Abstract
The purpose of this study was to examine exercise effects on muscle water T2 in patients with Duchenne muscular dystrophy (DMD). In 12 DMD subjects and 19 controls, lower leg muscle fat (%) was measured by Dixon and muscle water T2 and R2 (1/T2) by the tri-exponential model. Muscle water R2 was measured again at 3 hours after an ankle dorsiflexion exercise. The muscle fat fraction was higher in DMD participants than in controls (p < .001) except in the tibialis posterior muscle. Muscle water T2 was measured independent of the degree of fatty degeneration in DMD muscle. At baseline, muscle water T2 was higher in all but the extensor digitorum longus muscles of DMD participants than controls (p < .001). DMD participants had a lower muscle torque (p < .001) and exerted less power (p < .01) during exercise than controls. Nevertheless, muscle water R2 decreased (T2 increased) after exercise from baseline in DMD subjects and controls with greater changes in the target muscles of the exercise than in ankle plantarflexor muscles. Skeletal muscle water T2 is a sensitive biomarker of the disease status in DMD and of the exercise response in DMD patients and controls.
Keywords: Duchenne muscular dystrophy, magnetic resonance imaging, exercise, skeletal muscle, fat fraction, water T2
1. Introduction
Duchenne muscular dystrophy (DMD) is the most common X-linked lethal childhood disease, with an incidence of about 1 in 5000 newborn boys [1,2]. Even with clinical intervention boys and young men lose independent mobility during teen years. The pelvic and thigh muscles are involved early and are severely affected with fatty degeneration. The disease progression is slower in the lower leg muscles than in the proximal leg muscles. Among the lower leg muscles, the ankle dorsiflexor muscles including the tibialis anterior and extensor digitorum longus are relatively spared in younger boys with DMD [3–5].
In recent years promising new therapeutics have been entering in clinical trials for DMD. Robust biomarkers are needed to measure disease progression and therapeutic response in a smaller number of individuals over a shorter timeframe in clinical trials. Expression of dystrophin, the protein product of the DMD gene [6], has been used as an outcome measure in clinical trials. However, accurate quantification of dystrophin has been challenging and requires the invasive procedure of a muscle biopsy [7]. The magnetic resonance imaging (MRI) measure of the water transverse relaxation time (T2) was identified as a biomarker of dystrophin expression to therapeutic levels in a dog model of DMD [8].
It is well established that dystrophin provides mechanical stability to the sarcolemma [9, 10]. Absence of dystrophin leads to sarcolemmal fragility with consequent muscle fiber degeneration and progressive replacement of muscle by fat and connective tissue [11]. Environmental factors including contractile stress during exercise appear to exacerbate the damage in dystrophin-deficient muscle fibers [12]. Muscle water T2 is sensitive to changes of muscle injury or edema after exercise and can be used to identify the muscles from which creatine kinase, an indicator of sarcolemmal leak, is released into the circulation [13]. Muscle water T2 abnormalities have been shown to relate to membrane leakiness and inflammation in animal models of DMD. An increase in muscle T2 has been reported in the hindlimb muscles of mdx mice after downhill running [14]. However, in a study of DMD patients muscle T2 was not sensitive and gadolinium contrast administration was required to demonstrate exercise effects by MRI [15]. In these studies, muscle T2 was calculated based on a monoexponential model, which is increased by both fatty infiltration and changes in the water component in skeletal muscle. Mdx mice do not develop significant fatty infiltration, and consequently T2 directly reflects the muscle water component. In contrast fatty infiltration masks increases in muscle water T2 in human dystrophic muscle [16,17].
A recently proposed tri-exponential model [18] enables extraction of water T2 from the multi-echo signal decay by taking into account the large T2 differences between the fat and water components. Pilot data showed that the tri-exponential model measures muscle water T2 independent of fat values in skeletal muscle [18]. In this study, we examined volitional ankle dorsiflexion exercise effects on skeletal muscle water T2 as measured by the tri-exponential model in the lower leg muscles of DMD subjects participating in a clinical trial and in age-matched healthy volunteers.
2. Materials and methods
2.1. Participants and study design
In this cross-sectional study, MRI of the lower leg skeletal muscles was acquired at baseline and at 3 hours post-exercise in ambulatory subjects with DMD and age-matched healthy volunteers. All patients were on oral corticosteroids with a dose equivalent to prednisone 0.75 mg/kg/day. The DMD participants traveled to the NIH Clinical Center during the screening phase of a clinical trial evaluating an oligonucleotide therapy (NCT01462292). The healthy volunteer boys were recruited from the NIH Clinical Research Volunteer Program registry. Subject eligibility, inclusion and exclusion criteria have been described elsewhere [19]. All subjects were asked to avoid excessive physical activity beyond their normal levels for a week prior to the study visit.
The NIH imaging study was registered on clinicaltrials.gov (NCT01451281) and was in compliance with the NIH Privacy Act and approved by an NIH Institutional Review Board. Informed written assent and consent were obtained from each subject and parent or guardian before participation in the study.
2.2. The ankle dorsiflexion exercise
A portable device (Ankle IntelliStretch device, RehabTek, Chicago, IL) was used for volitional concentric ankle dorsiflexion exercise and to measure the biomechanical data. The device has FDA Class I approval and has been used in children for both passive stretching and voluntary exercise [20, 21]. It is equipped with a torque sensor, a servomotor, and a digital controller. The device was connected to a computer for display and user interface. The user interface allowed adjustment of the applied torque value, motion velocity, and difficulty levels of the exercise games, such as assistance level and resistance level, according to each participant’s ability. The participant was seated in a comfortable chair with his upper body strapped against the backrest of the chair and the device in front at an appropriate distance to keep the knee flexed at 30 degrees to eliminate the restraining effect of the gastrocnemius muscle on ankle dorsiflexion [22]. The leg was secured by the leg support and the foot was attached onto the footplate with the ankle joint aligned to the rotation axis of the device. The participant was asked to play computer games by voluntary ankle dorsiflexion movements that began from the neutral position of the ankle joint. Plantarflexion movement was restricted to avoid muscle injury related to eccentric lengthening. Before exercise ankle passive range of motion, active range of motion, and maximum dorsiflexion torque at neutral position were assessed using the IntelliStretch device. The range of the moving target in the computer games was scaled to fit the range of motion of the ankle dorsiflexion in each child. The resistance level was determined by each child’s ability to maximally dorsiflex his ankle as a measure of his ankle dorsiflexion strength. The resistance level added by the device was never greater than 50% of maximal ankle dorsiflexion strength in each participant. The duration of the exercise for each participant was limited by his exercise tolerance (generally 25–30 minutes). None of the participants reported muscle soreness or fatigue either during or after exercise. Note that the ankle device allowed to set the exercise parameters according to the abilities of a participant and physical exhaustion was avoided during muscle activity.
Exercise performance data were analyzed using in-house MATLAB scripts. The total work performed by the subject during each period of exercise was computed by a two-step process. First, linear regression was used to estimate the foot sagittal plane moment of inertia from the individual’s age, height, and body mass [23, 24]. Work performed during exercise was calculated as the area under the angular acceleration-angle curve multiplied by the estimated moment of inertia. Power was computed as the work divided by the time of movement execution. For each subject, the work (J) and power (W) reported are aggregate totals summed across the entire exercise session.
2.3. MRI acquisition
MR images of the lower leg were acquired on a 3T Verio scanner (Siemens, Erlangen, Germany) at the NIH Clinical Center. An 8-element knee coil was used for the lower leg. Subjects were placed in the feet-first supine position inside the magnet bore and the legs were secured with foam pads. All scans were performed on the same scanner. T1-weighted turbo spin echo images were acquired consisting of 21 slices of 5 mm thickness with a slice gap of 5 mm and 1.5 mm in-plane resolution. For water T2 determination, a standard non-fat saturated multi-slice multi-echo sequence was acquired with a TR = 5000 ms, nominal flip angles = 90° and 180°, and a train of 12 echoes with TEs ranging from 14.3 ms to 171.6 ms with 14.3 ms echo-spacing. The field of view was equal to 126×180 mm2, with a pixel size of 0.7 mm2, covering 15 slices of 5 mm thickness with a slice gap of 6.5 mm. Fat quantification was obtained using a standard 3D gradient echo two-point Dixon technique with the following parameters: TR = 7.2 ms, TE1 = 2.5 ms, TE2 = 3.7 ms, T. flip angle = 9°, field of view = 151 × 220 × 140 mm3 with a voxel size of 0.43 × 0.43 × 5 mm3.
2.4. MRI analysis
Regions of interest (ROIs) were drawn manually on multi-slice multi-echo images (10 slices covering 58.5 mm) according to an anatomical atlas for the seven muscles in the lower legs: tibialis anterior, tibialis posterior, extensor digitorum longus, peroneus group, soleus, gastrocnemius medialis and gastrocnemius lateralis (Fig 1A). ROIs delineated the interior of the muscle avoiding fasciae and blood vessels. The mean water T2 relaxation times (ms) from ROIs were averaged prior to fitting using the tri-exponential model, as described [18]. We used T2 relaxation rates or R2 = 1/T2 (s−1) to show the effects of exercise on changes in muscle water component because the relaxation rates are additive, not the relaxation times. This multi-exponential model accounts for both water and fat component within the muscle tissue in contrast to the techniques based on fat saturation which are sensitive to field inhomogeneity artifact. This model is defined as the following:
| (1) |
Where S(TE) is the signal for a given echo time TE. T2fl and T2fs are the long and short relaxation times of the fat component respectively. T2m is the relaxation time of the muscle water component. Af, Am, are coefficient that reflect the proportion of water and fat component in the signal. cl and cs are coefficients of the bi-exponential model of the fat. For each subject, the subcutaneous fat signal was measured and then a bi-exponential fit was performed to estimate fat parameters (cl, cs, T2fl and T2fs). These parameters were fixed during the estimation of water T2 component inside the ROIs. It is important to point out, that for each ROI, the mean signal was computed and then the water T2 was estimated from the mean signal decay.
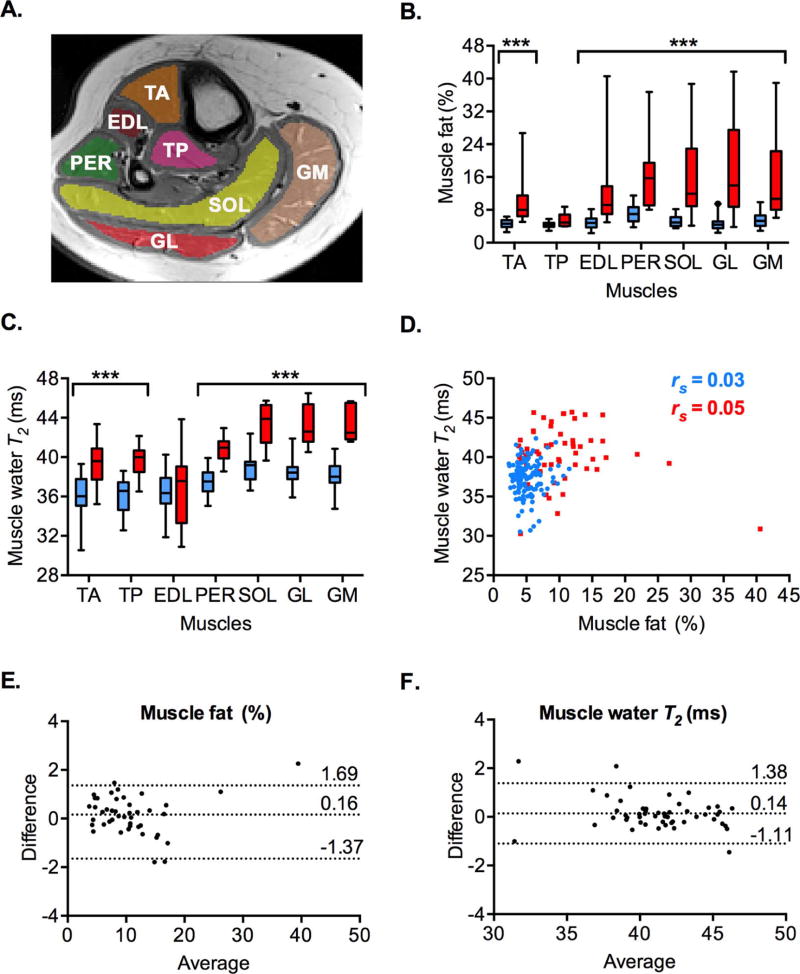
Figure 1.
MRI quantification of the lower leg muscle fat (%) and water T2 (ms) in DMD participants (red) and the healthy volunteers (blue). A. An axial T1 – weighted image of the lower leg of a healthy volunteer is shown. The regions-of-interest are drawn for the evaluation of muscle fat and water T2 on the tibialis anterior (TA), tibialis posterior (TP), extensor digitorum longus (EDL), peroneus (PER), soleus (SOL), gastrocnemius lateralis (GL), and gastrocnemius medialis (GM) muscles. B. Median muscle fat (%) is significantly increased in all but the tibialis posterior (TP) muscles of the DMD participants relative to the healthy volunteers. C. Median muscle water T2 (ms) is significantly increased in all but the extensor digitorum longus (EDL) muscles of the DMD participants relative to the healthy volunteers. D. Graph shows that muscle water T2 (ms) measurements were independent of the muscle fat (%) in the same muscle of the DMD participants (n = 56 muscles, p = 0.694) and the healthy volunteers (n = 133 muscles, p = 0.762). E–F. Bland-Altman plots demonstrate good agreement between the two independent blinded measurements of percent muscle fat (E) and muscle water T2 (F) in the same muscle (n = 45 and n = 52, respectively). Dotted horizontal lines represent mean difference and the 95% limits of agreement (mean ± 1.96 S.D.) (E–F). Box and whiskers represent 5th – 95th percentile values. Spearman’s r (rs) is shown. ***p < .001.
Dixon images were reconstructed using Siemens (Erlangen, Germany) product software producing a single water image (sw) and fat image (sf) generated from two gradient echo images and a field map, without T2* correction [25]. The fat fraction measured using Dixon was then calculated as 100 * |sf|/(|sf| + |sw|). The fat fraction maps were aligned with the T2 images, so that same ROIs were analyzed for muscle water T2 and muscle fat fraction quantification. The alignment was performed using an in-house MATLAB program that extracts the subject’s position and orientation and computes the adequate scaling factors and translations to be applied to the fat ratio map. A blinded investigator manually drew the ROIs and performed the analysis on duplicate sets of images with a distinct identity code for the assessment of inter-rater variability. Analyses were performed by investigators who were blinded to other clinical information.
2.5. Statistical analysis
Descriptive statistics were used to describe the subject characteristics. The least square means of height and weight were estimated adjusted for age, using the linear regression models, which were compared by two sample t-tests. Wilcoxon Rank Sum tests were used to compare muscle fat, muscle water T2 and ankle dorsiflexion biomechanics measurements between DMD participants and healthy volunteers. Two-sample F-test was used to compare the variance in power exerted during exercise between DMD participants and the healthy volunteers. The degree of inter-rater agreement was measured by the Bland-Altman method [26]. Spearman’s correlation coefficient (rs) was used to assess the association between the following variables: 1) percent muscle fat and water T2; 2) age and ankle dorsiflexion torque; 3) age and power exerted during exercise; 4) change in water R2 post-exercise from baseline (Δ water R2) between the 2 ankle dorsiflexor muscles; 5) percent muscle fat and Δ water R2; and 6) power exerted during exercise and Δ water R2. Two sided tests were performed for all statistical analyses and the level of significance was set at p < 0.05. Data analyses were done using Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Subject Characteristics
Subject demographics are outlined in Table 1. The lower leg skeletal muscle MRI was obtained from 12 ambulatory boys with DMD (age range: 6–14 years) and 19 healthy volunteer boys (age range: 5–14 years) at baseline and 3 hours post-exercise. The 2 groups were similar in age and weight. There was a significant difference in the age-adjusted height between subjects with DMD and the healthy volunteers (p < .001).
Table 1.
Demographics of subjects with Duchenne muscular dystrophy (DMD) and the healthy volunteers.
| DMD (n = 12) |
Healthy Volunteers (n = 19) |
P value2 | |
|---|---|---|---|
| Mean ± SE | Mean ± SE | ||
| Age (years) | 8.9 ± 0.7 | 10.0 ± 0.5 | 0.210 |
| Height (cm)1 | 127.4 ± 2.7 | 145.9 ± 2.1 | < .001 |
| Weight (kg)1 | 33.8 ± 2.8 | 39.3 ± 2.2 | 0.141 |
adjusted for age;
two sample t-test
Baseline muscle fat (%) in 3 DMD participants was not measured due to fat water swap or poor signal to noise ratio of the MR images. Baseline muscle water T2 (ms) of the peroneus and calf muscles could not be measured in 2 and 3 DMD participants, respectively due to poor tri- exponential fit (the confidence interval width of water T2 > 12 ms). A 10 year-old healthy volunteer was not included in the analysis of exercise effects on MRI measures because his data could not be retrieved from the ankle device due to computer malfunction.
3.2. MRI measures of muscle fat (%) and muscle water T2 (ms) in the lower leg muscles of DMD participants and healthy volunteers at baseline
The median muscle fat percentage was about 2–3 fold higher in the tibialis anterior, extensor digitorum longus, peroneus, soleus, and gastrocnemius muscles of DMD participants than in the healthy volunteers (p < .001) (Fig 1B, Table 2). The highest muscle fat percentage was in the peroneus muscles (as in ref 4), followed by the gastrocnemius lateralis and soleus muscles of DMD participants. In agreement with previous studies [3, 4], muscle fat percentage in the tibialis posterior muscle was not significantly different between the groups.
Table 2.
Skeletal muscle fat fraction (%) in the lower leg muscles of subjects with Duchenne muscular dystrophy (DMD) and the healthy volunteers at baseline.
| Muscles | DMD (n = 9) |
Healthy Volunteers (n =19) |
P value2 |
|---|---|---|---|
| Median (IQR1) | Median (IQR1) | ||
| Tibialis anterior | 8.0 (6.5 – 11.5) | 4.6 (3.8 – 5.5) | < .001 |
| Tibialis posterior | 4.9 (4.1 – 6.8) | 4.3 (3.9 – 4.8) | 0.129 |
| Extensor digitorum longus | 9.2 (7.0 – 13.8) | 4.8 (3.7 – 5.9) | < .001 |
| Peroneus | 15.7 (9.1 – 19.5) | 7.0 (5.2 – 8.6) | < .001 |
| Soleus | 11.9 (8.9 – 22.9) | 4.9 (4.1 – 6.2) | < .001 |
| Gastrocnemius lateralis | 13.9 (8.7 – 27.5) | 4.4 (3.5 – 5.1) | < .001 |
| Gastrocnemius medialis | 10.7 (8.0 – 22.3) | 5.3 (4.1 – 6.6) | < .001 |
IQR: interquartile range (25th – 75th percentile);
Wilcoxon Rank Sum test
We found that median muscle water T2 was higher in the tibialis anterior, tibialis posterior, peroneus, soleus and gastrocnemius muscles of DMD participants than in healthy volunteers at baseline (p < .001) (Fig 1C, Table 3). In contrast water T2 of the extensor digitorum longus muscle was not significantly different between the groups. Among DMD participants and the healthy volunteers, water T2 of the soleus and gastrocnemius muscles was higher than that of the tibialis anterior and extensor digitorum longus muscles (p < .001).
Table 3.
Skeletal muscle water T2 (ms) in the lower leg muscles of subjects with Duchenne muscular dystrophy (DMD) and the healthy volunteers at baseline.
| Muscles | DMD | Healthy Volunteers | P value2 |
|---|---|---|---|
| Median (IQR1) n | Median (IQR1) n | ||
| Tibialis anterior | 39.6 (37.7 – 40.9) 12 | 36.0 (35.1 – 37.8) 19 | < .001 |
| Tibialis posterior | 40.0 (38.5 – 40.7) 12 | 36.6 (34.6 – 37.4) 19 | < .001 |
| Extensor digitorum longus | 37.6 (33.3 – 39.1) 12 | 36.3 (35.3 – 37.9) 19 | 0.614 |
| Peroneus | 41.0 (39.9 – 41.6) 10 | 37.5 (36.5 – 38.4) 19 | < .001 |
| Soleus | 43.9 (41.8 – 45.3) 9 | 39.2 (37.7 – 39.5) 19 | < .001 |
| Gastrocnemius lateralis | 42.6 (41.6 – 45.4) 9 | 38.4 (37.8 – 39.0) 19 | < .001 |
| Gastrocnemius medialis | 42.5 (41.8 – 45.5) 9 | 38.0 (37.4 – 39.1) 19 | < .001 |
IQR: interquartile range (25th – 75th percentile);
Wilcoxon Rank Sum test
There was no significant correlation between muscle fat and water T2 in the same muscle of DMD participants and healthy volunteers (Fig 1D). Bland-Altman plots showed that the mean difference between two independent blinded measurements of percent muscle fat and water T2 values in the same muscle was 0.16 % and 0.14 ms, with the 95% limits of agreement (−1.37,1.69) and (−1.11, 1.38), respectively (Fig 1E–F).
3.3. Muscle work (J) and power (W) used during the volitional ankle dorsiflexion exercise by DMD participants and healthy volunteers
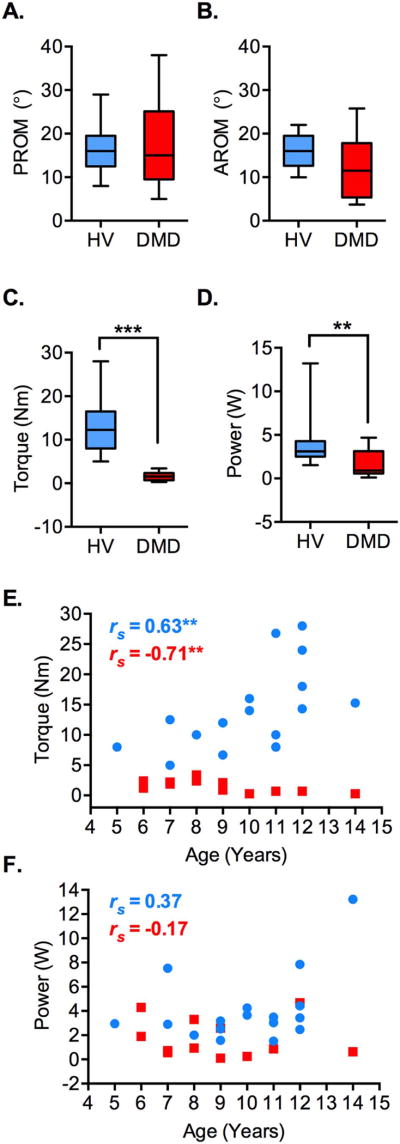
The median passive and active ankle dorsiflexion range of motion were not significantly different between the DMD participants and healthy volunteers (15° v. 16°, p = 0.958 and 11.5° v. 16°, p = 0.181, respectively; Fig 2A–B). The median isometric ankle dorsiflexion torque was significantly different between the DMD participants and healthy volunteers (1.5 Nm v. 12.2 Nm, p < .001; Fig 2C). There was an age-associated decrease in the isometric ankle dorsiflexion strength in the DMD participants, whereas there was an increase in ankle dorsiflexion torque with advancing age in healthy volunteers (rs = −0.71, p < .01 and rs = 0.63, p < .01, respectively; Fig 2E). Compared to the healthy volunteers, the DMD participants performed less work (139 J v. 562 J, p < .01) with less power (0.9 W v. 3.1 W, p < .01) over the course of the ankle dorsiflexion exercise (Fig 2D and F). There was no significant difference in the inter-subject variability of power exertion during exercise between the DMD participants and healthy volunteers (Two-sample F (17,11) = 3.16, p = 0.06).
Figure 2.
Biomechanics of the volitional ankle dorsiflexion exercise in DMD participants (DMD, n = 12, red) and the healthy volunteers (HV; n = 18, blue). A–B. The passive range-of-motion or PROM (A) and active range-of-motion or AROM (B) for ankle dorsiflexion were not significantly different between the DMD participants and the healthy volunteers (p = 0.958 and p = 0.181, respectively). C–D. The ankle dorsiflexion torque measured by the ankle device (C) and the power exerted over the course of ankle dorsiflexion exercise (D) were significantly reduced in the DMD participants relative to the healthy volunteers. E. Whereas ankle dorsiflexion torque decreased with age in the DMD participants there was a gain in the torque value with age in the healthy volunteers. Note that at all ages the ankle dorsiflexion torque values are lower in the DMD participants than the healthy volunteers. F. Graph shows no significant relation between age and power exerted during the ankle dorsiflexion exercise in the healthy volunteers (p = 0.127) and DMD participants (p = 0.581). Box and whiskers represent 5th – 95th percentile values (A–D). Spearman’s r (rs) is shown (E–F). **p < .01, ***p < .001.
3.4. Exercise effects on muscle water R2 (s−1) in the lower leg muscles of DMD participants and healthy volunteers
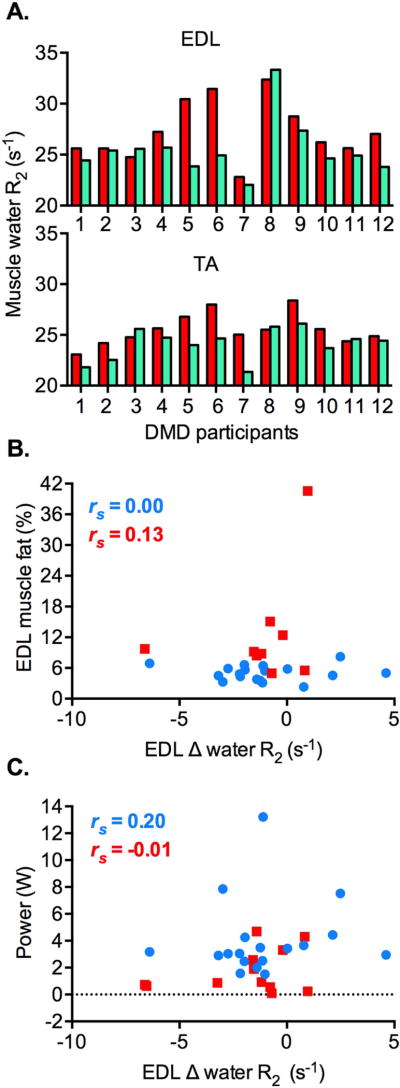
We found that the mean muscle water R2 decreased (= water T2 increased) at 3 hours post-exercise from baseline values in the lower leg muscles of the DMD participants and the healthy volunteers (Table 4). The mean Δ water R2 was greater in the target muscles of ankle dorsiflexion exercise, extensor digitorum longus and tibialis anterior, than the plantarflexor muscles in both groups. Whereas there was inter-subject variability for post-exercise R2 changes in the tibialis anterior and extensor digitorum muscles of DMD patients, we found a consistent trend in post-exercise R2 changes between muscles in individual patients (Fig 3A). Muscle water R2 decreased in both muscles of 10 DMD patients and increased in 2 patients. The Δ water R2 of the tibialis anterior muscle positively correlated with that of the extensor digitorum longus muscle in the DMD participants and the healthy volunteers (rs = 0.57, p = 0.05 and rs = 0.90, p < .001, respectively). The Δ water R2 of the lower leg muscles did not significantly correlate with muscle fat fraction or power exerted during exercise in both groups (Fig 3B–C; extensor digitorum longus muscle shown).
Table 4.
Changes in muscle water R2 (s−1) between baseline and 3 hours following the voluntary ankle dorsiflexion exercise (Δ water R2) in the lower leg muscles of subjects with Duchenne muscular dystrophy (A) and the healthy volunteers (B).
| (A) Subjects with Duchenne muscular dystrophy | ||||
|
Baseline Mean ± SE |
Post-Exercise Mean ± SE |
Δ water R2 Mean ± SE (n) |
||
| Ankle dorsiflexors | Extensor digitorum longus | 27.3 ± 0.8 | 25.5 ± 0.8 | −1.8 ± 0.7 (12) |
| Tibialis anterior | 25.5 ± 0.4 | 24.1 ± 0.4 | −1.4 ± 0.4 (12) | |
| Ankle plantarflexors | Soleus | 23.1 ± 0.4 | 22.8 ± 0.3 | −0.3 ± 0.3 (9) |
| Gastrocnemius lateralis | 23.1 ± 0.4 | 22.6 ± 0.2 | −0.5 ± 0.3 (9) | |
| Gastrocnemius medialis | 23.1 ± 0.3 | 22.5 ± 0.3 | −0.6 ± 0.4 (9) | |
| (B) Healthy Volunteers | ||||
|
Baseline Mean ± SE |
Post-Exercise Mean ± SE |
Δ water R2 Mean ± SE (n) |
||
| Ankle dorsiflexors | Extensor digitorum longus | 27.4 ± 0.4 | 26.5 ± 0.3 | −1.1 ± 0.6 (18) |
| Tibialis anterior | 28.1 ± 0.5 | 26.7 ± 0.4 | −1.4 ± 0.7 (18) | |
| Ankle plantarflexors | Soleus | 25.6 ± 0.2 | 25.1 ± 0.2 | −0.6 ± 0.3 (18) |
| Gastrocnemius lateralis | 25.9 ± 0.2 | 25.2 ± 0.2 | −0.9 ± 0.4 (18) | |
| Gastrocnemius medialis | 26.1 ± 0.2 | 25.1 ± 0.2 | −1.0 ± 0.4 (18) | |
Figure 3.
Changes in muscle water R2 (s−1) in the ankle dorsiflexor muscles of DMD participants and the healthy volunteers following the volitional ankle dorsiflexion exercise. A. Congruent changes in muscle water R2 between baseline (red) and 3 hours post-exercise (green) in the tibialis anterior (TA) and extensor digitorum longus (EDL) muscles of individual DMD participants (n = 12). A decrease in muscle water R2 is seen in 10 DMD patients, whereas an increase in water R2 is seen in 2 patients. B. There is no significant correlation between Δ muscle water R2 and muscle fat (%) in the EDL muscle of DMD patients (red squares; n = 9, p = 0.743) and the healthy volunteers (blue circles; n = 18, p = 0.990). C. Similarly, Δ water R2 of the EDL muscle is not significantly correlated with power exerted over the course of the exercise in the DMD participants (red squares; n = 12, p = 0.991) and healthy volunteers (blue circles; n = 18, p = 0.433). Spearman’s r (rs) is shown.
4. Discussion
Our findings demonstrate that the tri-exponential model of muscle water T2 measurement provides an efficient noninvasive method to measure responses to exercise and pathological changes related to underlying disease process in fatty-infiltrated muscles of an individual with DMD. We show that the tri-exponential model allows quantification of muscle water T2 independent of muscle fatty degeneration in the DMD participants. This finding is in agreement with a previous study which demonstrated the insensitivity of the triexponential method to muscle fat content in other skeletal myopathies [18].
MR spectroscopy is the most accepted method for quantification of muscle fat and water components. However, it is limited by poor spatial resolution and coverage and challenges in setting the spectroscopy volume registrations. The triexponential method provided better spatial resolution and coverage to allow assessment of multiple muscles simultaneously. We found that the baseline muscle water T2 was higher in most lower leg muscles of the DMD participants than the healthy volunteers. The pattern of muscle involvement for fatty degeneration in the lower leg of DMD participants was in agreement with previous studies [3,4]. We observed that the abnormalities of muscle water T2 had a different pattern than fatty degeneration in the lower leg of DMD patients. The extensor digitorum longus muscle of the DMD participants showed higher muscle fat (%) but had water T2 values similar to that in the healthy volunteers, whereas the tibialis posterior muscle had higher muscle water T2 in DMD participants than the healthy volunteers but did not show changes of fatty infiltration. During the preparation of this manuscript, Hooijmans et al also reported an increase in muscle water T2 in the non-fatty infiltrated lower leg muscles of DMD patients using tri-exponential model [27]. Minor differences in the muscle water T2 values between studies could be related to differences in the MR acquisition parameters for echo spacing and the length of echo-train, heterogeneity of disease status between patient groups and different steroid dosing regimen.
The baseline increase in muscle water T2 in DMD patients compared to age-matched controls indicates inflammation, necrosis, damage and other disease processes which lead to increase in intracellular and/or extracellular edema in dystrophic muscles (reviewed in ref 16, 17, 28). In patients with Becker muscular dystrophy the pathological changes of muscle inflammation are less compared to that in DMD patients [29] and it was observed that fat-adjusted muscle T2 (water T2) was not higher than that seen in age-matched controls [30]. In patients with facioscapulohumeral muscular dystrophy, the hyperintense muscles on T2-short tau inversion recovery sequence (elevated water T2) had pathological changes of muscle inflammation accompanied by a significant upregulation of genes involved in the muscle inflammatory disease process [31]. In adult patients with Pompe disease, the muscles with higher water T2 at baseline had a faster progression of fatty degeneration than muscles with normal water T2 [32]. Whereas muscle inflammation and related processes are non-specific events, they are thought to directly contribute to progressive muscle degeneration in DMD [33]. A decrease in muscle water T2 and subsequent slower rate of fatty degeneration in response to prednisone was seen in the lower leg muscles of 5 – 7 years old DMD patients compared to those who were not treated [34]. It has been demonstrated that muscle water T2 tends to normalize with disease progression in DMD [19, 35, 36]. However, a direct correlation between changes in muscle water T2 and a faster rate of progression in muscle degeneration towards fatty replacement has not yet been shown in skeletal muscles of DMD patients.
Importantly, our results demonstrate a decrease in the lower leg muscle water R2 at 3 hours after a voluntary dynamic concentric ankle dorsiflexion exercise in the DMD participants and healthy volunteers. These changes were prominent in the tibialis anterior and extensor digitorum longus muscles, the primary effectors of ankle dorsiflexion than ankle plantarflexor muscles. A previous study suggested that the changes in muscle T2 occurring < 1 hour post-exercise were proportional to exercise intensity in healthy individuals [37]. We did not find any significant relation between Δ water R2 3 hours post-exercise and power exerted during exercise in DMD patients and healthy volunteers. The DMD participants had a significantly lower ankle dorsiflexion muscle torque and performed exercise with significantly less power than the healthy volunteers. Nevertheless, they showed a similar Δ water R2 in the exercised muscles as the healthy volunteers. Whether DMD muscle is sensitive to a lower intensity of exercise compared to healthy muscle should be determined by setting the same exercise parameters for both groups in further studies. The inter-subject variability in Δ water R2 probably originated from the physiologic response to the exercise and not due to volitional effort because it did not correlate with the exercise intensity and we did not observe inter-subject variability in power exerted over the course of the exercise.
Muscle T2 changes after exercise may depend on the type of the muscle activity. We specifically avoided eccentric contractions during exercise because it is well-established that eccentric exercise leads to skeletal muscle damage in healthy individuals [38]. Increased permeability of muscle membrane is an early event in the eccentrically contracted muscle [39]. Indeed, dystrophin-deficient muscle fibers with fragile sarcolemma are more susceptible to damage than healthy muscle [40]. Large rises of plasma creatine kinase enzyme levels, an indirect indicator of muscle membrane leakiness, have been observed after eccentric (muscle elongates in response to a greater opposing force) but not concentric (muscle shortens thereby generating force) muscle activity in healthy individuals [41]. The T2 changes associated with muscle damage, inflammatory responses and edema occur at least 2 days following eccentric exercise [42]. Muscles performing concentric exercise have a significantly higher increase in muscle T2 than the muscles performing eccentric actions at the relatively same load in healthy volunteers [43]. However, post-exercise T2 changes do not last longer than 24 hours and the delayed T2 changes of eccentric activity are not known to occur following concentric exercise in healthy muscle [44]. A few studies have focused on concentric exercise effects on healthy skeletal muscles by MRI and have demonstrated that muscle injury was significantly less compared to eccentric lengthening exercise [45,46]. Electron microscopic studies of rabbit skeletal muscle fibers showed myofibrillar disruption after eccentric but not concentric exercise [47]. Therefore, muscle water R2 changes within hours of concentric exercise in our study likely do not represent exercise induced muscle injury or inflammation.
We do not yet know the underlying mechanism for R2 changes 3 hours post-exercise in healthy and DMD muscle. Muscle water T2 and R2 can be affected by muscle blood flow and hemoglobin oxygenation, pH and lactate changes and osmotically-driven bulk water diffusion into the myofibrillar and interstitial space. Requirement for muscle blood flow and aerobic ATP production are increased during dynamic exercise, however it has been established that the changes in muscle perfusion return to baseline within minutes post-exercise and muscle T2 changes are not dependent on the perfusion status of skeletal muscle during this early phase post-exercise [48,49]. Previous studies have demonstrated that post-exercise muscle T2 increase does not represent an increase in extracellular water component, and changes in intramuscular pH, lactate and other metabolites do not contribute to the changes in muscle R2 beyond 1 hour following submaximal resistance exercise [37, 50, 51]. In mice increased muscle T2 was associated with alternations in bulk water diffusion into muscle fibers and interstitial space and there was Evans blue dye uptake by muscle fibers, an indicator for loss of sarcolemmal integrity in vivo [52]. In DMD increased sarcolemmal fragility in dystrophin deficient muscle fibers may allow increase in water flux at a lower contractile stress relative to healthy individuals. The Δ water R2 did not relate to the degree of muscle fatty degeneration in DMD patients. Heterogeneous muscle damage, inflammation and necrosis within and across muscles of DMD participants may have contributed to the inter-subject variability in post-exercise muscle water R2 changes. Longitudinal measurements over time and following intervention in the same subjects would be good to pursue in clinical trials.
Ankle dorsiflexion is a critical component of the gait cycle [53]. The extensor digitorum longus and the tibialis anterior muscles concentrically contract to assist in toe clearance during the swing phase while maintaining a supinated position during heel strike. Instrumented gait analysis showed a reduced ankle dorsiflexion during terminal stance and pre-swing with a consequent reduction in the peak-to-peak excursion in 5 – 7 years old DMD boys [54]. Active movement training with the ankle device used in this study led to improvements in the functional abilities of balance and walking in children with cerebral palsy [20]. Randomized double blind intervention trials are needed to determine the effects of low resistance strength training on the functional abilities of patients with DMD [55]. The ankle device can provide well controlled goal-directed active movement training, and video games can be a motivation for children to actively engage in a selective voluntary movement task. Controlled settings for exercise are relevant for standardization across sites in a multisite intervention study. Muscle water T2 and R2 as measured in this study would be useful as sensitive and noninvasive outcome measures to compare the relative exercise effects on different skeletal muscles of DMD patients.
The study was conducted at a single site, although the DMD participants were enrolled from across the United States. A limitation of this study is the small number of participants. We could only examine a single time point at 3 hours post-exercise due to time constraints in the clinical trial. It would be important to assess the timeline of changes in muscle water T2 following concentric muscle exercise as it is anticipated to be different in DMD muscle with loss of sarcolemmal integrity. It would be prudent to examine the relation between muscle contractile content [3] and Δ water R2 of DMD muscle in further studies. We measured muscle water T2 from the same ROI and there was a good agreement between two blinded measurements. Whereas these methods should minimize variability in our measurements, errors could still occur related to where the edges of ROIs are drawn and image artifacts. We did not use the B1 map for pixel selection to overcome the B1 inhomogeneity error. This could be addressed in future studies by using extended phase graph fitting as proposed by Lebel and Wilman [56,57]. It should be noted that there is no identified rationale at present for fitting the lipids by 2 exponentials [18]. Also, we cannot exclude a possibility that changes in long T2 water component can potentially overlap with the lipids due to T2 similarities. A potential limitation of the 2 point Dixon method is that swaps between fat and water components can occur between limbs or inside limb segments [28]. We found that DMD muscle had a significantly higher muscle fat fraction than controls, where control muscle fat fraction was about 4.5 – 5%. The exact value of control muscle fat fraction can depend on whether the analysis model accounts for noise bias and T2* for example, control muscle fat values reported by Loughran et al [58] measured by incorporated R2* modeling were lower than Hooijmans et al [27], who did not.
In summary, we show that the MRI biomarker of muscle water T2 measured by the triexponential method is sensitive to the underlying disease status in DMD and is responsive to acute changes related to exercise in both patients and healthy individuals. Exploration of the role of this imaging biomarker in predicting the restoration of dystrophin activity in DMD skeletal muscle is warranted in future randomized clinical trials.
Highlights.
Triexponential method measures muscle water T2 in fatty-infiltrated muscles in DMD.
Muscle water T2 is a useful biomarker of disease status in clinical trials of DMD.
Muscle water T2 is responsive to exercise effects in DMD subjects as in controls.
Acknowledgments
The authors thank the study participants and their families; Donovan Stock, Elizabeth Hartnett, and Alice Schindler (NINDS) for help with coordinating study visits and scheduling MRI studies; Robert Evers and Larry Yao (Radiology, NIH Clinical Center) for help with installing MRI sequence protocols on the scanner; Carsten G. Bonnemann (NINDS) for critical reading of the manuscript and the Principal Investigators of the referring sites participating in the clinical trial (Barry Russman, Portland, OR; Benjamin Renfroe, Gulf Breeze, FL; Brenda Wong, Cincinnati, OH; Douglas Sproule, New York, NY; Edward Smith, Durham, NC; and Kathryn Wagner, Baltimore, MD) for sharing study participants.
Funding
The study was supported in part by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke and the National Institutes of Health Clinical Center. Drs. Azzabou, Reyngoudt and Carlier received funding from the French Association against Myopathies and European Union's Seventh Framework Program FP7/2013-2017/ Bioimage-NMD.
Abbreviations
- DMD
Duchenne muscular dystrophy
- MRI
Magnetic resonance imaging
- T2
The transverse relaxation time constant
- ROI
Region of interest
- R2
T2 relaxation rate
- Δ water R2
Difference between baseline R2 and post-exercise R2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conception and design of the study (AM, KF), data acquisition and analysis (AM, NA, TB, HR, HS, YR, EK, PC), and drafting the manuscript, making the figures and revising the manuscript for intellectual content (AM, NA, TB, HS, EK, KF, PC). AM had access to all the data and takes responsibility for the data, accuracy of the data analysis, and the conduct of the research.
Potential Conflicts of Interest
YR is employed by RehabTek, Inc. All other authors have nothing to report.
References
- 1.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–13. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 3.Vohra RS, Lott D, Mathur S, et al. Magnetic Resonance Assessment of Hypertrophic and Pseudo-Hypertrophic Changes in Lower Leg Muscles of Boys with Duchenne Muscular Dystrophy and Their Relationship to Functional Measurements. PLoS One. 2015;10:e0128915. doi: 10.1371/journal.pone.0128915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torriani M, Townsend E, Thomas BJ, Bredella MA, Ghomi RH, Tseng BS. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol. 2012;41:437–45. doi: 10.1007/s00256-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arpan I, Forbes SC, Lott DJ, et al. T(2) mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26:320–28. doi: 10.1002/nbm.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 7.Anthony K, Arechavala-Gomeza V, Taylor LE, et al. Dystrophin quantification: Biological and translational research implications. Neurology. 2014;83:2062–69. doi: 10.1212/WNL.0000000000001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota T, Lu QL, Partridge T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–76. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman EP, Dressman D. Molecular pathophysiology and targeted therapeutics for muscular dystrophy. Trends Pharmacol Sci. 2001;22:465–70. doi: 10.1016/s0165-6147(00)01770-3. [DOI] [PubMed] [Google Scholar]
- 10.Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81:S162–74. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 11.Dudley RW, Danialou G, Govindaraju K, Lands L, Eidelman DE, Petrof BJ. Sarcolemmal damage in dystrophin deficiency is modulated by synergistic interactions between mechanical and oxidative/nitrosative stresses. Am J Pathol. 2006;168:1276–87. doi: 10.2353/ajpath.2006.050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord. 1997;7:487–92. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 13.Larsen RG, Ringgaard S, Overgaard K. Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scand J Med Sci Sports. 2007;17:76–83. doi: 10.1111/j.1600-0838.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 14.Mathur S, Vohra RS, Germain SA, et al. Changes in muscle T2 and tissue damage after downhill running in mdx mice. Muscle Nerve. 2011;43:878–86. doi: 10.1002/mus.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrood P, Hollingsworth KG, Eagle M, et al. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J Magn Reson Imaging. 2009;30:1130–38. doi: 10.1002/jmri.21941. [DOI] [PubMed] [Google Scholar]
- 16.Carlier PG. Global T2 versus water T2 in NMR imaging of fatty infiltrated muscles: different methodology, different information and different implications. Neuromuscul Disord. 2014;24:390–92. doi: 10.1016/j.nmd.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Hollingsworth KG. Quantitative MRI in muscular dystrophy: An indispensable trial endpoint? Neurology. 2014;83:956–57. doi: 10.1212/WNL.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 18.Azzabou N, Loureiro de Sousa P, Caldas E, Carlier PG. Validation of a generic approach to muscle water T2 determination at 3T in fat-infiltrated skeletal muscle. J Magn Reson Imaging. 2015;41:645–53. doi: 10.1002/jmri.24613. [DOI] [PubMed] [Google Scholar]
- 19.Mankodi A, Bishop CA, Auh S, Newbould RD, Fischbeck KH, Janiczek RL. Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:650–58. doi: 10.1016/j.nmd.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YN, Hwang M, Ren Y, Gaebier-Spira D, Zhang LQ. Combined passive stretching and active movement rehabilitation of lower-limb impairments in children with cerebral palsy using a portable robot. Neurorehabil Neural Repair. 2011;25:378–85. doi: 10.1177/1545968310388666. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Wu YN, Hwang M, et al. Changes of calf muscle-tendon biomechanical properties induced by passive-stretching and active-movement training in children with cerebral palsy. J Appl Physiol. 2011;111:435–42. doi: 10.1152/japplphysiol.01361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumbach SF, Brumann M, Binder J, Mutschler W, Regauer M, Poizer H. The influence of knee position on ankle dorsiflexion - a biometric study. BMC Musculoskelet Disord. 2014;15:246. doi: 10.1186/1471-2474-15-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Leva P. Adjustments to Zatsiorsky-Seluyanov's segment inertia parameters. J Biomech. 1996;29:1223–30. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 24.Jensen RK. Body segment mass, radius and radius of gyration proportions of children. J Biomech. 1986;19:359–68. doi: 10.1016/0021-9290(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 25.Jellus V. Phase correction method. US20100201364. US Patent. 2010
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 27.Hooijmans MT, Niks EH, Burakiewicz J, Verschuuren JJGM, Webb AG, Kan HE. Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR Biomed. 2017;30:e3667. doi: 10.1002/nbm.3667. [DOI] [PubMed] [Google Scholar]
- 28.Carlier PG, Marty B, Scheidegger O, et al. Skeletal Muscle Quantitative Nuclear Magnetic Resonance Imaging and Spectroscopy as an Outcome Measure for Clinical Trials. J Neuromuscul Dis. 2016;3:1–28. doi: 10.3233/JND-160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennett X, Shield LK, Clingan LJ, Woolley DA. Becker and Duchenne muscular dystrophy: a cooperative morphological study. Aust Paediatr J. 1988;25:15–20. [PubMed] [Google Scholar]
- 30.Wokke BH, van den Bergen JC, Hooijmans MT, Verschuuren JJ, Niks EH, Kan HE. T2 relaxation times are increased in skeletal muscle of DMD but not BMD patients. Muscle Nerve. 2016;53:38–43. doi: 10.1002/mus.24679. [DOI] [PubMed] [Google Scholar]
- 31.Tasca G, Pescatori M, Monforte M, et al. Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One. 2012;7:e38779. doi: 10.1371/journal.pone.0038779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlier PG, Azzabou N, de Sousa PL, et al. Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis. 2015;38:565–72. doi: 10.1007/s10545-015-9825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YW, Nagaraju K, Bakay M, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–34. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 34.Arpan I, Wilcocks RJ, Forbes SC, et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology. 2014;83:974–80. doi: 10.1212/WNL.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes SC, Willcocks RJ, Triplett WT, et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: a multicenter cross sectional study. PLoS One. 2014;9:e106435. doi: 10.1371/journal.pone.0106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed. 2015;28:1150–62. doi: 10.1002/nbm.3352. [DOI] [PubMed] [Google Scholar]
- 37.Fisher MJ, Meyer RA, Adams GR, Foley JM, Potchen EJ. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest Radiol. 1990;25:480–85. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med. 1989;7:207–34. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- 39.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 40.Lovering RM, Brooks SV. Eccentric exercise in aging and diseased skeletal muscle: good or bad? J Appl Physiol. 2014;116:1439–45. doi: 10.1152/japplphysiol.00174.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newham DJ, Jones DA, Edwards RH. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve. 1986;9:59–63. doi: 10.1002/mus.880090109. [DOI] [PubMed] [Google Scholar]
- 42.Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 1999;87:2311–18. doi: 10.1152/jappl.1999.87.6.2311. [DOI] [PubMed] [Google Scholar]
- 43.Shellock FG, Fukunaga T, Mink JH, Edgerton VR. Acute effects of exercise on MR imaging of skeletal muscle: concentric vs eccentric actions. AJR Am J Roentgenol. 1991;156:765–68. doi: 10.2214/ajr.156.4.2003443. [DOI] [PubMed] [Google Scholar]
- 44.Ochi E, Tsuchiya Y, Nosaka K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur J Appl Physiol. 2016 doi: 10.1007/s00421-016-3462-3. Epub September 8. [DOI] [PubMed] [Google Scholar]
- 45.Sesto ME, Chourasia AO, Block WF, Radwin RG. Mechanical and magnetic resonance imaging changes following eccentric or concentric exertions. Clin Biomech. 2008;23:961–68. doi: 10.1016/j.clinbiomech.2008.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorichter S, Koller A, Haid C, et al. Light concentric exercise and heavy eccentric muscle loading: effects on CK, MRI and markers of inflammation. Int J Sports Med. 1995;16:288–92. doi: 10.1055/s-2007-973007. [DOI] [PubMed] [Google Scholar]
- 47.Lieber RL, Friden J. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988;133:587–88. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 48.Archer BT, Fleckenstein JL, Bertocci LA, et al. Effect of perfusion on exercised muscle: MR imaging evaluation. J Magn Reson Imaging. 1992;2:407–13. doi: 10.1002/jmri.1880020409. [DOI] [PubMed] [Google Scholar]
- 49.Schewzow K, Fiedler GB, Meyerspeer M, et al. Dynamic ASL and T-2*-Weighted MRI in Exercising Calf Muscle at 7 T-A Feasibility Study. Mag Reson Med. 2015;73:1190–95. doi: 10.1002/mrm.25242. [DOI] [PubMed] [Google Scholar]
- 50.Ploutz-Snyder LL, Nyren S, Cooper TG, Potchen EJ, Meyer RA. Different effects of exercise and edema on T2 relaxation in skeletal muscle. Magn Reson Med. 1997;37:676–82. doi: 10.1002/mrm.1910370509. [DOI] [PubMed] [Google Scholar]
- 51.Pan JW, Hamm JR, Hetherington HP, Rothman DL, Shulman RG. Correlation of lactate and pH in human skeletal muscle after exercise by 1H NMR. Magn Reson Med. 1991;20:57–65. doi: 10.1002/mrm.1910200107. [DOI] [PubMed] [Google Scholar]
- 52.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandernborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve. 2005;32:605–12. doi: 10.1002/mus.20398. [DOI] [PubMed] [Google Scholar]
- 53.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–81. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doglio L, Pavan E, Pernigotti I, Petralia Early signs of gait deviation in Duchenne muscular dystrophy. Eur J Phys Rehabil Med. 2011;47:587–94. [PubMed] [Google Scholar]
- 55.Gianola S, Pecoraro V, Lambiase S, Gatti R, Banfi G, Moja L. Efficacy of muscle exercise in patients with muscular dystrophy: a systematic review showing a missed opportunity to improve outcomes. PLoS One. 2013;8:e65414. doi: 10.1371/journal.pone.0065414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebel RM, Wilman AH. Transverse relaxometry with stimulated echo compensation. Magn Reson Med. 2010;64:1005–14. doi: 10.1002/mrm.22487. [DOI] [PubMed] [Google Scholar]
- 57.Marty B, Baudin PY, Reyngoudt H, et al. Simultaneous muscle water T2 and fat fraction mapping using transverse relaxometry with stimulated echo compensation. NMR Biomed. 2016;29:431–43. doi: 10.1002/nbm.3459. [DOI] [PubMed] [Google Scholar]
- 58.Loughran T, Higgins DM, McCallum M, Coombs A, Straub V, Hollingsworth KG. Improving highly acelerated fat fraction measurements for clinical trials in muscular dystrophy: origin and quantitative effect of R2* changes. Radiology. 2015;275:570–78. doi: 10.1148/radiol.14141191. [DOI] [PubMed] [Google Scholar]