Abstract
In a variety of normal and pathological cell types, Rho-kinases I and II (ROCKI/II) play a pivotal role in the organization of the nonmuscle and smooth muscle cytoskeleton and adhesion plaques as well as in the regulation of transcription factors. Thus, ROCKI/II activity regulates cellular contraction, motility, morphology, polarity, cell division, and gene expression. Emerging evidence suggests that dysregulation of the Rho-ROCK pathways at different stages is linked to cardiovascular, metabolic, and neurodegenerative diseases as well as cancer. This review focuses on the current status of understanding the multiple functions of Rho-ROCK signaling pathways and various modes of regulation of Rho-ROCK activity, thereby orchestrating a concerted functional response.
Keywords: coiled-coil-containing protein kinase, drug target, RhoA, Rho-associated, Rho-kinase, ROCK
Introduction
A hallmark of small GTPases, including Rho proteins, is the ability to undergo conformational changes in response to alternate binding of GDP and GTP. They act as molecular switches in the cell by cycling between a GDP-bound inactive state and a GTP-bound active state (Wittinghofer and Vetter, 2011). With a few exceptions (Jaiswal et al., 2013b), the conformational state and localization of Rho GTPases is regulated by three kinds of interacting molecules, guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (Dvorsky and Ahmadian, 2004).
Rho GTPases, in their activated states, regulate cellular structures by controlling the dynamics of microfilaments and microtubules through the binding and activation of their specific downstream effector proteins (Bishop and Hall, 2000; Dvorsky and Ahmadian, 2004). For RhoA, two classes of effector molecules have been described so far. These are scaffold proteins such as Rhophilin, Rhotekin, Kinectin, and Diaphanous (Dia), as well as serine/threonine protein kinases like protein kinase C-related kinase (PRK1, also called PKNα), citron kinase, and the Rho-associated coiled-coil kinases I (ROCKI, also called ROKβ/p160ROCK) and II (ROCKII, also known as ROKα/Rho-kinase) (Amano et al., 2010; Zou and Teitelbaum, 2010; Rath and Olson, 2012). Although both isoforms are ubiquitously expressed, ROCKI expression is enriched in the lung, liver, spleen, kidneys, and testes, whereas ROCKII is more prominent in the brain and heart (Morgan-Fisher et al., 2013). Although ROCKI and ROCKII share many downstream targets, some functional differences have been reported, such as the inhibition of pressure overload-induced cardiac fibrosis in ROCKI null mice (Zhang et al., 2006) or the ability to bind myosin; the regulatory subunit of myosin phosphatase binds ROCKII but not ROCKI, yet both can regulate myosin phosphatase and regulatory light-chain phosphorylation (Wang et al., 2009). For convenience, we do not distinguish between the ROCK isoforms and generally refer to ROCK in this review.
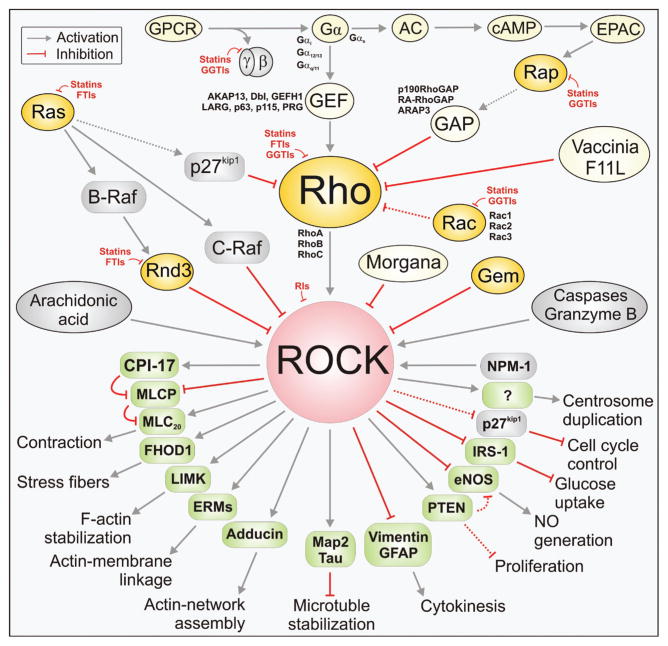
More than 8000 articles on Rho-ROCK are currently available in the PubMed database, including approximately 900 reviews. Here, we summarize the current knowledge about the crucial role of the Rho-ROCK pathways in human diseases and therapeutic strategies to interfere with their functions. In this review, we will point out various, in part reciprocal, control mechanisms orchestrating a concerted functional response as collectively shown in Figure 1.
Figure 1. Regulation, functions, and inhibition of the Rho-ROCK-controlled cellular processes.
Broad ranges of ROCK substrates are responsible for diverse cellular functions, which are controlled both positively and negatively by multiple mechanisms. As indicated, statin, GGTI, and FTI treatments as therapeutic strategies abrogate membrane localization of various proteins, including Rho, Rac, Ras, Rnd, and Gγ subunit, and thus interfere with the Rho-ROCK signaling in various types of cells and diseases. Solid arrows are known direct signal cascades, whereas dashed line arrows indicate the putative ones.
Functional repertoire and molecular pathways
Following activation by Rho, ROCK becomes a regulator, especially of cytoskeletal remodeling, for example, actin filament stabilization, assembly of the actin network and the actomyosin fibers, actin-membrane linkage, and microtubule dynamics through the phosphorylation of a number of downstream target proteins (Figure 1).
Smooth muscle and nonmuscle myosin II actomyosin ATPase activity and myosin cross-bridge cycling are regulated by Ca2+-calmodulin-activated myosin light-chain kinase (MLCK) phosphorylation of the regulatory myosin light chain (MLC20) and its dephosphorylation by myosin phosphatase (MLCP). At constant agonist-induced MLCK activity, the extent of MLC20 phosphorylation and contraction can be modulated through concomitant activation of RhoA/ROCK signaling, which regulates MLCP activity to Ca2+-sensitize or Ca2+-desensitize contraction (Somlyo and Somlyo, 2003). Indeed, one of the best investigated ROCK functions is the inhibitory phosphorylation of the myosin phosphatase-targeting subunit isoform 1 (MYPT1) at both Thr696 and Thr853 (Kimura et al., 1996; Somlyo and Somlyo, 2003; Loirand et al., 2006; Nunes et al., 2010; Walsh, 2011). This leads to an increase in MLC20 phosphorylation to promote contraction of smooth muscle and the formation of contractile actomyosin stress fibers in cultured cells (Somlyo and Somlyo, 2004). ROCK also inhibits MLCP activity by indirectly phosphorylating CPI-17, a potent inhibitor of the catalytic subunit of type 1 protein phosphatase (PP1c) that is not dependent on the phosphorylation of MYPT1 (Eto et al., 1995; Koyama et al., 2000). ROCK has been shown to directly phosphorylate MLC20 (Amano et al., 1996), but this contribution to total MLC20 phosphorylation in vivo is not clear.
ROCK controls the stabilization of actin filaments by phosphorylating LIM (LIN-11, ISL1, and MEC-3) kinases 1 and 2 at conserved threonines localized in their respective activation loops. LIM kinases phosphorylate cofilin, thereby inhibiting cofilin-mediated actin filament disassembly. Another ROCK substrate is adducin, a membrane skeletal phosphoprotein that associates with and promotes the association of spectrin with actin filaments, thereby increasing the contractile response (Kimura et al., 1998). The phosphorylation of formin homology domain protein 1 (FHOD1), a major endothelial formin leads to the formation of stress fibers (Takeya et al., 2008). ROCK activates ezrin/radixin/moesin proteins (ERMs) through phosphorylation in the actin-binding domain (Matsui et al., 1998), which in turn directly cross-link the actin cytoskeleton to the plasma membrane and allow the recruitment of multiple signaling proteins.
During cytokinesis, Rho and ROCK are involved in both the progression of the cleavage furrow formation and the disassembly of intermediate filaments such as vimentin and glial fibrillary acidic protein (GFAP) through the phosphorylation of their head domains, which ensures furrow completion (Goto et al., 1998; Yasui et al., 1998; Amano et al., 2010). Other ROCK substrates are the microtubule-associated proteins Tau and MAP2, which modulate microtubule structure and dynamics (Amano et al., 2010). By controlling these events, ROCK directly contributes to a number of cytoskeleton-mediated processes, including adhesion, contraction, polarity, cytokinesis, motility, permeability, phagocytosis, and neurite retraction (Somlyo and Somlyo, 2003; Tan et al., 2011; Tonges et al., 2011).
Further downstream effects of the Rho-ROCK pathway include the negative regulation of endothelial NO synthase (eNOS) and therefore the suppression of NO production in the endothelium, leading to an increase in vascular tone (Rikitake and Liao, 2005). ROCK directly phosphorylates eNOS at Thr495, thereby inhibiting its enzymatic activity (Sugimoto et al., 2007). In addition, Rho-ROCK signal transduction also regulates eNOS gene expression by affecting its mRNA stability (Eto et al., 2001). An indirect effect of ROCK on NO production is achieved by the negative regulation of the PI3K-Akt-eNOS-mediated signaling cascade. Here, phosphatase activity of phosphatase and tensin homologue (PTEN) is stimulated through phosphorylation (Li et al., 2005). Accumulated evidence also suggests that ROCK plays a pivotal role in the regulation of insulin- and PI3K-dependent translocation of glucose transporter 4 (GLUT4) to the plasma membrane, for example, in skeletal muscles (Lee et al., 2009). ROCK activation is essential for the normal action of insulin on glucose uptake, most likely due to ROCK-mediated phosphorylation and inhibition of insulin receptor substrate 1 (IRS-1) (Begum et al., 2002; Furukawa et al., 2005). A targeted disruption of ROCK causes insulin resistance in vivo (Lee et al., 2009).
In addition, Rho-ROCK signaling plays an important function in gene expression, cell cycle progression, proliferation, differentiation, and apoptosis (Olson, 2008; Fukasawa, 2011; Street and Bryan, 2011; David et al., 2012). ROCK regulates the level of the cell cycle regulatory proteins, e.g., by elevating cyclin D1 and reducing p27Kip1 protein levels (Croft and Olson, 2006). Another remarkable link of ROCK to cell cycle progression has been implicated by the interaction between ROCK and the multifaceted nucleolar phosphoprotein nucleophosmin (NPM-1). Following phosphorylation by cyclin-dependent kinase 2 (CDK2)/cyclin E, NPM-1 tightly associates with and activates ROCK, a critical event for the timely initiation of centrosome duplication and the coupling of centrosome duplication and DNA replication during S-phase (Ma et al., 2006; Hanashiro et al., 2011). Interestingly, Morgana (also called cysteine- and histidine-rich domain-containing protein 1), which is strongly downregulated in breast and lung cancer samples, directly binds ROCK in a complex with heat shock protein 90 (HSP90) and thereby inhibits centrosome duplication and tumorigenesis (Ferretti et al., 2010).
The selectivity of Rho/ROCK inhibition in human diseases
The strong interest in the Rho-ROCK pathway for drug targeting is based on the observation that the abnormal activation of this pathway plays a crucial role in numerous and diverse human diseases. These include tumor invasion, angiogenesis, and metastasis (Narumiya et al., 2009; Baranwal and Alahari, 2011; Mardilovich et al., 2012; Morgan-Fisher et al., 2013; Schofield and Bernard, 2013); cardiovascular disorders such as coronary vasospasm, cerebral cavernous malformation, hypertension, atherosclerosis, pulmonary hypertension, cardiac hypertrophy, and stroke (Shimokawa and Rashid, 2007; Olson, 2008; Nunes et al., 2010; Satoh et al., 2011; Shi et al., 2011; Zhou et al., 2011; Noma et al., 2012; Wang and Liao, 2012); insulin resistance, metabolic diseases, and diabetic nephropathy (Komers, 2011; Zhou and Li, 2012; Richardson et al., 2013); and neurodegenerative disorders (Mueller et al., 2005; Schmandke and Strittmatter, 2007; Salminen et al., 2008; Tonges et al., 2011). Advances in understanding the role of Rho-ROCK signaling in various human disorders originate from extensive experimental studies using predominantly different pharmacological inhibitors (Somlyo, 1997; Uehata et al., 1997; Olson, 2008; Hahmann and Schroeter, 2010; Miyamoto et al., 2010; Zhou and Liao, 2010; Tonges et al., 2011; Mardilovich et al., 2012) and also to some extent from other independent tools, including the use of bacterial toxins and dominant negative variants of Rho GTPases (Bishop and Hall, 2000). Moreover, ROCK inhibitors have been shown to be useful tools for cultivation of human embryonic stem cells and induced pluripotent stem cells (Rizzino, 2010; Ohgushi and Sasai, 2011).
The activation process of the Rho-ROCK pathway underlies several different regulatory mechanisms: (i) posttranslational lipid modification and translocation of Rho to the cellular membrane, (ii) receptor-dependent GEF-catalyzed GDP/GTP exchange of Rho, (iii) allosteric mode of ROCK activation upon direct association with Rho, and (iv) ATP-dependent phosphorylation of various ROCK substrates. Interference with any of the above processes has been proven to inhibit Rho-ROCK-stimulated cellular responses (Mardilovich et al., 2012). The most frequently used pharmacological inhibitors for the Rho-ROCK pathway can be categorized into three classes, including substances inhibiting ROCK [ROCK inhibitors (RIs)], geranylgeranyl transferase 1 (GGTase), or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (also known as statins). An issue of debate, however, is that in many studies, these compounds, especially statins and GGTase inhibitors (GGTIs), have been often regarded as ‘specific’ inhibitors of the Rho-ROCK activity, which is actually rather improper.
Y-27632 and fasudil (also known as HA-1077) are by far the most widely used ROCK inhibitors that target its ATP-binding site of the kinase domain and competitively inhibit phosphorylation of various substrates (Uehata et al., 1997; Somlyo, 1997; Suzuki et al., 1999; Mueller et al., 2005; Liao et al., 2007; Hahmann and Schroeter, 2010; Miyamoto et al., 2010; Mardilovich et al., 2012). A systematic in vitro analysis of Y-27632 and fasudil targets has revealed that they also inhibit PRK2/PKNγ, another Rho-regulated kinase, almost as potently as ROCK itself (Davies et al., 2000). Y-27632 had a minimal effect on other kinases in this screen of a large panel of protein kinases. However, at high concentrations, Y-27632 inhibits several other serine/threonine-specific protein kinases with wide cellular functions, including ERK2, GSK3β, JNK1α, p38α, PKA, PKBα, PKCα, and S6K1 (Davies et al., 2000). Therefore, the judicious use of low concentrations of Y-27632 and fasudil as well as the evaluation of the activity of these possible off-target kinases is important. Of note, fasudil is known as a prodrug, which has to be metabolized to hydroxyfasudil in vivo that then displays a more potent inhibitory effect than its precursor fasudil (Rikitake et al., 2005).
Subcellular localization of Rho proteins to different cellular membranes, which is known to be critical for their biological activity, is achieved by posttranslational modifications at a distinct cysteine residue in the C-terminal CAAX motif (C is cysteine, A is any aliphatic amino acid, and X is any amino acid) (Roskoski, 2003; Roberts et al., 2008). Thus, Rho proteins serve as substrates for isoprenyl-transferring enzymes, such as GGTase 1 and in a few cases also farnesyl transferase (FTase). A covalent and irreversible attachment of a 20-carbon geranylgeranyl or a 15-carbon farnesyl moiety by these enzymes to the cysteine residue of the CAAX motif, which is present in more than 100 proteins, is necessary for eukaryotic cell growth, differentiation, and morphology (Lane and Beese, 2006). These two lipids are synthesized from the activated cholesterol precursors, farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP) by the mevalonate pathway. Two post-prenylation enzymatic steps are critical for proper localization, including proteolytic cleavage of the AAX residues by the protease Rce1 and methylation of the terminal isoprenylcysteine by the methyltransferase ICMT (Winter-Vann and Casey, 2005).
Geranylgeranylation is required for Rho protein functions and is thus a prerequisite for their involvement in pathogenesis of human diseases. This prompted the development of potential GGTase 1 inhibitors (GGTIs) (Gelb et al., 2006; Triola et al., 2012). By preventing geranylgeranylation, GGTIs are able to inhibit proliferation and induce apoptosis in various biological systems (Sebti and Hamilton, 2000a; Khwaja et al., 2006) and to interfere with the progression of atherosclerosis via the inhibition of plaque angiogenesis (Park et al., 2006). GGTIs have been shown to block subcellular localization and consequently the signaling function of several Rho proteins, including RhoA, Cdc42, and Rac1 (Sebti and Hamilton, 2000b; Joyce and Cox, 2003; Khan et al., 2011). Importantly, the fact that a range of geranylgeranylated proteins besides Rho proteins, for example, the γ-subunits of heterotrimeric G proteins represent substrates of GGTase 1 (Marrari et al., 2007), scale down the selectivity spectrum of GGTIs (Konstantinopoulos et al., 2007).
Major recent efforts focused on blocking prenylation of Ras oncogenes have been first directed to the development of FTase inhibitors (FIs) (Blum et al., 2008; Mardilovich et al., 2012). By blocking the FTase activity, Ras was instead geranylgeranylated. This stimulated the development of dual-targeting FTase and GGTase 1 inhibitors, such as AZD3409, which has been shown to inhibit farnesylation to a higher extent than geranylgeranylation (Appels et al., 2011). However, the inhibition of both farnesylation and geranylgeranylation could not be correlated with the antiproliferative activity of this drug (Appels et al., 2011).
In addition, it was recently shown that protein geranylgeranylation is required for the dimerization and activation of the epidermal growth factor receptor, a key player in a signaling pathway, whose activity is upregulated in more than 30% of human cancers (Zhao et al., 2010). Another example is geranylgeranylation of Rab27B, which has been shown to be required for breast cancer growth, invasion, and metastasis (Hendrix et al., 2010). Thus, geranylgeranyl pyrophosphate (GGPP) synthase has emerged as a new therapeutic target that has been suggested to provide another platform for interfering with protein prenylation in cells (Wiemer et al., 2011). Cellular GG-PP depletion, by inhibiting this enzyme, affects all geranylgeranylated proteins, including also the regulatory functions of Rab proteins in vesicle trafficking (Stenmark, 2009). Rab GTPases consisting of at least 60 different family members are modified by GGTase 2, which recognizes C-terminal CCXX, CC, or CXC motifs (Brunsveld et al., 2006; Itzen and Goody, 2011). It will be interesting to see whether GG-PP synthase inhibitors will develop also into widely used drugs comparable to the HMG-CoA reductase inhibitors (statins).
Statins are clinically approved for the treatment of hypercholesterolemia (Faiz et al., 2012; Raper et al., 2012). However, increasing clinical and experimental evidence demonstrates a variety of beneficial effects beyond the reduction of serum cholesterol (Lopez-Pedrera et al., 2012). These cholesterol-independent ‘pleiotropic’ effects are mediated by the depletion of crucial isoprenoid intermediates of the cholesterol biosynthetic pathway, namely FPP and GGPP. Statin treatment has been shown to abrogate membrane localization of small GTPases and interfere with, among others, Rho-ROCK signaling in various types of cells and diseases, including cancer (Riganti et al., 2008; Wiemer et al., 2009; Roy et al., 2011; Mardilovich et al., 2012), diabetes (Zhou and Li, 2011), cardiovascular disease (Reddy et al., 2005; Nakamura et al., 2006; Zhou and Liao, 2009; Zhou et al., 2011; Lopez-Pedrera et al., 2012), endothelial dysfunction (Tesfamariam, 2006; Noma et al., 2012), pulmonary hypertension (Oka et al., 2008; Antoniu, 2012), heart failure and ischemic stroke (Sawada and Liao, 2009; Miyamoto et al., 2010), bronchial asthma (Chiba et al., 2010), Alzheimer disease (Tang, 2005), and kidney disease (Fried, 2008). To what extent Rho-ROCK activity is inhibited in these respective patients upon statin therapy is unclear. It is a fact that inhibition of HMG-CoA reductase and consequently the depletion of GGPP and FPP may conceivably compromise diverse cellular functions and processes controlled by the families of Ras, Rho, and Rab GTPases; the γ subunits of the heterotrimeric G proteins involved in signaling by G-protein-coupled receptors (GPCRs); and many other prenylated proteins (Figure 1).
It is important to note that targeting protein prenylation in human diseases, despite the large number of different prenylated proteins, has reached an immense area of applications, ranging from cancer and cardiovascular diseases to also very rare genetic disorders, for example, Hutchinson-Gilford progeria syndrome (Resh, 2012; Young et al., 2013). The usefulness of such a clinical approach remains in many cases a matter of debate. In this context, it is important to note that statins have negative side effects on mood states, including depression, anxiety, anger, hostility, fatigue, confusion, skeletal muscle pain, and vigor (While and Keen, 2012).
Control mechanisms regulating the activity of the Rho-ROCK pathway
As discussed above, the Rho-ROCK pathway is involved in multiple biochemical and pathobiochemical processes, and not surprisingly, Rho and ROCK proteins are subjected to several regulatory mechanisms that influence their activation, thereby controlling the kinase activity of ROCK. The first step is a proper subcellular localization of these proteins that is highly dependent on the cell type and consequently on the controlled process, ranging from changes in contractility, permeability, motility, proliferation, to apoptosis.
The activity of Rho proteins is controlled through the activation of various cell surface receptors, including tyrosine kinase receptors, GPCRs, and cell-cell and cell-matrix adhesion molecules, such as cadherins and integrins (Wettschureck and Offermanns, 2002; Iden and Collard, 2008; Tybulewicz and Henderson, 2009; Zou and Teitelbaum, 2010; Litosch, 2011; Raptis et al., 2011; Momotani and Somlyo, 2012). Receptor signaling recruits and activates a large variety of the Dbl family proteins (so-called RhoGEFs), which have been recently classified on the basis of their selectivity for different Rho proteins as substrate into distinct subfamilies (Jaiswal et al., 2013a). In this regard, multiple Rho-selective members of the Dbl family have been reported to specifically link G-protein-coupled signals to Rho activation, including AKAP13/Lbc, Dbl, GEFH1/Lfc, LARG, p63, p115, and PRG (Jin and Exton, 2000; Diviani et al., 2001; Loirand et al., 2006; Vanni et al., 2007; Wirth et al., 2008; Meiri et al., 2009; Momotani et al., 2011; Momotani and Somlyo, 2012; Mikelis et al., 2013; Takefuji et al., 2013). Dbl proteins catalyze the GDP/GTP exchange of the three Rho isoforms, RhoA, RhoB, and RhoC, and therefore act as positive regulators (Jaiswal et al., 2011, 2013a; Rossman et al., 2005).
The interaction of the active (GTP-bound) Rho isoforms with a large and functionally diverse number of effectors is the basis for signal transduction into different pathways (Bishop and Hall, 2000; Karnoub et al., 2004). It is very likely that different RhoGEFs contribute to directing Rho signaling to these different pathways (Wirth et al., 2008; Momotani et al., 2011). In addition to the competition of multiple effectors in binding to a single GTPase, the utilization of multiple contact sites adds another level of complexity toward comprehending the molecular mechanisms underlying RhoA-mediated effector activation (Blumenstein and Ahmadian, 2004). The most common mechanism of effector activation by RhoA appears to be the disruption of intramolecular autoinhibitory interactions to release functional domains within the effector protein. The activity of the kinase domain of ROCK, for example, has been proposed to be autoinhibited by a segment at the C-terminus of ROCK, encompassing a Rho-binding domain (RBD) (Dvorsky et al., 2004) and a split PH domain that is bisected by a cysteine-rich C1 domain (PHn-C1-PHc) (Amano et al., 1999). Under resting conditions, inactive ROCK may exist in a tetrameric state (Chen et al., 2002; Doran et al., 2004). Activated RhoA has been shown to bind to three different domains in the central coiled-coil region of ROCK, such as the RBD, the Rho-interacting domain (RID), and the homology region 1 (HR1) (Blumenstein and Ahmadian, 2004). Therefore, it has been proposed that Rho-mediated activation of ROCK may operate through an allosteric binding mechanism. Accordingly, Rho might successively associate with RBD, RID, and HR1, inducing a conformational change that displaces the autoinhibitory C-terminus, generating ROCK dimers. The activity of the released kinase domain is most probably further potentiated via transphosphorylation and other components, including arachidonic acid binding and lipid membrane binding via the unconventional C-terminal PHn-C1-PHc (Somlyo and Somlyo, 2000; Riento and Ridley, 2003; Wen et al., 2008; Morgan-Fisher et al., 2013). ROCK is a substrate of proteases, such as granzyme B or caspases 3 and 8, which cleave off the PH domain and generate a constitutively active ROCK (Morgan-Fisher et al., 2013). ROCK cleavage by caspase-3 during apoptosis generates a truncated active form and induces MLC phosphorylation and apoptotic membrane blebbing (Coleman et al., 2001). Caspase-8-mediated ROCK cleavage leads to the remodeling of the actin cytoskeleton, resulting in an amoeboid-shaped cell associated with cell migration and in enhanced invasiveness of tumor cells in response to constitutively active PI3K signaling, which regulates cell proliferation, growth and mobility, and signaling through the cell death ligands, TRAIL and CD95L (Ehrenschwender et al., 2010).
In contrast to activating upstream regulators, there are only a few signaling pathways known that negatively control Rho-ROCK-mediated cellular processes through the activation of inactivating regulatory molecules, including the Rho GTPase-activating proteins (RhoGAPs) (Ligeti et al., 2012). p190RhoGAP, for example, antagonizes the Rho-ROCK-mediated regulation of actomyosin contractility by stimulating GTP hydrolysis and reducing the activity of RhoA in tumor cells. This mechanism has been shown to depend on the association of p190RhoGAP with Rnd3/RhoE, a Rho-related GTP-binding protein (Jaiswal et al., 2013b), which is controlled by the assembly of the DDR1-Par3/Par6 complex (Hidalgo-Carcedo et al., 2011). p190RhoGAP has been also shown to be an integral component in the Rac1-induced inactivation of Rho signaling (Nimnual et al., 2003; Herbrand and Ahmadian, 2006). Contrary to Rho-induced cell contractility, Rac promotes rather cellular protrusion and thus counteracts Rho signaling. In this regard, the reciprocal balance between these GTPases determines morphology and migratory behavior of cells (Heasman and Ridley, 2008). Recently, a cAMP-mediated PKA-independent signaling through the Epac/Rap1 pathway has been shown to induce a significant relaxation of RhoA-mediated smooth muscle contraction (Zieba et al., 2011). In this context, the Rap1-activated RhoGAPs, such as RA-RhoGAP or ARAP3, have been suggested to downregulate RhoA activity in the smooth muscle (Zieba et al., 2011). Moreover, blocking Rho-ROCK interaction and signaling by directly targeting Rho protein is another mechanism. Phosphorylation of the cell cycle inhibitor p27Kip1 by p90 ribosomal S6 kinase (RSK1) downstream of Ras, has been shown to directly bind RhoA and inhibit Rho-ROCK pathway (Larrea et al., 2009). Vaccinia virus utilizes its F11L protein to interfere with the Rho interaction with ROCK. This virus blocks stress fiber formation of the host cells through its F11L protein, reported to directly bind RhoA and inhibit RhoA-mediated ROCK activation (Valderrama et al., 2006).
Rho-ROCK signaling can be specifically abrogated by the inhibition of the kinase domain of ROCK. This Ras-dependent mechanism includes a link between C-Raf and ROCK (Niault and Baccarini, 2010). Accordingly, the N-terminal regulatory domain of C-Raf binds physically to the kinase domain of ROCK and directly inhibits its enzymatic activity. ROCK inhibition by C-Raf has been proposed to be necessary for the development and maintenance of Ras-induced epidermal tumors. In addition, B-Raf has been shown to positively control Rnd3/RhoE expression, which in turn regulates the cross-talk between the RAF/MEK/ERK and Rho/ROCK signaling pathways and contributes to oncogene-mediated reorganization of the actin cytoskeleton (Klein et al., 2008). Rnd3/RhoE induces stress fiber disassembly by directly binding ROCK and inhibiting it from phosphorylating downstream targets (Riento et al., 2003). ROCK can in turn phosphorylate Rnd3/RhoE as well as p190RhoGAP to downregulate GAP activity, leading to a further increase in RhoA activity (Madigan et al., 2009). Gem and Rad are other GTP-binding proteins that act as negative regulators of the Rho-ROCK pathway (Ward et al., 2002). Gem binds to the coiled-coil region of ROCK independently of RhoA and modifies the substrate specificity of ROCK. Taken together, RhoA, RhoB, and RhoC associate with and activate ROCK, whereas other GTP-binding proteins inhibit ROCK either directly, as has been found for Rnd3/RhoE and Gem, or indirectly, as has been reported for Rac and Ras signals.
Conclusion
RhoA-ROCK has emerged as a central signal-integrating node that senses and responds to extracellular and intracellular cues and thus regulates a wide range of fundamental cell functions such as contraction, motility, proliferation, and apoptosis. Abnormal activation of the RhoA-ROCK pathway has been observed in cancer, neurodegenerative diseases, and notably in major cardiovascular disorders, including hypertension, atherosclerosis, cerebral cavernous malformations leading to stroke, postangioplasty restenosis, pulmonary hypertension, and cardiac hypertrophy. Great effort has been expended over the past years in the development of pharmacological inhibitors interfering with RhoA-ROCK signal transduction. A large number of studies have shown that statins, GGTIs, FIs, and RIs are valuable tools for elucidating the physiological and pathophysiological roles of pathways and processes involving prenylated proteins, such as RhoA, B, and C and protein kinases, including ROCK.
Despite almost 20 years of intensive research on therapeutic-relevant small GTPase signaling, only two approaches for drug design have been thoroughly exploited, which are kinase and prenylation inhibitors. A common problem with kinase inhibitors is their tendency toward nonselectivity because the majority of these inhibitors interact with highly conserved proteins domains, in particular the catalytic domain. For known reasons, the structure-function relationship of the kinase domains has been very well investigated in the last decade, and other mechanisms have been largely disregarded. Targeting protein prenylation in human diseases, despite the large number of different prenylated proteins, has been mostly described for Ras and Rho proteins. There is, therefore, an urgent need for alternative approaches that specifically target Rho-ROCK signal transduction. Although ROCK belongs to one of the best investigated small GTPase effectors in both fundamental and clinical research, the molecular basis of its regulation has remained a matter of speculation. Thus, it is required to identify new mechanisms, which may offer great potential for defining new drug target sites and for attempting a novel strategy for more selective therapeutic intervention.
It is of major importance to note that apart from Rho isoforms, which bind to and activate ROCK, there are, in addition to the RhoGEFs and RhoGAPs, also Rnd3/RhoE, Gem, C-Raf, p27Kip1, F11L, Morgana, NPM-1, and the Gγ subunits as well as Rac and Ras isoforms that directly or indirectly control the activity of Rho-ROCK signaling pathways (Figure 1). Thus, understanding the mechanisms underlying the negative regulation of Rho-ROCK signaling could lead to the development of novel therapeutic approaches for the treatment of these diseases. In addition, one has to take into account the fact that the function of these negative regulators, except for Rad, also depends on prenylation. Rnd3 and Ras proteins are farnesylated, whereas RhoA, RhoC, and the Rac isoforms as well as Gγ proteins are geranylgeranylated. RhoB exists in two populations that are either farnesylated or geranylgeranylated. It is therefore crucial that future studies examining associations between prenylation inhibitors and various ROCK-associated human diseases also focus on small GTPases other than only the Rho isoforms.
Acknowledgments
We thank our colleagues Astrid Hoeppner, Georg Groth, Cordula Kruse, Sander H. Smits, and Jürgen Scheller for their support and the discussions. We apologize for not being able to cite all the relevant publications due to space limits. We gratefully acknowledge the support and training from the International NRW Research School BioStruct, granted by the Ministry of Innovation, Science and Research of the State North Rhine-Westphalia, the Heinrich-Heine-University of Düsseldorf, and the Entrepreneur Foundation at the Heinrich-Heine-University of Düsseldorf. We also thank the Research Committee of the Medical Faculty of the Heinrich-Heine University of Düsseldorf, the NGFNplus program of the German Ministry of Science and Education (BMBF; grant 01GS08100), USA National Institutes of Health grant R01GM086457 and the International Research Training Group 1902 (IRGT1902) of the German Research Foundation (DFG).
Contributor Information
Ehsan Amin, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Badri Nath Dubey, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Si-Cai Zhang, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Lothar Gremer, Institut für Strukturbiochemie (ICS-6), Forschungszentrum Jülich, Jülich, Germany; and Institut für Physikalische Biologie, Mathematisch-Naturwissenschaftliche Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Radovan Dvorsky, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Jens M. Moll, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany
Mohamed S. Taha, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany
Luitgard Nagel-Steger, Institut für Strukturbiochemie (ICS-6), Forschungszentrum Jülich, Jülich, Germany; and Institut für Physikalische Biologie, Mathematisch-Naturwissenschaftliche Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
Roland P. Piekorz, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany
Avril V. Somlyo, Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22908, USA
Mohammad R. Ahmadian, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät der Heinrich-Heine-Universität, D-40255 Düsseldorf, Germany.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH-terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355–363. doi: 10.1517/14728222.2012.671811. [DOI] [PubMed] [Google Scholar]
- Appels NM, Bolijn MJ, van Eijndhoven MA, Stephens TC, Beijnen JH, Schellens JH. Characterization of the in vitro activity of AZD3409, a novel prenyl transferase inhibitor. Cancer Chemother Pharmacol. 2011;67:137–145. doi: 10.1007/s00280-010-1300-6. [DOI] [PubMed] [Google Scholar]
- Baranwal S, Alahari SK. Rho GTPase effector functions in tumor cell invasion and metastasis. Curr Drug Targets. 2011;12:1194–1201. doi: 10.2174/138945011795906534. [DOI] [PubMed] [Google Scholar]
- Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-a) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:6214–6222. doi: 10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Blum R, Cox AD, Kloog Y. Inhibitors of chronically active ras: potential for treatment of human malignancies. Recent Pat Anticancer Drug Discov. 2008;3:31–47. doi: 10.2174/157489208783478702. [DOI] [PubMed] [Google Scholar]
- Blumenstein L, Ahmadian MR. Models of the cooperative mechanism for Rho effector recognition: implications for RhoA-mediated effector activation. J Biol Chem. 2004;279:53419–53426. doi: 10.1074/jbc.M409551200. [DOI] [PubMed] [Google Scholar]
- Brunsveld L, Kuhlmann J, Alexandrov K, Wittinghofer A, Goody RS, Waldmann H. Lipidated ras and rab peptides and proteins – synthesis, structure, and function. Angew Chem. 2006;45:6622–6646. doi: 10.1002/anie.200600855. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKa implication of the pleckstrin homology domain in ROKa function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Matsusue K, Misawa M. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. J Pharmacol Sci. 2010;114:239–247. doi: 10.1254/jphs.10r03cr. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Petit D, Bertoglio J. Cell cycle regulation of Rho signaling pathways. Cell Cycle. 2012;11:3003–3010. doi: 10.4161/cc.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Ga 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- Doran JD, Liu X, Taslimi P, Saadat A, Fox T. New insights into the structure-function relationships of Rho-associated kinase: a thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem J. 2004;384:255–262. doi: 10.1042/BJ20040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorsky R, Ahmadian MR. Always look on the bright site of Rho: structural implications for a conserved intermolecular interface. EMBO Rep. 2004;5:1130–1136. doi: 10.1038/sj.embor.7400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem. 2004;279:7098–7104. doi: 10.1074/jbc.M311911200. [DOI] [PubMed] [Google Scholar]
- Ehrenschwender M, Siegmund D, Wicovsky A, Kracht M, Dittrich-Breiholz O, Spindler V, Waschke J, Kalthoff H, Trauzold A, Wajant H. Mutant PIK3CA licenses TRAIL and CD95L to induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death Differ. 2010;17:1435–1447. doi: 10.1038/cdd.2010.36. [DOI] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem (Tokyo) 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, Luscher TF. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89:583–590. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- Faiz F, Hooper AJ, van Bockxmeer FM. Molecular pathology of familial hypercholesterolemia, related dyslipidemias and therapies beyond the statins. Crit Rev Clin Lab Sci. 2012;49:1–17. doi: 10.3109/10408363.2011.646942. [DOI] [PubMed] [Google Scholar]
- Ferretti R, Palumbo V, Di Savino A, Velasco S, Sbroggio M, Sportoletti P, Micale L, Turco E, Silengo L, Palumbo G, et al. Morgana/chp-1, a ROCK inhibitor involved in centrosome duplication and tumorigenesis. Dev Cell. 2010;18:486–495. doi: 10.1016/j.devcel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Fried LF. Effects of HMG-CoA reductase inhibitors (statins) on progression of kidney disease. Kidney Int. 2008;74:571–576. doi: 10.1038/ki.2008.231. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Aberrant activation of cell cycle regulators, centrosome amplification, and mitotic defects. Horm Cancer. 2011;2:104–112. doi: 10.1007/s12672-010-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Therapeutic intervention based on protein prenylation and associated modifications. Nat Chem Biol. 2006;2:518–528. doi: 10.1038/nchembio818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67:171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashiro K, Brancaccio M, Fukasawa K. Activated ROCK II by-passes the requirement of the CDK2 activity for centrosome duplication and amplification. Oncogene. 2011;30:2188–2197. doi: 10.1038/onc.2010.607. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010;102:866–880. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrand U, Ahmadian MR. p190-RhoGAP as an integral component of the Tiam1/Rac1-induced downregulation of Rho. Biol Chem. 2006;387:311–317. doi: 10.1515/BC.2006.041. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Itzen A, Goody RS. Covalent coercion by Legionella pneumophila. Cell Host Microbe. 2011;10:89–91. doi: 10.1016/j.chom.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Gremer L, Dvorsky R, Haeusler LC, Cirstea IC, Uhlenbrock K, Ahmadian MR. Mechanistic insights into specificity, activity, and regulatory elements of the regulator of G-protein signaling (RGS)-containing Rho-specific guanine nucleotide exchange factors (GEFs) p115, PDZ-RhoGEF (PRG), and leukemia-associated RhoGEF (LARG) J Biol Chem. 2011;286:18202–18212. doi: 10.1074/jbc.M111.226431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Dvorsky R, Ahmadian MR. Deciphering the molecular and functional basis of Dbl family proteins: a novel systematic approach toward classification of selective activation of the Rho family proteins. J Biol Chem. 2013a;288:4486–4500. doi: 10.1074/jbc.M112.429746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Fansa EK, Dvorsky R, Ahmadian MR. New insight into the molecular switch mechanism of human Rho family proteins: shifting a paradigm. Biol Chem. 2013b;394:89–95. doi: 10.1515/hsz-2012-0207. [DOI] [PubMed] [Google Scholar]
- Jin S, Exton JH. Activation of RhoA by association of Ga(13) with Dbl. Biochem Biophys Res Commun. 2000;277:718–721. doi: 10.1006/bbrc.2000.3744. [DOI] [PubMed] [Google Scholar]
- Joyce PL, Cox AD. Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 2003;63:7959–7967. [PubMed] [Google Scholar]
- Karnoub AE, Symons M, Campbell SL, Der CJ. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat. 2004;84:61–71. doi: 10.1023/B:BREA.0000018427.84929.5c. [DOI] [PubMed] [Google Scholar]
- Khan OM, Ibrahim MX, Jonsson IM, Karlsson C, Liu M, Sjogren AK, Olofsson FJ, Brisslert M, Andersson S, Ohlsson C, et al. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. J Clin Invest. 2011;121:628–639. doi: 10.1172/JCI43758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Sharpe CC, Noor M, Hendry BM. The role of geranylgeranylated proteins in human mesangial cell proliferation. Kidney Int. 2006;70:1296–1304. doi: 10.1038/sj.ki.5001713. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- Klein RM, Spofford LS, Abel EV, Ortiz A, Aplin AE. B-RAF regulation of Rnd3 participates in actin cytoskeletal and focal adhesion organization. Mol Biol Cell. 2008;19:498–508. doi: 10.1091/mbc.E07-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komers R. Rho kinase inhibition in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2011;20:77–83. doi: 10.1097/MNH.0b013e32834131f8. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Larrea MD, Hong F, Wander SA, da Silva TG, Helfman D, Lannigan D, Smith JA, Slingerland JM. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci USA. 2009;106:9268–9273. doi: 10.1073/pnas.0805057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim YB. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeti E, Welti S, Scheffzek K. Inhibition and termination of physiological responses by GTPase activating proteins. Physiol Rev. 2012;92:237–272. doi: 10.1152/physrev.00045.2010. [DOI] [PubMed] [Google Scholar]
- Litosch I. RhoA co-ordinates with heterotrimeric G proteins to regulate efficacy. Biochem Biophys Res Commun. 2011;415:215–219. doi: 10.1016/j.bbrc.2011.10.063. [DOI] [PubMed] [Google Scholar]
- Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrera C, Ruiz-Limon P, Valverde-Estepa A, Barbarroja N, Rodriguez-Ariza A. To cardiovascular disease and beyond: new therapeutic perspectives of statins in autoimmune diseases and cancer. Curr Drug Targets. 2012;13:829–841. doi: 10.2174/138945012800564112. [DOI] [PubMed] [Google Scholar]
- Ma Z, Kanai M, Kawamura K, Kaibuchi K, Ye K, Fukasawa K. Interaction between ROCK II and nucleophosmin/B23 in the regulation of centrosome duplication. Mol Cell Biol. 2006;26:9016–9034. doi: 10.1128/MCB.01383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan JP, Bodemann BO, Brady DC, Dewar BJ, Keller PJ, Leitges M, Philips MR, Ridley AJ, Der CJ, Cox AD. Regulation of Rnd3 localization and function by protein kinase Ca-mediated phosphorylation. Biochem J. 2009;424:153–161. doi: 10.1042/BJ20082377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardilovich K, Olson MF, Baugh M. Targeting Rho GTPase signaling for cancer therapy. Future Oncol. 2012;8:165–177. doi: 10.2217/fon.11.143. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry (Mosc) 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri D, Greeve MA, Brunet A, Finan D, Wells CD, LaRose J, Rottapel R. Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation. Mol Cell Biol. 2009;29:5963–5973. doi: 10.1128/MCB.01268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H, et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem. 2013;288:12232–12243. doi: 10.1074/jbc.M112.428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Del Re DP, Xiang SY, Zhao X, Florholmen G, Brown JH. Revisited and revised: is RhoA always a villain in cardiac pathophysiology? J Cardiovasc Transl Res. 2010;3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momotani K, Somlyo AV. p63RhoGEF: a new switch for Gq-mediated activation of smooth muscle. Trends Cardiovasc Med. 2012;22:122–127. doi: 10.1016/j.tcm.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momotani K, Artamonov MV, Utepbergenov D, Derewenda U, Derewenda ZS, Somlyo AV. p63RhoGEF couples Ga(q/11)-mediated signaling to Ca2+ sensitization of vascular smooth muscle contractility. Circ Res. 2011;109:993–1002. doi: 10.1161/CIRCRESAHA.111.248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185–198. doi: 10.1369/0022155412470834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- Niault TS, Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis. 2010;31:1165–1174. doi: 10.1093/carcin/bgp337. [DOI] [PubMed] [Google Scholar]
- Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- Noma K, Kihara Y, Higashi Y. Striking crosstalk of ROCK signaling with endothelial function. J Cardiol. 2012;60:1–6. doi: 10.1016/j.jjcc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21:274–282. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155:444–454. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Zhang Y, Georgescu SP, Johnson KL, Kong D, Galper JB. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2:93–102. doi: 10.1007/s12015-006-0015-x. [DOI] [PubMed] [Google Scholar]
- Raper A, Kolansky DM, Cuchel M. Treatment of familial hypercholesterolemia: is there a need beyond statin therapy? Curr Atheroscler Rep. 2012;14:11–16. doi: 10.1007/s11883-011-0215-y. [DOI] [PubMed] [Google Scholar]
- Raptis L, Arulanandam R, Geletu M, Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res. 2011;317:1787–1795. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Chahoud G, Mehta JL. Modulation of cardiovascular remodeling with statins: fact or fiction? Curr Vasc Pharmacol. 2005;3:69–79. doi: 10.2174/1570161052773915. [DOI] [PubMed] [Google Scholar]
- Resh MD. Targeting protein lipidation in disease. Trends Mol Med. 2012;18:206–214. doi: 10.1016/j.molmed.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BT, Dibble CF, Borikova AL, Johnson GL. Cerebral cavernous malformation is a vascular disease associated with activated RhoA signaling. Biol Chem. 2013;394:35–42. doi: 10.1515/hsz-2012-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Aldieri E, Doublier S, Bosia A, Ghigo D. Statins-mediated inhibition of rho GTPases as a potential tool in anti-tumor therapy. Mini Rev Med Chem. 2008;8:609–618. doi: 10.2174/138955708784534436. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A. Stimulating progress in regenerative medicine: improving the cloning and recovery of cryopreserved human pluripotent stem cells with ROCK inhibitors. Regen Med. 2010;5:799–807. doi: 10.2217/rme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Protein prenylation: a pivotal posttranslational process. Biochem Biophys Res Commun. 2003;303:1–7. doi: 10.1016/s0006-291x(03)00323-1. [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Roy M, Kung HJ, Ghosh PM. Statins and prostate cancer: role of cholesterol inhibition vs. prevention of small GTP-binding proteins. Am J Cancer Res. 2011;1:542–561. [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Suuronen T, Kaarniranta K. ROCK, PAK, and Toll of synapses in Alzheimer’s disease. Biochem Biophys Res Commun. 2008;371:587–590. doi: 10.1016/j.bbrc.2008.04.148. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- Sawada N, Liao JK. Targeting eNOS and beyond: emerging heterogeneity of the role of endothelial Rho proteins in stroke protection. Expert Rev Neurother. 2009;9:1171–1186. doi: 10.1586/ern.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013;48:301–316. doi: 10.3109/10409238.2013.786671. [DOI] [PubMed] [Google Scholar]
- Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors in cancer therapy: important mechanistic and bench to bedside issues. Expert Opin Invest Drugs. 2000a;9:2767–2782. doi: 10.1517/13543784.9.12.2767. [DOI] [PubMed] [Google Scholar]
- Sebti SM, Hamilton AD. Inhibition of Rho GTPases using protein geranylgeranyltransferase I inhibitors. Methods Enzymol. 2000b;325:381–388. doi: 10.1016/s0076-6879(00)25459-1. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang L, Wei L. Rho-kinase in development and heart failure: insights from genetic models. Pediatr Cardiol. 2011;32:297–304. doi: 10.1007/s00246-011-9920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Somlyo AP. Signal transduction. Rhomantic interludes raise blood pressure. Nature. 1997;389:908–909. 911. doi: 10.1038/40002. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Street CA, Bryan BA. Rho kinase proteins – pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31:3645–3657. [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yamamoto M, Wada H, Ito M, Nakano T, Sasaki Y, Narumiya S, Shiku H, Nishikawa M. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood. 1999;93:3408–3417. [PubMed] [Google Scholar]
- Takefuji M, Kruger M, Sivaraj KK, Kaibuchi K, Offermanns S, Wettschureck N. RhoGEF12 controls cardiac remodeling by integrating G protein- and integrin-dependent signaling cascades. J Exp Med. 2013;210:665–673. doi: 10.1084/jem.20122126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27:618–628. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HB, Zhong YS, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol. 2011;4:652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. Alzheimer’s disease: channeling APP to non-amyloidogenic processing. Biochem Biophys Res Commun. 2005;331:375–378. doi: 10.1016/j.bbrc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B. The effects of HMG-CoA reductase inhibitors on endothelial function. Am J Cardiovasc Drugs. 2006;6:115–120. doi: 10.2165/00129784-200606020-00005. [DOI] [PubMed] [Google Scholar]
- Tonges L, Koch JC, Bahr M, Lingor P. ROCKing regeneration: Rho kinase inhibition as molecular target for neurorestoration. Front Mol Neurosci. 2011;4:39. doi: 10.3389/fnmol.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triola G, Waldmann H, Hedberg C. Chemical biology of lipidated proteins. ACS Chem Biol. 2012;7:87–99. doi: 10.1021/cb200460u. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science. 2006;311:377–381. doi: 10.1126/science.1122411. [DOI] [PubMed] [Google Scholar]
- Vanni C, Mancini P, Ottaviano C, Ognibene M, Parodi A, Merello E, Russo C, Varesio L, Zheng Y, Torrisi MR, et al. Ga13 regulation of proto-Dbl signaling. Cell Cycle. 2007;6:2058–2070. doi: 10.4161/cc.6.16.4574. [DOI] [PubMed] [Google Scholar]
- Walsh MP. Vascular smooth muscle myosin light chain diphosphorylation: mechanism, function, and pathological implications. IUBMB Life. 2011;63:987–1000. doi: 10.1002/iub.527. [DOI] [PubMed] [Google Scholar]
- Wang QM, Liao JK. ROCKs as immunomodulators of stroke. Expert Opin Ther Targets. 2012;16:1013–1025. doi: 10.1517/14728222.2012.715149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Liu W, Yan J, Zhang M. Structure basis and unconventional lipid membrane binding properties of the PH-C1 tandem of rho kinases. J Biol Chem. 2008;283:26263–26273. doi: 10.1074/jbc.M803417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- While A, Keen L. The effects of statins on mood: a review of the literature. Eur J Cardiovasc Nurs. 2012;11:85–96. doi: 10.1016/j.ejcnurse.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wiemer AJ, Hohl RJ, Wiemer DF. The intermediate enzymes of isoprenoid metabolism as anticancer targets. Anticancer Agents Med Chem. 2009;9:526–542. doi: 10.2174/187152009788451860. [DOI] [PubMed] [Google Scholar]
- Wiemer AJ, Wiemer DF, Hohl RJ. Geranylgeranyl diphosphate synthase: an emerging therapeutic target. Clin Pharmacol Ther. 2011;90:804–812. doi: 10.1038/clpt.2011.215. [DOI] [PubMed] [Google Scholar]
- Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A, Vetter IR. Structure-function relationships of the G domain, a canonical switch motif. Annu Rev Biochem. 2011;80:943–971. doi: 10.1146/annurev-biochem-062708-134043. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol. 1998;143:1249–1258. doi: 10.1083/jcb.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Yang SH, Davies BS, Jung HJ, Fong LG. Targeting protein prenylation in progeria. Sci Transl Med. 2013;5:171–173. doi: 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sawashita J, Fu X, Korenaga T, Yan J, Mori M, Higuchi K. Transmissibility of mouse AApoAII amyloid fibrils: inactivation by physical and chemical methods. FASEB J. 2006;20:1012–1014. doi: 10.1096/fj.05-4890fje. [DOI] [PubMed] [Google Scholar]
- Zhao TT, Le Francois BG, Goss G, Ding K, Bradbury PA, Dimitroulakos J. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: potential regulation by targeting rho proteins. Oncogene. 2010;29:4682–4692. doi: 10.1038/onc.2010.219. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li Y. Long-term diabetic complications may be ameliorated by targeting Rho kinase. Diabetes Metab Res Rev. 2011;27:318–330. doi: 10.1002/dmrr.1182. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li YJ. Rho kinase inhibitors: potential treatments for diabetes and diabetic complications. Curr Pharm Des. 2012;18:2964–2973. doi: 10.2174/138161212800672688. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15:467–478. doi: 10.2174/138161209787315684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liao JK. Pleiotropic effects of statins. Basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, et al. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem. 2011;286:16681–16692. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Teitelbaum SL. Integrins, growth factors, and the osteoclast cytoskeleton. Ann NY Acad Sci. 2010;1192:27–31. doi: 10.1111/j.1749-6632.2009.05245.x. [DOI] [PubMed] [Google Scholar]