Abstract
We have recently shown that the aggregation factor (AF) from the sponge Geodia cydonium stimulates DNA synthesis in quiescent, dissociated cells from the same organism; this event was correlated with the release of the two second messengers: inositol trisphosphate and diacylglycerol. Here we describe that after binding of the AF to the plasma membrane-bound aggregation receptor, a rapid and drastic increase in the incorporation of 32Pi into a series of proteins in the pore complex-lamina fraction occurs. Addition of the tumor promoter, 12-O-tetradecanoylphorbol-13-acetate, to quiescent cells resulted in a similar stimulation of phosphorylation of nuclear proteins. Among them we have selected one protein with a polypeptide Mr of 170,000 (pp170) for detailed studies. By immunoblotting pp170 was identified as DNA topoisomerase II. In vitro studies with nuclei and purified, homogeneous protein kinase C together with the required activators of this enzyme also showed a phosphorylation of pp170. After phosphorylation, DNA topoisomerase II activity was found to be 2.5-fold that of the non-phosphorylated enzyme. From these data we conclude that protein kinase C is involved in AF induced transmembrane signalling, ultimately leading to an initiation of DNA synthesis.
Full text
PDF

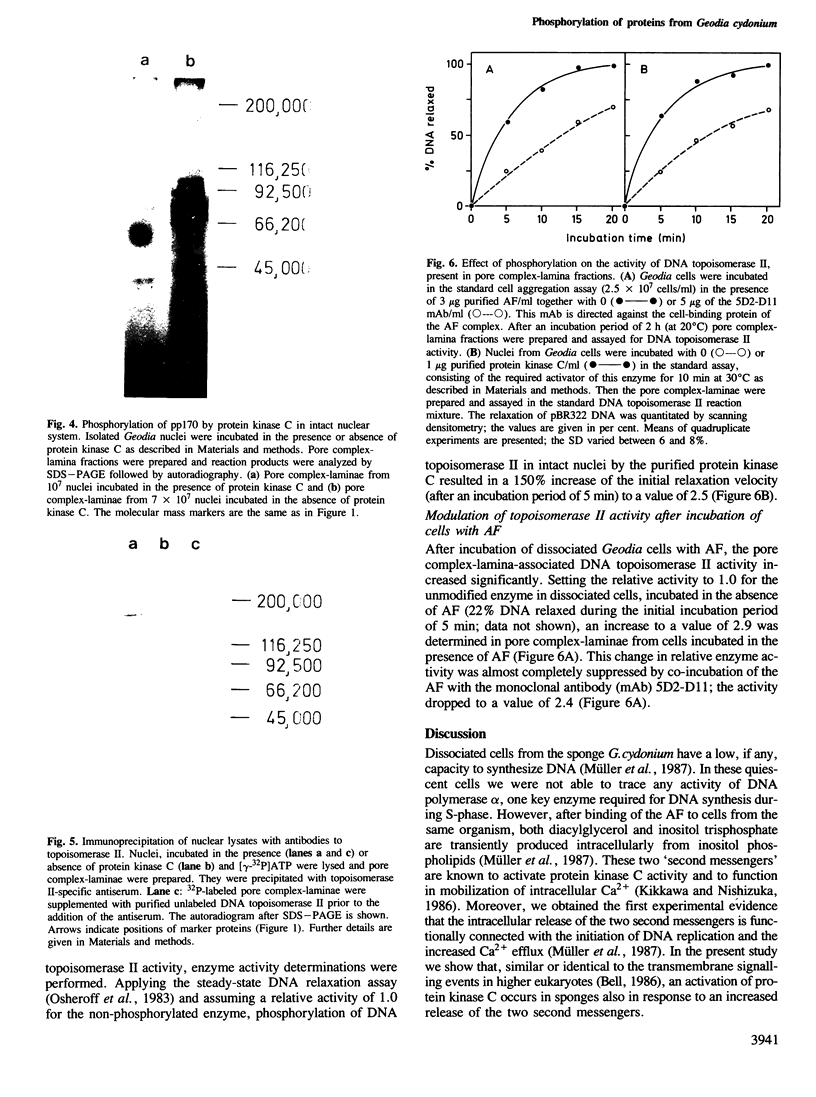



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc Natl Acad Sci U S A. 1985 May;82(10):3164–3168. doi: 10.1073/pnas.82.10.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M., Falke D., Preuhs J., Schröder H. C., Pfeifer K., Müller W. E. Occurrence of novel small RNAs with concomitant inhibition of host cellular U small nuclear RNA synthesis in Vero cells infected with herpes simplex virus type 1. J Gen Virol. 1986 Dec;67(Pt 12):2587–2594. doi: 10.1099/0022-1317-67-12-2587. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Jumblatt J. Membrane involvement in cell-cell interactions: a two-component model system for cellular recognition that does not require live cells. Soc Gen Physiol Ser. 1977;32:155–172. [PubMed] [Google Scholar]
- Burger M. M., Turner R. S., Kuhns W. J., Weinbaum G. A possible model for cell-cell recognition via surface macromolecules. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):379–393. doi: 10.1098/rstb.1975.0059. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gramzow M., Bachmann M., Uhlenbruck G., Dorn A., Müller W. E. Identification and further characterization of the specific cell binding fragment from sponge aggregation factor. J Cell Biol. 1986 Apr;102(4):1344–1349. doi: 10.1083/jcb.102.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzow M., Dorn A., Steffen R., Müller W. E. Sponge aggregation factor: in situ localization by fluorescent monoclonal antibody techniques. J Cell Biochem. 1986;31(4):251–258. doi: 10.1002/jcb.240310402. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. CHEMICAL DISSOLUTION AND IN VITRO RECONSTRUCTION OF SPONGE CELL ADHESIONS. I. ISOLATION AND FUNCTIONAL DEMONSTRATION OF THE COMPONENTS INVOLVED. Dev Biol. 1963 Aug;8:27–47. doi: 10.1016/0012-1606(63)90024-1. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Henkart P., Humphreys S., Humphreys T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3045–3050. doi: 10.1021/bi00740a016. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. STUDIES ON CELL AGGREGATION: DEMONSTRATION OF MATERIALS WITH SELECTIVE CELL-BINDING ACTIVITY. Proc Natl Acad Sci U S A. 1963 May;49(5):742–747. doi: 10.1073/pnas.49.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Maidhof A., Zahn R. K., Shannon W. M. Effect of 9-beta-D-arabinofuranosyladenine on DNA synthesis in vivo. Cancer Res. 1977 Jul;37(7 Pt 1):2282–2290. [PubMed] [Google Scholar]
- Müller W. E., Müller I., Zahn R. K., Kurelec B. Species-specific aggregation factor in sponges. VI. Aggregation receptor from the cell surface. J Cell Sci. 1976 Jul;21(2):227–241. doi: 10.1242/jcs.21.2.227. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Müller I., Zahn R. K. Species-specific aggregation factor in sponges. V. Influence on programmed syntheses. Biochim Biophys Acta. 1976 Jan 19;418(2):217–225. doi: 10.1016/0005-2787(76)90071-x. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Rottmann M., Diehl-Seifert B., Kurelec B., Uhlenbruck G., Schröder H. C. Role of the aggregation factor in the regulation of phosphoinositide metabolism in sponges. Possible consequences on calcium efflux and on mitogenesis. J Biol Chem. 1987 Jul 15;262(20):9850–9858. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Kurelec B., Müller I., Uhlenbruck G., Vaith P. Aggregation of sponge cells. A novel mechanism of controlled intercellular adhesion, basing on the interrelation between glycosyltransferases and glycosidases. J Biol Chem. 1979 Feb 25;254(4):1280–1287. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K. Metabolism of 1-beta-D-arabinofuranosyluracil in mouse L5178Y cells. Cancer Res. 1979 Mar;39(3):1102–1107. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Müller I., Kurelec B., Uhlenbruck G., Vaith P. Cell aggregation of the marine sponge Geodia cydonium. Identification of lectin-producing cells. Eur J Cell Biol. 1981 Apr;24(1):28–35. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K. Purification and characterization of a species-specific aggregation factor in sponges. Exp Cell Res. 1973 Jul;80(1):95–104. doi: 10.1016/0014-4827(73)90279-6. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Sahyoun N., Wolf M., Besterman J., Hsieh T., Sander M., LeVine H., 3rd, Chang K. J., Cuatrecasas P. Protein kinase C phosphorylates topoisomerase II: topoisomerase activation and its possible role in phorbol ester-induced differentiation of HL-60 cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1603–1607. doi: 10.1073/pnas.83.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H. C., Bachmann M., Diehl-Seifert B., Müller W. E. Transport of mRNA from nucleus to cytoplasm. Prog Nucleic Acid Res Mol Biol. 1987;34:89–142. doi: 10.1016/s0079-6603(08)60494-8. [DOI] [PubMed] [Google Scholar]
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Wells W. W. Solubilization and reconstitution of a nuclear envelope-associated ATPase. Synergistic activation by RNA and polyphosphoinositides. J Biol Chem. 1984 Oct 10;259(19):11890–11894. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillyan J. M., Kulkarni R. K., Byus C. V. Tumor-promoting phorbol esters stimulate the phosphorylation of ribosomal protein S6 in quiescent Reuber H35 hepatoma cells. J Biol Chem. 1984 Jan 25;259(2):897–902. [PubMed] [Google Scholar]
- Udvardy A., Schedl P., Sander M., Hsieh T. S. Novel partitioning of DNA cleavage sites for Drosophila topoisomerase II. Cell. 1985 Apr;40(4):933–941. doi: 10.1016/0092-8674(85)90353-8. [DOI] [PubMed] [Google Scholar]
- Weigele M., DeBernardo S., Leimgruber W. Fluorometric assay of secondary amino acids. Biochem Biophys Res Commun. 1973 Jan 23;50(2):352–356. doi: 10.1016/0006-291x(73)90847-4. [DOI] [PubMed] [Google Scholar]
- Weinbaum G., Burger M. M. Two component system for surface guided reassociation of animal cells. Nature. 1973 Aug 24;244(5417):510–512. doi: 10.1038/244510a0. [DOI] [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]