Abstract
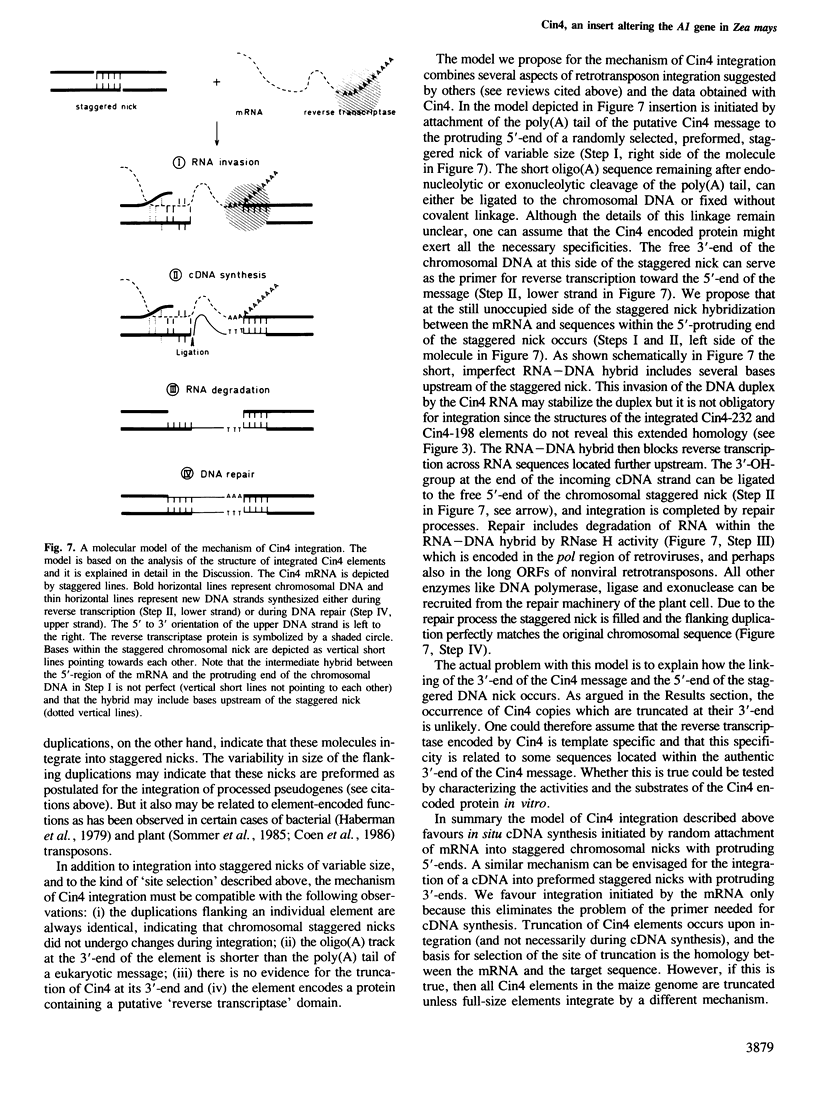
A wild-type allele of the A1 gene of Zea mays contains a 1.1-kb-long insert termed Cin4-1, which alters the structure of the transcription unit compared to other A1 alleles. The Cin4-1 element is a member of a family of elements occurring in 50–100 copies in the maize genome. Genomic cloning and sequence analysis of several family members and their flanking regions allowed classification of Cin4 as a nonviral retrotransposon. Individual Cin4 elements terminate in an oligo(A) track of variable size (6–11 residues) at their 3'-end. The 5'-ends of family members are heterogeneously truncated with respect to the longest Cin4 element. Cin4 elements are flanked by small direct duplications, the size of which varies between 3 and 16 bp. On the basis of a comparison of the target sequence and the sequence of Cin4 we suggest and discuss a model of the mechanism of Cin4 integration via in situ cDNA synthesis on an RNA template. The longest Cin4 element analysed so far has two non-overlapping open reading frames (ORFs) comprising 2793 nucleotides (ORF1) and 3489 nucleotides (ORF2). The putative 1163 amino acid long Cin4 protein derived from the sequence of ORF2 has the capacity to encode a reverse transcriptase-like protein and a DNA-binding domain. The conservation pattern of these two domains and the overall organisation of Cin4 is similar to that detected in nonviral retrotransposons in animals. The origin and function of Cin4 are discussed.
Keywords: plant, retrotransposition, mechanism of integration
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986 Oct 24;47(2):285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
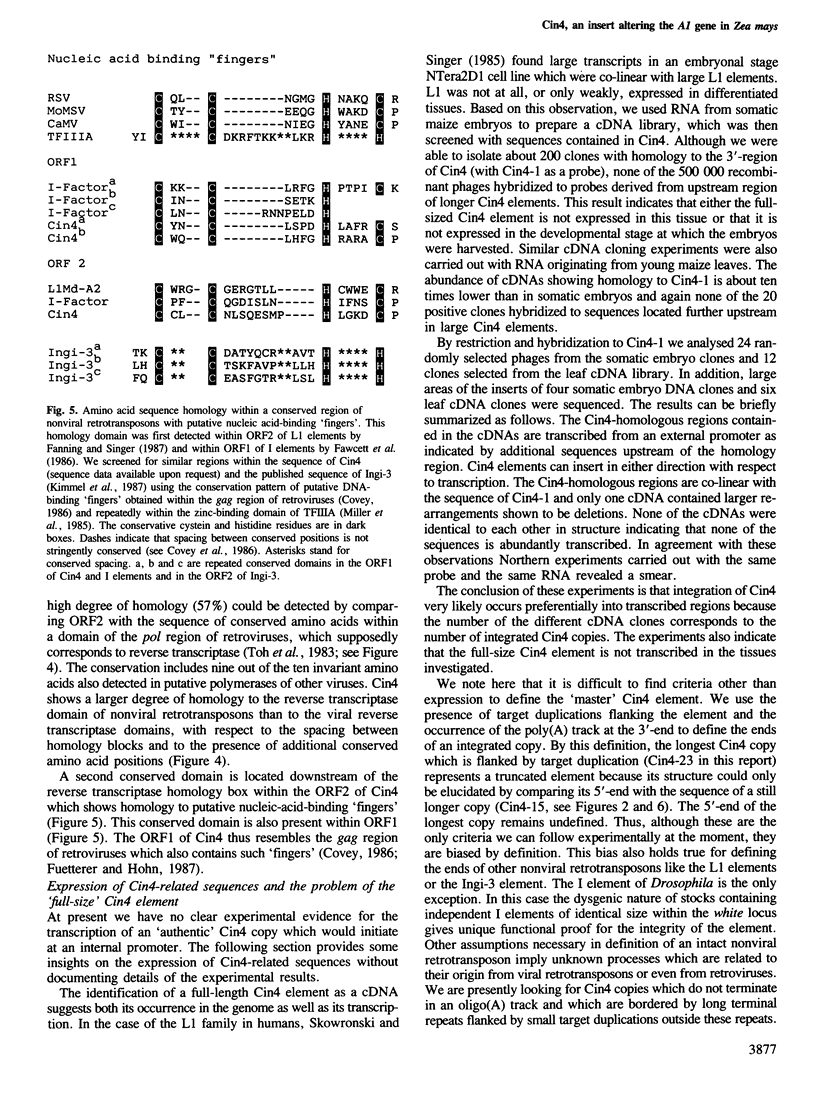
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers G. W., Brech K., Hardison R. C. Long interspersed L1 repeats in rabbit DNA are homologous to L1 repeats of rodents and primates in an open-reading-frame region. Mol Biol Evol. 1986 May;3(3):179–190. doi: 10.1093/oxfordjournals.molbev.a040390. [DOI] [PubMed] [Google Scholar]
- Fanning T., Singer M. The LINE-1 DNA sequences in four mammalian orders predict proteins that conserve homologies to retrovirus proteins. Nucleic Acids Res. 1987 Mar 11;15(5):2251–2260. doi: 10.1093/nar/15.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. H., Lister C. K., Kellett E., Finnegan D. J. Transposable elements controlling I-R hybrid dysgenesis in D. melanogaster are similar to mammalian LINEs. Cell. 1986 Dec 26;47(6):1007–1015. doi: 10.1016/0092-8674(86)90815-9. [DOI] [PubMed] [Google Scholar]
- Fink G. R. Pseudogenes in yeast? Cell. 1987 Apr 10;49(1):5–6. doi: 10.1016/0092-8674(87)90746-x. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Transposable elements in eukaryotes. Int Rev Cytol. 1985;93:281–326. doi: 10.1016/s0074-7696(08)61376-5. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann P., Klaer R., Kühn S., Starlinger P. IS4 is found between eleven or twelve base pair duplications. Mol Gen Genet. 1979 Oct 1;175(3):369–373. doi: 10.1007/BF00397237. [DOI] [PubMed] [Google Scholar]
- Hattori M., Kuhara S., Takenaka O., Sakaki Y. L1 family of repetitive DNA sequences in primates may be derived from a sequence encoding a reverse transcriptase-related protein. Nature. 1986 Jun 5;321(6070):625–628. doi: 10.1038/321625a0. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Mottinger J., Freeling M. A low copy number, copia-like transposon in maize. EMBO J. 1985 May;4(5):1093–1101. doi: 10.1002/j.1460-2075.1985.tb03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel B. E., ole-MoiYoi O. K., Young J. R. Ingi, a 5.2-kb dispersed sequence element from Trypanosoma brucei that carries half of a smaller mobile element at either end and has homology with mammalian LINEs. Mol Cell Biol. 1987 Apr;7(4):1465–1475. doi: 10.1128/mcb.7.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Hohn T. Involvement of reverse transcription in the replication of cauliflower mosaic virus: a detailed model and test of some aspects. Cell. 1983 Jul;33(3):781–789. doi: 10.1016/0092-8674(83)90020-x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Saigo K., Kugimiya W., Matsuo Y., Inouye S., Yoshioka K., Yuki S. Identification of the coding sequence for a reverse transcriptase-like enzyme in a transposable genetic element in Drosophila melanogaster. Nature. 1984 Dec 13;312(5995):659–661. doi: 10.1038/312659a0. [DOI] [PubMed] [Google Scholar]
- Sakaki Y., Hattori M., Fujita A., Yoshioka K., Kuhara S., Takenaka O. The LINE-1 family of primates may encode a reverse transcriptase-like protein. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):465–469. doi: 10.1101/sqb.1986.051.01.056. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Cuypers H., Peterson P. A., Saedler H. Plant transposable elements generate the DNA sequence diversity needed in evolution. EMBO J. 1985 Mar;4(3):591–597. doi: 10.1002/j.1460-2075.1985.tb03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Klösgen R. B., Wienand U., Peterson P. A., Saedler H. The Spm (En) transposable element controls the excision of a 2-kb DNA insert at the wx allele of Zea mays. EMBO J. 1984 May;3(5):1021–1028. doi: 10.1002/j.1460-2075.1984.tb01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Shepherd N., Tacke E., Gierl A., Rohde W., Leclercq L., Mattes M., Berndtgen R., Peterson P. A., Saedler H. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 1987 Feb;6(2):287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. The abundant LINE-1 family of repeated DNA sequences in mammals: genes and pseudogenes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):457–464. doi: 10.1101/sqb.1986.051.01.055. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wilde C. D. Pseudogenes. CRC Crit Rev Biochem. 1986;19(4):323–352. [PubMed] [Google Scholar]