Abstract
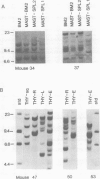
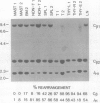
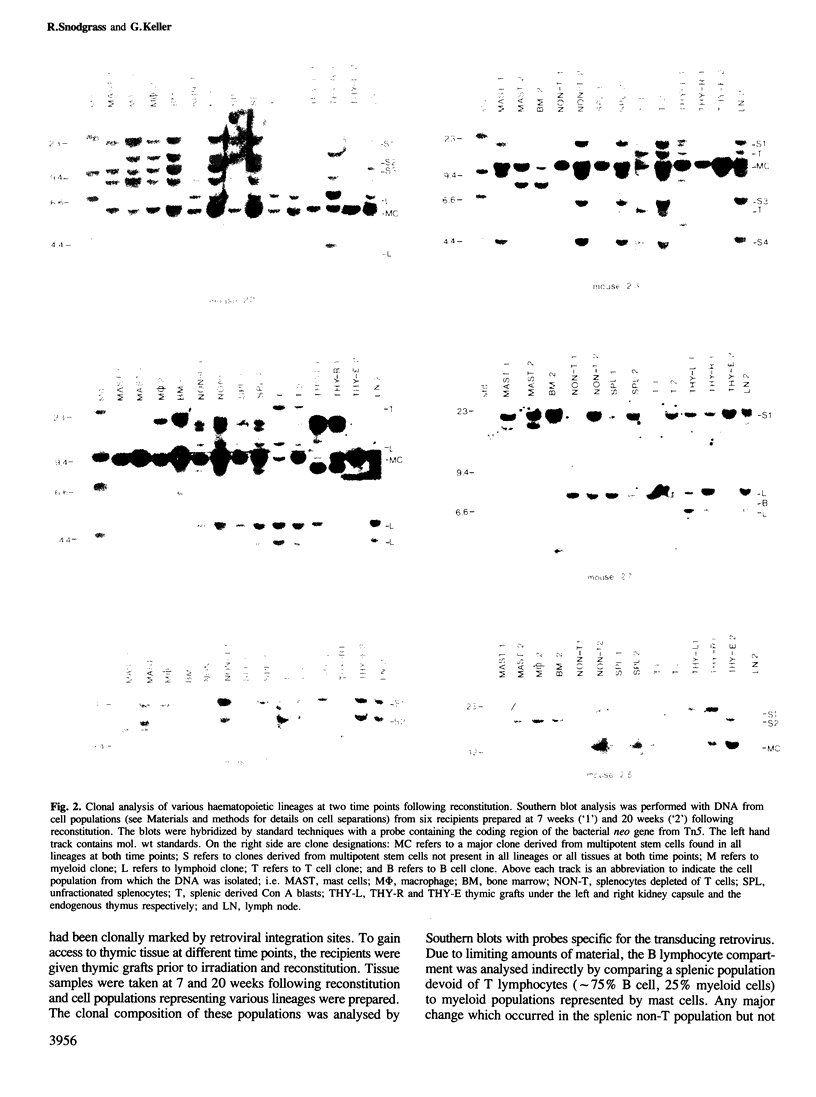
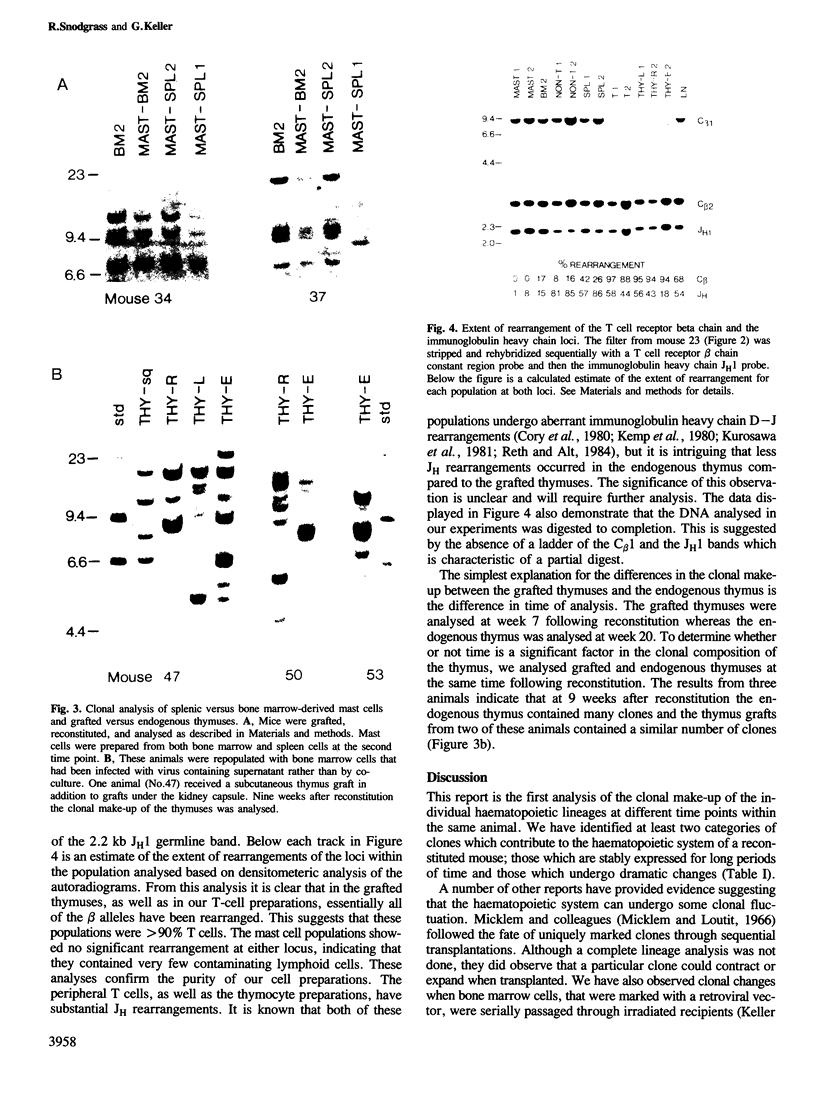
The clonal make-up of the haematopoietic system of mice reconstituted with retrovirus-infected bone marrow cells was analysed at two different points in time following reconstitution. We have found that under these conditions, the haematopoietic system consists of clones that persist throughout the 5 month course of the experiment as well as those which undergo temporal changes. The various changes that we have observed included the appearance of a new clone(s) in all lineages, the loss of a clone from some lineages and the shift in the appearance of a clone from one lineage to another. In addition, we provide evidence which suggests that the clonal make-up of the thymus changes with time; early after reconstitution it consists of many clones, whereas at the later time-points it contains a limited number of predominant clones. These studies document the dramatic clonal changes which occur within the various lineages for a long time following reconstitution and highlight the difficulty in demonstrating lineage-specific stem cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Gough N. M. Nucleotide sequence of immunoglobulin heavy chain joining segments between translocated VH and mu constant regions genes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3630–3634. doi: 10.1073/pnas.77.6.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronkite E. P. Kinetics of granulocytopoiesis. Natl Cancer Inst Monogr. 1969 May;30:51–62. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Miller R. G., Phillips R. A. Differentiation of rosette-forming cells from myeloid stem cells. J Immunol. 1970 Sep;105(3):719–729. [PubMed] [Google Scholar]
- Ezine S., Weissman I. L., Rouse R. V. Bone marrow cells give rise to distinct cell clones within the thymus. Nature. 1984 Jun 14;309(5969):629–631. doi: 10.1038/309629a0. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Rocha B., Coutinho A. A. Lymphocyte population kinetics in the mouse. Immunol Rev. 1986 Jun;91:5–37. doi: 10.1111/j.1600-065x.1986.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Kadish J. L., Basch R. S. Hematopoietic thymocyte precursors. I. Assay and kinetics of the appearance of progeny. J Exp Med. 1976 May 1;143(5):1082–1099. doi: 10.1084/jem.143.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Wagner E. F. Efficient expression of foreign genes in mice reconstituted with retrovirus-infected bone marrow cells. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1053–1058. doi: 10.1101/sqb.1986.051.01.122. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Mann R., Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNAs. J Virol. 1985 May;54(2):401–407. doi: 10.1128/jvi.54.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Mintz B., Anthony K., Litwin S. Monoclonal derivation of mouse myeloid and lymphoid lineages from totipotent hematopoietic stem cells experimentally engrafted in fetal hosts. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7835–7839. doi: 10.1073/pnas.81.24.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol. 1973 Apr;7(1):40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- Wallis V. J., Leuchars E., Chwalinski S., Davies A. J. On the sparse seeding of bone marrow and thymus in radiation chimaeras. Transplantation. 1975 Jan;19(1):2–11. doi: 10.1097/00007890-197501000-00002. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]