Abstract
Scope
Enhancing the formation and function of brown adipose tissue (BAT) increases thermogenesis and hence reduces obesity. Thus, we investigate the effects of resveratrol (Resv) on brown adipocyte formation and function in mouse interscapular BAT (iBAT).
Methods and results
CD1 mice and stromal vascular cells (SVCs) isolated from iBAT were treated with Resv. Expression of brown adipogenic and thermogenic markers, and involvement of AMP-activated protein kinase (AMPK)α1 were assessed. In vivo, Resv enhanced expression of brown adipogenic markers, PR domain-containing 16 (PRDM16) and thermogenic genes, uncoupling protein 1 (UCP1) and cytochrome C in iBAT, along with smaller lipid droplets, elevated AMPKα activity and increased oxygen consumption. Meanwhile, Resv promoted expression of PRDM16, UCP1, PGC1α, cytochrome C and pyruvate dehydrogenase (PDH) in differentiated iBAT SVCs, suggesting that Resv enhanced brown adipocyte formation and function in vitro. In addition, Resv stimulated AMPKα and oxygen consumption in differentiated iBAT SVCs. However, the promotional effects of Resv were diminished by AMPK inhibition or AMPKα1 knockout, implying the involvement of AMPKα1 in this process.
Conclusion
Resv enhanced brown adipocyte formation and thermogenic function in mouse iBAT by promoting the expression of brown adipogenic markers via activating AMPKα1, which contributed to the anti-obesity effects of Resv.
Keywords: AMP-activated protein kinase (AMPK) α1, brown adipocyte formation and function, obesity resveratrol, stromal vascular cells (SVCs), uncoupling protein 1 (UCP1)
Introduction
The global epidemic of obesity is increasingly viewed as a serious public health challenge and a major risk factor for the development of metabolic syndromes such as type 2 diabetes mellitus, dyslipidemia, and cardiovascular disease [1]. In mammals, brown adipose tissue (BAT) is the major tissue of non-shivering thermogenesis, dissipating energy as heat due to its high mitochondrial content and rich in uncoupling protein 1 (UCP1) [2, 3]. Although BAT has long been believed to be absent or negligible in adult humans, recent studies show that adult humans have substantial amounts of functional BAT [4, 5]. The amount of BAT is negatively correlated with obesity in adults [6]. Thus, enhancement of BAT formation and function is a useful strategy to prevent obesity and metabolic disease [7, 8].
While there are many transcriptional regulators affecting brown adipocyte formation and function [9–11], PR domain-containing 16 (PRDM16) is an indispensable transcriptional factor specifying brown adipogenic program [12, 13]. The expression of PRDM16 leads to the activation of mitochondrial biogenesis and the expression of genes associated with BAT function, including UCP1, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), pyruvate dehydrogenase (PDH), and cytochrome c [14]. Hormones and cytokines including T3, norepinephrine, bone morphogenetic protein 7 (BMP7), fibroblast growth factor 21 (FGF21), and natriuretic peptides (NP) can regulate brown adipogenesis through altering PRDM16 expression [10, 15]. Furthermore, certain dietary compounds also regulate BAT formation and function, which may reduce obesity [16–18].
Dietary supplementation of resveratrol (Resv), a natural polyphenol present in grapes, red wine, peanuts and some berries, has been shown to exert anti-obesity effects [19]. Resv is able to protect against high-fat diet induced obesity in mice [20, 21], to decrease fat mass in pigs [22, 23] and to reduce body weight and adipocyte size in obese persons [24, 25]. The anti-obesity property of Resv may stem from its effects on inhibiting adipogenesis or lipogenesis [26], promoting lipolysis [27, 28], and inducing browning of white adipose tissue (WAT) [29]. Resv-induced browning of WAT upregulates UCP1 and elevates thermogenesis [11, 29]. In addition, Resv increases UCP1 protein level in BAT and skeletal muscle of rat fed an obesogenic diet [30], consistent with a recent report showing that Resv promotes BAT thermogenic gene expression and energy metabolism in mice fed a standard diet [31]. However, the effects of Resv on brown adipocyte formation and function remain largely untested.
A number of studies have demonstrated that AMP-activated protein kinase (AMPK), a master regulator of energy metabolism [32, 33], is involved in adipose tissue metabolism [21, 34, 35]. More recently, our study demonstrated that Resv induces browning of WAT through activation of AMPKα1 [29], a predominant isoform expressed and accounted for the majority of the total AMPK activity of in adipose tissue [36–38]. However, it is unclear whether AMPK participates in Resv-mediated regulation of brown adipocyte formation and function.
Thus, the objective of the present study is to elucidate the role of Resv in regulating brown adipocyte formation and function and to explore the underlying mechanism. Our data show that Resv enhances brown adipocyte formation and function through activation of AMPKα1.
Materials and methods
Animals and in vivo study
Twelve 5-month-old CD1 female mice were randomly divided into control and Resv groups. The mice in control group were fed a high-fat diet (HFD; 45% energy from fat, D12451, Research Diet, New Brunswick, NJ), while the mice in Resv group were fed a HFD containing 0.1% (w/w) Resv (Resveratrol 500, purchased from SWANSON ULTRA® and each capsule contains 500 mg resveratrol). Mice were housed in an environmentally controlled room on a 12-h light-dark cycle with free access to food and water. The body weight and food intake were recorded, and metabolic efficiency was subsequently calculated. After 4 weeks of treatments, mice were euthanized by carbon dioxide anesthesia. Interscapular BAT (iBAT) was rapidly isolated and weighed. Left side of the iBAT was frozen in liquid nitrogen and stored at −80°C until further analyses. A portion of the right side was fixed in 4% paraformaldehyde for sectioning and staining. Another portion of the right side was used for tissue oxygen consumption measurement. All animal experiments and care procedures were performed according to protocols pre-approved by the Institutional Animal Care and Use Committees (IACUC) at Washington State University.
Stromal vascular cell (SVC) isolation and in vitro differentiation
SVCs were isolated from iBAT as previously described [39]. Briefly, iBAT were dissected from weaned wild type or RosaCre/AMPKα1flox/flox C57BL/6 mice obtained by crossing Rosa-Cre mice (Stock No: 004847, Jackson Lab, Bar Harbor, Maine) with AMPKa1 flox/flox (Stock no: 014141, Jackson Lab) [40]), washed with phosphate-buffered saline (PBS), minced and digested in DMEM/F12 (Life Technologies) containing collagenase D (0.75 U/ml), dispase II (1 U/ml) (Roche Diagnostics), and 10 mM CaCl2 at 37 °C for 30~40 min with frequent shaking. Digesta was centrifuged at 400 g for 5 min and the pellet was re-suspended in DMEM/F12 containing 10% fetal bovine serum (FBS) (Life Technologies), and then filtered through a 40 µm nylon strainer. The filtrate was centrifuged at 400 g for 5 min, re-suspended in erythrocyte lysis buffer (NH4Cl 154 mM, KHCO3 10 mM, EDTA 0.1 mM, pH 7.4) and incubated at room temperature for 5 min. The resulting suspension was centrifuged at 400 g for 5 min again, re-suspended in DMEM/F12 containing 10% FBS, 1% penicillin-streptomycin solution and grown at 37 °C in 5% CO2. The medium was changed every other day [41].
For knockout of AMPKα1, the SVCs isolated from iBAT of weaning Rosa26Cre/AMPKα1flox/flox mice were treated with 250 nM 4-hydroxytamoxifen (4-OHT) for 2 days to induce acute deletion of AMPKα1 before being induced to undergo brown adipogenic differentiation [29]. Brown adipogenic differentiation of iBAT SVCs was induced as previously described [29]. For AMPK inhibitor experiment, the confluent iBAT SVCs were incubated in differentiated medium supplemented with 10 µM Resv (Sigma) and/or 1 µM Compound C (CC) for 7 days.
In vitro O2 consumption assay
In vitro O2 consumption of differentiated SVCs or iBAT was measured with Thermo Scientific Orion 3-Star Dissolved Oxygen meter and probe (Thermo Electron Corporation, Madison, WI) as previously described [29]. O2 consumption was calculated as the rate of decrease in dissolved oxygen (DO).
Oil-Red O staining
Lipid accumulation in differentiated iBAT SVCs was detected by Oil-Red O staining as we previously described [29].
H&E and immunostaining
H&E staining of iBAT and immunostaining of the differentiated iBAT SVCs and iBAT sections were conducted as previously described [29]. The anti-UCP1 polyclonal antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and used at a dilution of 1:200. Adipocyte diameters were measured by Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD).
Real-time quantitative PCR
The mRNA expression of genes was determined by real-time quantitative PCR as previously described [42]. Primer sequences (with their respective PCR fragment lengths) were shown in Table 1.
Table 1.
The primer sequences used for real-time quantitative PCR
| Gene | Forward (5’-3’) | Reverse (3’-5’) | Amplicon size (bp) |
Gene access number |
|---|---|---|---|---|
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 | NC_000083.6 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | 136 | NM_007702.2 |
| PGC1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC | 161 | XM_006503779.1 |
| PRDM16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG | 87 | NM_001291029.1 |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG | 123 | NM_009463.3 |
Western blot analysis
Western blot analysis was performed as previously described [29]. Primary antibodies used include UCP-1 (1:500, Santa Cruz Biotechnology), PRDM16 (1:1000, Millipore), Cyto C (1:1000, Cell Signaling), PDH (1:1000, Cell Signaling), AMPKα (1:1000, Cell Signaling), p-AMPKα at Thr172 (1:1000, Cell Signaling), or β-actin (1:1000, Cell Signaling). Band density was normalized to the β-actin content.
Statistical analysis
Data are presented as means ± standard error of the means (SEM). In the mouse feeding trial, individual animal was considered as an experimental unit and six mice in each group were used. For in vitro cell culture studies, three independent experiments were conducted with 3 parallel measurements in each experiment. Statistical analysis was performed using SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA). Differences between means were determined using Student’s t-test or one-way analysis of variance (ANOVA) followed by Duncan’s multiple test when appropriate and a confidence level of P < 0.05 was considered to be statistically significant.
RESULTS
Dietary resveratrol enhanced iBAT formation and function and activated AMPKα in mice fed HFD
In our previous study, we found that dietary supplementation of 0.1% Resv induced browning of inguinal subcutaneous WAT (iWAT) in mice fed HFD [29]. Here, we examined the effects of Resv on the formation and function of iBAT. The average daily food intake (ADFI) was comparable between control and 0.1% Resv mice (Fig. 1A). However, the average daily weight gain (ADWG) of 0.1% Resv group was significantly lower than that of control group (Fig. 1B). Thus, the metabolic efficiency (ADWG/ADFI) was remarkably decreased in Resv treated mice (Fig. 1C). Although the iBAT index (iBAT mass/body weight) was not significantly altered between 0.1% Resv and control mice (Fig. 1D), noticeable morphological changes in iBAT were observed in Resv treated mice as follows.
Fig. 1.
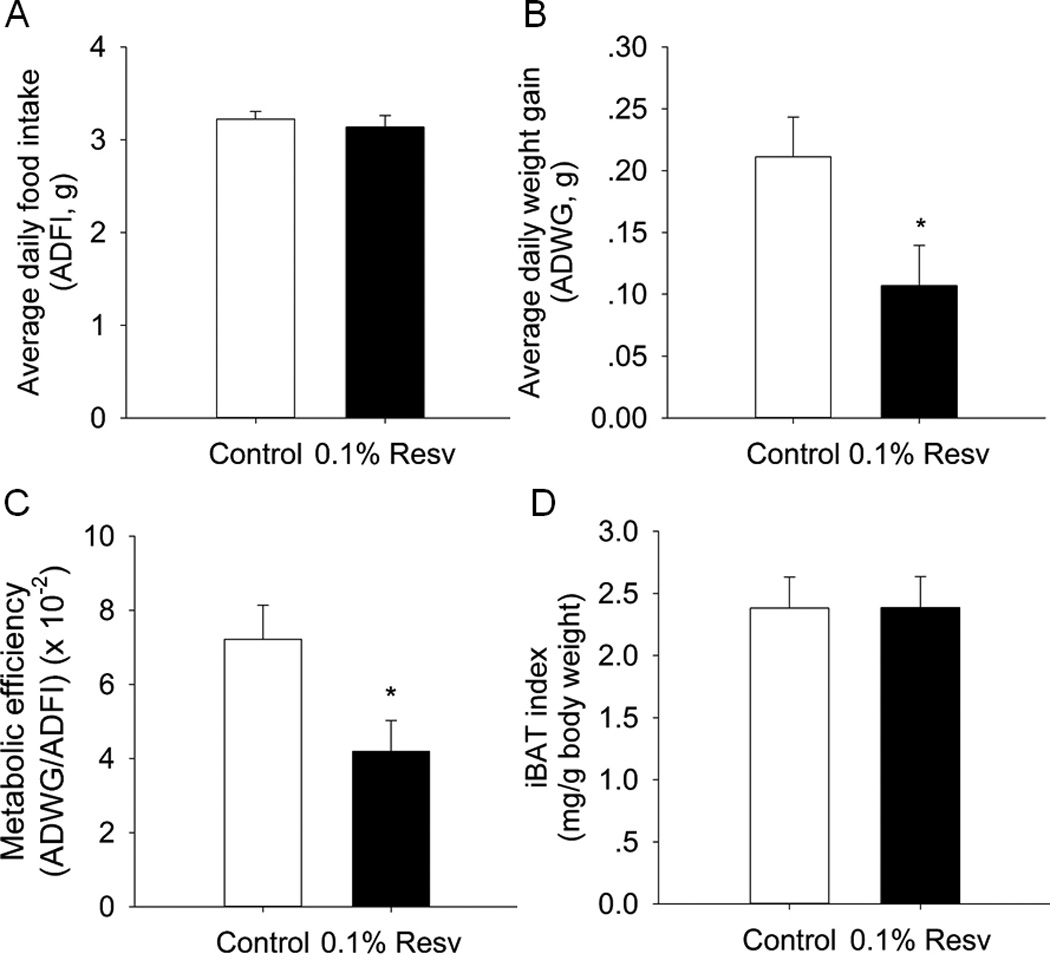
Effects of dietary 0.1% resveratrol on average daily food intake (ADFI, A), average daily weight gain (ADWG, B), metabolic efficiency (ADWG/ADFI, C), and iBAT index (D) in mice fed HFD. *P < 0.05 versus control.
Morphologically, there were smaller lipid droplets in the iBAT of Resv-treated mice, with the diameter of brown adipocytes lower than 15 µm. In contrast, the adipocyte diameter in the iBAT of control group mice was higher than 18 µm, with the biggest diameter of about 35 µm (see arrow in Fig. 2A). In addition, we analyzed the number of adipocytes in iBAT by counting nuclei in microscopic fields selected at random and found that the number of nuclei per field of 0.1% Resv group was significantly more than that of control group, implying that Resv enhanced the formation of brown adipocytes in vivo. Accordingly, Resv supplementation increased protein expression of PRDM16 (2.0-fold versus control, P < 0.001) (Fig. 2C, D), a master gene involved in brown adipocyte formation.
Fig. 2.
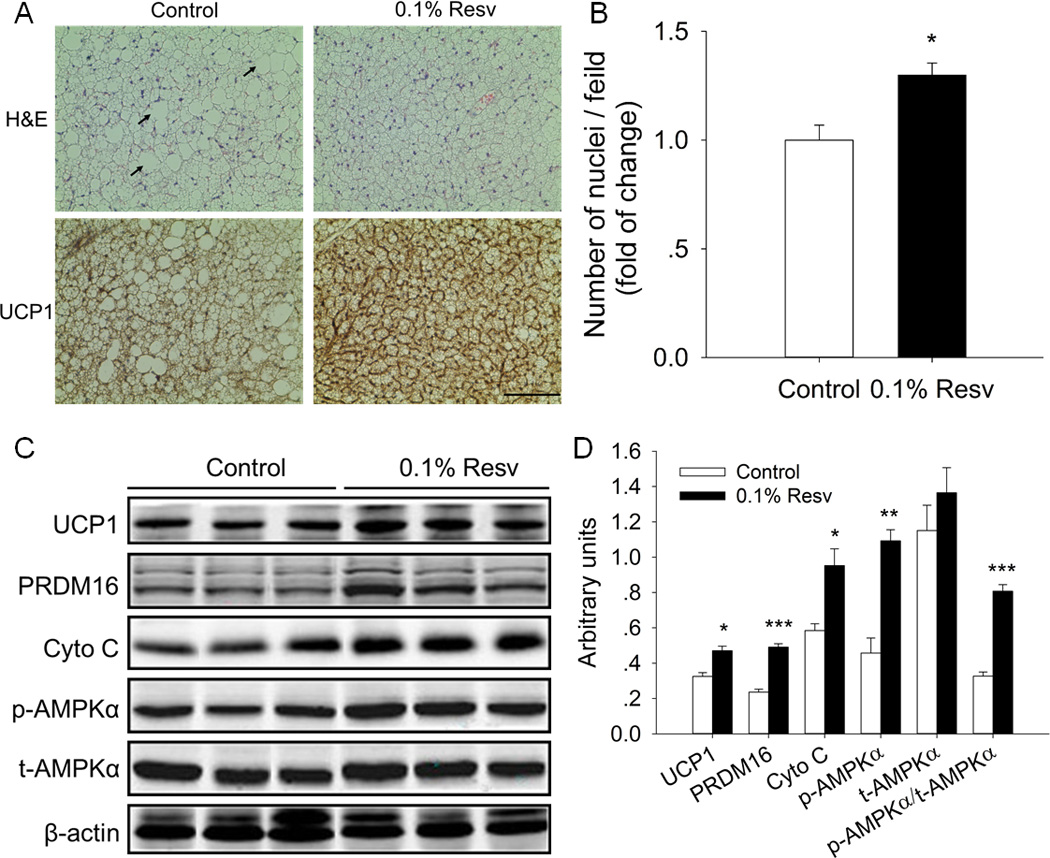
Dietary supplementation of resveratrol enhanced iBAT formation and function in mice fed HFD. A) Representative images of H&E and UCP1 IHC staining in sections of iBAT of control and 0.1% Resv treated mice (scale bar, 100 µm). Arrow indicates the large lipid droplets (diameter ~35 µm) in control group. B) The number of nuclei in microscopic fields selected at random from H&E stained sections were compared between control and 0.1% Resv groups. C) Western blot analyses of brown adipogenic marker gene (PRDM16), thermogenic genes (UCP-1, Cyto C), p-AMPKα and t-AMPKα, and β-actin was used as the loading control. D) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units. *P < 0.05, **P < 0.01 and *** P < 0.001 versus control.
We next compared the function of iBAT between control and Resv-treated mice by examining the expression of thermogenic genes. By immunohistochemical (IHC) staining of UCP1, we found a markedly increased UCP1 content in the iBAT of Resv treated mice (Fig. 2A). In agreement, the UCP1 protein content in iBAT of the 0.1% Resv group was 1.4-fold higher than that of the control group (P < 0.05) (Fig. 2C, D). Furthermore, Resv supplementation elevated the protein level of cytochrome C (Cyto C) (1.6-fold increase versus control, P < 0.05).
We have demonstrated that AMPKα mediates resveratrol-induced browning of iWAT [29]. Thus, we further examined whether AMPKα was activated in the iBAT by Resv. There was an elevated content of p-AMPKα (2.4-fold versus control, P < 0.01) and an increase in the ratio of p-AMPKα to t-AMPKα (p-AMPKα/t-AMPKα) (2.5-fold versus control, P < 0.001) in Resv-treated group (Fig. 2C, D), with no difference of total AMPKα (t-AMPKα). These data suggested that the enhanced formation and function of iBAT induced by dietary Resv was associated with AMPK activation in mice fed HFD.
Resveratrol exerted dose dependent effects on brown adipogenic differentiation of iBAT SVCs
To investigate whether Resv indeed promoted brown adipocyte formation and function in vitro, iBAT SVCs were induced brown adipogenic differentiation, with presence of various concentrations of Resv. Using Oil-Red O staining, we found that, at high concentrations (20 µM or 40 µM), Resv significantly (P < 0.001) inhibited lipid accumulation in the differentiated iBAT SVCs after 7-day brown adipogenic differentiation (Fig. 3A, B). Consistently, high concentrations of Resv significantly reduced the protein contents of adipogenic markers (PPARγ and aP2) (Fig. 3C, D). In contrast, at the concentration of 10 µM or lower, Resv had no effect on lipid accumulation and protein expression of PPARγ and aP2 (Fig. 3C, D). Thus, we used 10 µM Resv in the following in vitro experiments.
Fig. 3.
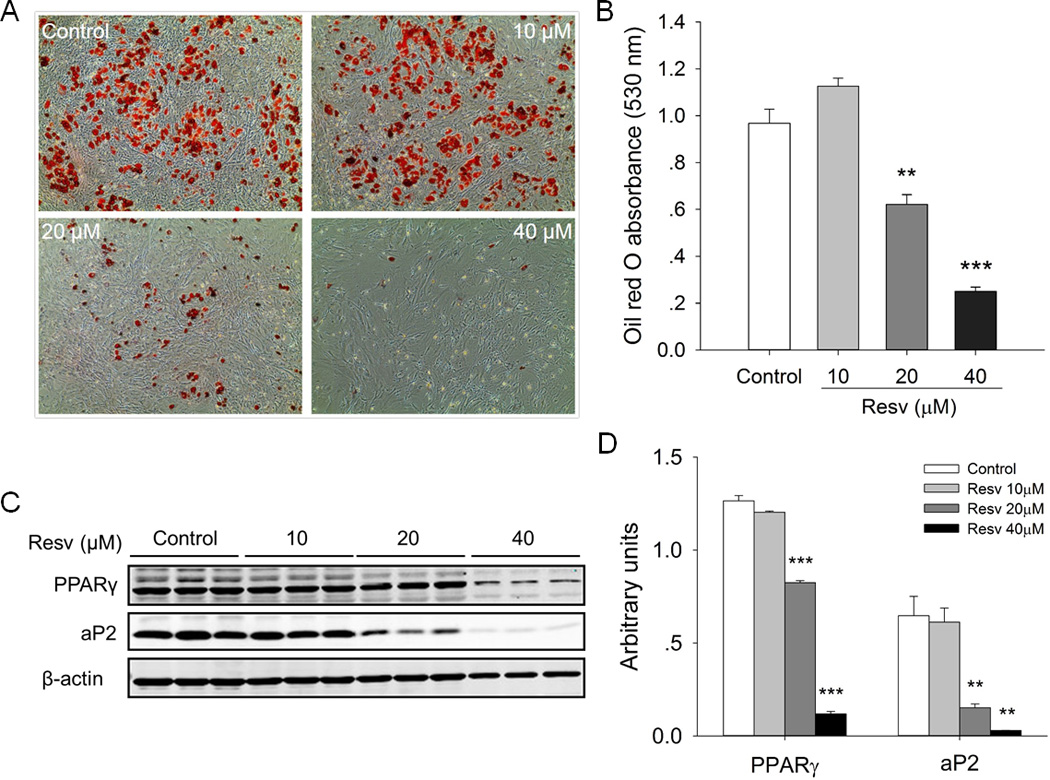
Effects of resveratrol on the lipid accumulation and the expression of adipogenic marker genes in iBAT SVCs after 7-day brown adipogenic differentiation. A) Oil-Red O staining of differentiated iBAT SVCs B) The absorbance of the extracted Oil-Red O measured spectrophotometrically at 530 nm. C) Western blot analysis of adipogenic marker genes (PPARγ and aP2) of differentiated iBAT SVCs, and β-actin was used as a loading control. D) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units. **P < 0.01 and *** P < 0.001 versus control.
Resveratrol enhanced formation and function of brown adipocytes in the differentiated iBAT SVCs
Although low concentrations of Resv had no effect on lipid accumulation during brown adipogenic differentiation of iBAT SVCs, we further determined the effects of Resv on the formation and function of brown adipocytes in vitro by examining the expression of brown adipogenic marker genes. As shown in Fig. 4A, 10 µM Resv significantly increased the mRNA level of PRDM16 (1.9-fold versus control, P<0.01) in differentiated iBAT SVCs of wild-type. In agreement, the protein level of PRDM16 was also markedly elevated in the Resv treatment group (1.6-fold versus control, P < 0.05) (Fig. 4C, D). In addition, the expression of Cidea, a gene predominantly expressed in brown adipocytes increased 6.4-fold (P < 0.01) in the Resv group. Furthermore, Resv stimulated the mRNA expression of PGC1α (2.1-fold, P < 0.01), the master regulator of mitochondrial biogenesis and oxidative phosphorylation.
Fig. 4.
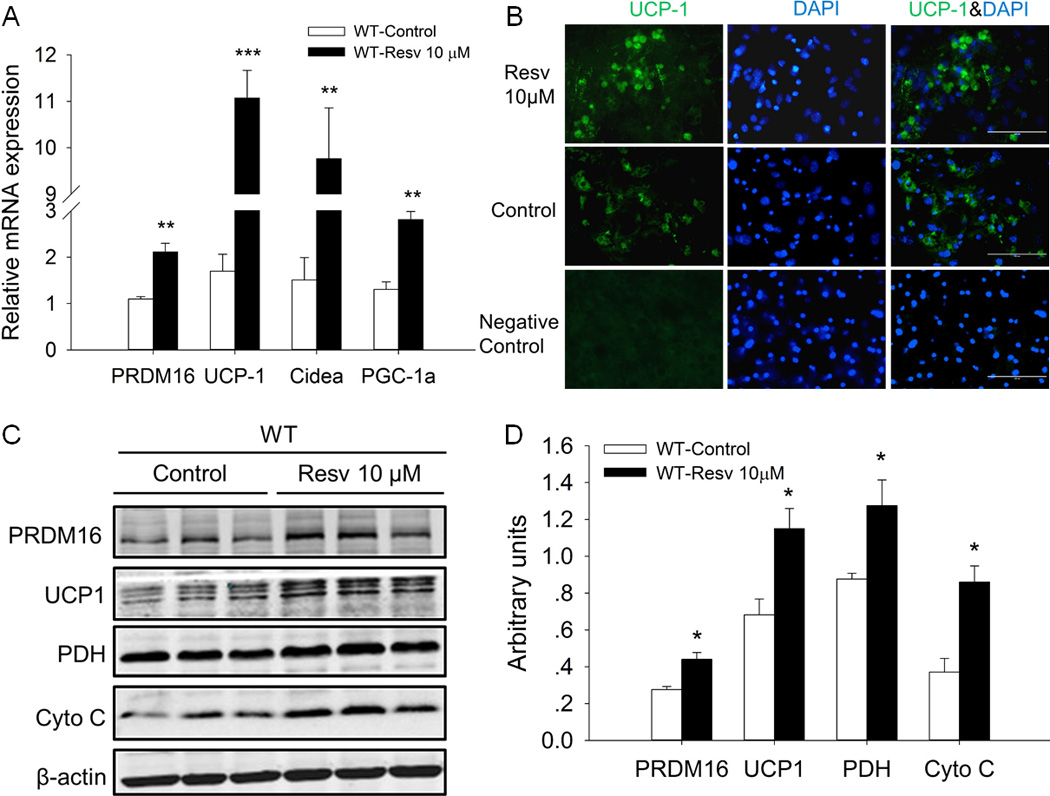
Resveratrol enhanced the expression of brown adipogenic marker genes in differentiated iBAT SVCs after 7-day brown adipogenic differentiation. A) Relative mRNA expression of PRDM16, UCP-1, Cidea and PGC-1a in differentiated iBAT SVCs of wild-type. B) UCP1 immunofluorescence staining of differentiated iBAT SVCs. Nuclei were stained with DAPI (scale bar, 100 µm). C) Western blot analysis of PRDM16UCP1, PDH and Cyto C of differentiated iBAT SVCs. β-actin was used as loading control. D) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units. *P < 0.05, **P < 0.01 and *** P < 0.001 versus control.
Mitochondrial UCP1 is the key regulator of thermogenesis in the BAT. In the present study, we found that the mRNA expression of UCP1 was remarkably boosted by Resv in differentiated iBAT SVCs (6.5-fold versus control, P < 0.001) (Fig. 4A). In addition, based on immunofluorescence staining, UCP1 expression was higher in the Resv-treated group than that in control group (Fig. 4B), which was confirmed by western blotting (UCP1 protein level was 1.7-fold higher in Resv-treated cells than that of control cells, P < 0.05) (Fig. 4C, D). Moreover, the protein levels of Cyto C (2.3-fold versus control, P < 0.05) and pyruvate dehydrogenase (PDH) (1.4-fold versus control, P < 0.05), which represent the mitochondrial content and metabolic flux, were also elevated by Resv treatment (Fig. 4C, D).
Resveratrol activated AMPKα in differentiated iBAT SVCs of the wild-type but not AMPKα1 knockout
In vivo, we found that AMPKα was activated by 0.1% Resv supplementation. We further examined whether AMPKα was activated by Resv in vitro. As shown in Fig. 5, Resv increased the p-AMPKα level (1.3-fold versus control, P < 0.05) and the ratio of p-AMPKα to t-AMPKα (1.4-fold versus control, P < 0.05) in differentiated iBAT SVCs of wild type, with no effect on t-AMPKα. To confirm which isoform of AMPK α-catalytic subunit participated in this process, the confluent iBAT SVCs isolated from Rosa26Cre/AMPKα1flox/flox mice were treated with 4-OHT to knockout AMPKα1 acutely before brown adipogenic differentiation. Following AMPKα1 knockout (KO), the expression of p-AMPKα and t-AMPKα in differentiated iBAT SVCs was much lower than in wild-type cells. Moreover, we found that Resv had no effect on the protein levels of p-AMPKα and t-AMPKα, and the p-AMPKα/t-AMPKα ratio was unalterated in differentiated iBAT SVCs with AMPKα1 KO (Fig. 5A, B).
Fig. 5.
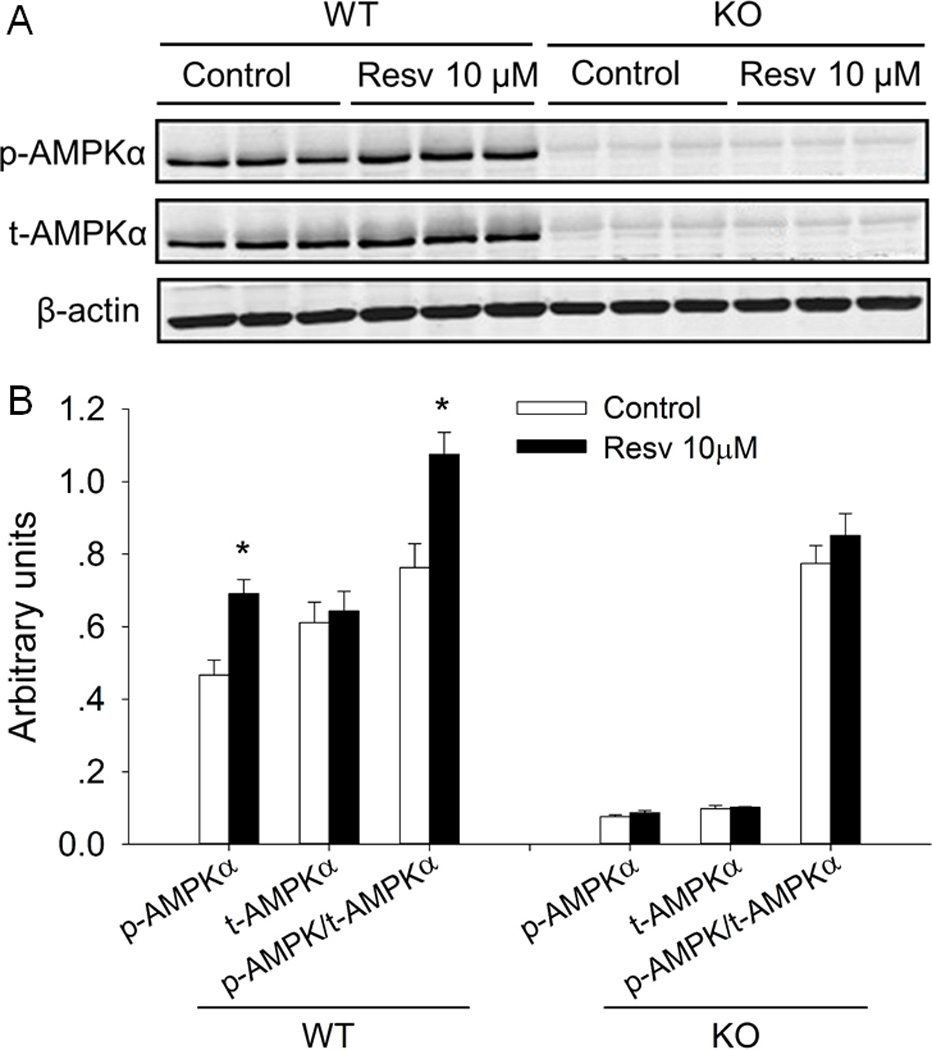
Resveratrol activated AMPKα in differentiated iBAT SVCs separated from the wild-type but not AMPKα1 conditional knockout mice. A) Western blot analysis of p-AMPKα, t-AMPKα of differentiated iBAT SVCs. β-actin was used as loading control. B) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units. *P < 0.05 versus control.
AMPK inhibition or AMPKα1 KO abolished the enhanced brown adipocyte formation and function induced by resveratrol in differentiated iBAT SVCs
We used Compound C (CC), an inhibitor of AMPK, to further examine the role of AMPK in regulating Resv-enhanced formation and function of brown adipocytes in differentiated iBAT SVCs. We found that CC (1µM) had no significant effect on the expression of brown adipogenic marker (PRDM16) and thermogenic genes (UCP1 and Cyto C). However, CC eliminated the stimulatory effects of Resv on the expression of PRDM16, UCP1, Cyto C and PDH (Fig. 6A, B). These findings suggested that AMPKα activation was involved in Resv-mediated enhancement of brown adipocyte formation and function in differentiated mouse iBAT SVCs.
Fig. 6.
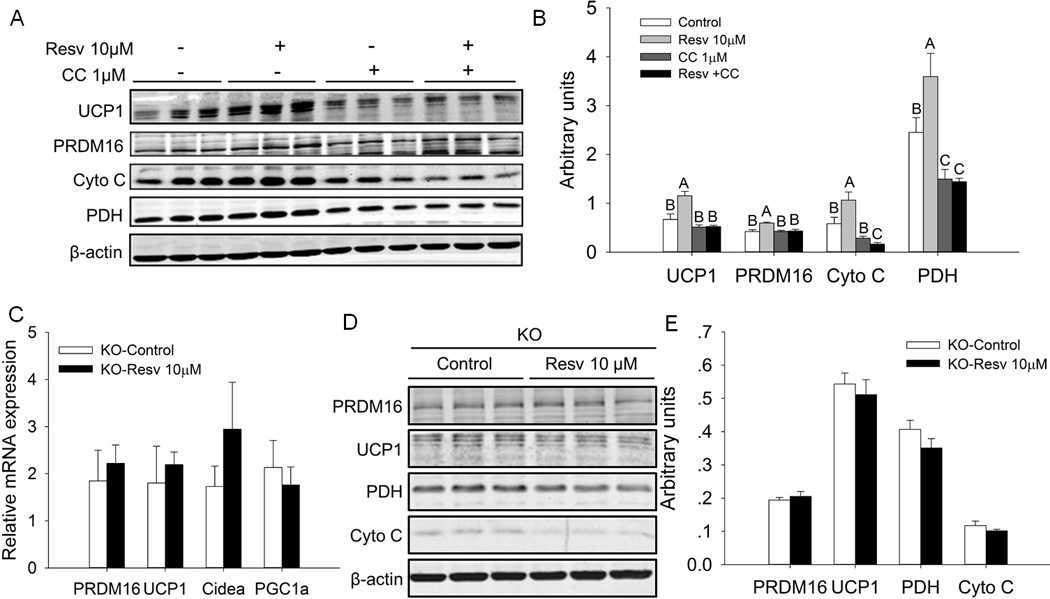
AMPK inhibition or AMPKα1 knockout (KO) eliminated resveratrol-enhanced expression of brown adipogenic marker genes in differentiated iBAT SVCs. A) Effects of resveratrol and AMPK inhibitor Compound C (CC) on the protein contents of PRDM16, UCP-1, Cyto C and PDH in differentiated iBAT SVCs. β-actin was used as loading control. B) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units. Bars that do not share the same letter are significantly different (P < 0.05). C) Relative mRNA expression of PRDM16, UCP-1, Cidea and PGC-1a in differentiated iBAT SVCs with AMPKα1 knockout. D) Western blot analysis of brown adipogenesis marker genes PRDM16, UCP1, PDH and Cyto C in differentiated iBAT SVCs of AMPKα1 KO. β-actin was used as loading control. D) Mean ± SEM of immunoblotting bands and the intensities of the bands were expressed as arbitrary units.
We further determined which isoform of AMPK α subunit was associated with the promotional action of Resv on brown adipocyte formation and function in differentiated iBAT SVCs through acute AMPKα1 KO. In the absence of AMPKα1, Resv had no effects on the mRNA expression of PRDM16, UCP1, Cidea and PGC1a (Fig. 6C). Consistently, after deletion of AMPKα1, the protein levels of PRDM16, UCP1, Cyto C and PDH in the Resv group did not differ from those in the control group (Figure 6D, E). These results further showed that AMPKα1 has a mediatory role in this process.
Resveratrol increased basal oxygen consumption of iBAT and differentiated iBAT SVCs
Previously, we found a significant increase of the oxygen consumption (VO2) in Resv treated mice [29]. Here, we further explored the effects of Resv on the basal oxygen consumption of iBAT and differentiated iBAT SVCs in vitro. As shown in Fig. 7A, Resv significantly increased the basal oxygen consumption of iBAT (1.4-fold versus control, P<0.01). Similarly, the basal oxygen consumption of differentiated iBAT SVCs in Resv group was 1.8-fold higher than that of control group (P<0.001) (Fig. 7B). In contrast, after AMPKα1 KO, the basal oxygen consumption of differentiated iBAT SVCs was not affected by Resv treatment. Aggregate, these results suggested that AMPKα1 had an essential role in mediating the stimulatory effect of Resv on oxygen consumption.
Fig. 7.
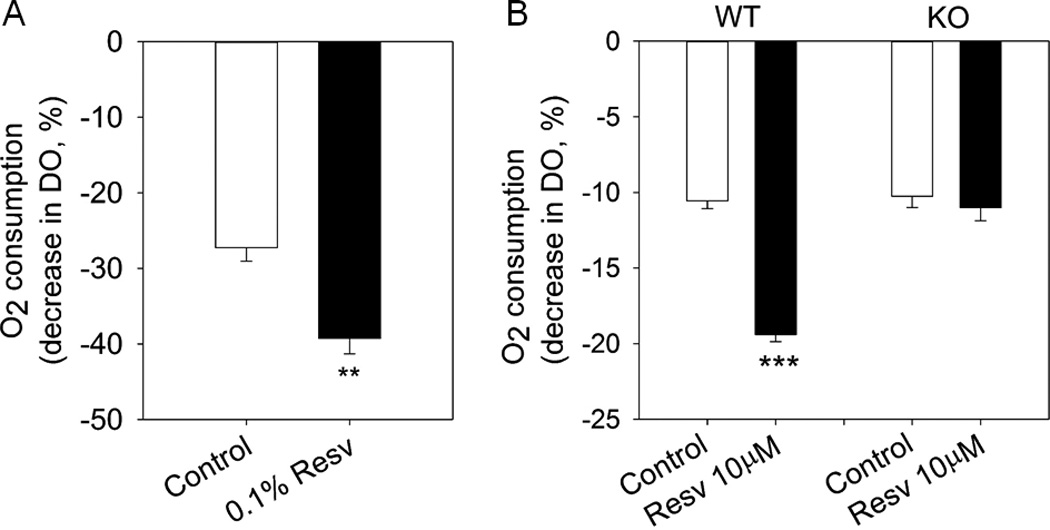
Basal O2 consumption of iBAT and differentiated iBAT SVCs. A) Basal O2 consumption of iBAT of control and 0.1% resveratrol treated mice measured as the decrease in dissolved oxygen (DO). B) Basal O2 consumption of differentiated iBAT SVCs of wild type and AMPKα1 knockout (KO) from control and resveratrol treated groups. **P < 0.01 and *** P < 0.001 versus control.
Discussion
Brown and beige adipose tissues have been identified as important therapeutic targets for combating diet-induced obesity and metabolic disease [43, 44]. In this paper, we investigated the effects of Resv on brown adipocyte formation and function in mouse iBAT and explored the underlying mechanism. Our results demonstrated that Resv enhanced the brown adipocyte formation and function by promoting the expression of brown adipogenic marker genes through the activation of AMPKα1. This study was an extension of our previous study [29], where we explored the role and mechanism of Resv in the formation of beige adipocytes (browning) in inguinal WAT (iWAT). It has been reported that brown and beige adipocytes have different developmental origins, with brown adipocytes derived from MYF5+ myogenic cells [45] while beige adipocytes are derived from PDGFRα+ cells [46, 47]. Thus, the possible effects of Resv on brown adipocyte formation and function need to be further examined.
The dose of Resv (0.1%, 1 g Resv per kg diet) used for in vivo study was selected based on the previous reports in rat [30, 48], mice [20, 31, 49], pigs [22] and humans [25, 50]. It has been shown that Resv protects against high-fat diet induced obesity in mice [20, 21]. In agreement with the previous findings, we found that Resv decreased the average daily weight gain compared with the control mice when challenged with an obesogenic diet. In the current study, although the iBAT index was not significantly altered between mice of control and 0.1% Resv group, obvious morphological changes of iBAT between these two groups were observed. The iBAT of Resv-treated mice contained considerably smaller lipid droplets than those seen in the iBAT of control mice. More importantly, there were more brown adipocytes in the iBAT of Resv-treated mice than control mice, implying that Resv could enhance the formation of brown adipocytes in vivo. And this was further confirmed by the upregulation of brown adipogenic marker gene PRDM16 by Resv supplementation.
The change of iBAT morphology might alter the function of brown adipocytes. Indeed, the expression of thermogenic genes such as UCP1 and Cyto C was higher in the iBAT of Resv treated mice, suggesting that Resv enhanced brown adipocyte thermogenesis and protected against obesity. In line with our results, it has been reported that thermogenesis was involved in the body-fat lowering effects of Resv [30, 31]. Alternatively, the anti-obesity effects of Resv might also result from its ability to inhibit adipogenesis, suppress lipogenesis, stimulate lipolysis or induce browning of WAT [29]. Together, our in vivo data strongly suggest that Resv enhances the formation and function of iBAT in mice fed HFD.
Subsequently, we further performed in vitro studies to address the effects of Resv on brown adipocyte formation and function in differentiated iBAT SVCs. High concentrations (20 or 40 µM) of Resv elicited inhibitory effects on lipid accumulation and the expression of adipogenic marker genes (PPARγ and aP2) during brown adipogenic differentiation of iBAT SVCs, reminiscent of previous reports on white adipogenesis [26], which could be due to cytotoxicity, because at concentrations higher than 20 µM, Resv had a significant cytotoxic effect on adipocytes such as 3T3-L1 and MEF-derived adipocytes [51, 52]. In addition, it was reported that the plasma Resv concentration was 1.56 ±0.28 µM in rat fed a HFD containing 4 g Resv per kg diet [48]. While in mice fed a HFD containing 0.4% Resv, the highest plasma Resv concentration was about 0.5 µM [20]. Thus, to exclude the possible cytotoxic effect of Resv and consider the serum level of Resv, we selected the relative low concentration (10 µM) to conduct in vitro experiments, which is closer to the physiological concentration [20, 48]. Resv indeed affected brown adipocyte formation and function by elevating the expression of brown adipogenic marker gene (PRDM16) and thermogenic genes such as UCP1, Cidea, PGC1α, PDH and Cyto C, with no influence on lipid content. These data strongly suggested that, in agreement with the in vivo findings, Resv could enhance brown adipocyte formation and function in differentiated mouse iBAT SVCs in vitro.
Enhanced brown adipocyte formation and function might be associated with increased oxygen consumption and heat production. In our study, we observed a significantly decreased metabolic efficiency in Resv-treated mice, which might be, at least in part, due to the elevated energy expenditure. In accordance, our previous study showed that Resv increased oxygen consumption (VO2) and heat production while decreased respiratory exchange ratio (RER) (CO2 production/O2 uptake) in mice [29]. To further confirm the role of iBAT in the increase of oxygen consumption of the whole body, we measured ex vivo oxygen consumption of iBAT and differentiated iBAT SVCs. We found that the oxygen consumption of tissue (iBAT) and cells (differentiated iBAT SVCs) in Resv treated group was significantly higher than those of control group. In aggregate, these data suggest that Resv promotes brown adipocyte function by increasing oxygen consumption and heat production, contributing to its anti-obesity effects.
Several studies have reported that Resv may elicit its anti-obesity effects in part through the activation of AMPK [48, 53], consistent with our observation of AMPK activation by Resv. In addition, our in vitro study also revealed that Resv elevated AMPKα activity in differentiated iBAT SVCs of wild type but not AMPKα1 knockout. Furthermore, AMPKα inhibition by Compound C completely reversed the stimulatory effects of Resv on the expression of brown adipogenic marker genes. These results showed that AMPKα was involved in Resv enhanced formation and function of brown adipocytes.
The predominant isoform of α catalytic subunit expressed in adipose tissue is α1 [36, 37] and Resv-induced increase of metabolic rate and decrease of fat mass require AMPKα1 but not AMPKα2 [21]. Thus, we speculated that AMPKα1 but not AMPKα2 participates in Resv enhanced formation and function of brown adipocyte. To verify, we isolated iBAT SVCs from Rosa26Cre/Ampkα1flox/flox mice, induced acute AMPKα1 deletion with 4-OHT, and then induced brown adipogenic differentiation. As expected, only trace amount of p-AMPKα and t-AMPKα was detected in SVCs after the acute knockout of AMPKα1, showing that α1 isoform accounts for most of the total activity of AMPKα in differentiated iBAT SVCs [37, 38]. More importantly, after knockout of AMPKα1, the promotional effects of Resv on the mRNA and/or protein expression of brown adipogenic marker genes were totally abolished. Furthermore, the increased oxygen consumption was absent in the differentiated iBAT SVCs with AMPKα1 knockout. Together, these findings showed that AMPKα1 mediated the Resv stimulated brown adipocyte formation and thermogenic function.
In conclusion, Resv enhanced brown adipocyte formation and function in mice by stimulating the expression of brown adipogenic marker genes and oxygen consumption, which appeared to be associated with AMPKα1 activation. These data provided new insights into the regulation of brown adipocyte formation and function by dietary polyphenols (Resv) and suggested the potential application of Resv as a nutritional intervention/therapeutic strategy for the prevention and treatment of obesity and related diseases.
Acknowledgments
This work was supported by grants from National Institutes of Health (R01HD067449 and R21AG049976), the National Natural Science Foundation of China (31372397 and 31672508) and the National Science Foundation (1147275). This activity was also funded, in part, with an Emerging Research Issues Internal Competitive Grant from the Agricultural Research Center at Washington State University, College of Agricultural, Human, and Natural Resource Sciences.
Abbreviations
- 4-OHT
4-hydroxytamoxifen
- AMPK
AMP-activated protein kinase
- aP2
adipocyte protein 2
- BAT
brown adipose tissue
- CC
Compound C
- Cidea
Cell death-inducing DFFA-like effector A
- Cre
cre recombinase
- Cyto C
cytochrome C
- H&E
hematoxylin and eosin
- HFD
high fat diet
- IHC
immunohistochemical
- iBAT
interscapular brown adipose tissue
- PDH
pyruvate dehydrogenase
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PPARγ
peroxisome proliferator-activated receptor γ
- PRDM16
PR domain-containing 16
- Resv
resveratrol
- SVCs
stromal vascular cells
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Footnotes
Author contributions
S.W. designed, performed experiments, analyzed data, and wrote the manuscript. X.L., Q.Y., and X.F. performed experiments. All authors contributed to discussion and review of the manuscript. M.D. designed the experiments, interpreted the data and edited the manuscript.
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1.Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Annals of nutrition & metabolism. 2015;66(Suppl 2):7–12. doi: 10.1159/000375143. [DOI] [PubMed] [Google Scholar]
- 2.Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes & metabolism journal. 2013;37:22–29. doi: 10.4093/dmj.2013.37.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vosselman MJ, van Marken Lichtenbelt WD, Schrauwen P. Energy dissipation in brown adipose tissue: from mice to men. Mol Cell Endocrinol. 2013;379:43–50. doi: 10.1016/j.mce.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virtanen KA, Lidell ME, Orava J, Heglind M, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Plutzky J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes & metabolism journal. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Frontiers in physiology. 2015;6:4. doi: 10.3389/fphys.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poekes L, Lanthier N, Leclercq Isabelle A. Brown adipose tissue: a potential target in the fight against obesity and the metabolic syndrome. Clinical Science. 2015;129:933–949. doi: 10.1042/CS20150339. [DOI] [PubMed] [Google Scholar]
- 9.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Jun H, McDermott JR. Formation and activation of thermogenic fat. Trends in genetics : TIG. 2015;31:232–238. doi: 10.1016/j.tig.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016 doi: 10.1038/nrm.2016.96. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva Claudio J, Vergnes L, Wang J, Drew Brian G, et al. Adipose Subtype-Selective Recruitment of TLE3 or Prdm16 by PPARγ Specifies Lipid Storage versus Thermogenic Gene Programs. Cell metabolism. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seale P, Kajimura S, Yang W, Chin S, et al. Transcriptional control of brown fat determination by PRDM16. Cell metabolism. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seale P. Transcriptional control of brown adipocyte development and thermogenesis. Int J Obes. 2010;34:S17–S22. doi: 10.1038/ijo.2010.178. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Jung YS, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Experimental & molecular medicine. 2014;46:e78. doi: 10.1038/emm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Moustaid-Moussa N, Chen L, Mo H, et al. Novel insights of dietary polyphenols and obesity. The Journal of nutritional biochemistry. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlin J, Evans BA, Dehvari N, Sato M, et al. Could burning fat start with a brite spark? Pharmacological and nutritional ways to promote thermogenesis. Mol Nutr Food Res. 2016;60:18–42. doi: 10.1002/mnfr.201500251. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends in Food Science & Technology. 2014;35:114–128. [Google Scholar]
- 19.Wang S, Zhu M-J, Du M. Prevention of obesity by dietary resveratrol: how strong is the evidence? Expert Review of Endocrinology & Metabolism. 2015;10:561–564. doi: 10.1586/17446651.2015.1096771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Um JH, Park SJ, Kang H, Yang S, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Luo J, Yu B, Chen J, Chen D. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J Funct Foods. 2015;14:590–595. [Google Scholar]
- 23.Zhang C, Luo J, Yu B, Zheng P, et al. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat science. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Mendez-del Villar M, Gonzalez-Ortiz M, Martinez-Abundis E, Perez-Rubio KG, Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metabolic syndrome and related disorders. 2014;12:497–501. doi: 10.1089/met.2014.0082. [DOI] [PubMed] [Google Scholar]
- 25.Konings E, Timmers S, Boekschoten MV, Goossens GH, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. International journal of obesity (2005) 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 26.Mitterberger MC, Zwerschke W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013;68:1356–1376. doi: 10.1093/gerona/glt019. [DOI] [PubMed] [Google Scholar]
- 27.Lasa A, Schweiger M, Kotzbeck P, Churruca I, et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. The Journal of nutritional biochemistry. 2012;23:379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Zorita S, Treguer K, Mercader J, Carpene C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. Journal of physiology and biochemistry. 2013;69:585–593. doi: 10.1007/s13105-012-0229-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Liang X, Yang Q, Fu X, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes (Lond) 2015;39:967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, et al. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food chemistry. 2013;141:1530–1535. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 31.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. European journal of nutrition. 2014;53:1503–1510. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 32.Lam YY, Peterson CM, Ravussin E, Resveratrol vs. calorie restriction: data from rodents to humans. Experimental gerontology. 2013;48:1018–1024. doi: 10.1016/j.exger.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Current opinion in cell biology. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond) 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 35.Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. The Journal of physiology. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daval M, Diot-Dupuy F, Bazin R, Hainault I, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. The Journal of biological chemistry. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 37.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochemical and biophysical research communications. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 38.Gaidhu MP, Ceddia RB. The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exercise and sport sciences reviews. 2011;39:102–108. doi: 10.1097/JES.0b013e31820ac03e. [DOI] [PubMed] [Google Scholar]
- 39.Aune UL, Ruiz L, Kajimura S. Isolation and differentiation of stromal vascular cells to beige/brite cells. Journal of visualized experiments : JoVE. 2013:50191. doi: 10.3791/50191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Liang X, Sun X, Zhang L, et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016;24:1–13. doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Zhou G, Shu G, Wang L, et al. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31:981–996. doi: 10.1159/000350116. [DOI] [PubMed] [Google Scholar]
- 42.Liang X, Yang Q, Fu X, Rogers CJ, et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. The Journal of physiology. 2016;594:4453–4466. doi: 10.1113/JP272123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kissig M, Shapira SN, Seale P. SnapShot: Brown and Beige Adipose Thermogenesis. Cell. 2016;166:258.e251–258.e251. doi: 10.1016/j.cell.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian S, Huang H, Tang Q. Brown and beige fat: the metabolic function, induction, and therapeutic potential. Frontiers of medicine. 2015;9:162–172. doi: 10.1007/s11684-015-0382-2. [DOI] [PubMed] [Google Scholar]
- 45.Seale P, Bjork B, Yang W, Kajimura S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 47.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 48.Higashida K, Kim SH, Jung SR, Asaka M, et al. Effects of Resveratrol and SIRT1 on PGC-1alpha Activity and Mitochondrial Biogenesis: A Reevaluation. PLoS biology. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Timmers S, Konings E, Bilet L, Houtkooper Riekelt H, et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell metabolism. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. The Journal of nutritional biochemistry. 2011;22:828–834. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytotherapy research : PTR. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 53.Price NL, Gomes AP, Ling AJ, Duarte FV, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell metabolism. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


