfinger A, Craft S, Shyken J. Visual attention in children with prenatal cocaine exposure. J Int Neuropsychol Soc. 1997;3:237–245. [PubMed] [Google Scholar]
- Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: implications for behavioral regulation. J Int Neuropsychol Soc. 2002;8:12–21. [PubMed] [Google Scholar]
- Henderson MG, McMillen BA. Effects of prenatal exposure to cocaine or related drugs on rat developmental and neurological indices. Brain Res Bull. 1990;24:207–212. doi: 10.1016/0361-9230(90)90207-g. [DOI] [PubMed] [Google Scholar]
- Henderson MG, McMillen BA. Changes in dopamine, serotonin and their metabolites in discrete brain areas of rat offspring after in utero exposure to cocaine or related drugs. Teratology. 1993;48:421–430. doi: 10.1002/tera.1420480506. [DOI] [PubMed] [Google Scholar]
- Henderson MG, McConnaughey MM, McMillen BA. Long-term consequences of prenatal exposure to cocaine or related drugs: effects on rat brain monoaminergic receptors. Brain Res Bull. 1991;26:941–945. doi: 10.1016/0361-9230(91)90261-h. [DOI] [PubMed] [Google Scholar]
- Hess CW, Hahn ME, Benno RH, Schanz N. Prenatal cocaine exposure alters maternal retrieval behavior in mice. Behav Genet. 2002;32:259–266. doi: 10.1023/a:1019776729821. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Chen WJ, Miller J, et al. Prenatal cocaine exposure induces deficits in Pavlovian conditioning and sensory preconditioning among infant rat pups. Behav Neurosci. 1990;104:955–963. doi: 10.1037//0735-7044.104.6.955. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Goodwin GA, Moody CA, Spear LP. Prenatal cocaine exposure attenuates cocaine-induced odor preference in infant rats. Pharmacol Biochem Behav. 1992a;42:169–173. doi: 10.1016/0091-3057(92)90461-n. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Miller JS, Spear NE, Spear LP. Prenatal exposure to cocaine disrupts cocaine-induced conditioned place preference in rats. Neurotoxicol Teratol. 1992b;14:57–64. doi: 10.1016/0892-0362(92)90029-a. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Rajachandran L, Spear NE, Spear LP. Responsiveness to cocaine challenge in adult rats following prenatal exposure to cocaine. Psychopharmacology. 1994;116:45–55. doi: 10.1007/BF02244870. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Prenatal cocaine exposure enhances long-term potentiation induction in rat medial prefrontal cortex. Int J Neuropsychopharmacol. 2011;14:431–443. doi: 10.1017/S1461145710000258. [DOI] [PubMed] [Google Scholar]
- Huber J, Darling S, Park K, Soliman KF. Altered responsiveness to stress and NMDA following prenatal exposure to cocaine. Physiol Behav. 2001;72:181–188. doi: 10.1016/s0031-9384(00)00410-8. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta JM, Korczykowski M, et al. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J Pediatr. 2008;152:371–377. doi: 10.1016/j.jpeds.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Fico TA, Dow-Edwards DL. Prenatal cocaine: maternal toxicity, fetal effects and locomotor activity in rat offspring. Neurotoxicol Teratol. 1989;11:65–69. doi: 10.1016/0892-0362(89)90087-1. [DOI] [PubMed] [Google Scholar]
- Ismail ZI, Bedi KS. Rats exposed to cocaine during late gestation and early postnatal life show deficits in hippocampal pyramidal and granule cells in later life. J Anat. 2007;210:749–760. doi: 10.1111/j.1469-7580.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, et al. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996;129:581–590. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR. Prenatal cocaine exposure affects social behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1995;17:569–576. doi: 10.1016/0892-0362(95)00017-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Means LW, Means MJ, McMillen BA. Prenatal exposure to cocaine. I: effects on gestation, development, and activity in Sprague-Dawley rats. Neurotoxicol Teratol. 1992a;14:337–342. doi: 10.1016/0892-0362(92)90040-h. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Anderson DR, et al. Prenatal exposure to cocaine. II: effects on open-field activity and cognitive behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1992b;14:343–349. doi: 10.1016/0892-0362(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, et al. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and in their prenatally exposed offspring. Ann N Y Acad Sci. 1998;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Lieberman JA, Lauder JM. Developmental effects of prenatal cocaine exposure on 5-HT1A receptors in male and female rat offspring. Dev Neurosci. 2002;24:522–530. doi: 10.1159/000069363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AL, Morrow CE, Accornero VH, et al. Maternal cocaine use: estimated effects on mother-child play interactions in the preschool period. J Dev Behav Pediatr. 2002;23:191–202. doi: 10.1097/00004703-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Fischer I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb Cortex. 1996;6:431–445. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- Jones LB, Stanwood GD, Reinoso BS, et al. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ZD, Lourenco F, Byrne ME, et al. Brain-derived neurotrophic factor genotype impacts the prenatal cocaine-induced mouse phenotype. Dev Neurosci. 2012;34:184–197. doi: 10.1159/000337712. [DOI] [PubMed] [Google Scholar]
- Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PLoS One. 2013;8:e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ZD, Kennedy B, Katzman A, et al. Effects of prenatal cocaine exposure on social development in mice. Dev Neurosci. 2014;36:338–346. doi: 10.1159/000360524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RW, Jr, Johnson KS, Snyder-Keller AM, et al. Effects of prenatal cocaine exposure on the mesocorticolimbic dopamine system: an in vivo microdialysis study in the rat. Brain Res. 1996a;742:71–79. doi: 10.1016/s0006-8993(96)00959-6. [DOI] [PubMed] [Google Scholar]
- Keller RW, Jr, LeFevre R, Raucci J, et al. Enhanced cocaine self-administration in adult rats prenatally exposed to cocaine. Neurosci Lett. 1996b;205:153–156. doi: 10.1016/0304-3940(96)12409-5. [DOI] [PubMed] [Google Scholar]
- Kistin N, Handler A, Davis F, Ferre C. Cocaine and cigarettes: a comparison of risks. Paediatr Perinatal Epidemiol. 1996;10:269–278. doi: 10.1111/j.1365-3016.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE. Cocaine-induced alterations in neuro-development. Semin Speech Lang. 1998;19:109–121. doi: 10.1055/s-2008-1064040. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS. A mouse model of transplacental cocaine exposure. Clinical implications for exposed infants and children. Ann N Y Acad Sci. 1998;846:248–261. [PubMed] [Google Scholar]
- Kramer LD, Locke GE, Ogunyemi A, Nelson L. Neonatal cocaine-related seizures. J Child Neurol. 1990;5:60–64. doi: 10.1177/088307389000500115. [DOI] [PubMed] [Google Scholar]
- Kubrusly RCC, Bhide PG. Cocaine exposure modulates dopamine and adenosine signaling in the fetal brain. Neuropharmacology. 2010;58:436–443. doi: 10.1016/j.neuropharm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunko PM, Moyer D, Robinson SE. Intravenous gestational cocaine in rats: effects on offspring development and weanling behavior. Neurotoxicol Teratol. 1993;15:335–344. doi: 10.1016/0892-0362(93)90035-m. [DOI] [PubMed] [Google Scholar]
- Kunko PM, Wallace MJ, Robinson SE. Gestational cocaine and ethanol exposure alter spontaneous and cocaine-induced behavior in weanling rats. Pharmacol Biochem Behav. 1996;55:559–564. doi: 10.1016/s0091-3057(96)00283-3. [DOI] [PubMed] [Google Scholar]
- Laferriere A, Ertug F, Moss IR. Prenatal cocaine alters open-field behavior in young swine. Neurotoxicol Teratol. 1995;17:81–87. doi: 10.1016/0892-0362(94)00056-j. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, et al. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5:e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Lehrmann E, Hayashi T, et al. Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. J Addict Med. 2009;3:218–226. doi: 10.1097/ADM.0b013e318199d863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Worden LT, Freed WJ. Cocaine causes deficits in radial migration and alters the distribution of glutamate and GABA neurons in the developing rat cerebral cortex. Synapse. 2011;65:21–34. doi: 10.1002/syn.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Jung AB, et al. Effects of prenatal cocaine exposure upon postnatal development of neostriatal dopaminergic function. Synapse. 1994;17:210–215. doi: 10.1002/syn.890170311. [DOI] [PubMed] [Google Scholar]
- Lester BM, Corwin MJ, Sepkoski C, et al. Neurobehavioral syndromes in cocaine-exposed newborn infants. Child Dev. 1991;62:694–705. doi: 10.1111/j.1467-8624.1991.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- Levin ED, Seidler FJ. Sex-related spatial learning differences after prenatal cocaine exposure in the young adult rat. Neurotoxicology. 1993;14:23–28. [PubMed] [Google Scholar]
- Lewis BA, Singer LT, Short EJ, et al. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Kirchner HL, Short EJ, et al. Prenatal cocaine and tobacco effects on children's language trajectories. Pediatrics. 2007;120:e78–e85. doi: 10.1542/peds.2006-2563. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Minnes S, Short EJ, et al. The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol Teratol. 2011;33:17–24. doi: 10.1016/j.ntt.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Effect of cocaine on cell proliferation in the cerebral wall of monkey fetuses. Cereb Cortex. 2001a;11:545–551. doi: 10.1093/cercor/11.6.545. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001b;435:263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Trakht T, Howard RL. Cocaine-induced alterations in the density of monoaminergic receptors in the embryonic guinea pig cerebral wall. Synapse. 1999;32:225–237. doi: 10.1002/(SICI)1098-2396(19990601)32:3<225::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Bozian D, Song ZM. Cocaine affects cerebral neocortical cytoarchitecture in primates only if administered during neocortical neuronogenesis. Brain Res Dev Brain Res. 2001;128:45–52. doi: 10.1016/s0165-3806(01)00139-0. [DOI] [PubMed] [Google Scholar]
- Lin D, Kellogg CK. Neonatal exposure to cocaine enhances the reward-potentiating properties of the drug in young adult animals. Behav Neurosci. 1996;110:791–801. doi: 10.1037//0735-7044.110.4.791. [DOI] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippard ET, Jarrett TM, McMurray MS, et al. Early postpartum pup preference is altered by gestational cocaine treatment: associations with infant cues and oxytocin expression in the MPOA. Behav Brain Res. 2015;278:176–185. doi: 10.1016/j.bbr.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JW, Davidson TL, Carvey PM, Weese-Mayer DE. Prenatal cocaine: effect on hypoxic ventilatory responsiveness in neonatal rats. Respir Physiol. 1996;106 doi: 10.1016/s0034-5687(96)00075-8. 161 Respiration 169. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM. Brain growth among fetuses exposed to cocaine in utero: asymmetrical growth retardation. Obstet Gynecol. 1991;77:361–364. [PubMed] [Google Scholar]
- Liu J, Lester BM, Neyzi N, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 2013;167:348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu H, Lim B, Poo MM. Cocaine exposure in utero alters synaptic plasticity in the medial prefrontal cortex of postnatal rats. J Neurosci. 2009;29:12664–12674. doi: 10.1523/JNEUROSCI.1984-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Liu X, Long H, Ma L. Effects of prenatal cocaine and heroin exposure on neuronal dendrite morphogenesis and spatial recognition memory in mice. Neurosci Lett. 2012;522:128–133. doi: 10.1016/j.neulet.2012.06.023. [DOI] [PubMed] [Google Scholar]
- MacGregor SN, Keith LG, Chasnoff IJ, et al. Cocaine use during pregnancy: adverse perinatal outcome. Am J Obstet Gynecol. 1987;157:686–690. doi: 10.1016/s0002-9378(87)80029-7. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicol Teratol. 1999;21:539–550. doi: 10.1016/s0892-0362(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Malanga CJ. Still no time for complacency: developmental effects of prenatal methamphetamine exposure. Neurology. 2009;72:2062–2063. doi: 10.1212/WNL.0b013e3181a92c98. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Brain Res Dev Brain Res. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Pejchal M, Kosofsky BE. Prenatal exposure to cocaine alters the development of conditioned place-preference to cocaine in adult mice. Pharmacol Biochem Behav. 2007;87:462–471. doi: 10.1016/j.pbb.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry. 2008;63:214–221. doi: 10.1016/j.biopsych.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowski VP, Cox C, Preston R, Weiss B. Effects of age and gender but not prenatal cocaine on random ratio and delayed spatial alternation responding in rats. Neurotoxicol Teratol. 2000;22:421–428. doi: 10.1016/s0892-0362(99)00080-x. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995:539–545. [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, et al. Impaired regulation of arousal in 3-month-old infants exposed prenatally to cocaine and other drugs. Dev Psychopathol. 1996;8:29–42. [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann N Y Acad Sci. 1998;846:126–143. doi: 10.1111/j.1749-6632.1998.tb09731.x. [DOI] [PubMed] [Google Scholar]
- Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychol. 2007;13:205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG. Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ratio in the medial prefrontal cortex. Dev Neurosci. 2012;34:174–183. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Zhang X, Darnell SB, et al. Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J Neurosci. 2011;31:13400–13411. doi: 10.1523/JNEUROSCI.2944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Brown AN, Bhide PG. Regulation of BDNF expression by cocaine. Yale J Biol Med. 2012a;85:437–446. [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Gioioso V, Zhang X, et al. Neurogenesis and neuronal migration in the forebrain of the TorsinA knockout mouse embryo. Dev Neurosci. 2012b;34:366–378. doi: 10.1159/000342260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Kabir ZD, Bhide PG, Kosofsky BE. Effects of prenatal exposure to cocaine on brain structure and function. Prog Brain Res. 2014;211:277–289. doi: 10.1016/B978-0-444-63425-2.00012-X. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, et al. Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress. 2008;11:398–410. doi: 10.1080/10253890701850239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick SM, Dow-Edwards DL. Differential behavioral responses to chronic amphetamine in adult male and female rats exposed to postnatal cocaine treatment. Pharmacol Biochem Behav. 2001;69:219–224. doi: 10.1016/s0091-3057(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Sherlock JD, Macdonald NR. Effects of prenatal cocaine on behavioral responses to a cocaine challenge on postnatal day 11. Neurotoxicol Teratol. 1992;14:183–189. doi: 10.1016/0892-0362(92)90014-2. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Robinson P, Todtenkopf MS. Prenatal cocaine treatment reduces haloperidol-induced catalepsy on postnatal day 10. Neurotoxicol Teratol. 1994;16:193–199. doi: 10.1016/0892-0362(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shearman LP, Collins LM. Monoamine transporters and the neurobehavioral teratology of cocaine. Pharmacol Biochem Behav. 1996;55:585–593. doi: 10.1016/s0091-3057(96)00280-8. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Garavan HP, Mactutus CF, et al. Enduring effects of prenatal cocaine exposure on attention and reaction to errors. Behav Neurosci. 2002;116:624–633. doi: 10.1037//0735-7044.116.4.624. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, et al. The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol. 1996a;18:147–154. doi: 10.1016/0892-0362(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Morris P, Gillam MP, Allen RR, Paule MG. The effect of chronic cocaine exposure during pregnancy on the acquisition of operant behaviors by rhesus monkey offspring. Neurotoxicol Teratol. 1996b;18:155–166. doi: 10.1016/0892-0362(95)02031-4. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Male rats exposed to cocaine in utero demonstrate elevated expression of Fos in the prefrontal cortex in response to environment. Neuropsychopharmacology. 2002a;26:275–285. doi: 10.1016/S0893-133X(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Prenatal cocaine exposure disrupts non-spatial, short-term memory in adolescent and adult male rats. Behav Brain Res. 2002b;129:217–223. doi: 10.1016/s0166-4328(01)00338-2. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Axo-axonic structures in the medial prefrontal cortex of the rat: reduction by prenatal exposure to cocaine. J Neurosci. 2003a;23:5227–5234. doi: 10.1523/JNEUROSCI.23-12-05227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CE, Bandstra ES, Anthony JC, et al. Influence of prenatal cocaine exposure on early language development: longitudinal findings from four months to three years of age. J Dev Behav Pediatr. 2003b;24:39–50. doi: 10.1097/00004703-200302000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CE, Vogel AL, Anthony JC, et al. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Prenatal exposure to cocaine selectively disrupts the development of parvalbumin containing local circuit neurons in the medial prefrontal cortex of the rat. Synapse. 2005;56:1–11. doi: 10.1002/syn.20121. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Hajszan T, Leranth C, et al. Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse. 2007;61:862–865. doi: 10.1002/syn.20430. [DOI] [PubMed] [Google Scholar]
- Moss IR, Laferriere A, Faltus RE. Prenatal cocaine alters diaphragmatic EMG responses to hypoxia in developing swine. Am J Respir Crit care Med. 1995;152:1961–1966. doi: 10.1164/ajrccm.152.6.8520763. [DOI] [PubMed] [Google Scholar]
- Murphy EH, Fischer I, Friedman E, et al. Cocaine administration in pregnant rabbits alters cortical structure and function in their progeny in the absence of maternal seizures. Exp Brain Res. 1997;114:433–441. doi: 10.1007/pl00005652. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Evrard P, Courtoy PJ. Selective neuronal toxicity of cocaine in embryonic mouse brain cocultures. Proc Natl Acad Sci USA. 1995;92:11029–11033. doi: 10.1073/pnas.92.24.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassogne MC, Louahed J, Evrard P, Courtoy PJ. Cocaine Induces Apoptosis in Cortical Neurons of Fetal Mice. J Neurochem. 1997;68:2442–2450. doi: 10.1046/j.1471-4159.1997.68062442.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, et al. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, et al. Cocaine-induced changes in the expression of apoptosis-related genes in the fetal mouse cerebral wall. Neurotoxicol Teratol. 2005a;27:3–14. doi: 10.1016/j.ntt.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Lidow MS. Neuropathology of the cerebral cortex observed in a range of animal models of prenatal cocaine exposure may reflect alterations in genes involved in the Wnt and cadherin systems. Synapse. 2005b;56:105–116. doi: 10.1002/syn.20134. [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Greenbaum R, et al. Neurodevelopment of adopted children exposed in utero to cocaine: the Toronto Adoption Study. Clin Invest Med. 2001;24:129–137. [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Brady M, Gause S, et al. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- Overstreet DH, Moy SS, Lubin DA, et al. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70:149–156. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Binienda Z, Morris P. Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann N Y Acad Sci. 1996;801:301–309. doi: 10.1111/j.1749-6632.1996.tb17450.x. [DOI] [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Allen RR, Chelonis JJ. Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann N Y Acad Sci. 2000;914:412–417. doi: 10.1111/j.1749-6632.2000.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Peeke HV, Dark KA, Salamy A, et al. Cocaine exposure pre-breeding to weaning: maternal and offspring effects. Pharmacol Biochem Behav. 1994;48:403–410. doi: 10.1016/0091-3057(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Peris J, Coleman-Hardee M, Millard WJ. Cocaine in utero enhances the behavioral response to cocaine in adult rats. Pharmacol Biochem Behav. 1992;42:509–515. doi: 10.1016/0091-3057(92)90146-7. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-Cadherin Regulates Ingrowth and Laminar Targeting of Thalamocortical Axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Cadherins: molecular codes for axon guidance and synapse formation. Int J Dev Neurosci. 2000;18:643–651. doi: 10.1016/s0736-5748(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, et al. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Raum WJ, McGivern RF, Peterson MA, et al. Prenatal inhibition of hypothalamic sex steroid uptake by cocaine: effects on neurobehavioral sexual differentiation in male rats. Brain Res Dev Brain Res. 1990;53:230–236. doi: 10.1016/0165-3806(90)90011-m. [DOI] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, et al. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Richardson GA. Prenatal cocaine exposure: a longitudinal study of developmenta. Ann N Y Acad Sci. 1998;846:144–152. doi: 10.1111/j.1749-6632.1998.tb09732.x. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol Teratol. 1996a;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. The effects of prenatal cocaine use on neonatal neurobehavioral status. Neurotoxicol Teratol. 1996b;18:519–528. doi: 10.1016/0892-0362(96)00062-1. [DOI] [PubMed] [Google Scholar]
- Riley J, Brodsky N, Porat R. Risk for SIDS in infants with in utero cocaine exposure: a prospective study. Pediatr Res. 1988;23:454A. [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology. 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- Rodning C, Beckwith L, Howard J. Characteristics of attachment organization and play organization in prenatally drug-exposed toddlers. Dev Psychopathol. 1989;1:277–289. [Google Scholar]
- Romano AG, Harvey JA. Elicitation and modification of the rabbit's nictitating membrane reflex following prenatal exposure to cocaine. Pharmacol Biochem Behav. 1996;53:857–862. doi: 10.1016/0091-3057(95)02095-0. [DOI] [PubMed] [Google Scholar]
- Romano AG, Harvey JA. Prenatal cocaine exposure: long-term deficits in learning and motor performance. Ann N Y Acad Sci. 1998;846:89–108. [PubMed] [Google Scholar]
- Ronnekleiv OK, Naylor BR. Chronic cocaine exposure in the fetal rhesus monkey: consequences for early development of dopamine neurons. J Neurosci. 1995;15:7330–7343. doi: 10.1523/JNEUROSCI.15-11-07330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv OK, Fang Y, Choi WS, Chai L. Changes in the midbrain-rostral forebrain dopamine circuitry in the cocaine-exposed primate fetal brain. Ann N Y Acad Sci. 1998;846:165–181. [PubMed] [Google Scholar]
- Ryan L, Ehrlich S, Finnegan L. Cocaine abuse in pregnancy: effects on the fetus and newborn. Neurotoxicol Teratol. 1987;9:295–299. doi: 10.1016/0892-0362(87)90018-3. [DOI] [PubMed] [Google Scholar]
- Salas-Ramirez KY, Frankfurt M, Alexander A, et al. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169:1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Sasaki A, Constantinof A, Pan P, et al. Cocaine exposure prior to pregnancy alters the psychomotor response to cocaine and transcriptional regulation of the dopamine D1 receptor in adult male offspring. Behav Brain Res. 2014;265:163–170. doi: 10.1016/j.bbr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Scalzo FM, Ali SF, Frambes NA, Spear LP. Weanling rats exposed prenatally to cocaine exhibit an increase in striatal D2 dopamine binding associated with an increase in ligand affinity. Pharmacol Biochem Behav. 1990;37:371–373. doi: 10.1016/0091-3057(90)90350-q. [DOI] [PubMed] [Google Scholar]
- Scher MS, Richardson GA, Day NL. Effects of prenatal cocaine/crack and other drug exposure on electroencephalographic sleep studies at birth and one year. Pediatrics. 2000;105:39–48. doi: 10.1542/peds.105.1.39. [DOI] [PubMed] [Google Scholar]
- Schneider JW, Chasnoff IJ. Motor assessment of cocaine/polydrug exposed infants at age 4 months. Neurotoxicol Teratol. 1992;14:97–101. doi: 10.1016/0892-0362(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Schroder MD, Snyder PJ, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cogn. 2004;55:409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Sparber SB. Embryonic “binge” cocaine exposure alters neural-immune and neural-endocrine interactions in young chickens: involvement of serotonin(2) receptors. Brain Res Dev Brain Res. 2001;130:99–107. doi: 10.1016/s0165-3806(01)00217-6. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Getty ME, Wacnik PW, Sparber SB. Open-field and LPS-induced sickness behavior in young chickens: effects of embryonic cocaine and/or ritanserin. Pharmacol Biochem Behav. 1998;61:9–17. doi: 10.1016/s0091-3057(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, et al. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih L, Cone-Wesson B, Reddix B. Effects of maternal cocaine abuse on the neonatal auditory system. Int J Pediatr Otorhinolaryngol. 1988;15:245–251. doi: 10.1016/0165-5876(88)90079-1. [DOI] [PubMed] [Google Scholar]
- Simansky KJ, Kachelries WJ. Prenatal exposure to cocaine selectively disrupts motor responding to D-amphetamine in young and mature rabbits. Neuropharmacology. 1996;35:71–78. doi: 10.1016/0028-3908(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, et al. Developing language skills of cocaine-exposed infants. Pediatrics. 2001;107:1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, et al. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithisarn T, Bada HS, Dai H, et al. Effects of perinatal cocaine exposure on open field behavior and the response to corticotropin releasing hormone (CRH) in rat offspring. Brain Res. 2011;1370:136–144. doi: 10.1016/j.brainres.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller AM, Keller RW., Jr Prenatal cocaine increases striatal serotonin innervation without altering the patch/matrix organization of intrinsic cell types. Dev Brain Res. 1993;74:261–267. doi: 10.1016/0165-3806(93)90012-y. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Burton LE, Robinson NL, et al. Neurobehavioral and immunological effects of prenatal cocaine exposure in rat. Pharmacol Biochem Behav. 1990;35:617–629. doi: 10.1016/0091-3057(90)90299-w. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Ali SF, Slikker W, Jr, Holson RR. Interactive effects of prenatal cocaine and nicotine exposure on maternal toxicity, postnatal development and behavior in the rat. Mol Neurobiol. 1995;11:121–143. doi: 10.1007/BF02740690. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:501–518. doi: 10.1016/S0278-5846(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Spear LP, Frambes NA, Kirstein CL. Fetal and maternal brain and plasma levels of cocaine and benzoylecgonine following chronic subcutaneous administration of cocaine during gestation in rats. Psychopharmacology. 1989a;97:427–431. doi: 10.1007/BF00439542. [DOI] [PubMed] [Google Scholar]
- Spear LP, Kirstein CL, Bell J, et al. Effects of prenatal cocaine exposure on behavior during the early postnatal period. Neurotoxicol Teratol. 1989b;11:57–63. doi: 10.1016/0892-0362(89)90086-x. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Repeated i.v. cocaine exposure produces long-lasting behavioral sensitization in pregnant adults, but behavioral tolerance in their offspring. Neuroscience. 2003;122:579–583. doi: 10.1016/j.neuroscience.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27:152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001a;11:430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001b;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Parlaman JP, Levitt P. Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J Comp Neurol. 2005;487:270–282. doi: 10.1002/cne.20548. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CW, Scalzo FM, Valentine J, et al. Gestational exposure to cocaine or pharmacologically related compounds: effects on behavior and striatal dopamine receptors. Life Sci. 1998;63:2015–2022. doi: 10.1016/s0024-3205(98)00479-2. [DOI] [PubMed] [Google Scholar]
- Strother WN, Vorhees CV, Lehman MN. Long-term effects of early cocaine exposure on the light responsiveness of the adult circadian timing system. Neurotoxicol Teratol. 1998;20:555–564. doi: 10.1016/s0892-0362(98)00014-2. [DOI] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal cocaine exposure specifically alters spontaneous alternation behavior. Behav Brain Res. 2005;164:107–116. doi: 10.1016/j.bbr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne N, Cai G, Friedman E. Attenuation of cocaine-induced genomic and functional responses in prenatal cocaine-exposed rabbits. Pharmacol Biochem Behav. 2001;69:225–232. doi: 10.1016/s0091-3057(01)00534-2. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Harrison RH, Galler JR. Differential effects of prenatal protein malnutrition and prenatal cocaine on a test of homing behavior in rat pups. Physiol Behav. 1996;60:1013–1018. doi: 10.1016/0031-9384(96)00152-7. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Prenatal cocaine dampened behavioral responses to methylphenidate in male and female adolescent rats. Neurotoxicol Teratol. 2006;28:165–172. doi: 10.1016/j.ntt.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Laing FC, Jeffrey RB. Placental abruption associated with cocaine abuse. Am J Roentgenol. 1988;150:1339–1340. doi: 10.2214/ajr.150.6.1339. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kabir ZD, Kaur G, et al. Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Front Psychiatry. 2011;2:67. doi: 10.3389/fpsyt.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathy I, Marson L. Effects of prenatal morphine and cocaine exposure on spinal sexual reflexes in male and female rats. Physiol Behav. 1998;63:445–450. doi: 10.1016/s0031-9384(97)00467-8. [DOI] [PubMed] [Google Scholar]
- Vathy I, Katay L, Mini KN. Sexually dimorphic effects of prenatal cocaine on adult sexual behavior and brain catecholamines in rats. Brain Res Dev Brain Res. 1993;73:115–122. doi: 10.1016/0165-3806(93)90053-d. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Acuff-Smith KD, et al. Long-term learning deficits and changes in unlearned behaviors following in utero exposure to multiple daily doses of cocaine during different exposure periods and maternal plasma cocaine concentrations. Neurotoxicol Teratol. 1995;17:253–264. doi: 10.1016/0892-0362(94)00061-h. [DOI] [PubMed] [Google Scholar]
- Wang HY, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J Pharmacol Exp Ther. 1995a;273:492–498. [PubMed] [Google Scholar]
- Wang HY, Yeung JM, Friedman E. Prenatal cocaine exposure selectively reduces mesocortical dopamine release. J Pharmacol Exp Ther. 1995b;273:1211–1215. [PubMed] [Google Scholar]
- Wang XH, Levitt P, Grayson DR, Murphy EH. Intrauterine cocaine exposure of rabbits: persistent elevation of GABA-immunoreactive neurons in anterior cingulate cortex but not visual cortex. Brain Res. 1995c;689:32–46. doi: 10.1016/0006-8993(95)00528-x. [DOI] [PubMed] [Google Scholar]
- Wang XH, Jenkins AO, Choi L, Murphy EH. Altered neuronal distribution of parvalbumin in anterior cingulate cortex of rabbits exposed in utero to cocaine. Exp Brain Res. 1996a;112:359–371. doi: 10.1007/BF00227942. [DOI] [PubMed] [Google Scholar]
- Wang XH, Levitt P, O'Brien Jenkins A, Murphy EH. Normal development of tyrosine hydroxylase and serotonin immunoreactive fibers innervating anterior cingulate cortex and visual cortex in rabbits exposed prenatally to cocaine. Brain Res. 1996b;715:221–224. doi: 10.1016/0006-8993(96)00012-1. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins RC, Ruiz B. Development under the influence of cocaine. I. A comparison of the effects of daily cocaine treatment and resultant undernutrition on pregnancy and early growth in a large population of rats. Metab Brain Dis. 1990a;5:85–99. doi: 10.1007/BF01001049. [DOI] [PubMed] [Google Scholar]
- Wiggins RC, Ruiz B. Development under the influence of cocaine. II. Comparison of the effects of maternal cocaine and associated undernutrition on brain myelin development in the offspring. Metab Brain Dis. 1990b;5:101–109. doi: 10.1007/BF01001050. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Jones K, Kosofsky BE. Transplacental cocaine exposure. 2: effects of cocaine dose and gestational timing. Neurotoxicol Teratol. 1998a;20:227–238. doi: 10.1016/s0892-0362(97)00127-x. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Marota JJ, Tabit E, Kosofsky BE. Transplacental cocaine exposure. 3: Mechanisms underlying altered brain development. Neurotoxicol Teratol. 1998b;20:239–249. doi: 10.1016/s0892-0362(97)00128-1. [DOI] [PubMed] [Google Scholar]
- Williams SK, Johns JM. Prenatal and gestational cocaine exposure: effects on the oxytocin system and social behavior with implications for addiction. Pharmacol Biochem Behav. 2014;119:10–21. doi: 10.1016/j.pbb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SK, Lauder JM, Johns JM. Prenatal cocaine disrupts serotonin signaling-dependent behaviors: implications for sex differences, early stress and prenatal SSRI exposure. Curr Neuropharmacol. 2011;9:478–511. doi: 10.2174/157015911796557957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD, Spear LP. Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci. 1998;112:419–431. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]
- Wood RD, Bannoura MD, Johanson IB. Prenatal cocaine exposure: effects on play behavior in the juvenile rat. Neurotoxicol Teratol. 1994;16:139–144. doi: 10.1016/0892-0362(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wood RD, Molina VA, Wagner JM, Spear LP. Play behavior and stress responsivity in periadolescent offspring exposed prenatally to cocaine. Pharmacol Biochem Behav. 1995;52:367–374. doi: 10.1016/0091-3057(95)00120-l. [DOI] [PubMed] [Google Scholar]
- Yan QS. Reduced serotonin release and serotonin uptake sites in the rat nucleus accumbens and striatum after prenatal cocaine exposure. Brain Res. 2002;929:59–69. doi: 10.1016/s0006-8993(01)03378-9. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Yan SE. Prenatal cocaine exposure decreases brain-derived neurotrophic factor proteins in the rat brain. Brain Res. 2004;1009:228–233. doi: 10.1016/j.brainres.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Yolton KA, Bolig R. Psychosocial, behavioral, and developmental characteristics of toddlers prenatally exposed to cocaine. Child Study J. 1994;24:49. [Google Scholar]
- Yu X, Malenka RC. beta]-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zeskind PS, McMurray MS, Cox Lippard ET, et al. Translational analysis of effects of prenatal cocaine exposure on human infant cries and rat pup ultrasonic vocalizations. PLoS One. 2014;9:e110349. doi: 10.1371/journal.pone.0110349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Torres C, Wang HY, Friedman E. Prenatal exposure to cocaine disrupts D1A dopamine receptor function via selective inhibition of protein phosphatase 1 pathway in rabbit frontal cortex. J Neurosci. 2001;21:9160–9167. doi: 10.1523/JNEUROSCI.21-23-09160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmitrovich AC, Hutchings DE, Dow-Edwards DL, et al. Effects of prenatal exposure to cocaine on the rest-activity cycle of the preweanling rat. Pharmacol Biochem Behav. 1992;43:1059–1064. doi: 10.1016/0091-3057(92)90481-t. [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Frank DA, Hingson R, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]


