Abstract
Multiplexed point-of-care testing (xPOCT), which is simultaneous on-site detection of different analytes from a single specimen, has recently gained increasing importance for clinical diagnostics, with emerging applications in resource-limited settings (such as in the developing world, in doctors’ offices, or directly at home). Nevertheless, only single-analyte approaches are typically considered as the major paradigm in many reviews of point-of-care testing. Here, we comprehensively review the present diagnostic systems and techniques for xPOCT applications. Different multiplexing technologies (e.g., bead- or array-based systems) are considered along with their detection methods (e.g., electrochemical or optical). We also address the unmet needs and challenges of xPOCT. Finally, we critically summarize the in-field applicability and the future perspectives of the presented approaches.
Keywords: lab-on-a-chip (LOC), microfluidics, multianalyte analysis, multiplexing, on-site testing, point-of-care testing (POCT)
Trends
Simultaneous on-site measurement of different substances from a single sample, called multiplexed point-of-care testing, has recently become more and more important for in vitro diagnostics.
The major aim for the development of xPOCT systems is the smart combination of a high-performing device with a low system complexity. Thus, the on-site tests are realized in a short time by non-experts and ensure comparable results with clinical and central laboratory findings.
A multiplexing capability of up to 10 analytes has been sufficient for many recent xPOCT applications.
The future of xPOCT devices will be driven by novel biotechnologies (e.g., aptamers) or targets (e.g., circulating RNAs or tumor cells, exosomes, and miRNAs), as well as applications like personalized medicine, homecare monitoring, and wearables.
Multiplexed Point-of-Care Testing – xPOCT
An early and accurate diagnosis of a specific disease plays a decisive role for its effective treatment. Especially at the point of care, where an immediate decision on treatment most needs to be made, such as in cases of stroke or sepsis, the rapid and precise confirmation of clinical findings is vital [1]. However, in many instances (e.g., to distinguish between the different types of sepsis), clinical evidence based on a single biomarker is not adequate for an appropriate diagnosis of a disease or for monitoring its treatment.
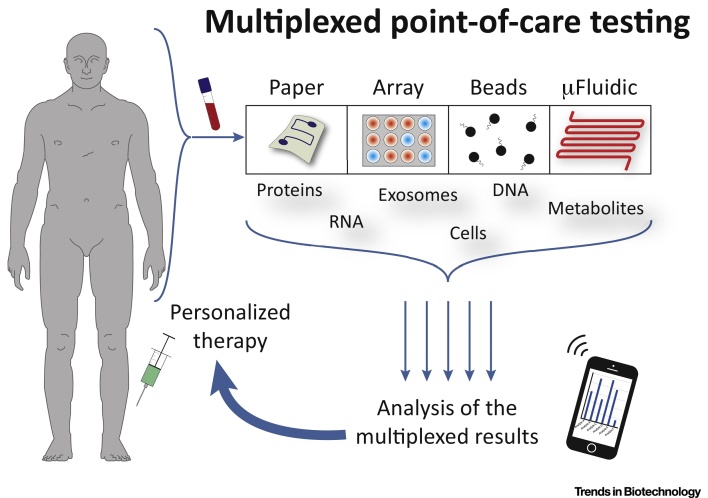
In addition, it is highly desirable to screen various analytes simultaneously, enabling a rapid, low-cost, and reliable quantification. Therefore, multiplexing (see Glossary) has become more important for point-of-care testing in the last decade [2] (Figure 1, Key Figure). In this context, there is a great demand for xPOCT devices, which ensure the quality and performance requirements of in vitro diagnostics, performed in a short period by non-experts [3]. This will also pave the way for novel home health monitoring systems and add valuable information for personalized medicine.
Figure 1.
Key Figure: Multiplexed Point-of-Care Testing (xPOCT)
Requires Novel (Appropriate, Powerful, Low-Cost and Simple) Strategies for Sampling, Analysis and Data Interpretation. Therefore, it will pave the way for personalized therapies or on-site disease diagnostics in resource-limited settings in the future.
For clinical xPOCT applications, there already exist many different commercial devices for the simultaneous detection of clinical chemistry parameters, including blood gases, and electrolytes, or acute metabolites (e.g., Abbott i-STAT system, Abaxis Piccolo Xpress, or Nova Biomedical StatSensor), or immunoassays (e.g., Radiometer AQT90 Radiometer and Mitsubishi PATHFAST analyzer). However, these systems are either bulky and expensive bench-top bioanalyzers, or only capable of detecting a limited amount or type of analytes.
To cover the requirements for xPOCT appropriately, diagnostic devices must rigorously complete the following tasks: (i) low sample consumption (e.g., blood from a finger prick), or the possibility to use easily accessible (noninvasive) samples, like urine, saliva, sweat and breath condensate, along with a maximum amount of information obtained from valuable specimens; (ii) simple or automated system operation requiring minimal user intervention; (iii) rapid turnaround times – within 10 minutes to 2 hours – allowing an immediate treatment; (iv) prolonged reagent storage and shelf life; (v) accurate and quantitative results in accordance with clinical and central laboratory findings, adhering to international quality standards (ISO 15189); and (vi) low-cost and portable readout devices, equipped with disposable test cartridges or strips, satisfying the in vitro diagnostics guidelines (EU Directive 98/79/EC or FDA regulations). Finally, equipment-free or cell-phone-based systems are highly preferred in the developing world or other resource-poor settings 4, 5. Ideally, the xPOCT device should also be able to analyze different types of compounds simultaneously, e.g., RNAs, metabolites, proteins, exosomes, and cells.
Current Multiplexing Technologies
Multiplexing is mainly realized through three different approaches: (i) spatial separation of detection sites, by means of different spots or wells, (ii) regional separation using discrete regions of a channel network or electrode arrays, or (iii) the use of various labels (e.g., enzymes, redox molecules, beads, and dyes). In particular, optical and electrochemical detection techniques are employed for the signal readout 6, 7.
Alternatively, mass spectrometry (MS) can directly identify many molecules, often even without prior separation [8]. For example, matrix-assisted laser desorption/ionization (MALDI)-MS is frequently employed in hospitals to rapidly characterize pathogens. Other types of MS and ion mobility spectrometry are used to monitor the volatile compounds in breath. Since these instruments are still bulky and expensive, they are not further considered in the following discussion of xPOCT.
To date, spatial separation of various analytes has been the most often applied method for multiplexed on-site bioanalysis systems. Yet, they mainly suffer from complexity due to device fabrication, assay preparation, and/or consecutive signal readout. Another frequently applied technique for multiplexing is the use of different labels, which generally results in high readout complexity by increasing the number of analytes. On the other hand, the regional separation of different detection sites (e.g., diversely functionalized electrodes or channel areas) has been increasingly employed in recent xPOCT applications. Their main drawbacks are their limited multiplexing levels and possible cross-sensitivity between single detection sites through diffusion 6, 9, 10.
Here, we present a survey of the existing diagnostic devices in academia and industry for xPOCT (Table 1). Moreover, we draw particular attention to lab-on-a-chip systems, especially to those including microfluidics, since the number of such devices for xPOCT approaches has been rapidly increasing thanks to the remarkable technical advances in recent years. The application of microfluidics facilitates the miniaturization of sophisticated laboratory procedures onto a tiny chip with several advantages, including rapid turnaround times as well as low sample/reagent consumption 10, 11, 12.
Table 1.
Brief Summary of Recent xPOCT Systems
| Multiplexing capability | Detection technique | System flexibility | System complexity | On-site applicability | Commercially available | Refs | |
|---|---|---|---|---|---|---|---|
| Paper-based systems | |||||||
| μPADs | 2 analytes, extendable | Colorimetric readout by naked eye | Low | Low | Yes | – | [24] |
| Triage | Up to 3 analytes | Lateral flow test with optical detection | Low | Low | Yes | Alere Inc. | [20] |
| Array-based systems | |||||||
| ElectraSense | Up to 12 544 analytes | Optical and electrochemical detection | High | Middle | Yes | CustomArray Inc. | [44] |
| Bead-based systems | |||||||
| xMAP | Up to 500 analytes | Flow cytometry | High | High | No | Luminex Corp. | [46] |
| GeneXpert Omni | Up to 6 analytes | Real-time PCR | Low | Low | Yes | Cepheid | [54] |
| Microfluidic multiplexed systems | |||||||
| MChip | Up to 5 analytes, extendable | Colorimetric detection | Low | Low | Yes | OPKO Diagnostics | 68, 84 |
| DxBox | 2 analytes, extendable | Colorimetric detection | Low | Middle | Yes | – | [70] |
| MultiLab | Up to 8 analytes, extendable | Amperometry combined with stop-flow protocols | High | Low | Yes | – | [73] |
Paper-Based Systems
In the field of classical on-site diagnostics, lateral flow assays (LFAs), e.g., at-home pregnancy tests, are by far the best-established commercial products because they are simple, fast and low-cost. A good place for the interested reader to learn more about multiplexing options in lateral flow biosensors is the excellent review recently published by Li and Macdonald [13].
Multiplexed LFAs mainly rely on an optical signal readout 14, 15, 16, 17. However, a few examples employ electrochemical detection 18, 19. A significant contribution of lateral flow biosensors to xPOCT was introduced by Alere Inc. (USA) with its Triage platform, which combines the simplicity of LFAs with quantitative multianalyte immunoassays, using a portable fluorometer [20]. Currently, it offers up to 20 different single analytes, but only a limited number of multianalyte immunoassays, such as for cardiac biomarkers and drug screening. The working principle of the Alere Triage test panel is similar to that of classical LFAs. It delivers quantitative results within approximately 20 minutes after sample introduction. Depending on the targeted analyte, either plasma or whole blood for the diagnosis of myocardial infarction or urine for drug testing can be analyzed.
In our opinion, LFAs are still the best method for qualitative xPOCT due to their appreciable benefits, including (i) easy sample loading by capillary forces, (ii) instrument-free readout by the naked eye, (iii) facile operability, even by a patient, and (iv) cheap price. Yet, they require a readout device for highly sensitive and quantitative detection. The main disadvantages of LFAs are the low reproducibility of disposable test panels, comparably high sample consumption, limited flexibility in assay design, and the need for a control line for each parameter.
Microfluidic Paper-Based Analytical Devices – μPADs
In the last decade, the POCT applications of paper-based 3D microfluidics have expanded considerably because of their tremendous capability to manipulate liquids at a high level in combination with a low-cost and rapid fabrication. Different fluidic operations, including mixing, splitting, separation, and filtration, can be easily adapted to the paper-based microfluidics by means of various design elements. However, the conventional paper-based devices (e.g., LFAs) can quantify single or multiple analytes on site only with a certain degree of assay diversity. In contrast, μPADs are capable of high-degree multiplexing, along with improved assay performance and flexibility. Interested readers are referred to recent reviews about μPADs 21, 22, 23.
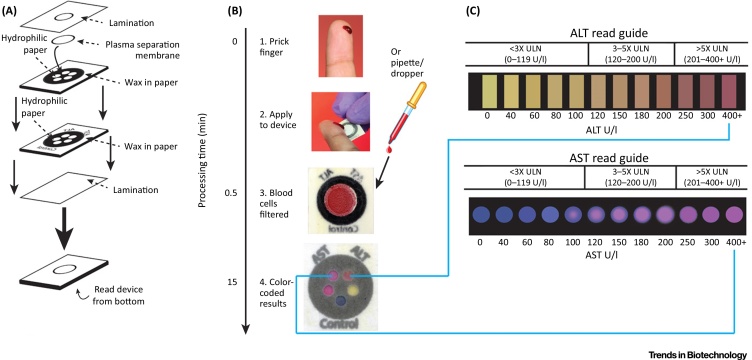
Low-cost xPOCT solutions have attracted particular interest in the developing world, e.g., for hepatotoxicity screening. Liver damage is one of the common side effects of AIDS treatment, including antiretroviral medicines. Moving beyond proof-of-concept, Pollock et al. [24] introduced a multiplexed μPAD system, extended from an earlier prototype [25], for on-site liver function testing by gauging the levels of two liver enzymes in whole blood, aspartate aminotransferase and alanine aminotransferase. The schematics of its fabrication procedure and working principle are summarized in Figure 2. This cheap and easy-to-handle μPAD device offers a colorimetric readout with both qualitative and quantitative data analysis within 15 min after the sample introduction (less than 35 μl blood).
Figure 2.
Schematic Description of the Microfluidic Paper-Based xPOCT Device for the Control of Liver Function. (A) Fabrication process describing the different assembly steps. (B) Measurement procedure. (C) Color readout guides for the multiplexed measurement of transaminase enzyme levels. Reproduced, with permission from AAAS, from [24]. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Compared to numerous LFAs in this field, μPADs have not made a breakthrough yet in the diagnostic market; to commercialize them, there are still various challenges and needs for (i) increased efforts in fabrication, particularly for mass production, (ii) improved system reproducibility, (iii) simple user interventions for untrained operators (e.g., sample introduction and handling of the measuring devices), and (iv) a killer application like at-home pregnancy tests by LFAs.
Instrument-free μPADs, employing colorimetric detection, are mainly limited to qualitative or a semiquantitative data evaluation. However, recent developments in the integration of μPADs to cell phones or portable scanners will likely lead to significant improvements in the near feature.
Alternatively, electrochemical μPADs were presented for the first time by Dungchai et al. [26] for the simultaneous detection of different metabolites (glucose, lactate, and uric acid) in human serum, using the respective oxidase enzymes (e.g., glucose oxidase). This device is fabricated by first patterning microfluidics with SU-8 photolithography, and subsequently screen-printing electrodes on a filter paper. Three years later, Ge et al. [27] introduced an electrochemiluminescence μPAD for the investigation of four tumor markers within 30 min from human plasma.
Nevertheless, electrochemical μPADs are still in the research and development phase. Different approaches described in the literature 28, 29, 30, 31, 32 do not meet the needs of xPOCT, particularly in terms of multiplexing and assay performances [4].
Array-Based Systems
For high-throughput multiplexing, array-based platforms are one of the most popular techniques in clinical diagnostics. Based on the latest technological advances, especially in genomics, microarray preparation and detection tools have become among the standard laboratory equipment. Nowadays, high-density arrays of microspots (down to picoliter volumes) are easily implemented by inkjet printing and analyzed for diagnostic purposes. Here, the signal readout is mainly achieved by optical or electrochemical methods [33].
Optical microarray systems often rely on fluorescence [34] or chemiluminescence [35] detection. They have a similar measurement setup allowing automatic assay preparation. The intensities of the bound fluorescently or chemiluminescently labeled biomolecules are measured via laser scanning or observed with a scanning charge-coupled device (CCD) or complementary metal oxide semiconductor (CMOS) camera. Moreover, there are a few examples of label-free, array-based, microfluidic biosensor platforms employing a localized surface plasmon resonance (LSPR) technique along with metallic nanoparticles 36, 37, 38. Despite their high system performance, they are still too complex and too expensive to be adopted in xPOCT.
Array-based electrochemical systems depend on individually addressable microelectrode arrays 39, 40, 41. Different biomolecules are directly immobilized on the electrodes and detected electrochemically via enzymatic reactions by potentiometry or amperometry. Hence, the choice of electrode material is crucial for the assay performance and needs to be considered for the system design [42]. To date, various metals (e.g., gold, platinum), semiconductors (e.g., indium tin oxide, iridium oxide), or carbon-based materials (e.g., carbon paste, glassy carbon, graphite) have been utilized as electrode materials for xPOCT [43].
An impressive example for commercialized array-based systems is the ElectraSense platform (CustomArray Inc., USA) with multimodal signal processing. It employs CMOS-based CustomArray 12 K chips, which can individually address single platinum microelectrodes on the very-large-scale integration (VLSI) sensor array (56 × 224). Additionally, it offers a wide assay flexibility along with an easy, reusable (up to four times), and universal immobilization procedure for various biomolecules (e.g., DNAs, antibodies) via oligonucleotide hybridization. Herein, the multimodal signal readout is performed in less than 1 minute with a handheld reader, using both fluorescent and electrochemical detection 44, 45.
Array-based systems ensure simple, rapid and high-throughput quantification of different assay technologies for clinical diagnostics. Yet, there still exist several bottlenecks that limit their xPOCT application: (i) relatively high sample consumption to prevent evaporation, (ii) complex and expensive chip production (e.g., ElectraSense platform), and (iii) bulky analytical instruments (mainly in optical systems).
Bead-Based Systems
The application of beads in bioanalytics as a substrate material is one of the cornerstones of analytical biotechnology. A wide variety of beads can be acquired nowadays in various sizes, materials, and surface functionalities 2, 6. In bead-based systems, three different conceptual approaches are primarily favored for multiplexing: (i) distinction of beads by either their size/shape or color [46], (ii) labeling of beads with different enzymes, metal ions [47], redox tags [48], and quantum dots 49, 50, 51, or (iii) spatial separation of beads in different channel sections 52, 53.
Recently, the company Cepheid introduced a portable cartridge-based system, GeneXpert Omni, for molecular xPOCT applications [54]. It employs a bead-based real-time PCR using multiple (up to 6 different) colors and thus, is capable of performing multianalyte diagnostic tests (e.g., for tuberculosis, drug-resistant tuberculosis, AIDS, and Ebola virus) within 2 hours. This closed-loop system offers a single sample-processing step, followed by automated protocols for DNA extraction, PCR amplification, and detection, which reduces any cross-contamination risks. The disadvantages of GeneXpert Omni are its high operating costs and its limited assay flexibility as well as its limited multiplexing capability.
Flow Cytometry
In flow cytometry, the biological information is gathered by gauging mostly fluorescently labelled particles (e.g., cells or beads), while they are flowing through a narrow channel, individually via a laser beam. To achieve high-level multiplexing, various beads with either different sizes/shapes or internal color barcodes are employed. A conventional flow cytometer comprises a microfluidic flow system, an optical excitation unit and an optical detection system along with high-speed digital signal processing 55, 56.
Presently, many commercial platforms are available for bead-based flow cytometry enabling multianalyte detection in clinical diagnostics [2]. One of the pioneers is the Luminex xMAP system (Luminex Corp., USA). It can simultaneously quantify many different analytes from the same specimen by using 5.6 μm polystyrene beads with two different fluorescent color barcodes. Multiplexed bioassays on these microbeads are measured consecutively by using a reporter biomolecule (e.g., DNA, antibody or protein) conjugated with a third fluorophore 46, 57.
In summary, bead-based flow cytometry offers high-throughput multianalyte detection (up to 500 analytes) with low operating costs for clinical diagnostics at central laboratories. Yet, the long turnaround times, and the size and high acquisition costs of measurement instruments, still prohibit their xPOCT applicability 55, 56. To this end, the development and evaluation of LOC-based flow cytometers and microflow cytometers for different approaches are in the focus of current research 58, 59.
Microfluidic Multiplexed Systems
Microfluidic multiplexing in bioanalyses is achieved in spatially distinct sections (either in parallel or in a row) of microchannel networks. Here, different assay fluids have to be directed accurately to these detection sites by means of individual inlets or valves. Specific channel regions are branched with Y- or T-junctions to a microfluidic array [6]. In most of the microfluidic multiplexed platforms, fluids are manipulated by a number of pneumatic valves that are integrated into polydimethylsiloxane (PDMS)-based devices 60, 61, 62.
One method of microfluidic multiplexing is the use of channel networks, combined with individual valve actuation paths for each fluid channel. Yet, such an inefficient approach limits its scale-up capacity. To solve this problem, Thorsten et al. introduced an analogy to VLSI technology, microfluidic large-scale integration (mLSI), in 2002 [63]. Here, microfluidic multiplexors, combinatorial arrays of binary valves, are employed to simplify the control of large-scale channel networks. With this respect, a microfluidic network with N channels, requires only 2log2N valve channels. This technology combines rapid and facile manufacturing with high-throughput multiplexing, using a minimum number of channel inputs. Its applicability for simultaneous multianalyte measurements in proteomics 64, 65, cellomics [66] and genomics [67] was demonstrated. Although they have a great potential for high-level multiplexing, mLSI-based systems are yet not applicable to xPOCT due to their system complexity, as well as bulky instrumentation for the pneumatic control and the respective fluid connection.
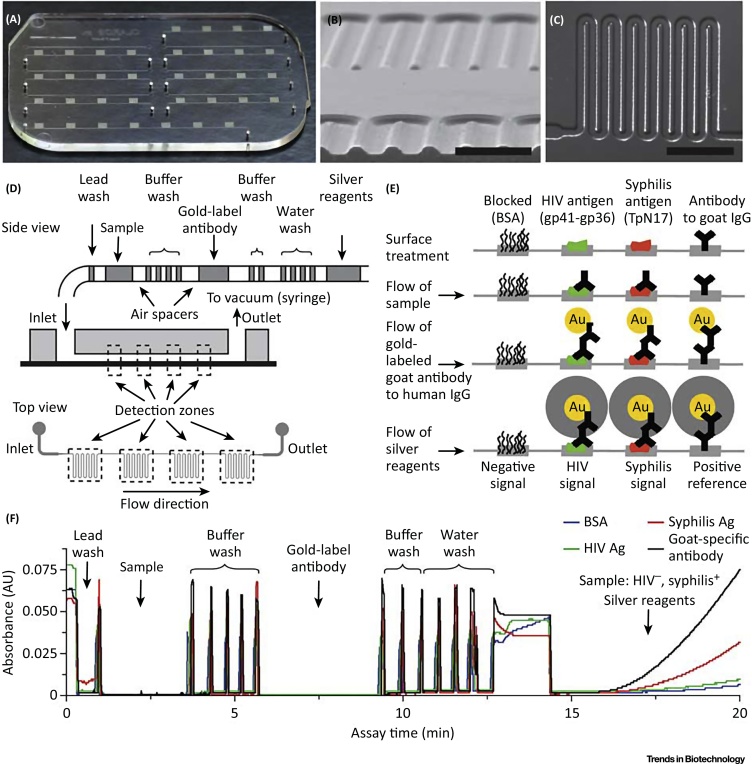
In the developing world, infectious diseases cause a high mortality and thus, there is a great and urgent need for low-cost and easy-to-use xPOCT platforms in order to distinguish diseases with similar symptoms in low-resource settings. For this purpose, Chin and colleagues presented a microfluidic multiplexed strategy for xPOCT of different infectious diseases, as depicted in Figure 3 [68]. The mChip platform consists of a disposable microfluidic cartridge with preloaded reagents, and a completely automated handheld analyzer. The low-cost plastic cassettes, fabricated with injection molding, can analyze up to seven samples (one per channel) at the same time. Each channel has four meandering detection sites, located in series, for the multianalyte detection. Assay preparation is realized manually or automatically by dispensing. Here, signal amplification is achieved by the reduction of silver ions onto gold nanoparticles, which are tagged to the detection antibodies. A compact and low-cost readout device, utilizing light-emitting diodes and photodetectors, measures the resulting optical density. Moreover, an integrated micropump and data communication unit minimize the user intervention [69]. Its multiplexing applicability was demonstrated by the xPOCT of HIV and syphilis from only 1 μl of whole blood from a finger prick within 20 min. Thereby, the mChip platform assay performed similarly to lab-based reference tests. Yet, there are several limitations, including its flexibility in assay design, and its multiplexing capability of high-throughput applications.
Figure 3.
mChip Platform for Multiplexed On-Site Diagnostics. (A) Photo of the microfluidic polystyrene cassette with seven measurement units. (B) SEM image of channel cross-section (scale bar: 500 μm). (C) Transmitted light micrograph of a single detection site (scale bar: 1 mm). (D) Passive delivery of preloaded sequence of different reagents. (E) Schematics of assay reactions in different detection sites at different incubation steps. The on-chip signal detection is achieved by reduction of silver ions on secondary antibodies tagged with gold nanoparticles. (F) Measurement procedure and resulting optical density of a HIV-syphilis duplex test. Reproduced, with permission from Macmillan Publishers Ltd: Nature Medicine, from [68].
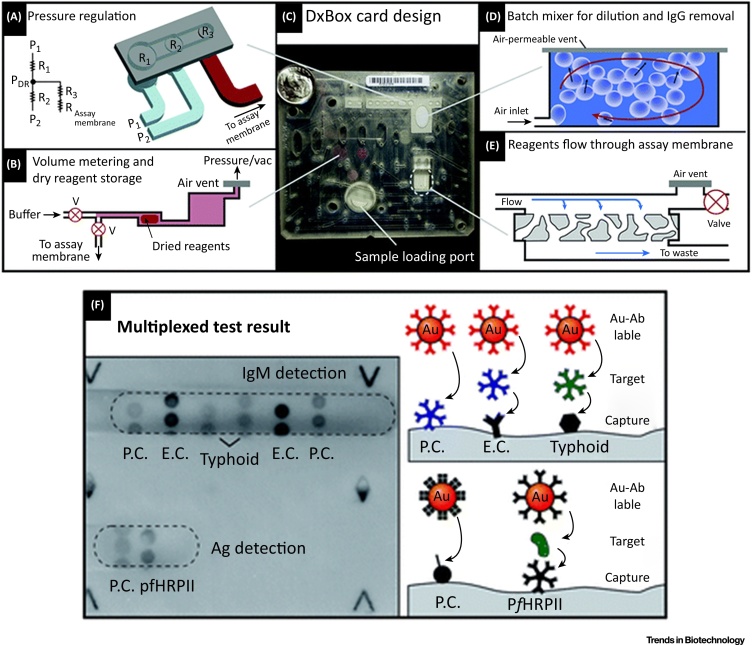
In a similar manner, Lafleur et al. [70] introduced a microfluidic card DxBox (Figure 4), comprising a paper-based measurement unit, for xPOCT of malaria pfHRPII antigen and IgM antibodies to Salmonella typhi within 30 min in whole blood. Here, the sample and dried reagents, stored on-chip, are delivered by pneumatic actuation over different detection sites to the waste reservoir. A flatbed scanner takes an image of each spot to measure the obtained intensities for quantitative analysis. Its main drawbacks for xPOCT include limited assay diversity, complex and expensive device fabrication, and poor management of air bubbles.
Figure 4.
DxBox Integrated Microfluidic Card with Its Main Features. (A) Illustration of the pneumatic regulation for the fluid manipulation. (B) On-card volume metering and freeze-dried biomolecule storage. (C) Photograph of integrated microfluidic cartridge. (D) Schematics of the employed bath mixer for sample dilution and IgG removal. (E) Incubation procedure on the assay membrane. The application of an air vent and a valve removes the air between reagent deliveries, and the reagents itself between different incubation steps. (F) On-chip multianalyte detection of IgM antibodies against typhoid infection and malaria pfHRPII antigen from human plasma. Reproduced, with permission from The Royal Society of Chemistry, from [70]. Abbreviations: E.C., endogenous control; P.C., process control.
Electrochemical approaches for microfluidic multiplexing enable the realization of compact analytical devices, which combine automated assay and measurement procedures along with rapid and sensitive detection. Herein, most of the systems employ separate electrochemical measurement cells within a channel network 40, 71, 72. However, the application of a single electrochemical measurement cell with one or multiple working electrodes is highly beneficial for the system design of xPOCT devices.
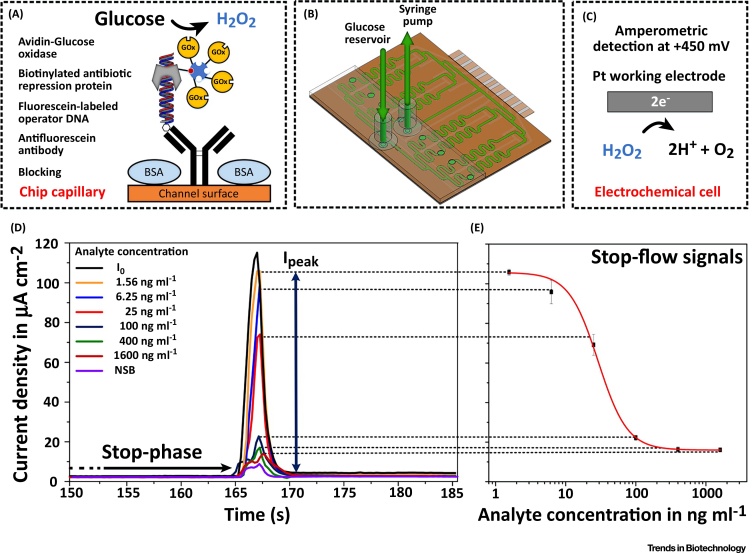
In this context, Kling et al. introduced an electrochemical biosensor ‘MultiLab’ platform with multiplexed microfluidics, based on dry film photoresist technology [73]. This device can simultaneously detect up to eight analytes. It consists of a microfluidic channel network of up to eight discrete immobilization sections, arranged in parallel, combined with a single electrochemical measurement cell comprising multiple working electrodes for the amperometric multianalyte detection. Each immobilization capillary can be addressed by individual channel inlets. Thus, different bioassay formats (e.g., competitive assay), omics technologies (e.g., genomics) and various combinations of those can be implemented on the same system. To amplify the enzyme-mediated signal, a microfluidic stop-flow protocol, similar to flow injection analysis, is employed [74]. The working principle of the MultiLab system is illustrated in Figure 5. Its feasibility for xPOCT was demonstrated by the simultaneous measurement of two different antibiotics in human plasma within 15 min. This approach provides (i) a facile and low-cost fabrication, (ii) simple reagent filling by capillarity, (iii) low sample/reagent consumption, and (iv) a high flexibility in assay design. However, its main drawback is the lack of a handheld analyzer, enabling fully automated sample incubation, measurement and analyzing procedures.
Figure 5.
Graphical Abstract Illustrating the Working Principle of ‘MultiLab’ Platform. (A) Schematics of the competitive enzyme-linked assay for the multianalyte antibiotic detection. For an easy and universally applicable ‘plug and play’ assay immobilization, antifluorescein antibodies are used as spacer and capture biomolecules. Glucose oxidase is employed as the labeling enzyme with glucose for its appropriate substrate. (B) CAD drawing of the microfluidic multiplexed biosensor. For the measurement, individual channel inlets are sealed with a PMMA piece and double-sided tape. (C) The subsequent electrochemical detection of hydrogen peroxide, generated by the competitive antibiotic assay, at the respective working electrode. (D) Stop-flow peaks from a simultaneous on-chip calibration measurement. (E) Resulting on single-chip calibration curve along with a four-parameter logistic fit. Reproduced, with permission from the American Chemical Society, from [73]. Abbreviations: CAD, computer-aided design; PMMA, poly(methyl methacrylate).
Centrifugal Microfluidic Platforms
Centrifugal-based microfluidic systems utilize centrifugal forces to navigate fluids through microchannel networks. Herein, the most frequently used design is known as ‘lab-on-a-disc’, using polymer compact discs (CDs) as a microfluidic platform. Thus, automated assay preparation and measurement procedures are realized by the CD rotation at different speeds. Herein, the signal readout relies mainly on optical and electrochemical detection techniques. Interested readers may find further information about the CD-based microfluidic systems in recent reviews 75, 76, 77.
In lab-on-a-disc applications, the spatial separation of various analytes in a channel network is the most common method of multiplexing. One commercialized example is the Gyrolab Bioaffy CDs (Gyros Protein Technologies AB, Sweden), which can simultaneously analyze a great number of samples or analytes on its workstation [78]. Here, the microfluidic lab-on-a-disc cartridge is fabricated by injection molding a cyclic olefin copolymer. The channel network consists of 112 microcolumns with a volume of 200 nl. The CD is sealed with a lid, which contains both individual and common inlets for the filling of these microstructures. Each single column includes a reaction chamber preloaded with streptavidin-coated beads as a solid phase. The fluid regulation is implemented by the combination of the centrifugal and capillary forces together with hydrophobic stopping barriers, located in the microcolumns. Thanks to its ability to address each microstructure via its individual inlet, this system provides furthermore great flexibility in assay design. To demonstrate its multiplexing performance, different cancer biomarkers (α-fetoprotein, interleukin-6, and carcinoembryonic antigen) were simultaneously detected within 50 min in human plasma.
Particularly, with regard to in vitro diagnostic applications, lab-on-a-disc platforms offer many advantages, including (i) short turnaround times, (ii) low sample consumption, and (iii) high flexibility in assay design. Nevertheless, they should be further improved for xPOCT in terms of system complexity and instrumentation. Therefore, the current trend in CD-based microfluidic systems is toward electrochemical approaches 79, 80, 81, 82, although the most often used signal detection is still the optical readout.
Concluding Remarks and Future Perspectives
In conclusion, multiplexed diagnostic systems, capable of high-throughput bioanalysis (of more than 100 parameters), such as array-based (e.g., CustomArray) or bead-based (e.g., xMAP and Gyrolab) platforms, have recently become standard equipment in central laboratories. In the near future, they will have significant impact on clinical diagnostics, especially in biomarker discovery and validation. Nevertheless, they are not suitable for xPOCT due to its rigorous requirements.
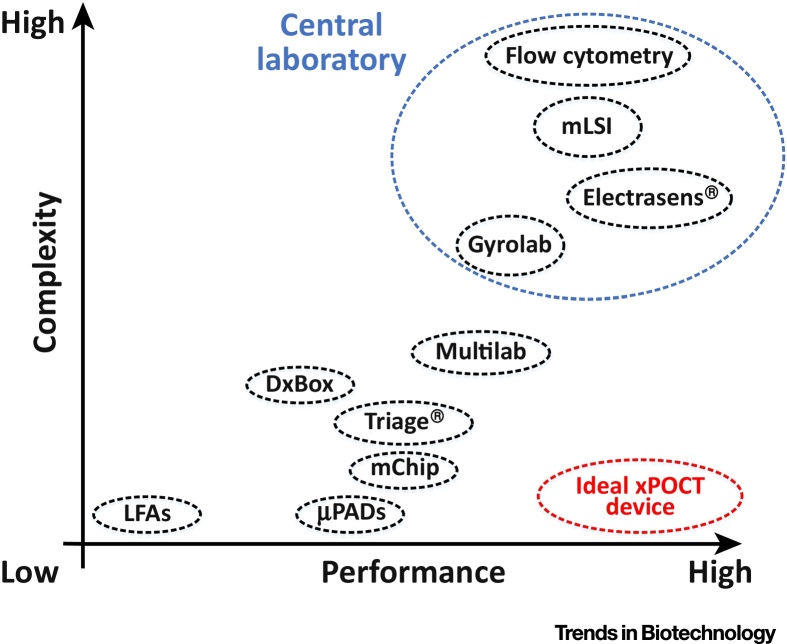
Derived from emerging medical demands, including personalized medicine (e.g., home health monitoring) and on-site diagnostics in resource-limited settings (e.g., in developing world, or directly at the bedside or in doctor’s offices), there is a great and urgent need for xPOCT systems. An ideal device for multiplexed point-of-care testing should offer a high sensor performance, like high sensitivity and multiplexing capability, as well as short turnaround times, at low system complexity, including low-cost fabrication and minimized user intervention. In order to provide a visual overview, the presented state-of-the-art systems for multiplexed bioanalyses are summarized in Figure 6, regarding their complexity and sensor performance. Thus, it appears that many challenges (see Outstanding Questions) still remain for the successful commercialization of xPOCT systems. Since many of them are yet at the early development stage, or are limited by their multiplexing capability (e.g., paper- or microfluidic-based devices), or system complexity (e.g., array- or bead-based platforms).
Figure 6.
Visual Overview of the Presented State-of-the-Art xPOCT Systems. Two important conceptual approaches are system complexity versus multiplexing performance. An ideal multiplexed on-site device needs to prove a high sensor performance at low system complexity. Abbreviations: LFAs, lateral flow assays; mLSI, microfluidic large-scale integration; μPADs, microfluidic paper-based analytical devices.
In general, it is desirable to combine a high-performing platform, requiring a minimum user intervention, with a low-cost fabrication. Thanks to their many advantages, microfluidic technologies play a crucial role in xPOCT devices. To design microfluidic LOC devices, primarily unnecessary features should be avoided in order to minimize their complexity. In addition, considerable effort has to be put in the integration of all required parts and liquids on disposable microfluidic cartridges or cassettes to eliminate fluid handling by the user.
To summarize, due to the recent substantial progress in microfluidics and detection techniques, the technological aspect for liquid handling and signal readout does not appear to be the limiting factor for the implementation of xPOCT devices anymore. Hence, the future technology challenges will be the standardization and further miniaturization of the system components and their facile and smart integration.
The presented applications comprise mainly instruments that would be used at a doctor’s office or in a hospital. Home-usable or wearable xPOCT devices are not yet available, although simple readout instruments – cell phones – are readily available in every home and apps for optical analysis or even microscopic image analysis exist [83]. One of the main reasons is that for the approval xPOCT devices need to satisfy the strict requirements of international regulations (e.g., EU Directive 98/79/EC or FDA requirements) on in vitro diagnostic medical devices. Other future challenges of xPOCT will be related to the internet of things, including regulatory requirements for handling and safety of the data between clinical settings and operators. Here, international standards for ethics and data security need to be fulfilled.
Most of the diagnostic methods rely on immunoassays or enzymatic reactions. The assay signal strongly depends on the sample (e.g., matrix effects, patient-to-patient variations) and the environment (e.g., temperature, humidity). In this context, novel and robust assay technologies enabling long-term storage are needed.
The emerging needs and demands for novel biotechnologies (e.g., aptamers) or targets (e.g., circulating RNAs or tumor cells, exosomes, and miRNAs) and their applications for diagnostic, prognostic, and therapeutic implications, including therapeutic drug monitoring towards personalized medicine, will shape the future of xPOCT systems.
Outstanding Questions.
What should be the degree or level of multiplexing? How many analytes should be tested in a single test simultaneously?
Does the multianalyte approach reduce the overall costs and turnaround times?
Can or should the multiplexed on-site testing be performed by non-trained users, such as the patients themselves?
In the future, would it possible to implement wearable multiplexed on-site testing devices for (quasi-) real-time monitoring of different biomolecules?
Can cell phones serve as readout instruments in xPOCT for home-use or wearables in the near future?
Which targets, including exosomes, circulating tumor cells or DNA/RNAs, and miRNAs, will dominate the future xPOCT applications?
Acknowledgments
C.D. and G.U. would like to thank the German Research Foundation (DFG) for partially funding this work under grant numbers UR 70/10-01 and UR 70/12-01. P.S.D. acknowledges financial support from the ETH Zurich Foundation and the European Research Council (ERC), Consolidator Grant ‘HybCell’ (Grant No. 681587).
Glossary
- Colorimetric readout
the visual comparison of the color intensity of the reaction spots by naked eye or mobile phones and portable scanners.
- Competitive assay
an assay capture format where the central event of the analyte detection is the competition between the labeled (tracer) and unlabeled (sample) analyte (e.g., antigen or antibody). The lower the analyte concentration in the sample is, the higher the measured signal. Thus, this technique is favorable for the quantification of antibodies or antigens in sample at very low concentrations.
- In vitro diagnostics
diagnostic tests performed in an artificial environment using blood, urine, and other body fluids (e.g., exhaled breath condensate, saliva, or nasal fluid) as sample.
- Lab-on-a-chip
general term for a miniaturized device that integrates one or several laboratory functions on a single chip.
- Localized surface plasmon resonance (LSPR)
an optical phenomenon when light interacts with conductive nanoparticles that are smaller than its wavelength. The resonance frequency of the occurring localized plasmon oscillations strongly depends on the size, composition and the dielectric environment of these nanoparticles. Thus, this can be used as a powerful detection technique for chemical and biological sensing applications.
- Matrix-assisted laser desorption/ionization (MALDI)
a soft ionization technique that is used in mass spectrometry, enabling the quantification of biomolecules (e.g., DNA and proteins).
- Microfluidic large-scale integration (mLSI)
the design and development of microfluidic devices integrated with thousands of micromechanical valves and control elements.
- Microfluidics
the manipulation and control of fluids in microchannels, where the surface related forces (e.g., surface tension and capillary forces) dominate and the impact of volume forces like inertia and gravity reduces.
- Microspot
the position of the targeted analyte immobilized on a planar microarray.
- Multiplexing
simultaneous detection of multiple analytes from a single sample.
- Personalized medicine
tailoring of medical treatment for patient cohorts to be treated in a unique manner depending on their health status and previous course of a disease.
- Polydimethylsiloxane (PDMS)
a silicon-based organic polymer, used mainly for the fabrication of microfluidic devices and contact lenses, or as sealing material.
- Point-of-care testing
on-site diagnostic tests performed near the patient or by the patient himself.
- SU-8
an epoxy-based negative photoresist that is often employed for the fabrication of microfluidic devices with high aspect ratios, or for isolation of electrodes, due to its outstanding chemical and thermal properties.
- Turnaround time
time interval between sample collection and result of a diagnostic test (also termed as sample-to-result time).
- Very-large-scale integration (VLSI)
the design and development of an integrated circuit which combines thousands of transistors into a single chip.
Footnotes
Supplemental information associated with this article can be found online at http://dx.doi.org/10.1016/j.tibtech.2017.03.013.
Supplemental Information
The following is the supplemental information to this article:
References
- 1.Jung W. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015;132:46–57. [Google Scholar]
- 2.Spindel S., Sapsford K.E. Evaluation of optical detection platforms for multiplexed detection of proteins and the need for point-of-care biosensors for clinical use. Sensors (Basel) 2014;14:22313–22341. doi: 10.3390/s141222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luppa P.B. Clinically relevant analytical techniques, organizational concepts for application and future perspectives of point-of-care testing. Biotechnol. Adv. 2016;34:139–160. doi: 10.1016/j.biotechadv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Vashist S.K. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015;33:692–705. doi: 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Gauglitz G. Point-of-care platforms. Annu. Rev. Anal. Chem. 2014;7:297–315. doi: 10.1146/annurev-anchem-071213-020332. [DOI] [PubMed] [Google Scholar]
- 6.Araz M.K. Microfluidic multiplexing in bioanalyses. J. Lab. Autom. 2013;18:350–366. doi: 10.1177/2211068213491408. [DOI] [PubMed] [Google Scholar]
- 7.Rusling J.F. Multiplexed electrochemical protein detection and translation to personalized cancer diagnostics. Anal. Chem. 2013;85:5304–5310. doi: 10.1021/ac401058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peacock P.M. Advances in ionization for mass spectrometry. Anal. Chem. 2017;89:372–388. doi: 10.1021/acs.analchem.6b04348. [DOI] [PubMed] [Google Scholar]
- 9.Gordon J., Michel G. Discerning trends in multiplex immunoassay technology with potential for resource-limited settings. Clin. Chem. 2012;58:690–698. doi: 10.1373/clinchem.2011.176503. [DOI] [PubMed] [Google Scholar]
- 10.Chin C.D. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 11.Sackmann E.K. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 12.Robinson T., Dittrich P.S. Microfluidic technology for molecular diagnostics. Adv. Biochem. Eng. Biotechnol. 2013;133:89–114. doi: 10.1007/10_2012_139. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Macdonald J. Multiplexed lateral flow biosensors: technological advances for radically improving point-of-care diagnoses. Biosens. Bioelectron. 2016;83:177–192. doi: 10.1016/j.bios.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Macdonald J. Multiplex lateral flow detection and binary encoding enables a molecular colorimetric 7-segment display. Lab Chip. 2016;16:242–245. doi: 10.1039/c5lc01323b. [DOI] [PubMed] [Google Scholar]
- 15.Song S. Multiplex lateral flow immunoassay for mycotoxin determination. Anal. Chem. 2014;86:4995–5001. doi: 10.1021/ac500540z. [DOI] [PubMed] [Google Scholar]
- 16.Taranova N.A. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens. Bioelectron. 2015;63:255–261. doi: 10.1016/j.bios.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Lafleur L.K. A rapid, instrument-free, sample-to-result nucleic acid amplification test. Lab Chip. 2016;16:3777–3787. doi: 10.1039/c6lc00677a. [DOI] [PubMed] [Google Scholar]
- 18.Mao X. Multiplex electrochemical immunoassay using gold nanoparticle probes and immunochromatographic strips. Electrochem. Commun. 2008;10:1636–1640. [Google Scholar]
- 19.Mao X. Rapid quantitative immunochromatographic strip for multiple proteins test. Sens. Actuators B Chem. 2013;186:315–320. [Google Scholar]
- 20.Clark T.J. The triage cardiac panel. Point Care J. Near Patient Test. Technol. 2002;1:42–46. [Google Scholar]
- 21.Ahmed S. Paper-based chemical and biological sensors: engineering aspects. Biosens. Bioelectron. 2016;77:249–263. doi: 10.1016/j.bios.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Rolland J.P., Mourey D.A. Paper as a novel material platform for devices. MRS Bull. 2013;38:299–305. [Google Scholar]
- 23.Yang Y. Paper-based microfluidic devices: emerging themes and applications. Anal. Chem. 2017;89:71–91. doi: 10.1021/acs.analchem.6b04581. [DOI] [PubMed] [Google Scholar]
- 24.Pollock N.R. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003981. 152ra129–152ra129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vella S.J. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal. Chem. 2012;84:2883–2891. doi: 10.1021/ac203434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dungchai W. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009;81:5821–5826. doi: 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 27.Ge L. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials. 2012;33:1024–1031. doi: 10.1016/j.biomaterials.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Liu X. A microfluidic paper-based origami nanobiosensor for label-free, ultrasensitive immunoassays. Adv. Healthc. Mater. 2016;5:1326–1335. doi: 10.1002/adhm.201501038. [DOI] [PubMed] [Google Scholar]
- 29.Li W. Multiplex electrochemical origami immunodevice based on cuboid silver-paper electrode and metal ions tagged nanoporous silver–chitosan. Biosens. Bioelectron. 2014;56:167–173. doi: 10.1016/j.bios.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014;52:180–187. doi: 10.1016/j.bios.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y. Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013;85:8661–8668. doi: 10.1021/ac401445a. [DOI] [PubMed] [Google Scholar]
- 32.Zang D. Electrochemical immunoassay on a 3D microfluidic paper-based device. Chem. Commun. 2012;48:4683. doi: 10.1039/c2cc16958d. [DOI] [PubMed] [Google Scholar]
- 33.Ling M.M. Multiplexing molecular diagnostics and immunoassays using emerging microarray technologies. Expert Rev. Mol. Diagn. 2007;7:87–98. doi: 10.1586/14737159.7.1.87. [DOI] [PubMed] [Google Scholar]
- 34.Chandra P.E. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res. Ther. 2011;13:R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadimisetty K. Automated multiplexed ecl immunoarrays for cancer biomarker proteins. Anal. Chem. 2015;87:4472–4478. doi: 10.1021/acs.analchem.5b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano. 2015;9:4173–4181. doi: 10.1021/acsnano.5b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masson J.-F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017;2:16–30. doi: 10.1021/acssensors.6b00763. [DOI] [PubMed] [Google Scholar]
- 38.Aćimović S.S. LSPR chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014;14:2636–2641. doi: 10.1021/nl500574n. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher S. Highly-integrated lab-on-chip system for point-of-care multiparameter analysis. Lab Chip. 2012;12:464–473. doi: 10.1039/c1lc20693a. [DOI] [PubMed] [Google Scholar]
- 40.Otieno B.A. Cancer diagnostics via ultrasensitive multiplexed detection of parathyroid hormone-related peptides with a microfluidic immunoarray. Anal. Chem. 2016;88:9269–9275. doi: 10.1021/acs.analchem.6b02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson M.S., Nie W. Multiplex measurement of seven tumor markers using an electrochemical protein chip. Anal. Chem. 2006;78:6476–6483. doi: 10.1021/ac060843u. [DOI] [PubMed] [Google Scholar]
- 42.Díaz-González M. Diagnostics using multiplexed electrochemical readout devices. Electroanalysis. 2014;26:1154–1170. [Google Scholar]
- 43.Wan Y. Development of electrochemical immunosensors towards point of care diagnostics. Biosens. Bioelectron. 2013;47:1–11. doi: 10.1016/j.bios.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 44.Ghindilis A.L. CombiMatrix oligonucleotide arrays: genotyping and gene expression assays employing electrochemical detection. Biosens. Bioelectron. 2007;22:1853–1860. doi: 10.1016/j.bios.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Roth K.M. Electrochemical detection of short DNA oligomer hybridization using the CombiMatrix ElectraSense Microarray reader. Electroanalysis. 2006;18:1982–1988. [Google Scholar]
- 46.Dunbar S.A. Applications of Luminex® xMAP™ technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta. 2006;363:71–82. doi: 10.1016/j.cccn.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng L.-N. Ultrasensitive multianalyte electrochemical immunoassay based on metal ion functionalized titanium phosphate nanospheres. Anal. Chem. 2012;84:7810–7815. doi: 10.1021/ac301438v. [DOI] [PubMed] [Google Scholar]
- 48.Tang J. Magneto-controlled graphene immunosensing platform for simultaneous multiplexed electrochemical immunoassay using distinguishable signal tags. Anal. Chem. 2011;83:5407–5414. doi: 10.1021/ac200969w. [DOI] [PubMed] [Google Scholar]
- 49.Kong F.-Y. Simultaneous electrochemical immunoassay using CdS/DNA and PbS/DNA nanochains as labels. Biosens. Bioelectron. 2013;39:177–182. doi: 10.1016/j.bios.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Wang J. Electrochemical coding technology for simultaneous detection of multiple DNA targets. J. Am. Chem. Soc. 2003;125:3214–3215. doi: 10.1021/ja029668z. [DOI] [PubMed] [Google Scholar]
- 51.Tang D. Multiplexed electrochemical immunoassay of biomarkers using metal sulfide quantum dot nanolabels and trifunctionalized magnetic beads. Biosens. Bioelectron. 2013;46:37–43. doi: 10.1016/j.bios.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 52.Sato K. Microchip-based immunoassay system with branching multichannels for simultaneous determination of interferon-γ. Electrophoresis. 2002;23:734–739. doi: 10.1002/1522-2683(200203)23:5<734::AID-ELPS734>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 53.Ko Y.-J. Microchip-based multiplex electro-immunosensing system for the detection of cancer biomarkers. Electrophoresis. 2008;29:3466–3476. doi: 10.1002/elps.200800139. [DOI] [PubMed] [Google Scholar]
- 54.Drain P.K., Garrett N.J. The arrival of a true point-of-care molecular assay – ready for global implementation? Lancet Glob. Health. 2015;3:e663–e664. doi: 10.1016/S2214-109X(15)00186-2. [DOI] [PubMed] [Google Scholar]
- 55.Ateya D.A. The good, the bad, and the tiny: a review of microflow cytometry. Anal. Bioanal. Chem. 2008;391:1485–1498. doi: 10.1007/s00216-007-1827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Godin J. Microfluidics and photonics for Bio-System-on-a-Chip: a review of advancements in technology towards a microfluidic flow cytometry chip. J. Biophotonics. 2008;1:355–376. doi: 10.1002/jbio.200810018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunbar S.a., Jacobson J.W. Parallel processing in microbiology: detection of infectious pathogens by Luminex xMAP multiplexed suspension array technology. Clin. Microbiol. Newsl. 2007;29:79–86. [Google Scholar]
- 58.Shriver-Lake L.C. Simultaneous assay for ten bacteria and toxins in spiked clinical samples using a microflow cytometer. Anal. Bioanal. Chem. 2013;405:5611–5614. doi: 10.1007/s00216-013-6980-4. [DOI] [PubMed] [Google Scholar]
- 59.Hashemi N. Optofluidic characterization of marine algae using a microflow cytometer. Biomicrofluidics. 2011;5:32009. doi: 10.1063/1.3608136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duncan P.N. Scaling of pneumatic digital logic circuits. Lab Chip. 2015;15:1360–1365. doi: 10.1039/c4lc01048e. [DOI] [PubMed] [Google Scholar]
- 61.Araci I.E., Brisk P. Recent developments in microfluidic large scale integration. Curr. Opin. Biotechnol. 2014;25:60–68. doi: 10.1016/j.copbio.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Shao H. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015;6:6999. doi: 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorsen T. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 64.Wu A.R. High throughput automated chromatin immunoprecipitation as a platform for drug screening and antibody validation. Lab Chip. 2012;12:2190–2198. doi: 10.1039/c2lc21290k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piraino F. A digital–analog microfluidic platform for patient-centric multiplexed biomarker diagnostics of ultralow volume samples. ACS Nano. 2016;10:1699–1710. doi: 10.1021/acsnano.5b07939. [DOI] [PubMed] [Google Scholar]
- 66.Wu X. In situ characterization of the mTORC1 during adipogenesis of human adult stem cells on chip. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4143–E4150. doi: 10.1073/pnas.1601207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalisky T., Quake S.R. Single-cell genomics. Nat. Methods. 2011;8:311–314. doi: 10.1038/nmeth0411-311. [DOI] [PubMed] [Google Scholar]
- 68.Chin C.D. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 69.Chin C.D. Mobile device for disease diagnosis and data tracking in resource-limited settings. Clin. Chem. 2013;59:629–640. doi: 10.1373/clinchem.2012.199596. [DOI] [PubMed] [Google Scholar]
- 70.Lafleur L. Progress toward multiplexed sample-to-result detection in low resource settings using microfluidic immunoassay cards. Lab Chip. 2012;12:1119. doi: 10.1039/c2lc20751f. [DOI] [PubMed] [Google Scholar]
- 71.Rossier J. GRAVI: robotized microfluidics for fast and automated immunoassays in low volume. J. Assoc. Lab. Autom. 2008;13:322–329. [Google Scholar]
- 72.Lawi W. A microfluidic cartridge system for multiplexed clinical analysis. J. Assoc. Lab. Autom. 2009;14:407–412. doi: 10.1016/j.jala.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kling A. Multianalyte antibiotic detection on an electrochemical microfluidic platform. Anal. Chem. 2016;88:10036–10043. doi: 10.1021/acs.analchem.6b02294. [DOI] [PubMed] [Google Scholar]
- 74.Dincer C. Designed miniaturization of microfluidic biosensor platforms using the stop-flow technique. Analyst. 2016;141:6073–6079. doi: 10.1039/c6an01330a. [DOI] [PubMed] [Google Scholar]
- 75.Strohmeier O. Centrifugal microfluidic platforms: advanced unit operations and applications. Chem. Soc. Rev. 2015;44:6187–6229. doi: 10.1039/c4cs00371c. [DOI] [PubMed] [Google Scholar]
- 76.Burger R. Detection methods for centrifugal microfluidic platforms. Biosens. Bioelectron. 2016;76:54–67. doi: 10.1016/j.bios.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 77.Gorkin R. Centrifugal microfluidics for biomedical applications. Lab Chip. 2010;10:1758. doi: 10.1039/b924109d. [DOI] [PubMed] [Google Scholar]
- 78.Honda N. Simultaneous multiple immunoassays in a compact disc-shaped microfluidic device based on centrifugal force. Clin. Chem. 2005;51:1955–1961. doi: 10.1373/clinchem.2005.053348. [DOI] [PubMed] [Google Scholar]
- 79.Andreasen S.Z. Integrating electrochemical detection with centrifugal microfluidics for real-time and fully automated sample testing. RSC Adv. 2015;5:17187–17193. [Google Scholar]
- 80.Li T. An electrochemical Lab-on-a-CD system for parallel whole blood analysis. Lab Chip. 2013;13:2634. doi: 10.1039/c3lc00020f. [DOI] [PubMed] [Google Scholar]
- 81.Nwankire C.E. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform. Biosens. Bioelectron. 2015;68:382–389. doi: 10.1016/j.bios.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 82.Kim T.-H. Flow-enhanced electrochemical immunosensors on centrifugal microfluidic platforms. Lab Chip. 2013;13:3747. doi: 10.1039/c3lc50374g. [DOI] [PubMed] [Google Scholar]
- 83.Contreras-Naranjo J.C. Mobile phone-based microscopy, sensing, and diagnostics. IEEE J. Sel. Top. Quantum Electron. 2016;22:392–405. [Google Scholar]
- 84.Nayak S. Microfluidics-based point-of-care test for serodiagnosis of Lyme disease. Sci. Rep. 2016;6:35069. doi: 10.1038/srep35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.