Abstract
A chicken calmodulin (CaM) gene has been expressed in mouse C127 cells using a bovine papilloma virus (BPV)-based vector (BPV-CM). The vector-borne genes produce a mature mRNA of the expected size that is present on cytoplasmic polyribosomes. In clonal cell lines transformed by BPV-CM, expression of the CaM gene produced CaM levels 2- to 4-fold above those observed in cells transformed by BPV alone. Increased intracellular CaM caused a reduction of cell cycle length that is solely due to a reduction in the length of the G1 phase. A comparison of six cell lines revealed a linear relationship between the intracellular CaM concentration and the rate of G1 progression. These data provide the first evidence that specific elevation of CaM levels directly affects the rate of cell proliferation.
Full text
PDF
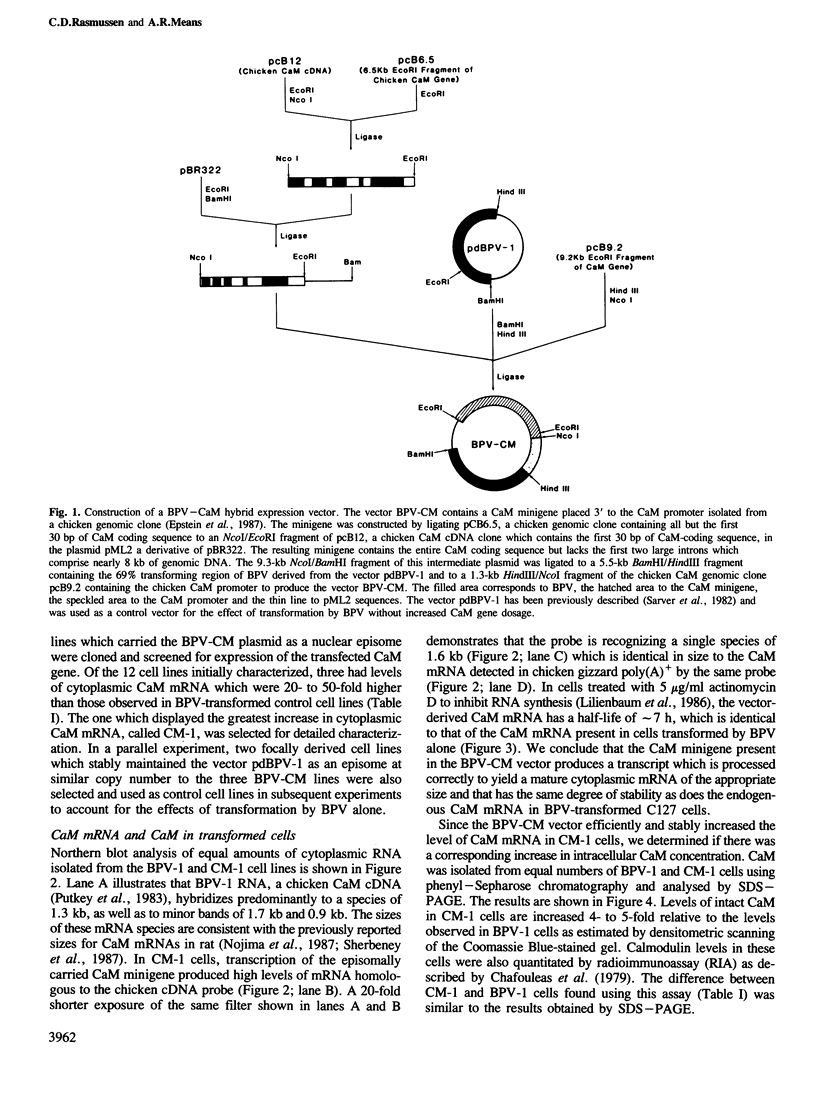
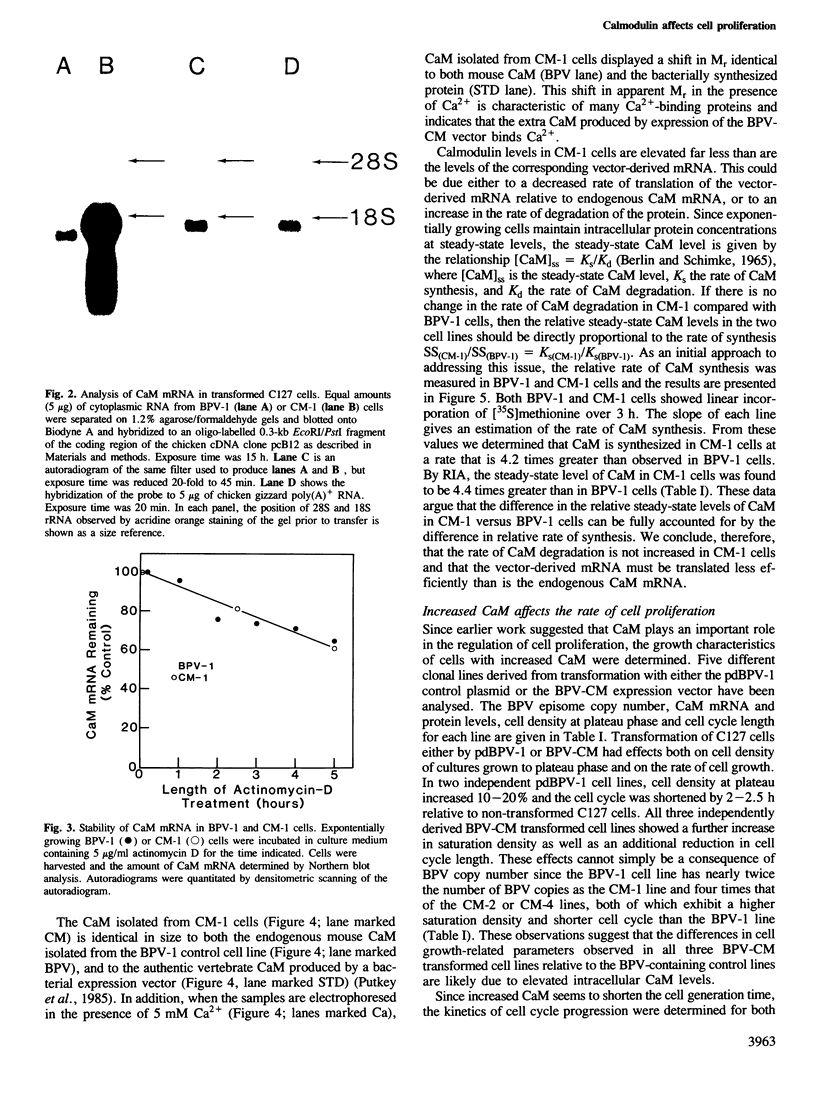
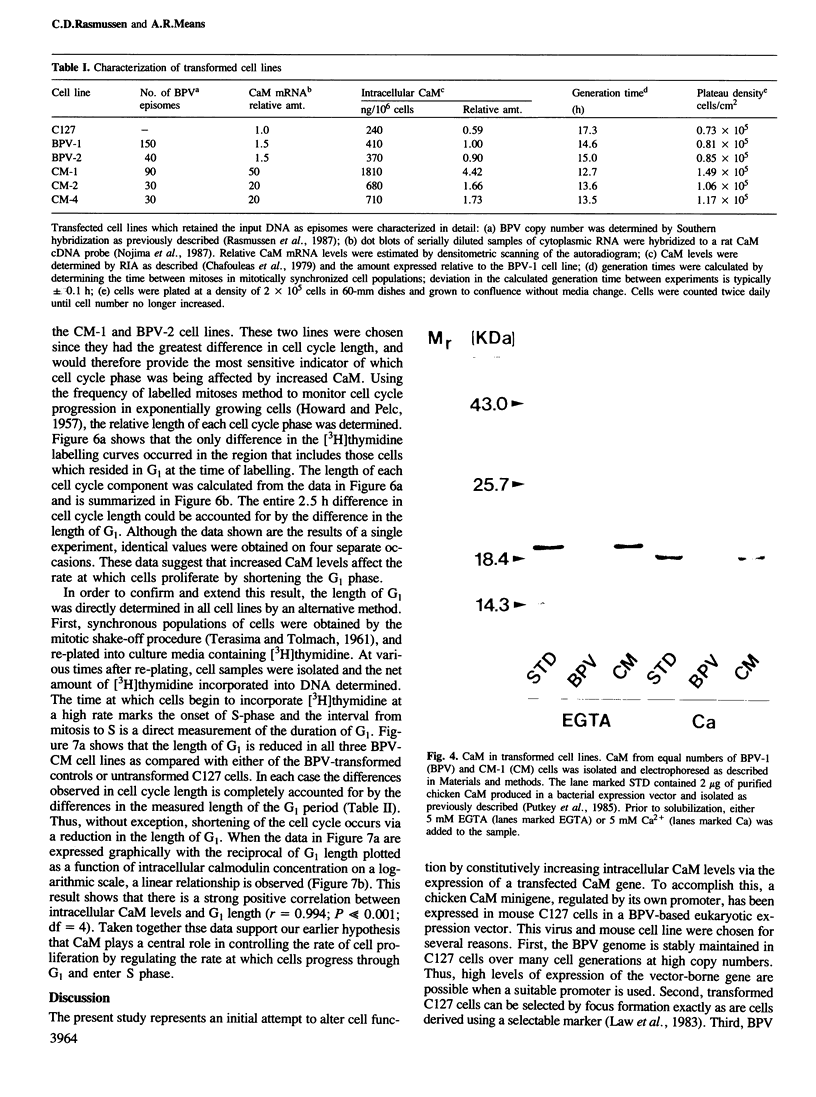



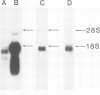
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Bolton W. E., Barranco S. C. Characterization of the cell kinetics and growth properties of cystic fibrosis diploid fibroblasts in vitro. Am J Hum Genet. 1975 May;27(3):394–409. [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Means A. R. Potentiation of bleomycin lethality by anticalmodulin drugs: a role for calmodulin in DNA repair. Science. 1984 Jun 22;224(4655):1346–1348. doi: 10.1126/science.6203171. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Pardue R. L., Brinkley B. R., Dedman J. R., Means A. R. Regulation of intracellular levels of calmodulin and tubulin in normal and transformed cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):996–1000. doi: 10.1073/pnas.78.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Connor C. G., Moore P. B., Brady R. C., Horn J. P., Arlinghaus R. B., Dedman J. R. The role of calmodulin in cell transformation. Biochem Biophys Res Commun. 1983 Apr 29;112(2):647–654. doi: 10.1016/0006-291x(83)91512-7. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Carney D. H. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981 Jan;23(1):61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Boynton A. L., Whitfield J. F. The src gene product (pp60 src) of avian sarcoma virus rapidly induces DNA synthesis and proliferation of calcium-deprived rat cells. Biochem Biophys Res Commun. 1981 Nov 16;103(1):233–239. doi: 10.1016/0006-291x(81)91684-3. [DOI] [PubMed] [Google Scholar]
- Epstein P., Simmen R. C., Tanaka T., Means A. R. Isolation and structural analysis of the chromosomal gene for chicken calmodulin. Methods Enzymol. 1987;139:217–229. doi: 10.1016/0076-6879(87)39088-3. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Murine dihydrofolate reductase transcripts through the cell cycle. Mol Cell Biol. 1986 Feb;6(2):365–371. doi: 10.1128/mcb.6.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goyns M. H., Hopkins C. R. Inhibition of epidermal growth factor-induced mitogenesis in bovine granulosa cells by trifluoperazine. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1107–1114. doi: 10.1016/s0006-291x(81)80126-x. [DOI] [PubMed] [Google Scholar]
- Greco A., Ittmann M., Basilico C. Molecular cloning of a gene that is necessary for G1 progression in mammalian cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1565–1569. doi: 10.1073/pnas.84.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait W. N., Grais L., Benz C., Cadman E. C. Inhibition of growth of leukemic cells by inhibitors of calmodulin: phenothiazines and melittin. Cancer Chemother Pharmacol. 1985;14(3):202–205. doi: 10.1007/BF00258116. [DOI] [PubMed] [Google Scholar]
- Hazelton B., Mitchell B., Tupper J. Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J Cell Biol. 1979 Nov;83(2 Pt 1):487–498. doi: 10.1083/jcb.83.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Fischer E. H., Margolis R. L. Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4679–4682. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C., DiPaola M., Maxfield F. R., Shelanski M. L. Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol. 1983 Dec;97(6):1918–1924. doi: 10.1083/jcb.97.6.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Gidwitz S., Weber M. J., Storm D. R. Relationship between changes in the calcium dependent regulatory protein and adenylate cyclase during viral transformation. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1169–1177. doi: 10.1016/0006-291x(79)90240-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. L., Hait W. N. Inhibition of growth of C6 astrocytoma cells by inhibitors of calmodulin. Life Sci. 1985 Jan 28;36(4):347–354. doi: 10.1016/0024-3205(85)90120-1. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A., Legagneux V., Portier M. M., Dellagi K., Paulin D. Vimentin gene: expression in human lymphocytes and in Burkitt's lymphoma cells. EMBO J. 1986 Nov;5(11):2809–2814. doi: 10.1002/j.1460-2075.1986.tb04572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. T., Gibson C. W., Hirschhorn R. R., Rittling S., Baserga R., Mercer W. E. Expression of thymidine kinase and dihydrofolate reductase genes in mammalian ts mutants of the cell cycle. J Biol Chem. 1985 Mar 25;260(6):3269–3274. [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., McClelland A., Roberts M. P., Ruddle F. H. Cell proliferation and expression of the transferrin receptor gene: promoter sequence homologies and protein interactions. J Cell Biol. 1986 Nov;103(5):1781–1788. doi: 10.1083/jcb.103.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Wang J. Y. Protein tyrosine phosphorylation in the cell cycle of BALB/c 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8191–8195. doi: 10.1073/pnas.83.21.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H., Kishi K., Sokabe H. Multiple calmodulin mRNA species are derived from two distinct genes. Mol Cell Biol. 1987 May;7(5):1873–1880. doi: 10.1128/mcb.7.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Slaughter G. R., Means A. R. Bacterial expression and characterization of proteins derived from the chicken calmodulin cDNA and a calmodulin processed gene. J Biol Chem. 1985 Apr 25;260(8):4704–4712. [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Rainteau D., Sharif A., Bourrillon R., Weinman S. Calmodulin in lymphocyte mitogenic stimulation and in lymphoid cell line growth. Exp Cell Res. 1987 Feb;168(2):546–554. doi: 10.1016/0014-4827(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. D., Simmen R. C., MacDougall E. A., Means A. R. Methods for analyzing bovine papilloma virus-based calmodulin expression vectors. Methods Enzymol. 1987;139:642–654. doi: 10.1016/0076-6879(87)39117-7. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, McDonald O. B., Cuatrecasas P. Specific postsynaptic density proteins bind tubulin and calmodulin-dependent protein kinase type II. J Biol Chem. 1986 Sep 15;261(26):12339–12344. [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Hidaka H. Calmodulin and cell proliferation. Biochem Biophys Res Commun. 1982 Jan 29;104(2):451–456. doi: 10.1016/0006-291x(82)90658-1. [DOI] [PubMed] [Google Scholar]
- Sherbany A. A., Parent A. S., Brosius J. Rat calmodulin cDNA. DNA. 1987 Jun;6(3):267–272. doi: 10.1089/dna.1987.6.267. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Kanno T., Nagata Y., Kato T. Inhibition of proliferative growth in glioma cells by calmodulin antagonists. J Neurosurg. 1986 Jul;65(1):74–79. doi: 10.3171/jns.1986.65.1.0074. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Changes in x-ray sensitivity of HeLa cells during the division cycle. Nature. 1961 Jun 24;190:1210–1211. doi: 10.1038/1901210a0. [DOI] [PubMed] [Google Scholar]
- Takeda T., Yamamoto M. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3580–3584. doi: 10.1073/pnas.84.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Ohmura T., Hidaka H. Hydrophobic interaction of the Ca2+-calmodulin complex with calmodulin antagonists. Naphthalenesulfonamide derivatives. Mol Pharmacol. 1982 Sep;22(2):403–407. [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Tripp M. L., Piñon R., Meisenhelder J., Hunter T. Identification of phosphoproteins correlated with proliferation and cell cycle arrest in Saccharomyces cerevisiae: positive and negative regulation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5973–5977. doi: 10.1073/pnas.83.16.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veigl M. L., Vanaman T. C., Sedwick W. D. Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta. 1984;738(1-2):21–48. doi: 10.1016/0304-419x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Wandosell F., Serrano L., Hernández M. A., Avila J. Phosphorylation of tubulin by a calmodulin-dependent protein kinase. J Biol Chem. 1986 Aug 5;261(22):10332–10339. [PubMed] [Google Scholar]
- Watterson D. M., Van Eldik L. J., Smith R. E., Vanaman T. C. Calcium-dependent regulatory protein of cyclic nucleotide metabolism in normal and transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2711–2715. doi: 10.1073/pnas.73.8.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. T., Church R. B., Wagenaar E. B. Changes in protein phosphorylation during the cell cycle of Chinese hamster ovary cells. J Biol Chem. 1985 Aug 25;260(18):10308–10313. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Woodford T. A., Pardee A. B. Histone H1 kinase in exponential and synchronous populations of Chinese hamster fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4669–4676. [PubMed] [Google Scholar]
- Zendegui J. G., Zielinski R. E., Watterson D. M., Van Eldik L. J. Biosynthesis of calmodulin in normal and virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1984 May;4(5):883–889. doi: 10.1128/mcb.4.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]