Abstract
Systemic inflammation was recognized as an essential factor contributing to the development of malignancies. This study aimed to investigate the prognostic value of pre-operative lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in patients with colorectal liver-only metastases (CLOM) undergoing hepatectomy. We retrospectively enrolled 150 consecutive patients with CLOM between 2000 and 2012. The optimal cutoff values of continuous LMR, NLR, and PLR were determined using the receiver operating characteristic curve analysis. Recurrence-free survival (RFS) and overall survival (OS) related to the LMR, NLR, and PLR were analyzed using both Kaplan–Meier and multivariate Cox regression methods. Elevated LMR (≥2.82) and lower NLR (<4.63) were significantly associated with better RFS and OS in patients with CLOM after hepatectomy, instead of lower PLR (<150.17). Multivariate Cox analysis identified elevated LMR as the only independent inflammatory factor for better RFS (hazard ratio, 0.591; 95% CI, 0.32–0.844; P=0.008) and OS (hazard ratio, 0.426; 95% CI, 0.254–0.716; P=0.001). In the subgroup analysis, elevated LMR was a significant favorable factor in both 5-year RFS and OS of patients with male gender, lymph node metastases, colon cancer, liver tumor with the largest diameter <5 cm, preoperative carcinoembryonic antigen level <200 ng/mL, negative hepatitis B virus infection, non-anatomic liver resection, postoperative chemotherapy, and non-preoperative chemotherapy. This study demonstrated that the preoperative LMR was an independent predictor of RFS and OS in patients with CLOM undergoing hepatic resection, and it appeared to be superior to the NLR and PLR.
Keywords: colorectal cancer, liver metastases, lymphocyte-to-monocyte ratio, survival
Introduction
With increasing incidence and mortality, colorectal cancer (CRC) has become the third leading cause of cancer deaths with an estimated mortality of 191,000 in People’s Republic of China, in 2015.1 The development of distantly metastatic disease is the major cause of death, in which the liver is usually the most frequent site of metastatic disease. Approximately 20%–25% patients were initially diagnosed as having synchronous metastases and approximately half of the cases developed the metachronous disease after resecting the primary tumor.2,3 Hepatic resection is known to represent the only chance of curative treatment for colorectal liver-only metastases (CLOM).4,5 Unfortunately, a large proportion of the population with the disease fails to achieve favorable long-term survival due to tumor recurrence.6,7 Thus, identifying relevant prognostic factors can readily screen out the high-risk subgroups and subsequently optimize therapeutic strategies for CLOM.
To date, tumor pathologic staging, differentiation, and types have been widely used for predicting CRC outcomes of patients.8,9 In addition to tumor factors, systemic inflammation has been recognized as an essential factor contributing to the development of malignancies.10 Recently, growing evidence elucidated that a systemic inflammatory response was associated with poor clinical outcomes in numerous cancers.11,12 Specifically, among multiple systemic inflammatory markers, the lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have been successfully identified as prognostic predictors for patients with advanced CRC receiving chemotherapy or surgical procedure.13–15 However, evidence for the prognostic predictive values of preoperative LMR, NLR, and PLR in patients with CLOM receiving hepatic resection remains limited.16,17 Therefore, this study hypothesized that some of these inflammatory indexes might exhibit a potential and effective prognostic value for CLOM after hepatic resection.
This study aimed to investigate the prognostic impact of preoperative LMR, NLR, and PLR in patients with CLOM undergoing hepatic resection. Moreover, it also aimed to find out an authentic biomarker for accurate prognostic prediction by comparing the prognostic values among these systemic inflammatory biomarkers.
Methods
Patients
This study retrospectively enrolled 150 patients from September 2000 to July 2012 at Sun Yat-sen University Cancer Center, People’s Republic of China, undergoing primary tumor resection and hepatectomy with curative intent. The enrolled patients met the following inclusion criteria: 1) histologically confirmed colorectal adenocarcinoma, 2) metastases limited to liver, 3) R0 or R1 resection, and 4) no percutaneous ablation. Patients were excluded from the analysis if they had other active malignancies, died in the postoperative period, or had missing preoperative data making it impossible to calculate the LMR, NLR, and PLR. Patient demographics, primary and metastatic tumor characteristics, preoperative treatment, and follow-up results were reviewed in detail from the medical records and the follow-up system of Sun Yat-sen University Cancer Center. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Institutional Review Board approval was also obtained from the independent ethics committee at the Sun Yat-sen University Cancer Center. Informed consent was waived in this noninterventional, observational, and retrospective study, in which the patient data were kept strictly confidential.
Treatments
The treatment strategy for every patient was determined according to the final agreement of the multidisciplinary team, including staff from the Department of Colorectal Surgery, Hepatobiliary Surgery, Medical Oncology, and Medical Imaging and Invasive Technology. The operability of liver metastases was assessed using an enhanced chest computed tomography and abdominal and pelvic nuclear magnetic resonance imaging. Patients whose tumors were deemed to be both resectable and low-risk recurrent were suggested to undergo surgery directly, while those considered as potentially resectable or high-risk recurrent were suggested to perform preoperative chemotherapy followed by tumor resection.
Inflammatory index calculation
The systemic inflammatory data (neutrophil, lymphocyte, monocyte, and platelet counts) were collected from the blood routine test between 8 and 10 am within 7 days before the hepatic operation. LMR was calculated by dividing the absolute number of circulating lymphocytes by the absolute number of monocytes. Likewise, the NLR or PLR was generated by dividing the absolute counts of circulating neutrophils or platelets by the absolute number of lymphocytes.
Follow-up
All patients were suggested to return for a subsequent visit every 3 months for 2 years and then semi-annually until 3 years after hepatic resection. The evaluation included clinical examination, carcinoembryonic antigen (CEA) level, abdominal ultrasonography, and chest radiograph. Chest computed tomography, abdominal/pelvic magnetic resonance imaging, and colonoscopy were performed annually. Overall survival (OS) was defined as the time length from operation to death for any cause, while recurrence-free survival (RFS) was defined as the interval from hepatic resection to disease recurrence or death. The last follow-up visit occurred in September 2015.
Statistical analysis
The receiver operating characteristic (ROC) curve analysis was applied to calculate the area under the ROC curve (AUC) and then determine the optimal cutoff values of continuous LMR, NLR, and PLR according to the 5-year OS. The correlation between clinical–pathologic parameters and LMR, NLR, and PLR was assessed by the chi-square test or Fisher’s exact test, as appropriate. Survival outcomes for the binary level of inflammatory indexes were compared using the Kaplan–Meier log-rank test. Potential effects of clinical variables on RFS and OS were examined using univariate Cox proportional hazards. Variables proving statistical significance in univariate Cox models were further assessed using multivariate Cox models. The multivariate Cox proportional hazard model was used to further identify independent prognostic factors for RFS and OS. The hazard ratios (HRs) and CIs were subsequently calculated. The statistical analysis and forest plot figure were performed using the IBM SPSS Statistics 22 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 6.01 (GraphPad Software, Inc, La Jolla, CA, USA), respectively. All statistical tests used in this study were two sided, and a P-value of <0.05 was considered as statistically significant.
Results
Optimal cutoff determination
The AUC of LMR, NLR, and PLR was 0.639 (95% CI, 0.550–0.728, P=0.004), 0.568 (95% CI, 0.477–0.660, P=0.152), and 0.518 (95% CI, 0.424–0.611, P=0.707), respectively (Figure 1). According to the highest Youden index (specificity + sensitivity–1), the optimal cutoff values chosen for the LMR, NLR, and PLR were 2.82, 4.63, and 150.17, respectively (0.27 for LMR, 0.13 for NLR, and 0.10 for PLR). In the subsequent analysis, the LMR, NLR, and PLR were dichotomized into high-level and low-level groups according to the optimal cutoff values.
Figure 1.
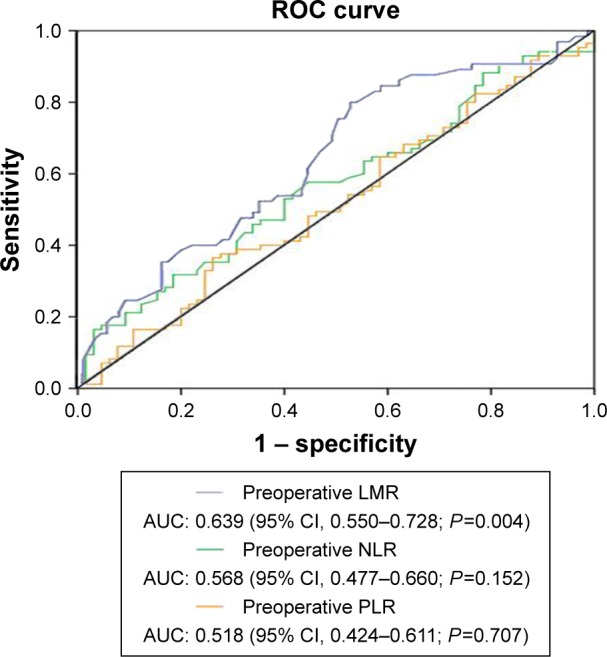
ROC curve for the preoperative LMR, NLR, and PLR according to the 5-year OS.
Abbreviations: AUC, area under the curve; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic.
Patient characteristics
Of the total patients, 64.7% (97/150) were male and 35.3% (53/150) were female, with a median age of 58 years (range 20–82). Among the patients with the primary tumor, 58% had colon cancer, while 42% had rectum cancer. In 84 (59.2%) patients, the primary tumors involved lymph node metastasis. The majority of patients (85/150, 56.7%) presented a solitary tumor, and 62.7% (94/150) had synchronous metastases at the time of diagnosis. As for the hepatic metastases, the median size of the largest tumor was 2.8 cm (range 0.3–12.1 cm). In addition, 46.7% (70/150) patients received synchronous resection for primary tumor and liver metastases. With respect to chemotherapy, 59 (39.3%) patients received neoadjuvant chemotherapy before hepatectomy and 110 (73.3%) patients received postoperative chemotherapy. The detailed characteristics of the patients in this study are described in Table 1.
Table 1.
Basic characteristics of total patients with colorectal cancer liver-only metastases
| Characteristics | Total patients (n, %) |
|---|---|
| Age (years), range | 58 (20–82) |
| Gender (n=150) | |
| Male | 97 (64.7) |
| Female | 53 (35.3) |
| Preoperative chemotherapy (n=150) | |
| No | 91 (60.7) |
| Yes | 59 (39.3) |
| Preoperative HBV infection (n=146) | |
| Negative | 128 (87.7) |
| Positive | 18 (12.3) |
| Preoperative CEA level (n=144), ng/mL | |
| <200 | 132 (91.7) |
| ≥200 | 12 (8.3) |
| Preoperative LMR (median, range) | 3.33 (0.16–11.0) |
| Preoperative NLR (median, range) | 2.0 (0.6–82.0) |
| Preoperative PLR (median, range) | 123.71 (39.05–990.0) |
| Timing of metastasis (n=150) | |
| Synchronous | 94 (62.7) |
| Metachronous | 56 (37.3) |
| Number of metastatic tumors (median, range) | 1 (1–11) |
| Largest tumor size (cm), range | 2.8 (0.3–12.1) |
| Tumor distribution (n=150) | |
| Unilobar | 104 (69.3) |
| Bilobar | 46 (30.7) |
| Liver resection (n=150) | |
| Anatomic | 19 (12.7) |
| Non-anatomic | 131 (87.3) |
| Resection margin (n=150) | |
| Negative | 91 (60.7) |
| Positive | 59 (39.3) |
| Postoperative chemotherapy (n=150) | |
| No | 40 (26.7) |
| Yes | 110 (73.3) |
Abbreviations: CEA, carcinoembryonic antigen; HBV, hepatitis B virus; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Relationships between preoperative LMR, NLR, and PLR and patient characteristics
A larger proportion of patients with an elevated LMR showed a lower preoperative CEA level compared with that of the LMR-low group (92.8% vs 79.2%, P=0.048), as shown in Table 2. Additionally, a higher proportion of the LMR-high group received postoperative chemotherapy (82.5% vs 56.6%, P<0.001). Patients in the NLR-high group were more likely to present multiple hepatic metastases than those in the NLR-low group (68.8% vs 40.3%, P=0.030). In the PLR-high group, 77.1% patients had a negative hepatic resection margin, while in the PLR-low group, only 52.9% patients had a negative resection margin (P=0.005). As to the different LMR, NLR, and PLR subgroups, clinical characteristics including gender distribution, lymph node metastases, primary tumor location, largest hepatic tumor size, metastatic tumor distribution, timing of metastasis, preoperative chemotherapy, and liver resection were comparable.
Table 2.
Clinical characteristics of the patients stratified by LMR, NLR, and PLR
| Characteristics | LMR
|
NLR
|
PLR
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| <2.82 n=53, n (%) | ≥2.82 n=97, n (%) | P-value | <4.63 n=134, n (%) | ≥4.63 n=16, n (%) | P-value | <150.17 n=102, n (%) | ≥150.17 n=48, n (%) | P-value | |
| Gender | 0.183 | 0.718 | 0.455 | ||||||
| Male | 38 (71.7) | 59 (60.8) | 86 (64.2) | 11 (68.8) | 68 (66.7) | 29 (60.4) | |||
| Female | 15 (28.3) | 38 (39.2) | 48 (35.8) | 5 (31.2) | 34 (33.3) | 19 (39.6) | |||
| Age, years | 0.896 | 0.734 | 0.500 | ||||||
| <58 | 24 (45.3) | 45 (46.4) | 61 (45.5) | 8 (50.0) | 45 (44.1) | 24 (50.0) | |||
| ≥58 | 29 (54.7) | 52 (53.6) | 73 (54.5) | 8 (50.0) | 57 (55.9) | 24 (50.0) | |||
| Preoperative chemotherapy | 0.451 | 0.702 | 0.080 | ||||||
| No | 30 (56.6) | 61 (62.9) | 82 (61.2) | 9 (56.3) | 57 (55.9) | 34 (70.8) | |||
| Yes | 23 (43.4) | 36 (37.1) | 52 (38.8) | 7 (43.7) | 45 (44.1) | 14 (29.2) | |||
| Preoperative HBV infection | 0.297 | 0.551 | 0.202 | ||||||
| Negative | 42 (79.2) | 86 (88.7) | 115 (85.8) | 13 (81.3) | 88 (86.3) | 40 (83.3) | |||
| Positive | 9 (17.0) | 9 (9.3) | 15 (11.2) | 3 (18.7) | 10 (9.8) | 8 (16.7) | |||
| Unknown | 2 (3.8) | 2 (2.1) | 4 (3.0) | 0 | 4 (3.9) | 0 | |||
| Primary tumor location | 0.928 | 0.493 | 0.955 | ||||||
| Colon | 31 (58.5) | 56 (57.7) | 79 (59.0) | 8 (50.0) | 59 (57.8) | 28 (58.3) | |||
| Rectum | 22 (41.5) | 41 (42.3) | 55 (41.0) | 8 (50.0) | 43 (42.2) | 20 (41.7) | |||
| Lymph node metastasis | 0.155 | 0.584 | 0.348 | ||||||
| No | 25 (47.2) | 33 (34.0) | 51 (38.1) | 7 (43.7) | 43 (42.2) | 15 (31.3) | |||
| Yes | 27 (50.9) | 57 (58.8) | 75 (56.0) | 9 (56.3) | 53 (52.0) | 31 (64.6) | |||
| Unknown | 1 (2.0) | 7 (7.2) | 8 (6.0) | 0 | 6 (5.9) | 2 (4.2) | |||
| Timing of metastasis | 0.066 | 0.595 | 0.075 | ||||||
| Synchronous | 28 (52.8) | 66 (68.0) | 83 (61.9) | 11 (68.8) | 59 (57.8) | 35 (72.9) | |||
| Metachronous | 25 (47.2) | 31 (32.0) | 51 (38.1) | 5 (31.2) | 43 (42.2) | 13 (27.1) | |||
| Number of metastatic tumors | 0.483 | 0.030 | 0.180 | ||||||
| 1 | 28 (52.8) | 57 (58.8) | 80 (59.7) | 5 (31.2) | 54 (52.9) | 31 (64.6) | |||
| >1 | 25 (47.2) | 40 (41.2) | 54 (40.3) | 11 (68.8) | 48 (47.1) | 17 (35.4) | |||
| Largest tumor size, cm | 0.120 | 0.518 | 0.455 | ||||||
| <5 | 36 (67.9) | 77 (79.4) | 102 (76.1) | 11 (68.8) | 75 (73.5) | 38 (79.2) | |||
| ≥5 | 17 (32.1) | 20 (20.6) | 32 (23.9) | 5 (31.2) | 27 (26.5) | 10 (20.8) | |||
| Tumor distribution | 0.309 | 0.076 | 0.302 | ||||||
| Unilobar | 34 (64.2) | 70 (72.2) | 96 (71.6) | 8 (50.0) | 68 (66.7) | 36 (75.0) | |||
| Bilobar | 19 (35.8) | 27 (27.8) | 38 (28.4) | 8 (50.0) | 34 (33.3) | 12 (25.0) | |||
| Preoperative CEA level, ng/mL | 0.048 | 0.680 | 0.558 | ||||||
| <200 | 42 (79.2) | 90 (92.8) | 119 (88.8) | 13 (81.3) | 90 (88.2) | 42 (87.5) | |||
| ≥200 | 7 (13.2) | 5 (5.2) | 10 (7.5) | 2 (12.5) | 9 (8.8) | 3 (6.3) | |||
| Unknown | 4 (7.5) | 2 (2.1) | 5 (3.7) | 1 (6.3) | 3 (2.9) | 3 (6.3) | |||
| Liver resection | 0.509 | 0.107 | 0.966 | ||||||
| Anatomic | 8 (15.1) | 11 (11.3) | 19 (14.2) | 0 | 13 (12.7) | 6 (12.5) | |||
| Non-anatomic | 45 (84.9) | 86 (88.7) | 115 (85.8) | 16 (100.0) | 89 (87.3) | 42 (87.5) | |||
| Resection margin | 0.146 | 0.214 | 0.005 | ||||||
| Negative | 28 (52.8) | 63 (64.9) | 79 (59.0) | 12 (75.0) | 54 (52.9) | 37 (77.1) | |||
| Positive | 25 (47.2) | 34 (35.1) | 55 (41.0) | 4 (25.0) | 48 (47.1) | 11 (22.9) | |||
| Postoperative chemotherapy | 0.001 | 0.102 | 0.635 | ||||||
| No | 23 (43.4) | 17 (17.5) | 33 (24.6) | 7 (43.8) | 26 (25.5) | 14 (29.2) | |||
| Yes | 30 (56.6) | 80 (82.5) | 101 (75.4) | 9 (56.2) | 76 (74.5) | 34 (70.8) | |||
Abbreviations: CEA, carcinoembryonic antigen; HBV, hepatitis B virus; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Survival outcomes and inflammatory index
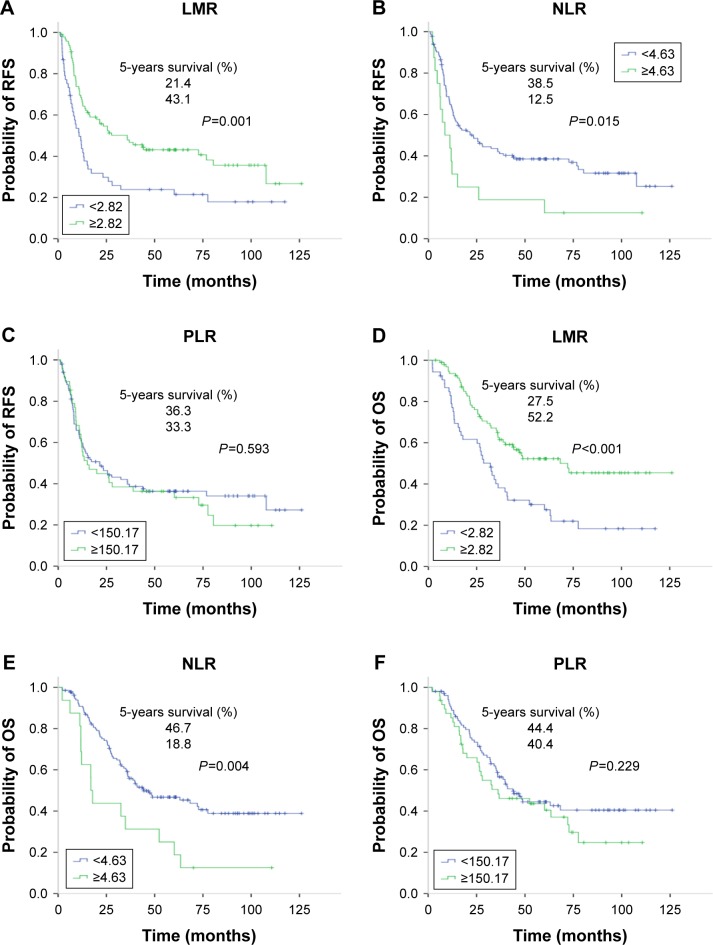
The median follow-up period for all the patients was 36 months (range 2–126 months). During the follow-up period, 68.0% (102/150) patients experienced tumor recurrence, including 65.7% (67/102) patients with intrahepatic recurrence, 13.7% (14/102) patients with lung metastases, 8.8% (9/102) patients with abdominal pelvic metastases, and 11.8% (12/102) patients with multiple organ metastases. As a result, 56.7% (85/150) patients died of disease progression. Regarding the entire study population, the 3- and 5-year RFS rates were 39.4% and 35.4%, respectively, and the 3- and 5-year OS rates were 56.8% and 43.2%, respectively. Patients with an elevated LMR had significantly better 5-year RFS and OS compared with those of the LMR-low group (RFS: 43.1% vs 21.4%, P=0.001; OS: 52.2% vs 27.5%, P<0.001; Figure 2A and D). On the contrary, the estimated 5-year RFS and OS for the NLR-high group were significantly inferior to those of NLR-low group (RFS: 12.5% vs 38.5%, P=0.015; OS: 18.8% vs 46.7%, P=0.004; Figure 2B and E). Regarding the PLR groups, neither the 5-year RFS rates (36.3% vs 33.3%, P=0.593; Figure 2C) nor the 5-year OS rates (44.4% vs 40.4%, P=0.229; Figure 2F) showed any statistical difference.
Figure 2.
Kaplan–Meier curves of LMR, NLR, and PLR for comparing the 5-year RFS and OS.
Notes: A, B, and C are the survival curves of LMR, NLR and PLR for RFS, respectively; D, E, and F are the survival curves of LMR, NLR, and PLR for OS, respectively.
Abbreviations: LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; RFS, recurrence-free survival.
The univariate analysis revealed that the high LMR was associated with better RFS (HR, 0.523; 95% CI, 0.35–0.782; P=0.002) and OS (HR, 0.475; 95% CI, 0.31–0.728; P=0.001), as shown in Table 3. On the contrary, high NLR, lymph node metastases, number of metastatic tumors >1, largest metastatic tumor size ≥5 cm, and bilobar tumor distribution were correlated with both worse RFS and OS. Additionally, patients receiving preoperative chemotherapy showed poorer RFS than those without preoperative treatment (HR, 1.665; 95% CI, 1.113–2.491; P=0.013).
Table 3.
Univariate and multivariate analyses of risk factors for recurrence-free survival and overall survival in the patients with colorectal liver-only metastases undergoing hepatectomy
| Characteristics | 5-year RFS survival (%) | RFS
|
5-year OS survival (%) | OS
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Gender | 0.123 | 0.176 | ||||||||
| Male | 31.0 | Reference | 38.9 | Reference | ||||||
| Female | 43.3 | 0.714 (0.466–1.095) | 50.9 | 0.727 (0.458–1.154) | ||||||
| Age, years | 0.238 | 0.070 | ||||||||
| <58 | 42.1 | Reference | 52.3 | Reference | ||||||
| ≥58 | 29.8 | 1.277 (0.851–1.915) | 35.4 | 1.494 (0.968–2.307) | ||||||
| Preoperative chemotherapy | 0.013 | 0.353 | 0.259 | |||||||
| No | 42.6 | Reference | 47.2 | Reference | ||||||
| Yes | 21.2 | 1.665 (1.113–2.491) | 1.238 (0.789–1.944) | 35.4 | 1.282 (0.833–1.974) | |||||
| Preoperative HBV infection | 0.079 | 0.125 | ||||||||
| Negative | 33.4 | Reference | 41.0 | Reference | ||||||
| Positive | 57.4 | 0.500 (0.231–1.084) | 60.6 | 0.521 (0.227–1.199) | ||||||
| Primary tumor location | 0.353 | 0.419 | ||||||||
| Colon | 40.1 | Reference | 46.4 | Reference | ||||||
| Rectum | 28.9 | 1.208 (0.811–1.80) | 39.0 | 1.193 (0.778–1.83) | ||||||
| Lymph node metastases | 0.042 | 0.011 | 0.011 | |||||||
| No | 48.9 | Reference | 60.7 | Reference | 0.001 | |||||
| Yes | 28.3 | 1.569 (1.017–2.420) | 1.785 (1.139–2.797) | 33.9 | 1.847 (1.151–2.962) | 2.271 (1.382–3.731) | ||||
| Timing of metastasis | 0.297 | 0.701 | ||||||||
| Synchronous | 36.2 | Reference | 44.9 | Reference | ||||||
| Metachronous | 33.9 | 1.24 (0.827–1.859) | 40.8 | 1.089 (0.705–1.681) | ||||||
| Number of metastatic tumors | 0.014 | 0.597 | 0.044 | |||||||
| 1 | 42.8 | Reference | 50.1 | Reference | 0.205 | |||||
| >1 | 25.2 | 1.645 (1.104–2.451) | 1.156 (0.693–1.929) | 34.8 | 1.55 (1.012–2.374) | 1.42 (0.826–2.442) | ||||
| Largest tumor size, cm | 0.001 | 0.005 | 0.006 | |||||||
| <5 | 39.9 | Reference | 47.5 | Reference | 0.028 | |||||
| ≥5 | 21.5 | 2.071 (1.343–3.194) | 1.928 (1.217–3.054 | 28.5 | 1.927 (1.206–3.079) | 1.754 (1.061–2.901) | ||||
| Tumor distribution | 0.001 | 0.051 | 0.021 | |||||||
| Unilobar | 41.6 | Reference | 48.7 | Reference | 0.431 | |||||
| Bilobar | 20.3 | 1.993 (1.304–3.047) | 1.712 (0.999–2.935) | 31.1 | 1.693 (1.082–2.65) | 1.26 (0.709–2.242) | ||||
| Liver resection | 0.455 | 0.485 | ||||||||
| Anatomic | 27.8 | Reference | 34.7 | Reference | ||||||
| Non-anatomic | 36.4 | 0.811 (0.467–1.406) | 44.5 | 0.81 (0.449–1.463) | ||||||
| Resection margin | 0.861 | 0.766 | ||||||||
| Negative | 32.8 | Reference | 41.9 | Reference | ||||||
| Positive | 39.3 | 0.964 (0.639–1.454) | 45.4 | 0.936 (0.605–1.449) | ||||||
| Preoperative CEA level, ng/mL | 0.577 | 0.637 | ||||||||
| <200 | 35.5 | Reference | 44.1 | Reference | ||||||
| ≥200 | 41.7 | 0.803 (0.371–1.737) | 41.7 | 1.205 (0.555–2.619) | ||||||
| Postoperative chemotherapy | 0.678 | 0.394 | ||||||||
| No | 29.1 | Reference | 36.8 | Reference | ||||||
| Yes | 37.9 | 0.912 (0.591–1.409) | 45.5 | 0.816 (0.512–1.302) | ||||||
| LMR | 0.002 | 0.008 | 0.001 | |||||||
| <2.82 | 21.4 | Reference | 27.5 | Reference | 0.001 | |||||
| ≥2.82 | 43.1 | 0.523 (0.35–0.782) | 0.591 (0.32–0.844) | 52.2 | 0.475 (0.31–0.728) | 0.426 (0.254–0.716) | ||||
| NLR | 0.017 | 0.452 | 0.005 | |||||||
| <4.63 | 38.5 | Reference | 46.7 | Reference | ||||||
| ≥4.63 | 12.5 | 2.001 (1.133–3.533) | 1.284 (0.67–2.46) | 18.8 | 2.28 (1.283–4.051) | 1.273 (0.654–2.476) | 0.477 | |||
| PLR | 0.594 | 0.230 | ||||||||
| <150.17 | 36.3 | Reference | 44.4 | Reference | ||||||
| ≥150.17 | 33.3 | 1.12 (0.739–1.699) | 40.4 | 1.31 (0.842–2.039) | ||||||
Abbreviations: CEA, carcinoembryonic antigen; HBV, hepatitis B virus; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; RFS, recurrence-free survival.
The multivariate analysis subsequently showed that lymph node metastases (HR, 1.785; 95% CI, 1.139–2.797; P=0.011), largest metastatic tumor size ≥5 cm (HR, 1.928; 95% CI, 1.217–3.054; P=0.005), and high LMR (HR, 0.591; 95% CI, 0.32–0.844; P=0.008) were meaningful prognostic factors for RFS. Similarly, lymph node metastases (HR, 2.271; 95% CI, 1.382–3.731; P=0.001), largest metastatic tumor size ≥5 cm (HR, 1.754; 95% CI, 1.061–2.901; P=0.028), and high LMR (HR, 0.426; 95% CI, 0.254–0.716; P=0.001) were also defined as independent factors for OS.
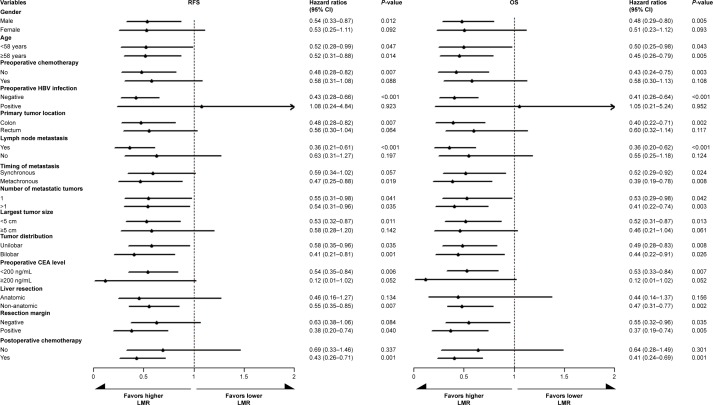
The LMR in the subgroups stratified by 14 clinical parameters was subjected to the univariate Cox model analysis to further verify the prognostic value of LMR. The prognostic value of LMR was consistent in both 5-year RFS and OS when considering the subgroups by age, tumor number, or distribution. In addition, the prognostic value of LMR was consistent in 5-year OS when considering the subgroups by timing of metastasis and resection margin (Figure 3). Thus, high LMR was demonstrated as the favorable prognostic factor for patients with male gender, lymph node metastases, colon cancer, metastatic tumor with the largest diameter <5 cm, preoperative CEA level <200 ng/mL, negative hepatitis B virus infection, non-anatomic liver resection, postoperative chemotherapy, and non-preoperative chemotherapy in both 5-year RFS and OS.
Figure 3.
Subgroup prognostic analysis for the 5-year RFS and OS according to preoperative LMR stratified by clinical characteristics and treatment parameters.
Abbreviations: CEA, carcinoembryonic antigen; HBV, hepatitis B virus; LMR, lymphocyte-to-monocyte ratio; OS, overall survival; RFS, recurrence-free survival.
Discussion
To date, mounting studies have mainly concentrated on the biologic behavior and presentation of tumor histopathologic parameters to screen out feasible cancer-related variables for predicting the prognosis of patients with CLOM after hepatic resection.18–20 However, these variables did not entirely present the complete potency for predicting and guiding the individual treatment appropriately. Recently, growing evidence suggested that systemic inflammation potentially contributed to the tumor development, and it is now considered as a hallmark of CRC.21–23 Therefore, this study evaluated the prognostic value of three common inflammation-based indexes (LMR, NLR, and PLR) and determined whether they could serve as alternative items to tailor different risk subgroups for patients with CLOM receiving hepatic resection.
After prognostic comparison, the LMR exhibited the highest prognostic diagnostic value (AUC =0.639; 95% CI, 0.550–0.728; P=0.004) and was confirmed as a superior predictor of RFS and OS in patients with CLOM undergoing curative hepatectomy, compared with the NLR and PLR. Furthermore, the findings of this study also demonstrated that preoperative LMR was the only independent inflammatory predictor for both RFS and OS. Additionally, the high LMR predicted a favorable prognosis in patients with male gender, lymph node metastases, colon cancer, liver tumor with the largest diameter <5 cm, preoperative CEA level <200 ng/mL, negative hepatitis B virus infection, non-anatomic liver resection, postoperative chemotherapy, and non-preoperative chemotherapy. Overall, these proved the hypothesis of this study and suggested that systemic inflammatory biomarkers, such as LMR, might be the same alternative indicator to further stratify patients with CLOM, compared with the conventional histopathologic parameters.
So far, the predictive value of the preoperative LMR in CRC has been identified, and the decreased LMR delivered an unfavorable impact on the long-term survival among various stage subgroups.24 Recently, one of the largest retrospective cohort studies revealed that the LMR appeared to be superior to the NLR, PLR, Glasgow prognostic score, BRAF mutation status, and mismatch repair status in prognostic prediction, which further indicated that the LMR was an independent predictor of OS in patients with nonmetastatic CRC undergoing curative resection.25 In patients with unresectable metastatic CRC who received palliative chemotherapy, previous studies have elucidated that the LMR was also a useful marker for predicting the long-term survival and the efficacy of chemotherapy.26,27 As for the patients with CLOM undergoing curative liver resection, Neofytou et al first identified that the preoperative LMR remained the only independent prognostic factor for cancer-specific survival, compared with the NLR and PLR.17 However, unlike the results of this study, the LMR was not proven an independent prognostic factor for RFS in their study. As a matter of fact, the recruited patients totally received preoperative chemotherapy in the study of Neofytou et al, while only 39.3% patients received the adjuvant therapy in this study. Consistent with the results of Neofytou et al’s study, high LMR was not significantly associated with better 5-year RFS of the patients receiving preoperative chemotherapy in this study (HR, 0.583; 95% CI, 0.314–1.084; P=0.088; Figure 3). However, low LMR indicated worse survival outcome in non-preoperative chemotherapy subgroup. We considered that neoadjuvant chemotherapy contributed to improve the RFS of the LMR-low group, thus reducing the differences among survival outcomes. In addition, recurrent tumors in the lower-LMR group might be more resistant to additional chemotherapy than those in the higher-LMR group, leading to decreased OS, thus resulting in a significant OS difference in the two groups.28 Together, it is worth further exploring the prognostic value of LMR in the extended cohort of patients with CLOM.
Several mechanisms could contribute to the outcome that the lower LMR compromised the long-term survival in patients with CLOM. The combination of two changes raised a very important role in the process: the relatively lower number of blood-circulating lymphocytes and excess monocytes. Previous studies have elucidated that lymphocytes played critical roles in the antitumor immunity of the host by infiltrating into the tumor microenvironment after being triggered by immunologic antitumor reaction.29,30 However, systemic inflammation significantly decreased cellular immunity, resulting in lymphocytopenia, which was marked as a decrease in CD4+ helper lymphocytes and an increase in CD8+ suppressor lymphocytes.31 A decreased number of circulating lymphocytes was, therefore, considered to be responsible for the insufficient immunologic reaction against the tumor, contributing to the poor cancer prognosis after surgical procedure in CRC.32 On the contrary, the excess circulating monocytes are recruited to the tumor sites and differentiated into macrophages, which become the tumor-associated macrophages (TAMs).33,34 It is generally accepted that the TAMs act like the “jack-of-all-trades”, being involved mostly in protumoral functions, including tumor cell growth, angiogenesis, migration, invasion, metastasis, and immune system suppression.35,36 Growing clinical evidence showed that an increased number of TAMs was correlated with therapy failure and poor prognosis in cancer patients.37,38 As a result, elevated circulating serum levels of monocytes may reflect increased production of tissue TAMs, high tumor burden, and immunodepression, entirely leading to worse survival outcomes.
This is a novel study that demonstrated the LMR to be superior to the NLR and PLR as an independent predictor for both RFS and OS in patients with CLOM. Determining the preoperative LMR might provide useful information for the therapeutic choices before surgery. For instance, patients with a higher LMR might achieve sufficient benefits from curative intent policy and radical hepatectomy, and the following adjuvant chemotherapy administration was considered as the optimal therapeutic strategy. On the contrary, for patients with a lower LMR, hepatectomy may be ill-advised and further systemic therapy would be preferable. As mentioned earlier, this study further demonstrated that the LMR was more feasible for prognostic prediction in several specific subgroups. For example, in a male patient with colon cancer and primary lymph node metastases, the LMR might be more powerful to distinguish the prognosis. Because of the strengths of LMR, such as reliability, reproducibility, and low cost, we believed that it is easy to be applied in clinical practice and provided a supplementary diagnosis for the patients.
This study has several limitations. First, this is a retrospective study with uncontrolled methodology and a limited number of patients from a single-institution experience. Second, the preoperative treatment is inconsistent in some patients, which might affect inflammation-based index evaluation and survival outcomes. Additionally, complete data on the postoperative treatments of patients, such as regimen chemotherapies, types of radiotherapy, and interventional therapy, were not available. Also, subsequent therapies after disease recurrence play a critical role in prolonging the OS. Furthermore, appropriate cutoff levels of LMR, NLR, and PLR were calculated for OS using the ROC analysis. In fact, these cutoff variables were not consistent with those in previous studies and were necessary to be determined in validation studies.17,28 Finally, several disease conditions, such as infection, ischemia, diabetes mellitus, and heart disease, which may bias the blood-circulating cell counts, could not be taken into consideration. Overall, large prospective studies should be performed to confirm the findings of this study.
Conclusion
This study demonstrated that in patients with CLOM undergoing hepatic resection, preoperative LMR is the optimal and independent predictor of RFS and OS compared with the NLR and PLR. Thus, the LMR may help surgeons evaluate the benefit from curative hepatectomy and formulate individualized strategies of preoperative treatment.
Acknowledgments
We would like to thank all the colleagues of the Department of Colorectal Surgery in Sun Yat-sen University Cancer Center who were involved in treating the patients of this study.
This work was supported by grants from Guangzhou Science and Technology Plan Projects (Health Medical Collaborative Innovation Program of Guangzhou; grant No 201400000001–4) and Sun Yat-sen University Clinical Research 5010 Program (2015024); Science and Technology Planning Project of Guangdong Province (No 2013B0218001462).
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval number as RDDA2017000107.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Cervantes A, ESMO Guidelines Working Group Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol. 2010;21(Suppl 5):v93–v97. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly DA, Poston GJ. Colorectal liver metastases: current and future perspectives. Future Oncol. 2006;2(4):525–531. doi: 10.2217/14796694.2.4.525. [DOI] [PubMed] [Google Scholar]
- 4.Akgul O, Cetinkaya E, Ersoz S, et al. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20(20):6113–6122. doi: 10.3748/wjg.v20.i20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102(9):1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigano L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: Risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276–1286. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 7.Chan KM, Chiang JM, Lee CF, et al. Outcomes of resection for colorectal cancer hepatic metastases stratified by evolving eras of treatment. World J Surg Oncol. 2011;9:174. doi: 10.1186/1477-7819-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61(5):561–569. doi: 10.1136/jcp.2007.054858. [DOI] [PubMed] [Google Scholar]
- 9.Mehrkhani F, Nasiri S, Donboli K, Meysamie A, Hedayat A. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Dis. 2009;11(2):157–161. doi: 10.1111/j.1463-1318.2008.01556.x. [DOI] [PubMed] [Google Scholar]
- 10.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Geng Q, Chen S, et al. Nomogram based on systemic inflammatory response markers predicting the survival of patients with resectable gastric cancer after D2 gastrectomy. Oncotarget. 2016;7(25):37556–37565. doi: 10.18632/oncotarget.8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 13.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You J, Zhu GQ, Xie L, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2016;7(18):25516–25527. doi: 10.18632/oncotarget.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. 2014;31(10):239. doi: 10.1007/s12032-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 17.Neofytou K, Smyth EC, Giakoustidis A, et al. The preoperative lymphocyte-to-monocyte ratio is prognostic of clinical outcomes for patients with liver-only colorectal metastases in the neoadjuvant setting. Ann Surg Oncol. 2015;22(13):4353–4362. doi: 10.1245/s10434-015-4481-8. [DOI] [PubMed] [Google Scholar]
- 18.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, Peng J, Wang Z, et al. High preoperative serum CA19–9 level is predictive of poor prognosis for patients with colorectal liver oligometastases undergoing hepatic resection. Med Oncol. 2016;33(11):121. doi: 10.1007/s12032-016-0838-5. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz L, Michel P, Scotte M. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983) Ann Surg. 2015;261(1):e28–e29. doi: 10.1097/SLA.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 21.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: Staging the tumor and staging the host. Ann Surg. 2016;263(2):326–336. doi: 10.1097/SLA.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 23.Shibutani M, Maeda K, Nagahara H, et al. Significance of markers of systemic inflammation for predicting survival and chemotherapeutic outcomes and monitoring tumor progression in patients with unresectable metastatic colorectal cancer. Anticancer Res. 2015;35(9):5037–5046. [PubMed] [Google Scholar]
- 24.Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget. 2016;7(22):31926–31942. doi: 10.18632/oncotarget.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JC, Chan DL, Diakos CI, et al. The Lymphocyte-to-Monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–546. doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21(34):9966–9973. doi: 10.3748/wjg.v21.i34.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin GN, Liu PP, Liu DY, Peng JW, Xiao JJ, Xia ZJ. Prognostic significance of the pre-chemotherapy lymphocyte-to-monocyte ratio in patients with previously untreated metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J Cancer. 2016;35:5. doi: 10.1186/s40880-015-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa T, Ishihara S, Kawai K, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Surg Res. 2015;199(2):386–392. doi: 10.1016/j.jss.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumor growth and spread. Br J Cancer. 2004;90(11):2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit Care Med. 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Liang L, Zhu J, Jia H, et al. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget. 2016;7(1):1014–1028. doi: 10.18632/oncotarget.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumor metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 35.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Rogers TL, Holen I. Tumor macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Caro G, Cortese N, Castino GF, et al. Dual prognostic significance of tumor-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2016;65(10):1710–1720. doi: 10.1136/gutjnl-2015-309193. [DOI] [PubMed] [Google Scholar]
- 38.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]