Summary
Background
Preclinical studies have found radiotherapy enhances antitumour immune responses. We aimed to assess disease control and pulmonary toxicity in patients who previously received radiotherapy for non-small-cell lung cancer (NSCLC) before receiving pembrolizumab.
Methods
We assessed patients with advanced NSCLC treated on the phase 1 KEYNOTE-001 trial at a single institution (University of California, Los Angeles, CA, USA). Patients were aged 18 years or older, had an Eastern Cooperative Oncology Group performance status of 1 or less, had adequate organ function, and no history of pneumonitis. Patients received pembrolizumab at a dose of either 2 mg/kg of bodyweight or 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks, until disease progression, unacceptable toxicity, or other protocol-defined reasons for discontinuation. Disease response and pulmonary toxicity were prospectively assessed by Immune-related Response Criteria and Common Terminology Criteria for Adverse Events version 4.0. The primary objective of the KEYNOTE-001 trial was to assess the safety, side-effect profile, and antitumour activity of pembrolizumab. For our secondary analysis, patients were divided into subgroups to compare patients who previously received radiotherapy with patients who had not. Our primary objective was to determine whether previous radiotherapy affected progression-free survival, overall survival, and pulmonary toxicity in the intention-to-treat population. The KEYNOTE-001 trial was registered with ClinicalTrials.gov, number NCT01295827.
Findings
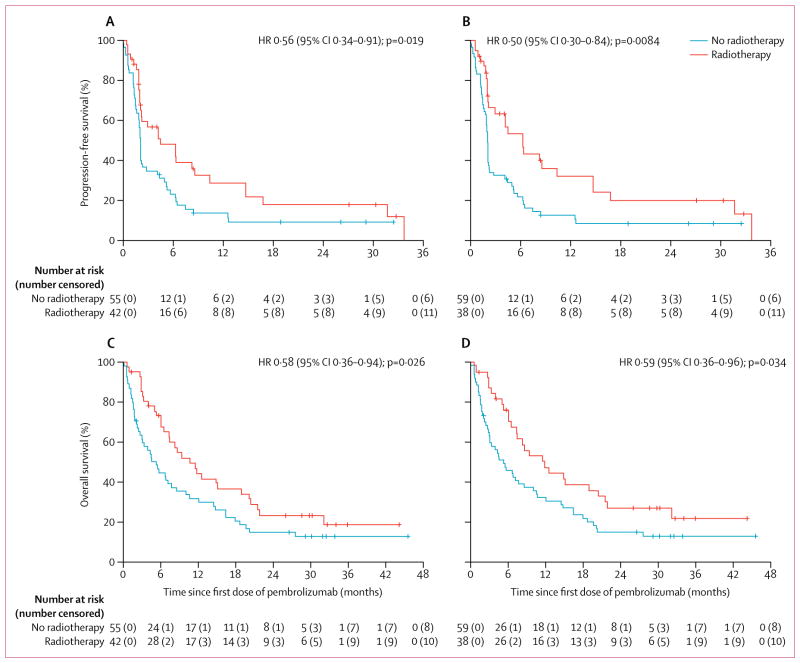
Between May 22, 2012, and July 11, 2014, 98 patients were enrolled and received their first cycle of pembrolizumab. One patient was lost to follow-up. 42 (43%) of 97 patients had previously received any radiotherapy for the treatment of NSCLC before the first cycle of pembrolizumab. 38 (39%) of 97 patients received extracranial radiotherapy and 24 (25%) of 97 patients received thoracic radiotherapy. Median follow-up for surviving patients was 32·5 months (IQR 29·8–34·1). Progression-free survival with pembrolizumab was significantly longer in patients who previously received any radiotherapy than in patients without previous radiotherapy (hazard ratio [HR] 0·56 [95% CI 0·34–0·91], p=0·019; median progression-free survival 4·4 months [95% CI 2·1–8·6] vs 2·1 months [1·6–2·3]) and for patients who previously received extracranial radiotherapy compared with those without previous extracranial radiotherapy (HR 0·50 [0·30–0·84], p=0·0084; median progression-free survival 6·3 months [95% CI 2·1–10·4] vs 2·0 months [1·8–2·1]). Overall survival with pembrolizumab was significantly longer in patients who previously received any radiotherapy than in patients without previous radiotherapy (HR 0·58 [95% CI 0·36–0·94], p=0·026; median overall survival 10·7 months [95% CI 6·5–18·9] vs 5·3 months [2·7–7·7]) and for patients who previously received extracranial radiotherapy compared with those without previous extracranial radiotherapy (0·59 [95% CI 0·36–0·96], p=0·034; median overall survival 11·6 months [95% CI 6·5–20·5] vs 5·3 months [3·0–8·5]). 15 (63%) of 24 patients who had previously received thoracic radiotherapy had any recorded pulmonary toxicity versus 29 (40%) of 73 patients with no previous thoracic radiotherapy. Three (13%) patients with previous thoracic radiotherapy had treatment-related pulmonary toxicity compared with one (1%) of those without; frequency of grade 3 or worse treatment-related pulmonary toxicities was similar (one patient in each group).
Interpretation
Our data suggest that previous treatment with radiotherapy in patients with advanced NSCLC results in longer progression-free survival and overall survival with pembrolizumab treatment than that seen in patients who did not have previous radiotherapy, with an acceptable safety profile. Further clinical trials investigating this combination are needed to determine the optimal treatment strategy for patients with advanced NSCLC.
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of death from cancer both worldwide and in the USA.1,2 Advances in immunotherapy have allowed for therapies directed against programmed cell death protein 1 (PD-1) signalling, which have shown considerable promise among patients with advanced NSCLC and have produced superior survival outcomes compared with cytotoxic chemotherapies in patients with metastatic NSCLC.3–6 Pembrolizumab is an antibody directed against PD-1, and stops inhibitory signalling, allowing for increased antitumour T-cell responses. Despite clinical trials of anti-PD-1 and anti-PD-ligand (L)-1 therapies producing unprecedented positive clinical outcomes, responses are achieved in about 17% to 19% of unselected patients,3,5 highlighting the need to identify strategies to convert non-responding patients to responders.
Research in context.
Evidence before this study
We searched PubMed with the terms “radiation and checkpoint blockade”, “radiation and anti-PD-1”, “pembrolizumab and radiation”, and “pembrolizumab and advanced lung cancer” for English language articles published between March 1, 2000, and March 25, 2017. This search produced limited clinical data for the effects of previous radiotherapy on the activity and toxicity of checkpoint inhibition immunotherapy. However, this search did produce several preclinical articles that showed radiotherapy enhanced the presentation and diversity of tumour-associated antigens, and preclinical data suggesting that the combination of radiotherapy with checkpoint inhibition immunotherapy produces synergistic antitumour responses. Prospective clinical studies on checkpoint inhibition immunotherapy in patients with advanced non-small-cell lung cancer (NSCLC) did show favourable results, and checkpoint blockade is becoming a standard treatment among patients with advanced NSCLC. However, the proportion of unselected patients who respond to checkpoint inhibition is estimated to be about 20%, suggesting the need for strategies to boost response.
Added value of this study
To our knowledge, this study is the largest to date to report the effects of previous radiotherapy on the activity and toxicity of checkpoint blockade immunotherapy. Our secondary analysis of the phase 1 KEYNOTE-001 study found that patients who previously received radiotherapy for NSCLC had significantly longer progression-free survival and overall survival with pembrolizumab treatment versus patients who did not receive radiotherapy. Three (13%) patients with previous thoracic radiotherapy had treatment-related pulmonary toxicity compared with one (1%) of those without; the incidence of grade 3 or worse pulmonary toxicity with pembrolizumab was not affected by previous thoracic radiotherapy.
Implications of all the available evidence
These data suggest that radiotherapy improves the activity of pembrolizumab in patients with advanced NSCLC with a clinically acceptable safety profile. These data corroborate previous preclinical findings about the interaction between radiotherapy and checkpoint inhibition immunotherapy and strengthen the need for further clinical trials investigating this combination.
Substantial data has accumulated showing that local radiotherapy stimulates a systemic immune response. Specifically, irradiation of a tumour results in the release of tumour-associated antigens and damage-associated molecular patterns (DAMPs) that can produce an immunogenic response, a process described as in-situ vaccination.7,8 Radiotherapy has also been found to enhance the presentation and diversity of tumour associated antigens in draining lymph nodes leading to an immune response with the potential for increased tumour recognition and antitumour activity.9–13 Additionally, preclinical studies14,15 that combined radiotherapy with PD-1 blockade have found synergistic antitumour responses, thought in part to be due to the enhanced diversity of the antitumour T-cells that result from radiotherapy.14,15 Therefore, because immune responses are long-lived and immunological memory is a hallmark of vaccination,16 we hypothesised that patients with NSCLC who previously received radiotherapy, either delivered to their primary thoracic disease or to another metastatic site, would have enhanced antitumour activity with anti-PD-1 treatment.
We aimed to assess the effect of previous radiotherapy on progression-free survival and overall survival in patients with advanced NSCLC treated with pembrolizumab in the phase 1 KEYNOTE-001 trial.3 We also aimed to determine whether previous thoracic radiotherapy influenced the frequency of pulmonary toxicity with pembrolizumab treatment.
Methods
Study design and participants
KEYNOTE-0013 was an international, multicentre, phase 1 trial of single agent pembrolizumab in patients with progressive locally advanced or metastatic NSCLC. Eligible patients had to be aged 18 years or older, have advanced NSCLC and an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or less, and have adequate organ function as previously described.3 Patients with a history of pneumonitis, systemic immunosuppressive therapy, or active autoimmune disease were excluded. PD-L1 status and expression was determined as previously described, and patients with membranous PD-L1 staining in at least 1% of cells were considered positive.3 Patients were assigned to multiple expansion cohorts allowing for the inclusion of patients who were naive to systemic therapy and those who had progression after at least one or at least two previous regimens as specified in the protocol (appendix p10). Previous progression was determined using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. All patients provided written informed consent based on the principles of the Declaration of Helsinki before any study-related procedures. The protocol was approved by site institutional review boards. Additional institutional review board approval was obtained for this secondary analysis.
We included patients enrolled at a single institution (University of California, Los Angeles, CA, USA) because radiotherapy records were not collected per the KEYNOTE-001 trial, but were obtained for this single institution population.
Procedures
Patients received intravenous pembrolizumab at a dose of 2 mg/kg or 10 mg/kg of bodyweight every 3 weeks or 10 mg/kg every 2 weeks until disease progression and clinical deterioration, unacceptable toxicity, withdrawal of consent, death, or other protocol-defined reasons for discontinuation. No dose modifications were allowed, but predefined dose delays were permitted for adverse events.
Patients were coded for having received radiotherapy if they received any radiotherapy for the treatment of NSCLC at any timepoint before the first cycle of pembrolizumab. Patients were coded for having received thoracic radiotherapy if they received radiotherapy to their lungs or intrathoracic lymph nodes. Radiotherapy intent was also determined, with definitive intent radiotherapy corresponding with previous curative intent thoracic radiotherapy. Receipt of either stereotactic body radiotherapy or stereotactic radiosurgery was also noted. No patients received radiotherapy during the study period per protocol.
PD-L1 status was determined using a prototype immunohistochemical assay using the anti-PD-L1 antibody clone 22C3 (Merck & Co, Kenilworth, NJ, USA) and commercially available reagents from the Dako EnVision FLEX+ HRP-Polymer Kit (Dako, Carpinteria, CA, USA) with PD-L1 positivity defined as membranous staining in at least 1% of cells (neoplastic and intercalated mononuclear inflammatory cells) as previously described.3 PD-L1 expression was determined using a clinical-trial assay developed by Dako that used the same 22C3 antibody as previously described.3
Safety was assessed on day 1 of each treatment cycle and at follow-up visits 30 days after the last dose. Toxicity was categorised and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Toxicity data was prospectively collected as previously described.3 All pulmonary toxicities were assessed, including dyspnoea, cough, wheezing, pneumonitis, and respiratory failure. Per study protocol, patients underwent scheduled CT or MRI every 9 weeks. Disease progression was prospectively determined using the investigator-assessed Immune-related Response Criteria (irRC).17
Outcomes
The primary objective of the KEYNOTE-001 trial was to evaluate the safety, side-effect profile, and antitumour activity of pembrolizumab. The primary objective of this secondary analysis was to determine the effect of previous radiotherapy on progression-free survival and overall survival with pembrolizumab treatment. An additional endpoint was to determine the effect of previous thoracic radiotherapy on pulmonary toxicity with pembrolizumab. Progression-free survival was defined as the time from the first dose of pembrolizumab to disease progression, according to investigator assessed irRC, or death from any cause. Overall survival was defined as the time from the first dose of pembrolizumab to the date of death from any cause.
Statistical analysis
We compared baseline differences in patient characteristics between subgroups using the χ2 test, Fisher’s exact test, or Student’s t test. We estimated progression-free survival, overall survival, median survival, and 95% CIs with Kaplan-Meier analysis, and we compared subgroups (previous radiotherapy versus no previous radiotherapy) using the log-rank test. We prospectively decided to do a separate analysis assessing only non-CNS radiotherapy (extracranial radiotherapy), because the blood-brain barrier potentially isolates the CNS from the systemic immune system.18,19 All analyses were done by intention to treat. We assessed patient clinical variables using univariate and multivariate Cox proportional hazards modelling to determine hazard ratios (HR), including 95% CIs, for progression-free survival and overall survival. The proportional hazards assumption was verified for the covariates using the empirical score process (appendix pp 1–4).20 Additionally, we used univariate and multivariate logarithmic regression to generate odds ratios (OR), including 95% CIs, for clinical factors that would predict for a minimum 3-month progression-free response to pembrolizumab. For comparisons, we assessed age, time interval between initial diagnosis and first dose of pembrolizumab, number of lines of systemic therapies, and PD-L1 expression as continuous variables. Variables included in the multivariate models had a p value of 0·1 or less in the univariate analysis.
We completed a separate analysis of pulmonary toxicities comparing patients who did and did not receive thoracic radiotherapy in the treatment of NSCLC. To provide the most sensitive analysis on the effect of thoracic radiotherapy on pulmonary toxicities with pembrolizumab, we evaluated all pulmonary toxicities recorded regardless of judgment about treatment association. We also included a separate analysis of toxicities that the treating investigator prospectively judged to be either possibly or probably treatment-related. We deemed results significant at p<0·05. We used SAS version 9.4 for all statistical analyses.
KEYNOTE-001 is registered with ClinicalTrials.gov, number NCT01295827.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. NS, AEL, KB, DV, JWG, EBG, and PL had full access to the raw data in the study. All authors had final responsibility for the decision to submit this report for publication.
Results
Between May 22, 2012, and July 11, 2014, 98 patients from the University of California, Los Angeles, were enrolled and received their first cycle of pembrolizumab on the KEYNOTE-001 trial. One patient transferred care to another institution 11 days after enrolment and was not included in this analysis because no follow-up data were available. All 97 remaining patients were included in this analysis. All patients presented with metastatic NSCLC at trial entry. Nine (9%) of 97 patients received 2 mg/kg pembrolizumab every 2 weeks, 35 (36%) received pembrolizumab 10 mg/kg every 2 weeks, and 53 (55%) received pembrolizumab 10 mg/kg every 3 weeks. Baseline patient characteristics are in table 1. Median patient age was 65 years (range 32–83) and most patients received several lines of systemic therapy before study enrolment (median 3, range 0–6). PD-L1 status (positive vs negative) was available for 85 (88%) of 97 patients and 17 (18%) of 76 patients who underwent testing had membranous PD-L1 expression of at least 50% (PD-L1 expression and PD-L1 status results are from two independent assays).
Table 1.
Baseline characteristics
| Previous radiotherapy
|
Previous extracranial radiotherapy
|
|||||
|---|---|---|---|---|---|---|
| No (n=55) | Yes (n=42) | p value | No (n=59) | Yes (n=38) | p value | |
| Age (years) | 66 (32·0–83·0) | 65 (36·0–77·0) | 0·36 | 66 (32·0–83·0) | 65 (36·0–77·0) | 0·36 |
|
| ||||||
| Sex | 0·79 | 0·56 | ||||
| Male | 29 (53%) | 21 (50%) | 29 (49%) | 21 (55%) | ||
| Female | 26 (47%) | 21 (50%) | 30 (51%) | 17 (45%) | ||
|
| ||||||
| ECOG performance status | 0·82 | 0·37 | ||||
| 0 | 21 (38%) | 17 (40%) | 21 (36%) | 17 (45%) | ||
| 1 | 34 (62%) | 25 (60%) | 38 (64%) | 21 (55%) | ||
|
| ||||||
| Histology | 0·24 | 0·11 | ||||
| Squamous cell | 8 (15%) | 11 (26%) | 8 (14%) | 11 (29%) | ||
| Adenocarcinoma or other | 47 (85%) | 31 (74%) | 51 (86%) | 27 (71%) | ||
|
| ||||||
| Time from initial diagnosis (months) | 17·3 (0·9–98·2) | 25·9 (2·6–107·0) | 0·042 | 17·4 (0·9–98·2) | 26·1 (2·9–107·0) | 0·033 |
|
| ||||||
| History of brain metastases | 0 | 8 (19%) | 0·0026 | 4 (7%) | 4 (11%) | 0·78 |
|
| ||||||
| Number of previous unique systemic therapies | 2 (0–5) | 3 (0–5) | 0·024 | 2 (0–5) | 3 (0–6) | 0·023 |
|
| ||||||
| No previous systemic therapies | 11 (20%) | 2 (5%) | 0·061 | 12 (20%) | 1 (3%) | 0·028 |
|
| ||||||
| PD-L1 status* | 0·75 | 0·51 | ||||
| Positive | 44 (80%) | 30 (71%) | 48 (81%) | 26 (68%) | ||
| Negative | 6 (11%) | 5 (12%) | 6 (10%) | 5 (13%) | ||
| Unknown | 5 (9%) | 7 (17%) | 5 (9%) | 7 (18%) | ||
|
| ||||||
| PD-L1 expression* | 0·46 | 0·74 | ||||
| <1% | 11 (20%) | 10 (24%) | 12 (20%) | 9 (24%) | ||
| 1–49% | 21 (38%) | 17 (40%) | 24 (41%) | 14 (37%) | ||
| ≥50% | 12 (22%) | 5 (12%) | 12 (20%) | 5 (13%) | ||
| Unknown | 11 (20%) | 10 (24%) | 11 (19%) | 10 (26%) | ||
|
| ||||||
| Smoking Status | 0·85 | 0·61 | ||||
| Non-smoker | 25 (45%) | 19 (45%) | 28 (47%) | 16 (42%) | ||
| Former smoker | 30 (55%) | 23 (55%) | 31 (53%) | 22 (58%) | ||
|
| ||||||
| Previous radiotherapy indent | ||||||
| Any | 42 (100%) | |||||
| Extracranial | 38 (91%) | 38 (100%) | ||||
| Thoracic | 24 (57%) | 24 (63%) | ||||
|
| ||||||
| Previous radiotherapy intent | ||||||
| Previous definitive radiotherapy only | 11 (26%) | 11 (29%) | ||||
| Previous palliative radiotherapy only | 27 (64%) | 23 (61%) | ||||
| Previous definitive and palliative radiotherapy | 4 (10%) | 4 (11%) | ||||
|
| ||||||
| Previous SBRT or SRS | ||||||
| Yes | 12 (29%) | 9 (24%) | ||||
| No | 25 (59%) | 24 (63%) | ||||
| Unknown | 5 (12%) | 5 (13%) | ||||
Data are median (range) or n (%). ECOG=Eastern Cooperative Oncology Group. PD-L1= programmed cell death protein ligand 1. SBRT or SRS=stereotactic body radiotherapy or stereotactic radiosurgery.
PD-L1 expression and PD-L1 status results are from two independent assays.
42 (43%) of 97 patients had previously received any radiotherapy for the treatment of their NSCLC before the first cycle of pembrolizumab. 38 (39%) of 97 patients received extracranial radiotherapy, and 24 (25%) of 97 patients received thoracic radiotherapy. Radiotherapy was delivered a median of 9·5 months (range 1·0–106·0, IQR 4·7–13·5) before the first cycle of pembrolizumab. Patients who received thoracic radiotherapy did so a median of 11·5 months (range 6·3–106·0, IQR 9·0–28·8) before the first cycle of pembrolizumab.
Patients were generally similar with regards to age, sex, ECOG performance status, tumour histology, PD-L1 status, PD-L1 expression, and smoking status, regardless of whether they had previously had radiotherapy (table 1). Patients with previous radiotherapy had a significantly greater frequency of brain metastases, received significantly more lines of unique systemic therapies, and had a significantly longer time interval between initial diagnosis and receipt of pembrolizumab than patients who had not previously had radiotherapy (table 1). Patients who received extracranial radiotherapy were generally comparable to patients who did not receive extracranial radiotherapy (table 1).
Median follow-up for surviving patients was 32·5 months (IQR 29·8–34·1). At the time of this analysis, 79 patients had died, 14 patients were alive, and the status of four patients who withdrew consent was unavailable. The immediate causes of death included malignant neoplasm progression in 66 patients; respiratory failure in four patients; unknown cause in three patients; infectious causes in two patients; diffuse alveolar damage in one patient; pneumothorax in one patient; iatrogenic intestinal perforation in one patient; and a thromboembolic event in one patient. All deaths were prospectively determined by the study investigators as unlikely to be treatment related.
Among all patients, after 80 progression events, median progression-free survival with pembrolizumab treatment was 2·1 months (95% CI 2·0–4·4) and 6-month progression-free survival was 33·7% (24·3–43·3). Among patients who received any previous radiotherapy for the treatment of NSCLC, there were 31 progression events versus 49 among patients with no previous radiotherapy. Patients who had previously received any radiotherapy had significantly longer progression-free survival with pembrolizumab than patients who did not receive radiotherapy (median progression-free survival 4·4 months [95% CI 2·1–8·6] vs 2·1 months [1·6–2·3]; 6-month progression-free survival 49% [95% CI 32–63] vs 23% [13–35]; figure, A). Among patients who received previous extracranial radiotherapy, there were 27 progression events versus 53 among patients with no previous extracranial radiotherapy. Patients who previously received extracranial radiotherapy also had significantly longer progression-free survival with pembrolizumab treatment than did patients who did not receive extracranial radiotherapy (median progression-free survival 6·3 months [95% CI 2·1–10·4] vs 2·0 months [1·8–2·1]; 6-month progression-free survival 54% [95% CI 37–69] vs 21% [12–33]; figure, B).
Figure. Effect of previous radiotherapy on progression-free survival and overall survival.
Progression-free survival in patients according to their history of (A) any radiotherapy and (B) extracranial radiotherapy. Overall survival in patients according to their history of (C) any radiotherapy and (D) extracranial radiotherapy. Hazards Ratios [HR] are shown.
Age, smoking history, ECOG performance status, any previous radiotherapy, and previous extracranial radiotherapy were significantly associated with longer progression-free survival on univariate analysis (table 2). The number of lines of previous systemic therapies, length of time from diagnosis to the first cycle of pembrolizumab, sex, histology and stage at diagnosis, and PD-L1 status and expression did not predict for progression-free survival (table 2). On multivariate analysis, any previous radiotherapy and previous extracranial radiotherapy were found to independently predict for significantly longer progression-free survival, as did an ECOG performance status of 0 (table 2). Additionally, both any previous radiotherapy and extracranial radiotherapy independently predicted for a minimum 3-month progression-free response with pembrolizumab (OR 2·86 [95% CI 1·06–7·72], p=0·039; 3·72 [1·33–10·36], p=0·012; appendix p 5).
Table 2.
Predictors of progression-free survival
| Progression-free survival* | Any previous radiotherapy and progression-free survival† | Previous extracranial radiotherapy and progression-free survival† | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age‡ | 0·98 (0·96–0·99) | 0·020 | 0·98 (0·96–1·00) | 0·053 | 0·98 (0·96–1·00) | 0·050 |
|
| ||||||
| Women vs men | 1·54 (0·98–2·41) | 0·060 | 1·48 (0·92–2·38) | 0·11 | 1·44 (0·90–2·32) | 0·13 |
|
| ||||||
| ECOG 1 vs ECOG 0 | 1·76 (1·09–2·82) | 0·020 | 1·80 (1·10–2·95) | 0·019 | 1·76 (1·07–2·88) | 0·025 |
|
| ||||||
| Former smokers vs non-smoker | 0·60 (0·38–0·95) | 0·031 | 0·78 (0·47–1·31) | 0·35 | 0·82 (0·49–1·37) | 0·45 |
|
| ||||||
| Adenocarcinoma and other vs squamous | 1·65 (0·92–2·95) | 0·092 | 1·11 (0·59–2·06) | 0·75 | 1·05 (0·56–1·98) | 0·87 |
|
| ||||||
| Any previous radiotherapy (yes vs no) | 0·59 (0·37–0·94) | 0·025 | 0·56 (0·34–0·91) | 0·019 | ·· | ·· |
|
| ||||||
| Previous extracranial radiotherapy (yes vs no) | 0·51 (0·32–0·82) | 0·0054 | ·· | ·· | 0·50 (0·30–0·84) | 0·0084 |
|
| ||||||
| PD-L1 status (positive vs negative) | 1·34 (0·64–2·79) | 0·44 | ·· | ·· | ·· | ·· |
|
| ||||||
| PD-L1 expression‡§ | 0·99 (0·99–1·00) | 0·28 | ·· | ·· | ·· | ·· |
|
| ||||||
| Number of previous systemic therapies‡ | 1·06 (0·93–1·21) | 0·37 | ·· | ·· | ·· | ·· |
|
| ||||||
| Time since diagnosis‡ | 1·00 (0·99–1·01) | 0·89 | ·· | ·· | ·· | ·· |
|
| ||||||
| Stage IV vs stage I–III at initial diagnosis¶ | 1·27 (0·78–2·09) | 0·34 | ·· | ·· | ·· | ·· |
Progression-free survival was defined as the time from the first dose of pembrolizumab. HR=hazard ratio. ECOG=Eastern Cooperative Oncology Group.
Univariate analysis.
Multivariate analysis.
Contiounous variable.
Percentage expression ranged from <1% to 100%.
All patients had metastatic disease at trial entry.
Among all patients, after 79 deaths, the median overall survival was 7·3 months (95% CI 5·3–10·7) and 6-month overall survival was 57% (95% CI 46–66). 32 patients who previously received any radiotherapy for the treatment of NSCLC had died and 47 of those who had not received radiotherapy had died. Patients who received any previous radiotherapy had significantly longer overall survival than patients who did not (median overall survival 10·7 months [95% CI 6·5–18·9] vs 5·3 months [2·7–7·7]; 6-month overall survival 73% [95% CI 56–84] vs 45% [32–57]; figure, C). Patients who previously received extracranial radiotherapy (28 deaths) for the treatment of their NSCLC also had significantly longer overall survival than did patients who did not receive extracranial radiotherapy (51 deaths; median overall survival 11·6 months [95% CI 6·5–20·5] vs 5·3 months [3·0–8·5]; 6-month overall survival 75% [95% CI 58–86] vs 45% [32–57]; figure, panel D). On univariate analysis, ECOG performance status, smoking history, receipt of any previous radiotherapy, and receipt of previous extracranial radiotherapy predicted for overall survival (table 3); however, the number of lines of previous systemic therapies, length of time from diagnosis to the first cycle of pembrolizumab, age, sex, histology and stage at diagnosis, and PD-L1 status and expression did not predict for overall survival (table 3). On multivariate analysis, any previous radiotherapy and previous extracranial radiotherapy were the only independent predictors for significantly longer overall survival (table 3).
Table 3.
Predictors of overall survival
| Overall survival* | Any previous radiotherapy and overall survival† | Previous extracranial radiotherapy and overall survival† | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age‡ | 0·98 (0·96–1·00) | 0·067 | 0·99 (0·96–1·01) | 0·18 | 0·99 (0·96–1·01) | 0·19 |
|
| ||||||
| Female vs male | 1·26 (0·81–1·98) | 0·30 | ·· | ·· | ·· | ·· |
|
| ||||||
| ECOG 1 vs ECOG 0 | 1·63 (1·01–2·62) | 0·044 | 1·62 (1·00–2·63) | 0·049 | 1·56 (0·96–2·51) | 0·072 |
|
| ||||||
| Former smoker vs non-smoker | 0·57 (0·36–0·90) | 0·015 | 0·69 (0·42–1·12) | 0·14 | 0·72 (0·44–1·19) | 0·19 |
|
| ||||||
| Adenocarcinoma and other vs squamous | 1·58 (0·87–2·86) | 0·14 | ·· | ·· | ·· | ·· |
|
| ||||||
| Any previous radiotherapy (yes vs no) | 0·62 (0·39–0·98) | 0·041 | 0·58 (0·36–0·94) | 0·026 | ·· | ·· |
|
| ||||||
| Previous extracranial radiotherapy (yes vs no) | 0·58 (0·36–0·93) | 0·024 | ·· | ·· | 0·59 (0·36–0·96) | 0·034 |
|
| ||||||
| PD-L1 status (positive vs negative) | 1·55 (0·71–3·40) | 0·27 | ·· | ·· | ·· | ·· |
|
| ||||||
| PD-L1 expression‡§ | 0·99 (0·99–1·00) | 0·39 | ·· | ·· | ·· | ·· |
|
| ||||||
| Number of previous systemic therapies‡ | 1·06 (0·93–1·21) | 0·37 | ·· | ·· | ·· | ·· |
|
| ||||||
| Time since diagnosis‡ | 1·00 (0·99–1·01) | 0·69 | ·· | ·· | ·· | ·· |
|
| ||||||
| Stage IV vs stage I–III at initial diagnosis¶ | 1·39 (0·84–2·29) | 0·20 | ·· | ·· | ·· | ·· |
HR=hazard ratio. ECOG=Eastern Cooperative Oncology Group.
Univariate analysis.
Multivariate analysis.
Contiounous variable.
Percentage expression ranged from <1% to 100%.
All patients had metastatic disease at trial entry. Overall survival was defined as the time from the first dose of pembrolizumab.
44 (45%) of 97 patients had any recorded pulmonary toxicity while on pembrolizumab treatment; no statistical differences in frequency of any pulmonary toxicity or frequency of grade 3 or worse pulmonary toxicity were noted between those who received previous thoracic radiotherapy and those who had not (table 4). Pulmonary toxicities stratified by grade are in the appendix (p 6).
Table 4.
Effect of previous thoracic radiotherapy on frequency of all recorded pulmonary toxicities and treatment-related pulmonary toxicities
| No previous thoracic radiotherapy (n=73) | Previous thoracic radiotherapy (n=24) | p value | |
|---|---|---|---|
| All recorded pulmonary toxicities* | |||
|
| |||
| Any pulmonary toxicity | 29 (40%) | 15 (63%) | 0·052 |
| Specific pulmonary toxicities | |||
| Dyspnoea | 15 (21%) | 6 (25%) | 0·64 |
| Cough | 16 (22%) | 7 (29%) | 0·46 |
| Wheezing | 3 (4%) | 1 (4%) | 0·99 |
| Pneumonitis | 1 (1%) | 2 (8%) | 0·15 |
| Respiratory failure† | 4 (6%) | 3 (13%) | 0·25 |
| Grade ≥3 pulmonary toxicity | 9 (12%) | 4 (17%) | 0·58 |
| Dyspnoea | 6 (8%) | 0 | ·· |
| Pneumonitis | 1 (1%) | 1 (4%) | ·· |
| Respiratory failure | 2 (3%) | 3 (13%) | ·· |
|
| |||
| Treatment-related pulmonary toxicities‡ | |||
|
| |||
| Any pulmonary toxicity | 1 (1%) | 3 (13%) | 0·046 |
| Specific pulmonary toxicities | |||
| Dyspnoea | 0 | 2 (8%) | 0·059 |
| Pneumonitis | 1 (1%) | 2 (8%) | 0·15 |
| Grade ≥ 3 pulmonary toxicity (pneumonitis) | 1 (1%) | 1 (4%) | 0·44 |
Toxicities were defined and graded by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
All pulmonary toxicities include any recorded toxicity regardless of judgment about treatment association.
Two cases of respiratory failure of less than grade 4 were prospectively reported by the trial investigators. These adverse events were incorrectly categorised according to CTCAE because respiratory failure can only be categorised as grade 4 or higher. Instead these adverse events should have been categorised as dyspnoea, but are shown as originally recorded.
Treatment-related pulmonary toxicities were prospectively judged to be treatment-related by the treating investigator.
A separate analysis considering only pulmonary toxicities that the treating investigator prospectively judged to be either possibly or probably treatment-related found three (13%) patients who received previous thoracic radiotherapy had treatment-related pulmonary toxicity compared with one (1%) patient who had not (table 4). The proportion of patients with specific treatment-related pulmonary events or events that were grade 3 or worse was not different between patients with or without a history of thoracic radiotherapy (table 4).
Discussion
In this secondary analysis of a subset of patients treated with pembrolizumab on the phase 1 KEYNOTE-001 study, we found that patients who had previously received radiotherapy for the treatment of NSCLC before receiving pembrolizumab had significantly longer progression-free survival and overall survival, compared with that in patients who had not received previous radiotherapy. We also found a higher number of patients with treatment-related pulmonary toxicity after pembrolizumab treatment among patients who previously received thoracic radiotherapy, but we did not find that these patients had more grade 3 or worse pulmonary toxicities. To our knowledge, these data represent the most compelling and largest clinical data to date on the effect of previous radiotherapy on enhancing the activity of anti-PD-1 therapy. Our findings are supported by dozens of preclinical studies showing synergistic antitumour activity with the combination of radiotherapy and immunotherapy,7,10,21 and suggest that the combination of radiotherapy with pembrolizumab has a clinically acceptable safety profile and shows promising activity among patients with advanced NSCLC.
For the immune system to attack tumour cells, several complex signalling cascades are required, including tumour recognition, antigen presentation, and T-cell activation. We hypothesise that by improving the priming of the antitumour immune response, radiotherapy allows for checkpoint inhibition with pembrolizumab to have increased clinical activity. Radiotherapy releases antigenic peptides from tumours,22 causes activation and migration of dendritic cells, and enhances antigen presentation by dendritic cells, leading to enhanced antitumour T-cell recognition and activity.10–13,23 Clinical work has also established that radiotherapy increases tumour-specific T cells in patients during and after treatment.24 Therefore, our findings of significantly improved pembrolizumab activity in patients who previously received radiotherapy, particularly extracranial radiotherapy, for NSCLC are supported by these previous studies showing that radiotherapy can enhance antitumour immune responses. However, baseline differences did exist between patients who did and did not previously receive radiotherapy. Patients who had radiotherapy received significantly more lines of unique systemic therapies and had a significantly longer time interval between diagnosis and the first cycle of pembrolizumab, suggesting that they might have had more indolent disease. Nevertheless, time since diagnosis and the number of lines of previous systemic therapies did not predict for progression-free survival with pembrolizumab; additionally, we found that patients presenting with stage I–III disease versus stage IV disease at initial diagnosis did not predict for improved outcomes with pembrolizumab. Therefore, this totality of evidence supports our hypothesis that previous radiotherapy enhanced the efficacy of pembrolizumab.
Patients with high PD-L1 expression have been found to be more likely to respond to anti-PD-1 therapy, but only about 23–28% of patients with advanced NSCLC have high PD-L1 expression.3,6 Among unselected patients with advanced NSCLC, responses to pembrolizumab have been achieved in between 17% to 19% of patients, with median progression-free survival ranging from 2·3 to 3·7 months.3,5 Responses to pembrolizumab were similar regardless of tumour histology, EGFR and KRAS mutation status, previous tyrosine-kinase inhibitor therapy, and number of previous lines of systemic therapies (1–2 vs ≥3); however, treatment-naive patients have been found to have longer progression-free survival with pembrolizumab compared with previously treated patients.3,5 More studies4,6 with pembrolizumab in patients with high PD-L1 expression have found comparably longer median progression-free survivals ranging from 5·0 to 10·3 months.4,6 Median progression-free survival in our cohort of patients was 2·1 months, which is in line with the literature because only 17·5% of patients had PD-L1 expression of at least 50%. Despite fewer patients having high PD-L1 expression in the subset who had received radiotherapy, these patients had significantly longer progression-free survival than those who had not received previous radiotherapy, suggesting the potential of radiotherapy to convert traditional non-responders to responders.
Pembrolizumab and other anti-PD-1 therapies are generally well tolerated in clinical trials;3–6 however, concerns exist about increased adverse events with radiotherapy and immunotherapy combinations.25,26 A particular consideration is high-grade pneumonitis, a shared toxicity with anti-PD-1 therapy and radiotherapy. Estimated incidence of all-grade pneumonitis among patients with NSCLC treated with anti-PD-1 therapies is 4·1%.27 We found patients who previously received thoracic radiotherapy were more likely to have any-grade pulmonary toxicity, pneumonitis, and respiratory failure, suggesting the need for close toxicity monitoring in this subset of patients. These data again highlight the role of radiotherapy in priming the immune response and thereby potentiating immune-mediated toxicity. Although high-grade pulmonary toxicity was similar between patients with and without previous thoracic radiotherapy, these data do suggest the need for closer toxicity monitoring among patients who previously received thoracic radiotherapy.
All patients included in this study were treated and followed per the phase 1 KEYNOTE-001 trial, providing a relatively homogeneous sample with prospectively gathered data, contributing to robustness of our findings. But, full details about radiotherapy dose, fractionation, and planning were not available for many patients, which limited more sensitive analyses, and PD-L1 status was not available for all patients. Although this study represented a single institution analysis, no information exists to indicate that this patient population was significantly different from the remainder of the study population. Additionally, although we have adjusted for and attempted to thoroughly investigate confounding factors, other unaccounted biases could still exist. Although this represents, to our knowledge, the largest clinical dataset for assessing the effect of previous radiotherapy on the activity of pembrolizumab, these data need to be further validated. Although these data suggest encouraging activity and warrant further trials, presently, radiotherapy should not be delivered with the sole purpose of improving the efficacy of immunotherapy outside of the context of clinical trials.28
In conclusion, preclinical studies have detailed the ability of radiotherapy to enhance antitumour immune responses. We show that patients who previously received radiotherapy for their NSCLC have significantly longer progression-free survival with pembrolizumab treatment than those who did not receive previous radiotherapy. Overall, these data suggest an acceptable safety profile and a promising activity for the combination of radiotherapy and pembrolizumab for the treatment of advanced NSCLC.
Supplementary Material
Acknowledgments
Funding: US National Institutes of Health.
We thank the patients and their families who participated in this study. This research was supported by National Institute of Health (grant RO1CA208403 [EBG]). We thank Xiaoyan Wang (University of California, Los Angeles) for her assistance with our statistical analyses.
Footnotes
Contributors
All authors contributed to study conception and design; data collection, assembly, analysis and interpretation; and drafting or critical revision of this article.
Declaration of interests
EBG reports grants from Merck during the conduct of the KEYNOTE-001 study; grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Genentech, Mirati, Pfizer, and Novartis outside the submitted work. All other authors declare no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Didkowska J, Wojciechowska U, Manczuk M, Lobaszewski J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Trans Med. 2016;4:150. doi: 10.21037/atm.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, bms-936558, ono-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84:879–80. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–32. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 9.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific pd-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 13.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–69. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 14.Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;16:e87415. doi: 10.1172/jci.insight.87415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–77. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 18.Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 21.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–22. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larmonier N, Merino D, Nicolas A, et al. Apoptotic, necrotic, or fused tumor cells: an equivalent source of antigen for dendritic cell loading. Apoptosis. 2006;11:1513–24. doi: 10.1007/s10495-006-8765-0. [DOI] [PubMed] [Google Scholar]
- 23.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 24.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14:4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2:831–38. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinov T, Fife BT. Fractionated radiotherapy combined with PD-1 pathway blockade promotes CD8 T cell-mediated tumor clearance for the treatment of advanced malignancies. Ann Transl Med. 2016;4:82. doi: 10.3978/j.issn.2305-5839.2016.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–16. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 28.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J ImmunoTherapy Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.