Abstract
While the normal functions of histamine (HA) in the central nervous system have gradually come into focus over the past 30 years, the relation of abnormalities in neurotransmitter HA to human disease has been slower to emerge. New insight came with the 2010 description of a rare nonsense mutation in the biosynthetic enzyme histidine decarboxylase (Hdc) that was associated with Tourette syndrome (TS) and related conditions in a single family pedigree. Subsequent genetic work has provided further support for abnormalities of HA signaling in sporadic TS. As a result of this genetic work, Hdc knockout mice, which were generated more than 15 years ago, have been reexamined as a model of the pathophysiology of TS and related conditions. Parallel work in these KO mice and in human carriers of the Hdc mutation has revealed abnormalities in the basal ganglia system and its modulation by dopamine (DA) and has confirmed the etiologic, face, and predictive validity of the model. The Hdc-KO model thus serves as a unique platform to probe the pathophysiology of TS and related conditions, and to generate of specific hypotheses for subsequent testing in humans. This chapter summarizes the development and validation of this model and summarize recent and ongoing work using it to further investigate pathophysiological changes that may contribute to TS and related conditions.
Keywords: histamine, histidine decarboxylase, Tourette syndrome, tic disorders, obsessive-compulsive disorder, animal model
INTRODUCTION
Histamine (HA) is a biogenic amine that has long been appreciated to have important roles in the periphery, particularly in the regulation of inflammation (Falus et al., 2004). It was recognized as a neurotransmitter in 1984, when Panula and colleagues identified HA-positive neurons in the posterior hypothalamus (Panula et al., 1984). Since that time, a substantial literature has examined the functions of neurotransmitter HA throughout the brain (Haas et al., 2008; Panula and Nuutinen, 2013).
A new window into the role of histamine dysregulation in neuropsychiatric disease was opened by a landmark 2010 genetic study. A combination of linkage analysis and exome sequencing in a family with an exceptionally high incidence of Tourette syndrome (TS), together with a range of comorbid conditions, identified a rare nonsense mutation in the gene histidine decarboxylase (Hdc) that segregated with the TS phenotype (Ercan-Sencicek et al., 2010). Hdc encodes the enzyme that converts the amino acid histidine into HA and is essential for HA biosynthesis in mammals (Haas et al., 2008). This genetic finding represented the first time that HA dysregulation had been associated with TS.
The TS-associated Hdc mutation has a number of characteristics that make it particularly well suited for study in animals, as further elaborated below. Hdc knockout mice were generated 15 years ago by Ohtsu and colleagues (Ohtsu et al., 2001) and had been studied in a variety of contexts, but they had not been conceived as a model of TS prior to 2010. Since then, a number of studies have examined these mice a potential model of the pathophysiology of TS. Studies to date have established the validity of the model at several levels (Castellan Baldan et al., 2014), motivating ongoing work to use these animals as a platform for further investigations of the pathophysiology of TS and related disorders. This work is summarized in this chapter.
Clinical features and pathophysiology of tic disorders
Tics are sudden, rapid, recurrent, non-rhythmic, semi-voluntary movements. Simple tics include such movements as blinking, sniffing, grunting, and turning the head; they are most common in the face but can affect any part of the body. Tics can also be more complex and can incorporate multi-step head, arm, or trunk movements and more complex utterances, including complete words or phrases. The spasmodic production of profanity, or coprolalia, is rare, but represents a particularly striking form of complex vocal tic. Tics are described as semi-voluntary, because most individuals (especially adults) are aware of a sense of tension or discomfort preceding the tic; this is known as a ‘premonitory urge’. A tic discharges this tension, much as a sneeze discharges a growing discomfort in the back of the nose. Most individuals with tics can suppress them to an extent; however, as with a sneeze, suppressing a tic requires effort and is typically accompanied by increasing discomfort. Tics are lessened by relaxation, sleep, and focused concentration; they are worsened by stress and sleep deprivation (Du et al., 2010; Leckman, 2002).
Tics are common, occurring in mild forms in approximately 20% of young people; clinically significant tics occur in about 5%. Tourette syndrome consists of chronic motor and vocal tics, beginning in childhood and persisting for at least a year; it affects ~1% of the population (Robertson et al., 2009; Scahill et al., 2001). Tics and TS are more common in males, with a sex ratio of ~3:1 (Scahill et al., 2001; Scharf et al., 2012). They are also more common in children; approximately 75% of children with a clinically significant tic disorder will improve to the point that they no longer have clinically significant tics by young adulthood (Leckman, 2002).
‘Pure’ TS is uncommon: up to 90% of individuals with a diagnosis of TS carry at least one additional diagnosis, most commonly obsessive-compulsive disorder (OCD) and attention deficit-hyperactivity disorder (ADHD) (Hirschtritt et al., 2015). Tics are also commonly seen in individuals with autism spectrum disorder (ASD) (Canitano and Vivanti, 2007). Given this high level of comorbidity, the pathophysiology of tics can be expected to overlap with that of some of these other conditions. A relationship with OCD is particularly clear and has been the subject of considerable study (Pittenger, 2017). TS and OCD often run together in families and have some shared genetic risk (Davis et al., 2013; Du et al., 2010). Both are associated with dysregulation of the cortico-basal ganglia circuitry (Leckman et al., 2010; Maia et al., 2008).
Current understanding of the neurobiology of TS is limited. Structural neuroimaging studies have implicated the striatum and afferent cortical areas: the caudate and putamen are slightly but significantly smaller in both children and adults with TS, and afferent sensorimotor cortical areas are thinner (Leckman et al., 2010; Pittenger, 2017). Functional neuroimaging suggests phasic abnormalities in activity in this circuitry; tics are associated with increased activity in motor and premotor areas and in the putamen, while effortful tic suppression is associated with activity in more anterior frontal areas and in the caudate. The supplementary area (SMA) is particularly clearly implicated in TS: activity in the SMA uniquely differentiates tics from topographically similar volitional movements (Hampson et al., 2009); and stimulation of the SMA in humans produces both tic-like movements and accompanying urges (Fried et al., 1991).
Several pathophysiological theories of TS, which are by and large not mutually exclusive with one another, have been advanced (Pittenger, 2017). One proposal is that TS is associated with elevated dopamine (DA) tone in the striatum. This proposal is based on several observations. First, dopamine D2R receptor blockers are the most efficacious pharmacotherapy for tics (though their use is limited by their side effects) (Bloch, 2008). Second, psychostimulant drugs and DA agonists can trigger stereotypic behaviors in rodents that have been interpreted as tic-like (Canales and Graybiel, 2000); supratherapeutic psychostimulant challenge can trigger or transiently worsen tics in patients (Denys et al., 2013; Feinberg and Carroll, 1979). Third, some neurochemical imaging studies, though not all, suggest elevated basal and evoked dopamine release in the striatum in patients with tics (Denys et al., 2013; Singer et al., 1992; Wong et al., 2008).
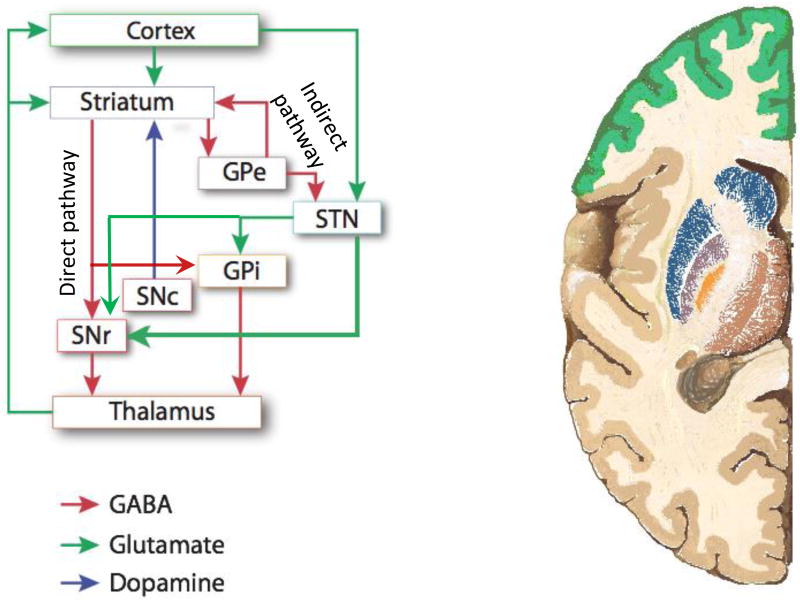
The striatum is the largest nucleus of the basal ganglia and their primary input; in primates, it consists of the caudate and putamen, though these are not discrete structures in rodents. Projections to the striatum and thence to the deeper components of the basal ganglia have classically been described as consisting of two parallel systems, termed the direct and indirect pathway. This scheme is a simplification but is of considerable heuristic value; it appears to be particularly applicable in the dorsal striatum (Figure 1). Striatal medium spiny neurons (MSNs) of the direct pathway (dMSNs) express D1R dopamine receptors and have a polysynaptic disinhibitory effect on the thalamic output of the basal ganglia system. MSNs of the indirect pathway (iMSNs) express D2R dopamine receptors and polysynaptically inhibit the thalamus. Recent data support the idea that these two pathways work in synergy in the process of action selection, with the direct pathway promoting a selected action through disinhibition of relevant thalamocortical feedback, while the indirect pathway inhibits off-target actions through thalamic inhibition (Cui et al., 2013; Hikosaka et al., 2000; Mink, 2003). In TS, a modest elevation of tonic DA is likely to primarily affect D2R receptors on MSNs of the indirect pathway, because the D2R receptor has a much higher affinity for DA than the D1R receptor. The D2R receptor reduces firing of MSNs of the indirect pathway, and so increased D2R tone is predicted to lead to disinhibition of off-target behaviors – which may, it has been proposed, manifest as tics (Albin et al., 1989; Mink, 2001, 2003; Pittenger, 2017).
Figure 1. Major pathways through the cortico-basal ganglia circuitry.
Dysregulation of the cortico-basal ganglia circuitry is implicated in TS and tic disorders, as well as in OCD and related conditions (Leckman et al, 2010; Maia et al, 2008; Pittenger, 2017). Projections from the cortex and thalamus through the nuclei of the basal ganglia can be conceptualized as traversing two pathways: the direct pathway, which polysynaptically disinhibits thalamic feedback to cortex, and the indirect pathway, which polysynaptically inhibits this feedback. Balance between these two pathways is regulated by dopamine and, perhaps, by histamine. In the Hdc-KO model of TS pathophysiology (and, it is proposed, in TS and tic disorders in humans), both DA dysregulation and HA deficiency lead to hyperactivity in the direct pathway and hypoactivity in the indirect pathway; the latter, in particular, may lead to deficient inhibition of off-target action patterns, which may manifest as tics and other repetitive behaviors. See text for further details. Adaptd from Pittenger, Bloch, and Williams, 2011.
A related model is that TS is associated with abnormal inhibition in the striatum. More specifically, localized foci of disinhibition have been proposed to produce domains of autonomous neuronal firing, which manifest as tics (Albin and Mink, 2006). This has been directly tested in animals: injection of GABA-A receptor antagonists into the monkey striatum produce tic-like movements of the contralateral limb and face (Bronfeld and Bar-Gad, 2013; McCairn et al., 2009). Similar phenomena have been documented in rats and mice (Bronfeld et al., 2013; Pogorelov et al., 2015). Post-mortem studies of individuals with severe, refractory tics have documented interneuronal abnormalities in the striatum, providing a potential explanation for deficient inhibition (Kalanithi et al., 2005; Kataoka et al., 2010; Lennington et al., 2016). And targeted disruption of inhibitory interneurons in otherwise normal mice enhances repetitive movements, providing support for a causal role for disrupted intrastriatal inhibition in the development of tics (Xu et al., 2015a; Xu et al., 2016).
A final perspective on the pathophysiology of TS, OCD, and related conditions, somewhat less well specified than the preceding, focuses on the dysregulation of neuroinflammatory processes. This focus derives from the observation that individuals with TS often exhibit other evidence of dysregulated immune function (Elamin et al., 2013). An extreme example of this is seen in the syndrome of pediatric autoimmune neuropsychiatric disorder associated with Streptococcus, in which an autoimmune reaction triggered in a susceptible host by a Streptococcal infection is thought to lead to basal ganglia inflammation (Williams and Swedo, 2015). But activation of microglia, the brain’s principle inflammatory cells, has also been seen in TS more generally, both in vivo (as measured by PET imaging using a marker of microglial activation (Kumar et al., 2015)) and post mortem (Lennington et al., 2016). Furthermore, individuals with TS, as a group, exhibit abnormalities in a number of peripheral immunological markers (Elamin et al., 2013). Several animal models have demonstrated that experimentally induced microglial abnormalities can produce repetitive behavior, typically elevated grooming (Chen et al., 2010; Zhan et al., 2014). Thus, while the details remain to be established, microglial activation and dysregulated neuroimmune interactions are an increasing focus of interest in the study of TS pathophysiology (Frick and Pittenger, 2017).
These various pathophysiological considerations allow us to enumerate a number of testable predictions that can be investigated in any model of TS. In addition to behavioral phenotypes (repetitive movements; prepulse inhibition deficits), a valid model of TS may be expected to exhibit modest elevations in tonic striatal DA, tonic or phasic alterations in striatal neuronal activity, abnormalities in striatal inhibition, and possibly abnormalities in neuroinflammatory processes.
Animal models of tic disorders
A number of studies over the past 30 years have sought to model tic pathophysiology in animal models (Godar et al., 2014; Pittenger, 2014). Analysis of the Hdc-knockout model, described in more detail below, has drawn on approaches and principles established in this previous work, which motivates discussion of past models here. It is important to acknowledge at the outset that no animal model of TS (or of OCD, or of any other complex neuropsychiatric condition) should be expected to recapitulate the human syndrome in its entirety (Pittenger, 2014; Pittenger et al., 2017).
There are several reasons for this. First, human neuropsychiatric syndromes are themselves complex and heterogeneous categories that may not represent natural kinds and are likely to be recharacterized and recategorized as understanding of pathophysiology advances (Insel and Cuthbert, 2015). Second, important aspects of TS are not readily assessed in an animal: for example, repetitive, tic-like behaviors can be observed and quantified, but it is impossible to assess whether they are associated with the premonitory urges that are characteristic of tics. Conversely, it may be unclear what human symptom (if any) a repetitive behavior in an animal best recapitulates: a repetitive behavior such as elevated grooming (Kalueff et al., 2016) could be homologous to tics, but it could as easily be argued to recapitulate symptoms of autism (Peca et al., 2011), OCD (Greer and Capecchi, 2002; Shmelkov et al., 2010; Welch et al., 2007), trichotillomania (Feusner et al., 2009), or some other condition. It is thus perilous to interpret the disease-relevance of an animal model or of a particular behavioral phenotype based solely on its resemblance to human symptomatology (that is, on its face validity) (Pittenger et al., 2017).
Finally, while the overall anatomical organization of the cortico-basal ganglia system is preserved between humans and rodents, there are key differences, such as the prominence of the globus pallidus interna (equivalent to the entopeduncular nucleus, which is fairly rudimentary, in rodents), and the fraction of basal ganglia output that projects to thalamus (predominant in humans) versus midbrain and brainstem structures (predominant in rodents). Because of these differences, even a rodent model that captures core pathophysiology perfectly might have behavioral consequences that are not perfectly isomorphic to tics.
For these reasons, it is better to speak of models that capture aspects of the pathophysiology of a disorder, rather than a disorder in its entirety. Such models are at their strongest when they are based on a clear causal hypothesis – that is, when they have clearly specified construct or etiologic validity (Pittenger et al., 2017). A series of such models have been described in TS and are contributing to increased understanding of the disorder (Godar et al., 2014; Pittenger, 2014, 2017). The most informative models can be understood as testing specific hypotheses of the pathophysiology of TS.
As noted above, pharmacological treatments that increased dopamine or dopamine receptor tone, such as psychostimulants, produce repetitive stereotypic behaviors that have some characteristics of tics (Iversen and Creese, 1975; Lyon and Robbins, 1975). This phenomenon provided early support for the idea that elevated dopamine levels may explain, or at least contribute to, the development of tics. These stereotypic movements after psychostimulant treatment have been observed to correlate with preferential activation of striosomes, neurochemically and synaptically distinct patches of cells within the striatum (Canales and Graybiel, 2000). Whether tics correspond to differential activity in striosomes in humans is difficult to test and has not been clearly established, and the validity of amphetamine-induced stereotypies as a model of tics has been questioned (Pittenger, 2014).
As noted above, neuroimaging data suggest that corticostriatal circuits are dysregulated and hyperactive in both TS and OCD (Leckman et al., 2010; Maia et al., 2008). Experimental activation of these circuits constitutes a test of the hypothesis that such dysregulation can lead to repetitive, tic-like behaviors. This was first done in a transgenic model described by Burton and colleagues almost 20 years ago (Campbell et al., 1999; Nordstrom and Burton, 2002). They expressed a transgene that increases neural activity – the alpha subunit of the cholera toxin – in a subset of D1R -expressing neurons in the forebrain. This leads to hyperactivity of both cortical and amygdalar projections to the striatum, and corresponding behavioral perseveration, grooming abnormalities, repetitive jumping, and other abnormalities. More recently, a more precise optogenetic approach has been used to perturb cortical projections to the striatum (from the orbitofrontal cortex, in this case); brief daily stimulation of striatal afferents has been found to result in persistently elevated repetitive behaviors (grooming) (Ahmari et al., 2013). In neither of these cases are the repetitive behaviors wholly isomorphic to tics; but the ability of experimentally induced dysregulation of the corticostriatal circuitry can produce repetitive behaviors supports the association of abnormal cortico-striatal activity with TS-relevant phenomenology.
Animal model evidence that disrupted local inhibition within the striatum can produce tic-like repetitive behavioral pathology (Bronfeld et al., 2013; McCairn et al., 2009; Pogorelov et al., 2015) or elevated grooming (Xu et al., 2015b; Xu et al., 2016) is reviewed above. These studies confirm the ability of inhibitory deficits within the basal ganglia circuitry to produce TS-relevant effects.
Genetics of TS: a focus on rare genes of large effect
TS is substantially genetic; recent estimates place heritability at approximately 50% (Davis et al., 2013). However, specific genetic risk factors have been slow to emerge (Fernandez et al., 2017). The one genome-wide association study (GWAS) reported to date identified a few suggestive associations, but none that reached the statistical threshold of genome-wide significance (Scharf et al., 2013).
Common risk alleles of small effect size will no doubt emerge from GWAS analyses as more subjects are studied. However, such mutations are of limited value in the modeling of pathophysiology in animals: recapitulation in an animal of a mutation that increases the risk of developing TS only modestly is likely to have very subtle effects. For a mutation to recapitulate pathophysiology in an animal model, it should ideally have a large effect size, such that carriers are extremely likely to develop disease (i.e., the mutation can be described as a cause of disease, not just a risk factor). Such mutations are invariably rare, and thus difficult or impossible to identify using GWAS methods. Despite their rarity and the attendant challenges of discovering and characterizing them, investigation of such rare mutations of large effect has proven to be of substantial value in other contexts (Geschwind and State, 2015).
In TS, several genes have been identified in which rare mutations of large effect are potentially causative (Fernandez et al., 2017). The first to be described, Slitrk1, was identified in a patient in which the gene was disrupted by a chromosomal translocation. Subsequent work identified a nonsense mutation and a mutation disrupting a 3’ regulatory site on the mRNA, both of which were associated with TS (Abelson et al., 2005). The functions of Slitrk1 are not well understood, but it is expressed at high levels in the developing brain (Stillman et al., 2009) and can regulate dendritic outgrowth (Abelson et al., 2005). Despite the genetic evidence that mutations in this gene can cause TS, knockout mice have mood and anxiety phenotype, and have not been reported to exhibit abnormal movements (Katayama et al., 2010). This animal model has yet to shed light on TS pathophysiology.
In contrast, a second rare mutation associated with TS, in the gene Hdc, has produced a highly informative animal model. This is the focus of the remainder of this chapter.
Histidine decarboxylase mutations and other disruptions of HA neurotransmission in TS
In 2010, State and colleagues described a two-generation pedigree in which a father and eight children all had chronic tics or TS (Ercan-Sencicek et al., 2010). The mother and her extended family had no history of TS, OCD, or related diagnoses. Linkage analysis in this pedigree identified a single interval, on chromosome 15, that segregated with the TS phenotype. Exome sequencing of this interval identified a single coding-frame mutation: a nonsense mutation, W317X, in histidine decarboxylase. This mutation truncates the protein and renders it catalytically inert – in fact, in vitro evidence suggests that the truncated protein functions as a dominant negative, inhibiting the ability of wild-type protein to catalyze the conversion of histidine into histamine (Ercan-Sencicek et al., 2010).
This study focused attention on the potential role of HA dysregulation in the development of TS for the first time (Bloch et al., 2011). However, the Hdc W317X mutation is vanishingly rare. Two subsequent genetic studies support the possibility that HA dysregulation contributes to TS more broadly (though still, most likely, in a minority of cases). First, Fernandez and colleagues performed a copy number variation (CNV) analysis in individuals with TS (Fernandez et al., 2012). While Hdc itself was not disrupted by any of the detected CNVs, unsupervised pathway analysis of genes affected by CNVs in TS implicated disruption of HA-mediated signaling. Second, Karagiannidis and colleagues examined markers of common variants at the Hdc locus in several hundred individuals with TS, and matched controls, and found overtransmission of a particular haplotype in patients; this suggests a contribution of common variants at this locus to disease risk (Karagiannidis et al., 2013). HA dysregulation is almost certainly still a rare cause of TS, but these findings suggest that it is not unique to the originally described Hdc-W317X family (Ercan-Sencicek et al., 2010).
To be harnessed for studying pathophysiology in an animal model, a disease-associated mutation should ideally have several characteristics; the Hdc-W317X mutation has all of them, and is thus particularly well suited for reverse translational analysis. First, as noted above, a disease-associated mutation is most likely to yield insights into pathophysiology if it has a large effect on disease risk. In the case of the Hdc-W317X mutation, every carrier who has been characterized to date (all in the originally described family) has TS or chronic tics, suggesting a large effect. Second, the mutation ideally has a known, quantifiable effect on a gene of known function. This is true in the case of Hdc-W317X: the function of the encoded enzyme is known (it is critical for the biosynthesis of HA), and the effect of the mutation is well established and quantifiable (it completely abrogates HA biosynthesis). Finally, a disease-associated mutation is more convincing if it implicates systems with a plausible link to established pathophysiology. While a link between HA neurotransmission and TS was not contemplated until a few years ago, the link is a priori plausible: as reviewed elsewhere (Haas et al., 2008; Panula and Nuutinen, 2013), including in other chapters in this volume, neurotransmitter HA modulates DA (Castellan Baldan et al., 2014; Schlicker et al., 1994) and basal ganglia function (Bolam and Ellender, 2015), both of which are implicated in TS.
The histidine decarboxylase knockout mouse as a model of TS pathophysiology: Initial validation
These considerations have motivated examination of mice in which the Hdc gene is mutated as a potential model of the pathophysiology of TS (Table 1). Initial work has not recapitulated the W317X mutation but rather has examined mice in which the Hdc gene is inactivated using conventional knockout technology; these mice were first described 15 years ago (Ohtsu et al., 2001). Hdc full knockout mice are unable to synthesize HA; while some studies have suggested low persistent levels of HA (Ohtsu et al., 2001), in these studies, HA levels in the brain are so low as to be undetectable (Castellan Baldan et al., 2014). Heterozygotes have intermediate levels of HA in brain (Castellan Baldan et al., 2014), which is important: while it has not been possible to directly assay HA levels in brain in human carriers of the Hdc W317X mutation, they are presumably reduced, but not zero. Therefore, while Hdc full knockout mice are useful probes of pathophysiology, heterozygotes may be closer to the human disease state; they have been included in some, but not all, of the analyses discussed here. This consideration also reduces the importance of any potential residual HA in the brains of KO mice: the presence of low levels of HA, below what the level of detection, does not undermine the utility of these animals as a tool to probe processes of potential relevance to TS pathophysiology.
| Characteristic | Patients w/ Hdc W317X mutation |
Hdc+/− & −/− mice |
References |
|---|---|---|---|
| Histamine biosynthesis | Reduced (in vitro) | Reduced in tissue and striatal microdialysate | Ercan-Sencicek et al, 2010; Castellan Baldan et al, 2014; Ohtsu et al, 2001 |
| Tics/stereotypy | Motor, phonic tics | Potentiated stereotypy after threshold-dose amphetamine and after stress | Ercan-Sencicek et al, 2010; Castellan Baldan et al, 2014; Xu et al, 2015b |
| Prepulse inhibition | Reduced | Reduced | Castellan Baldan et al, 2014 |
| Striatal dopamine | Not directly measured | Increased in active-phase microdialysate | Castellan Baldan et al, 2014; Rapanelli et al, 2014 |
| Striatal dopamine signaling | Not directly measured | Increased striatal Fos expression at baseline and after amphetamine | Castellan Baldan et al, 2014; Rapanelli et al, 2014 |
| Substantia nigra D2/D3 binding | Increased by in vivo PHNO PET imaging | Increased by in vitro raclopride binding | Castellan Baldan et al, 2014 |
| Dorsal striatal D2/D3 binding | No evident change, by in vivo PHNO PET imaging | Modest decrease, by in vitro raclopride binding | Castellan Baldan et al, 2014 |
As noted, Hdc knockout mice were generated years ago, and they have been characterized in a range of behavioral and neurochemical experiments, by a number of different authors (Schneider et al., 2014); they have also been extensively characterized in assays of inflammatory and immune processes (Ohtsu, 2010). Some findings may be interpreted, in retrospect, as being of relevance to the pathophysiology of TS. For example, Hdc-KO mice have been reported to have increased DA turnover in the striatum, suggestive of altered dopaminergic modulation (Dere et al., 2003). Other studies have examined anxiety-like, depression-like, learning, and other phenotypes, with variable results (Acevedo et al., 2006a; Acevedo et al., 2006b; Dere et al., 2004; Schneider et al., 2014).
At baseline, no tic-like movements, elevated grooming, or any other repetitive behavior of potential relevance to TS were evident in Hdc-KO mice. Exploratory behavior in an open field, rearing, anxiety-like behavior (Castellan Baldan et al., 2014), and fear conditioning (Xu et al., 2015b) were normal. This normal baseline behavior is at odds with some previous reports (Acevedo et al., 2006a; Dere et al., 2004). One possible explanation for this discrepancy is that different investigators have examined these mice on different genetic backgrounds (Schneider et al., 2014). These studies have been performed in males, extensively backcrossed (>N9) onto C57Bl/6. Regardless, normal baseline exploratory behaviors in these animals simplify interpretation of other behavioral phenotypes in these experiments.
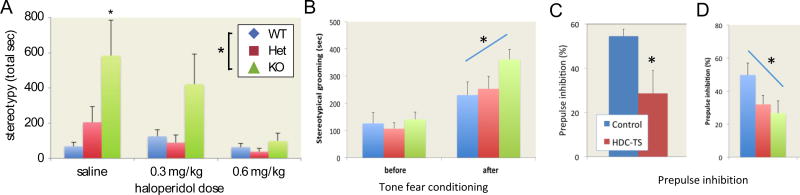
Tics in TS fluctuate dramatically (Leckman, 2002); they are potentiated by such factors as acute stress (Buse et al., 2014; Conelea and Woods, 2008), sleep deprivation, and supratherapeutic doses of psychostimulants (Denys et al., 2013; Feinberg and Carroll, 1979). To further investigate tic-like phenomenology in the Hdc-KO mice, therefore, mice were acute challenged with a high dose of the psychostimulant D-amphetamine. At a dose that produces locomotor activation but few stereotypies in a wild-type mouse (on this genetic background), stereotypies were markedly enhanced in the KO animals. At a slightly higher dose, many of the KO animals became completely immobile; heterozygotes had elevated stereotypies (Castellan Baldan et al., 2014). Pretreatment with the D2R antagonist haloperidol, which is an efficacious treatment for tics (Bloch, 2008), mitigated these stereotypies, endowing the model with a degree of predictive validity (Castellan Baldan et al., 2014) (Figure 2A). A similar interactive effect was seen after acute stress, induced by cued fear conditioning: KO animals showed elevated repetitive behavior (grooming, in this case) after the induction of stress, but not at baseline (Xu et al., 2015b) (Figure 2B).
Figure 2. Stereotypies in Hdc KO mice.
A. Stereotypies after D-amphetamine (8.5 mg/kg) were potentiated in Hdc KO and Het mice; pretreatment with haloperidol mitigated this effect. From Castellan Baldan et al, 2014, with permission. B. Stress, induced by tone fear conditioning, similarly increased stereotypical grooming. Adapted from Xu et al, 2015b. C. Prepulse inhibition (PPI), a measure of sensorimotor gating, was reduced in human carriers of the Hdc W317X mutation. D. PPI is similarly reduced in Hdc heterozygotes and knockouts. Data are shown for a 6 dB prepulse; similar effects were seen with larger prepulses. C and D from Castellan Baldan et al, 2014, with permission.
The face validity of these two repetitive behavioral phenotypes is open to question; certainly neither the repetitive stereotypic sniffing seen after amphetamine challenge nor the elevated grooming seen after stress are as clearly isomorphic to tics as the unilateral, spasmodic, non-rhythmic movements seen after focal striatal inhibition in other models (Bronfeld and Bar-Gad, 2013; Bronfeld et al., 2013; McCairn et al., 2009; Pogorelov et al., 2015). However, face validity is a fickle guide in the interpretation of animal models of tic pathophysiology (Pittenger, 2014); indeed, as argued above, both the complexity of neuropsychiatric phenotypes and the differences between human and rodent neuroanatomy suggest that even optimal recapitulation of tic pathophysiology in a mouse might produce behavioral effects that do not look identical to human tics. Therefore, in these experiments, and in other TS models (Xu et al., 2015b; Xu et al., 2016), a range of repetitive behaviors are accepted as tentatively confirmatory of relevance to TS. The claim to relevance to tics derives not from the specific topography of the behavior, but rather from the recapitulation of underlying pathophysiological processes – in this case, disruption of the Hdc gene. Put another way: an elevated grooming phenotype in isolation is difficult to interpret with respect to any particular neuropsychiatric diagnosis (Kalueff et al., 2016) and is unavoidably ambiguous; but an elevated grooming phenotype in conjunction with a clear recapitulation of a hypothesized causal factor, like Hdc gene disruption (Xu et al., 2015b), may be interpreted, at least provisionally, as confirmatory of the underlying causal hypothesis.
While tics are central to the diagnosis of TS, patients with tics typically have a range of other abnormalities, some of which are described above. Some, like the presence of premonitory urges before tics and the ability to effortfully suppress them, are difficult to assess in an animal model; but others can be assayed across species. A deficit in sensorimotor gating, indexed by prepulse inhibition (PPI), is in the latter category. Individuals with TS have deficient PPI (Castellanos et al., 1996; Kohl et al., 2013; Swerdlow et al., 2001), as do individuals with OCD (Ahmari et al., 2012; Hoenig et al., 2005; Kohl et al., 2013). PPI was tested both in human carriers of the Hdc-W317X mutation and in Hdc-KO mice. PPI of the acoustic startle reflex was impaired in both, compared to normal controls. In the mice, baseline startle was increased by Hdc knockout, but the deficit in PPI persisted after controlling for this effect. Importantly, heterozygotes – which, as noted above, may better recapitulate partial HA deficiency in the patients than do the KOs – showed an intermediate PPI deficit (Castellan Baldan et al., 2014). These PPI findings provide an additional behavioral parallel between TS patients and the Hdc-KO model (Figure 2C).
Pathophysiological mechanisms in the Hdc-KO model: Dopamine and dopamine receptors
With this validation in hand, candidate pathophysiological processes in the Hdc-KO model were investigated. The initial focus was on dopamine modulation of the striatum; as reviewed above, convergent evidence suggests a modest elevation in tonic striatal DA in patients with tics (Pittenger, 2017). Similar effects were predicted in the model.
Direct measurement of tonic extrasynaptic DA levels is possible using in vivo microdialysis. In the knockout animals, baseline striatal DA was elvated (Rapanelli et al., 2014). This baseline elevation was accentuated in the animals’ dark phase, when HA is normally elevated in mice (which are nocturnal); HA is of course absent in the KO animals, and thus this enhanced DA elevation in the dark cycle is consistent with negative regulation of DA by HA (Castellan Baldan et al., 2014). To directly test this, the effects of infusion of HA on DA levels were measured in vivo using microdialysis, in wild-type mice. As predicted, intacerebroventricular HA infusion reduced striatal DA levels (Castellan Baldan et al., 2014). This elevation in tonic extrasynaptic DA, which accords with current thinking about TS, provides further confirmation that the Hdc-KO model is recapitulating key aspects of pathophysiology.
What is the mechanism of this reduction in striatal DA levels by HA, and of the elevation in DA seen in the KO animals? Histamine binds to four G-protein-coupled receptors, H1R-H4R; H1R-H3R are expressed on neurons in the central nervous system, while H4R appears not to be (Haas et al., 2008; Schneider and Seifert, 2016). The initial focus was on H3R. This receptor couples to Galpha-i and has classically been considered to function primarily as a presynaptic inhibitor of transmitter release, both of histamine itself and of other transmitters (Haas et al., 2008). Ex vivo, it has been reported to inhibit DA release (Schlicker et al., 1994). Thus, loss of H3R tone on DA terminals in KO animals might lead to disinhibited DA release, and HA actions on H3R receptors on DA terminals might explain the reduced DA seen in vivo after HA infusion (Castellan Baldan et al., 2014).
However, recent data argue against this mechanism. The H3R agonist immepip has not been found to affect intra-striatal DA levels in wild-type mice (Alfaro-Rodriguez et al., 2013). Similarly, systemic administration of the specific agonist R-aminomethylhistamine (RAMH), at doses that produce behavioral effects (see below; Rapanelli et al., in press), does not produce the predicted reduction in striatal DA – in fact, in KO mice it produces a small but significant elevation in DA after RAMH challenge (Rapanelli et al., in press; Rapanelli et al., 2016). The ability of both endogenous and exogenous HA to reduce striatal DA levels (Castellan Baldan et al., 2014) can be concluded to depend on different receptors.
H1R is a candidate. H1R antagonists have been found to acutely increase intrastriatal DA (Dringenberg et al., 1998) and to produce rewarding effects in some behavioral paradigms (Halpert et al., 2002; Zimmermann et al., 1999), although the dependence of such effects on binding to H1R has been questioned (Oleson et al., 2012; Suzuki et al., 1999). The detailed mechanisms of HA regulation of striatal DA remain an important open question.
Elevated striatal DA is expected to produce a number of secondary effects. First, DA can activate of D1R -expressing dMSNs. Expression of the immediate early genes c-fos was modestly elevated at baseline in the striatum in Hdc-KO mice (Castellan Baldan et al., 2014), consistent with such an effect – and perhaps paralleling the dysregulation of the cortico-striatal circuitry seen in patients with TS (Leckman et al., 2010). C-fos is elevated following amphetamine challenge, as one would expect; interestingly, c-fos expression is particularly high in striosomes after amphetamine challenge in the knockout, relative to wild-type controls (Castellan Baldan et al., 2014). This parallels the specific role for striosomal MSN activity in stereotypy/tic generation suggested by fos-mapping investigations in wild-type mice (Canales and Graybiel, 2000).
Elevated striatal DA also has specific effects on molecular signaling within MSNs of both the direct and the indirect pathway (Girault, 2012). Selected signaling pathways were examined in Hdc-KO mice (Rapanelli et al., 2014). Signaling through the MAPK pathway was elevated in KO mice, consistent with a DA effect in D1R -expressing MSNs. The kinases Akt and Gsk3beta were relatively dephosphosphorylated, consistent with a DA effect in D2R -expresing MSNs. Both effects were further amplified by amphetamine treatment (Rapanelli et al., 2014). These results should be interpreted as preliminary; in particular, these initial studies did not differentiate between MSNs of the direct and indirect pathways. Work to better elucidate specific signaling alterations in these two pathways is ongoing. Additionally, the same pathways can be regulated by postsynaptic H3R receptors (Rapanelli et al., 2016); this complication is further addressed below.
A third effect of tonic elevation of striatal DA is the development of compensatory changes in DA receptor expression. In particular, treatment with both DA agonists and psychostimulants leads to decreased expression of D2R receptor in the dorsal striatum and elevated expression of the D3R receptor in the substantia nigra (Fauchey et al., 2000; Stanwood et al., 2000; Volkow et al., 2009). D2R and D3R receptors were examined in Hdc-KO mice using in vitro binding with the agonist raclopride. D2R/D3R receptor binding was downregulated in dorsal striatum, though the effect was subtle. More dramatic was the upregulation of D2R/D3R binding in the substantia nigra in KO mice (Castellan Baldan et al., 2014). These alterations are consistent with the predicted effects of chronic DA excess.
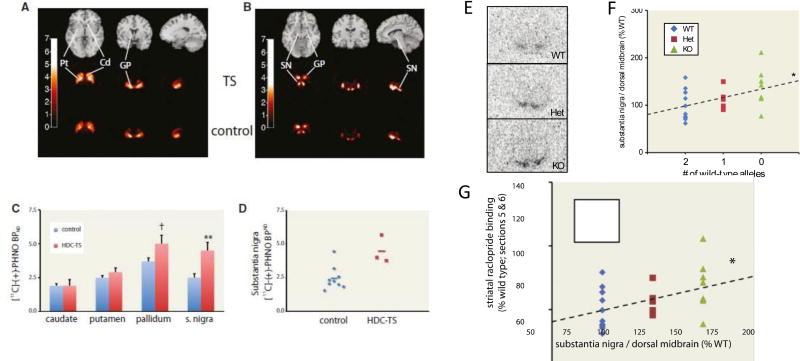
Importantly, while striatal DA levels cannot be directly assessed in humans, DA receptors can be. D2R/D3R receptors were examined in TS patients carrying the Hdc W317X mutation using positron emission tomography (PET) imaging with the agonist tracer PHNO. This investigation was limited to adult patients; after controlling for imaging quality, 3 adult carriers of the Hdc W317X mutation, and 9 matched healthy controls were included in this analysis. In this limited sample, there was no detectable alteration in striatal D2R/D3R binding. In the substantia nigra, in contrast, there was a striking upregulation of D2R/D3R receptor binding (Castellan Baldan et al., 2014). A similar pattern of increased PHNO binding in the nigra has been seen in human cocaine abusers, supporting the idea that it is a consequence of chronic DA receptor hyperstimulation (Matuskey et al., 2015; Payer et al., 2014). PHNO binding in the substantia nigra is thought to primarily reflect D3R receptor density (Rabiner et al., 2009; Tziortzi et al., 2011), although it cannot be concluded with complete certainty that the observed increase in PHNO binding is due solely to increased D3R expression. Regardless, the parallel increase in D2R/D3R binding in these patients and in Hdc-KO mice adds an additional validation of the mice as an informative model of pathophysiology (Figure 3).
Figure 3. D2/D3 receptors in humans and mice with a mutated Hdc gene.
A–D. D2/D3R receptor availability in TS patients carrying the Hdc-W317X mutation, relative to matched controls, measured using in vivo 11C-PHNO PET imaging. A,B composite radioligand binding images from patients (middle row) and controls (bottom row). C. Binding in subnuclei of the basal ganglia. D. Individual subject binding in the substantia nigra in patients and controls; group means are show by a horizontal line. E-G. D2/D3R receptor binding in mice measured ex vivo using 3H-raclopride binding. E Raclopride binding in the substantia nigra. F. Increased binding was seen in Hdc het and KO mice; individual data are shown. G. Reduced raclopride binding was seen in dorsal striatum; this correlated negatively, on an animal-by-animal basis, with the increased binding in the nigra. From Castellan Baldan et al, 2014, with permission.
Pathophysiological mechanisms in the Hdc-KO model: Histamine receptors
HA receptors have been previously examined in Hdc-KO mice; for example, H3R receptors have been reported to be downregulated in hippocampus and upregulated in hypothalamus in these animals (Chepkova et al., 2012). All four HA receptors were examined in the basal ganglia, using both radioligand binding and in situ quantification of mRNA expression (Frick et al., 2016; Rapanelli et al., in press). H2R receptors are decreased in the striatum in Hdc-KO mice, at the level of ligand binding, but not of mRNA expression; this suggests post-translational regulation of receptor level, alteration in affinity rather than expression, or decreased expression on afferents (with the corresponding mRNA alterations elsewhere in the brain). H4R receptors are also decreased, at the level of both mRNA and ligand binding; this alteration is further addressed below. H1R receptor are unchanged (Frick et al., 2016; Rapanelli et al., in press).
H3R receptors are increased in KO mice (Rapanelli et al., in press). H3R receptors have high constitutive activity, at least in histaminergic neurons themselves (Morisset et al., 2000). This raises the intriguing possibility that elevated H3R expression may influence striatal function in KO mice even in the absence of its ligand, HA. Systemic administration of the H3R agonist RAMH produced stereotypies in KO mice. The same effect is produced by the chemically dissimilar agonist immepip; it is blocked by the H3R antagonist JNJ5207852, further confirming the specificity of the effect (Rapanelli et al., in press). These observations provide further support for the idea that H3R activity contributes to tic-like phenomenology in these mice.
As noted above, H3R has classically been considered a presynaptic receptor negatively regulating transmitter release (Haas et al., 2008; Schlicker et al., 1994); and indeed there is evidence for such a role on glutamatergic afferents to the striatum (Ellender et al., 2011). However, it is increasingly evident that postsynaptic H3R receptors play a prominent and complex role in the striatum (Bolam and Ellender, 2015; Panula and Nuutinen, 2013). Postsynaptic H3R receptors interact physically and functionally with both D1R and D2R receptors, and their signaling properties are markedly different in different MSN types (Ferrada et al., 2008; Ferrada et al., 2009; Moreno et al., 2011).
These observations were confirmed and extended in MSNs in vivo, in wild-type mice (Rapanelli et al., 2016). After acute challenge with the H3R agonist RAMH, the MAPK signaling pathway is rapidly and transiently activated in D1R -expressing dMSNs, but not in D2R-expressing MSNs of the iMSNs. cAMP-dependent modulation of the key regulatory molecular DARPP-32 is not affected by RAMH in either cell type; this is surprising in light of the traditional concept of H3R as a Galpha-i-coupled receptor, which would be expected to reduce cAMP. Regulation of the Akt-GSK signaling pathway is particularly interesting. DA acting on D2R receptors in iMSNs inhibits Akt, thus dephosphorylating and thereby activating GSK (Beaulieu et al., 2005). H3R receptor activation recapitulates this effect. In dMSNs, on the other hand, DA has no effect on Akt-GSK signaling, but H3R activation activates Akt, thereby phosphorylating and inhibiting GSK (Rapanelli et al., 2016). This differential regulation of Akt/GSK signaling in such similar cell types by H3R may be unique; its importance is a topic of active investigation.
These abnormalities in signaling in vivo after RAMH challenge in wild-type mice are similar to the basal abnormalities seen in knockout animals (Rapanelli et al., 2014). These signaling abnormalities may relate to elevated tonic DA, as discussed above. However, since H3R receptors are upregulated in Hdc-KO mice, these changes may also result from constitutive effects of H3R (presuming that postsynaptic striatal H3R receptors have the same high constitutive activity that has been reported in other contexts; Morisset et al., 2000). These possibilities are not mutually exclusive; DA elevation and H3R upregulation may have additive or interactive effects, the details of which have yet to be worked out.
Pathophysiological mechanisms in the Hdc-KO model: modulation of microglia
As noted above, convergent evidence suggests an immune or neuroinflammatory contribution to TS, at least in some cases (Elamin et al., 2013; Frick and Pittenger, 2017; Kumar et al., 2015; Lennington et al., 2016; Williams and Swedo, 2015). HA is a regulator of allergic and inflammatory processes, and dysregulation of peripheral inflammatory processes has been extensively investigated in the Hdc-KO mice (Ohtsu, 2010). This motivated us to investigate the effects of HA on microglia, the primary inflammatory cells in the brain. Previous in vitro investigations of HA regulation of acutely isolated or cultured microglia have produced conflicting results.
These questions were further examined in vivo, in wild-type and Hdc-KO mice (Frick et al., 2016). HA infusion into the brain in vivo leads to an increased density of and marked morphological changes in microglia, particularly in the striatum and hypothalamus (Frick et al., 2016). This appears to be mediated by the H4R receptor (Frick et al., 2016), which is thought to be expressed on microglia but not on neurons (Schneider and Seifert, 2016). Conversely, in Hdc-KO mice, microglia are normal in number but reduced in their ramifications, and the H4R receptor is downregulated (Frick et al., 2016), suggesting that HA regulation is important under physiological conditions. (Of note, these studies used a relatively crude measure of microglial process density, the optical density of Iba1 immunostaining; while this measure efficiently reveals differences between groups and between conditions, its relationship to microglial functional ‘activation’ is unclear.)
This latter observation was initially puzzling, as it contrasts with what has been reported in patients with TS: increased activation of microglia, and increased expression of microglial markers (Frick and Pittenger, 2017; Kumar et al., 2015; Lennington et al., 2016). A resolution to this conundrum may be seen in the recent distinction between neuroprotective and inflammatory microglia (Olah et al., 2011). Some in vitro studies (Ferreira et al., 2012; Iida et al., 2015), though not all, suggest that HA-stimulated microglia may have a neuroprotective phenotype, and that HA may antagonize the classical inflammatory effects of stimuli such as lipopolysaccharide (LPS). In vivo, Hdc-KO mice to have a reduction in the fraction of microglia expressing the neurotrophin IGF-1, which is thought to be a marker of such neuroprotective microglia (Frick et al., 2016). This suggests an intriguing hypothesis, which may have pathophysiological significance: that absence of HA in Hdc-KO mice may lead to a deficit in neuroprotective microglia and, perhaps, to a consequent dysregulation of neuroinflammatory responses (Frick and Pittenger, 2017).
This hypothesis was tested by administering LPS to Hdc-KO mice. As predicted, Hdc-KOs showed an overexuberant microglial response to LPS challenge, apparent both in microglial morphology and in the production of the Th1 interleukin IL-1. As a consequence, microglial ramifications, which were reduced at baseline in KOs relative to WT controls, were increased after LPS (Frick et al., 2016). This observation provides a potential explanation for the discrepancy between the apparently quiescent microglia seen at baseline in the KO model and the activated microglia observed in vivo and post mortem in TS (Kumar et al., 2015; Lennington et al., 2016). With respect to microglial activation, Hdc deficiency (and analogous causal factors) may represent a vulnerability factor but may not fully recapitulate the disease state; in patients, who (unlike vivarium-raised mice) are subject to a lifetime of immune challenges, this may interact with viral infections and other pro-inflammatory stimuli to unmask neuroinflammatory dysregulation.
This ‘two-hit’ model of microglial dysregulation (Frick et al., 2016; Frick and Pittenger, 2017) suggests that face-valid behavioral phenotypes may be more evident after inflammatory challenge – perhaps even that behavioral stereotypy, elevated grooming, or other TS-relevant behavioral pathology might emerge spontaneously in LPS-challenged mice. Tests of this hypothesis to date have been equivocal (unpublished data); this work is ongoing.
Interpreting the Hdc-KO model: what human condition(s) are being recapitulated?
Several different lines of analysis in this model system are summarized above, one or more of which may prove to reflect events that are occurring in patients. Analysis in any such model system is best seen as recapitulating aspects of pathophysiology, and not as capturing TS, or any other particularly disease entity, in its entirety. With this caveat, it may be asked which patients are most likely to manifest similar mechanisms.
Patients carrying the Hdc W317X mutation all have TS (or at least chronic tics; in one of the two papers describing these patients, one subject was diagnosed with chronic tics rather than the full syndrome of TS) (Castellan Baldan et al., 2014; Ercan-Sencicek et al., 2010). But, as is typical for TS, most have comorbidities: 4 OCD (2 full syndrome and 2 subclinical); 3 depression; 1 ASD; 3 social phobia; 1 trichotillomania; 1 ADHD. Thus, the mutation is not associated with tics specifically, but rather with a more complex and somewhat heterogeneous clinical syndrome.
Certain abnormalities seen in the Hdc-KO model that can be assayed across species are seen in carriers of the W317X mutation: in particular, prepulse inhibition deficits and elevated D2R/ D3R binding in the substantia nigra (Castellan Baldan et al., 2014). But it remains possible that findings in the Hdc-KO model will generalize only to patients with this or similar rare mutations affecting brain histamine. Such limited generalizability would obviously reduce the clinical impact of work in the model. Alternatively, findings from the Hdc-KO system may generalize to some or all patients with tics, or more broadly, to OCD, ADHD, or other related conditions. This is, ultimately, an empirical question, which has yet to be resolved. The answer may differ for distinct findings in the model system: for example, some candidate pathophysiological mechanisms identified in the model may be seen only in patients with tics; others may be seen in a broader range of clinical groups; and still others may have no relevance to human disease at all.
Closing the loop: testing hypotheses from the Hdc-KO model in patients
The foregoing discussion reemphasizes that, from a translational perspective, such a model system is best considered a generator of pathophysiological hypotheses for testing in humans, and not as a veridical recapitulation of a particular disease or syndrome in its entirety. Ultimately, the translational value of such a pathophysiological model lies in its ability to generate hypotheses about human disease that would not otherwise have been considered, with the ultimate goal of advancing disease diagnosis, treatment, or prevention. With this in mind, it is important to identify abnormalities in the Hdc-KO mouse system (and especially in Hdc heterozygotes) that are testable in humans.
One of these is shown in Figure 2: elevated D2R/D3R availability can be measured in vivo in humans using 11C-PHNO PET imaging, and patients carrying the W317X mutation have an abnormality that parallels that seen in the KO mice (Castellan Baldan et al., 2014). It remains to be seen whether a similar abnormality is seen in patients with TS or tics more generally. The fact that similarly increased nigral D2R/D3R binding is seen in cocaine users (Matuskey et al., 2015; Payer et al., 2014) suggests that this may be a marker of chronic DA receptor hyperstimulation (Fauchey et al., 2000; Stanwood et al., 2000), and not of tic pathophysiology specifically: that is, elevated PHNO binding may be informative with regard to mechanism, but of limited clinical specificity.
Two other of the findings described above are potentially amenable to in vivo testing using PET imaging in humans. First, H3R upregulation in the striatum, which is seen in the Hdc-KO mice and may be of importance in the generation of their repetitive behavioral pathology (Rapanelli et al., in press), can be assayed in humans using the PET ligand 11C-GSK189254 (Gallezot et al., 2016). This has not yet been done in patients with TS, OCD, or related conditions. Interestingly, the H3R gene is nominally upregulated in post-mortem tissue from adults with TS, though not to a degree that emerges with statistical significance from the limited studies that have been reported to date (Lennington et al., 2016, supplemental data).
Another finding from the Hdc-KO model that can be tested in patients, in principle, is the activation of microglia seen after inflammatory challenge (Frick et al., 2016); this can be tested using in vivo PET imaging with the radioligands 11C-PBR28 (Sandiego et al., 2015) or 11C-PK11195 (Kumar et al., 2015), which bind to markers of microglial activation. Indeed, imaging in children with TS using 11C-PK11195 has revealed elevated binding in the basal ganglia, relative to healthy adult controls (Kumar et al., 2015). Further studies will be needed to establish the generality of this abnormality.
Clinically, one ultimate translational goal of such a model is the ability to identify novel therapeutic targets. One candidate target emerges from the studies described above: the histamine H3R receptor (Rapanelli and Pittenger, 2016). It is not yet clear how the H3R receptor might best be modulated to mitigate tic-like stereotypy; but the ability of an H3R agonist to elicit repetitive behavioral pathology in the Hdc-KO system (Rapanelli et al., in press) suggests that H3R antagonism might be therapeutic. Indeed, one recent clinical study has investigated the efficacy of an H3R antagonist/inverse agonist, AZD5213. Surprisingly, in this small clinical trial, H3R antagonism produced a small but statistically significant worsening of tics (www.clinicaltrials.gov:NCT01904773). This supports the relevance of the H3R receptor for the pathophysiology of TS beyond the original W317X family, but it indicates that further work is needed to clarify how this receptor might best be targeted to produce therapeutic benefit.
Conclusion
The association of HA dysregulation with TS and related conditions emerged only recently. Mechanistic work focusing on disease-relevant abnormalities in the Hdc-KO model has advanced significantly but remains in its early stages. The initial validation of the model has been summaized (Castellan Baldan et al., 2014), and recent advances in three areas have been described: dopamine dysregulation and abnormalities in DA receptors; abnormalities in HA receptors, especially in H3R; and dysregulation of microglia and neuroinflammatory processes. Many questions remain in each of these domains.
Most importantly, the translation of these observations back to clinical subjects is incomplete. In the coming years, it is to be hoped that pathophysiological hypotheses generated in the Hdc-KO system, and related models, will be testable in patients, and will lead to new insights into the fundamental nature of tic disorders and to new strategies for mitigation or prevention.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- Akt
Ak-thymoma protein kinase, also known as protein kinase B
- ASD
autism spectrum disorder
- AZD5213
an H3R antagonist
- C57Bl/6
C57 Black-6 inbred mouse line
- cAMP
cyclic adenosine monophophate
- CNV
copy number variation
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- DA
dopamine
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein
- dMSN
direct/striatonigral pathway medium spiny neuron
- GABA
gamma-aminobutyric acid
- GPe
globus pallidus, pars externa
- GPi
globus pallidus, pars interna
- GSK3beta
glycogen synthase kinase 3-beta
- 11C-GSK189254
an H3 receptor PET tracer
- GWAS
genome-wide association study
- H1R
histamine H1 receptor
- H2R
histamine H2 receptor
- H3R
histamine H3 receptor
- H4R
histamine H4 receptor
- HA
histamine
- Hdc
histidine decarboxylase gene
- Hdc-KO
histidine decarboxylase knockout mouse
- IGF-1
insulin-like growth factor 1
- IL-1
interleukin 1
- iMSN
indirect/striatopallidal pathway medium spiny neuron
- JNJ5207852
an H3R receptor antagonist
- LPS
lipopolysaccharide
- MAPK
mitogen-activited protein kinase
- mRNA
messenger ribonucleic acid
- MSN
medium spiny neuron
- OCD
obsessive-compulsive disorder
- PANDAS
pediatric autoimmune neuropsychiatric disorder associated with Streptococcus
- 11C-PBR28
a PET tracer that binds to the peripheral benzodiazepine receptor, PBR, a marker of activated microglia
- PET
positron emission tomography
- PHNO
(+)-4-propyl-9-hydroxynaphthoxazine
- 11C-PK11195
PET tracer that binds to activated microglia
- PPI
prepulse inhibition
- RAMH
R-aminomethylhistamine
- SMA
supplementary motor area
- SNc
substantia nigra, pars compacta
- SNr
substantia nigra, pars reticulata
- STN
subthalamic mucleus
- Th1
Type-1 T-helper cell
- TS
Tourette syndrome
References
- Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res. 2006a;1071:113–123. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Pfankuch T, Ohtsu H, Raber J. Anxiety and cognition in female histidine decarboxylase knockout (Hdc(−/−)) mice. Behav Brain Res. 2006b;168:92–99. doi: 10.1016/j.bbr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alfaro-Rodriguez A, Alonso-Spilsbury M, Arch-Tirado E, Gonzalez-Pina R, Arias-Montano JA, Bueno-Nava A. Histamine H3 receptor activation prevents dopamine D1 receptor-mediated inhibition of dopamine release in the rat striatum: a microdialysis study. Neuroscience letters. 2013;552:5–9. doi: 10.1016/j.neulet.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24:119–125. doi: 10.1097/WCO.0b013e328344648c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH. Emerging treatments for Tourette’s disorder. Curr Psychiatry Rep. 2008;10:323–330. doi: 10.1007/s11920-008-0052-z. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Ellender TJ. Histamine and the striatum. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Bar-Gad I. Tic disorders: what happens in the basal ganglia? Neuroscientist. 2013;19:101–108. doi: 10.1177/1073858412444466. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Front Syst Neurosci. 2013;7:50. doi: 10.3389/fnsys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J, Kirschbaum C, Leckman JF, Munchau A, Roessner V. The Modulating Role of Stress in the Onset and Course of Tourette’s Syndrome: A Review. Behavior modification. 2014;38:184–216. doi: 10.1177/0145445514522056. [DOI] [PubMed] [Google Scholar]
- Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, Sun LY, Burton FH. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. J Neurosci. 1999;19:5044–5053. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nature neuroscience. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism : the international journal of research and practice. 2007;11:19–28. doi: 10.1177/1362361307070992. [DOI] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, Anderson GM, Loring E, Gorczyca R, Billingslea E, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;82:1186–1187. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biological psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkova A, Yanovsky E, Parmentier R, Ohtsu H, Haas HL, Lin JS, Sergeeva OA. Histamine receptor expression, hippocampal plasticity and ammonia in histidine decarboxylase knockout mice. Cell Mol Neurobiol. 2012;32:17–25. doi: 10.1007/s10571-011-9730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette’s syndrome: a review. Journal of psychosomatic research. 2008;65:487–496. doi: 10.1016/j.jpsychores.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, Neale BM, Yang J, Lee SH, Evans P, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS genetics. 2013;9:e1003864. doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, van der Doef TF, Boellaard R, Westenberg H, van Balkom A, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23:1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Spieler RE, Lin JS, Ohtsu H, Haas HL, Huston JP. Changes in motoric, exploratory and emotional behaviours and neuronal acetylcholine content and 5-HT turnover in histidine decarboxylase-KO mice. Eur J Neurosci. 2004;20:1051–1058. doi: 10.1111/j.1460-9568.2004.03546.x. [DOI] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Topic B, Spieler RE, Haas HL, Huston JP. Histidine-decarboxylase knockout mice show deficient nonreinforced episodic object memory, improved negatively reinforced water-maze performance, and increased neo- and ventro-striatal dopamine turnover. Learn Mem. 2003;10:510–519. doi: 10.1101/lm.67603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, de Souza-Silva MA, Schwarting RK, Huston JP. Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:423–429. doi: 10.1007/pl00005274. [DOI] [PubMed] [Google Scholar]
- Du JC, Chiu TF, Lee KM, Wu HL, Yang YC, Hsu SY, Sun CS, Hwang B, Leckman JF. Tourette syndrome in children: an updated review. Pediatrics and neonatology. 2010;51:255–264. doi: 10.1016/S1875-9572(10)60050-2. [DOI] [PubMed] [Google Scholar]
- Elamin I, Edwards MJ, Martino D. Immune dysfunction in Tourette syndrome. Behav Neurol. 2013;27:23–32. doi: 10.3233/BEN-120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Bolam JP. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci. 2011;31:15340–15351. doi: 10.1523/JNEUROSCI.3144-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, Abbott T, Gupta A, King RA, Pauls DL, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falus As, Grosman N, Darvas Z. Histamine : biology and medical aspects. Basel, Switzerland; Budapest, Hungary: Karger; SpringMed Pub; 2004. [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Feinberg M, Carroll BJ. Effects of dopamine agonists and antagonists in Tourette’s disease. Archives of general psychiatry. 1979;36:979–985. doi: 10.1001/archpsyc.1979.01780090065007. [DOI] [PubMed] [Google Scholar]
- Fernandez T, State MW, Pittenger C. In: Tourette’s disorder and tic disorders. Neurogenetics DH, Geschwind, Paulson HL, editors. New York: Elsevier; 2017. [Google Scholar]
- Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan-Sencicek AG, Kim YS, Fishman DO, Raubeson MJ, Song Y, Yasuno K, Ho WS, et al. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biol Psychiatry. 2012;71:392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casado V, Bongers G, Cortes A, Mallol J, Canela EI, Leurs R, Ferre S, Lluis C, et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. British journal of pharmacology. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Goncalves J, Baltazar G, Ferreira L, Agasse F, Bernardino L. Histamine modulates microglia function. Journal of neuroinflammation. 2012;9:90. doi: 10.1186/1742-2094-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Phillips KA. The mouse who couldn’t stop washing: pathologic grooming in animals and humans. CNS spectrums. 2009;14:503–513. doi: 10.1017/s1092852900023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick L, Rapanelli M, Abbasi E, Ohtsu H, Pittenger C. Histamine regulation of microglia: Gene-environment interaction in the regulation of central nervous system inflammation. Brain Behav Immun. 2016;57:326–337. doi: 10.1016/j.bbi.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Pittenger C. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J Immunology Research. 2017 doi: 10.1155/2016/8606057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Planeta B, Nabulsi N, Palumbo D, Li X, Liu J, Rowinski C, Chidsey K, Labaree D, Ropchan J, et al. Determination of receptor occupancy in the presence of mass dose: [11C]GSK189254 PET imaging of histamine H3 receptor occupancy by PF-03654746. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16650697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, State MW. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 2015;14:1109–1120. doi: 10.1016/S1474-4422(15)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA. Integrating neurotransmission in striatal medium spiny neurons. Advances in experimental medicine and biology. 2012;970:407–429. doi: 10.1007/978-3-7091-0932-8_18. [DOI] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods. 2014;238:54–69. doi: 10.1016/j.jneumeth.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Halpert AG, Olmstead MC, Beninger RJ. Mechanisms and abuse liability of the anti-histamine dimenhydrinate. Neurosci Biobehav Rev. 2002;26:61–67. doi: 10.1016/s0149-7634(01)00038-0. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65:594–599. doi: 10.1016/j.biopsych.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–333. doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Iida T, Yoshikawa T, Matsuzawa T, Naganuma F, Nakamura T, Miura Y, Mohsen AS, Harada R, Iwata R, Yanai K. Histamine H3 receptor in primary mouse microglia inhibits chemotaxis, phagocytosis, and cytokine secretion. Glia. 2015;63:1213–1225. doi: 10.1002/glia.22812. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Brain disorders? Precisely. Science. 2015;348:499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Creese I. Behavioral correlates of dopaminergic supersensitivity. Advances in neurology. 1975;9:81–92. [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nature reviews Neuroscience. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis I, Dehning S, Sandor P, Tarnok Z, Rizzo R, Wolanczyk T, Madruga-Garrido M, Hebebrand J, Nothen MM, Lehmkuhl G, et al. Support of the histaminergic hypothesis in Tourette syndrome: association of the histamine decarboxylase gene in a large sample of families. Journal of medical genetics. 2013;50:760–764. doi: 10.1136/jmedgenet-2013-101637. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The Journal of comparative neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Yamada K, Ornthanalai VG, Inoue T, Ota M, Murphy NP, Aruga J. Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Molecular psychiatry. 2010;15:177–184. doi: 10.1038/mp.2008.97. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. Journal of psychiatric research. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kumar A, Williams MT, Chugani HT. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. 2015;30:749–756. doi: 10.1177/0883073814543303. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20:237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N, Leckman JF, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry. 2016;79:372–382. doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. The action of central nervous system stimuland drugs: a general theory concerning amphetamine effects. In: Essmann WB, Valzelli L, editors. Current Developments in Psychopharmacology. New York: Spectrum; 1975. pp. 80–163. [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gaiser EC, Gallezot JD, Angarita GA, Pittman B, Nabulsi N, Ropchan J, MaCleod P, Cosgrove KP, Ding YS, et al. A preliminary study of dopamine D2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [(11)C](+)PHNO. Drug Alcohol Depend. 2015;154:167–173. doi: 10.1016/j.drugalcdep.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepulveda M, Navarro G, Casado V, Cortes A, Mallol J, Vignes M, McCormick PJ, Canela EI, et al. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem. 2011;286:5846–5854. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Molecular psychiatry. 2002;7:617–625. doi: 10.1038/sj.mp.4001144. 524. [DOI] [PubMed] [Google Scholar]
- Ohtsu H. Histamine synthesis and lessons learned from histidine decarboxylase deficient mice. Adv Exp Med Biol. 2010;709:21–31. doi: 10.1007/978-1-4419-8056-4_3. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS & neurological disorders drug targets. 2011;10:108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Ferris MJ, Espana RA, Harp J, Jones SR. Effects of the histamine H(1) receptor antagonist and benztropine analog diphenylpyraline on dopamine uptake, locomotion and reward. European journal of pharmacology. 2012;683:161–165. doi: 10.1016/j.ejphar.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nature reviews Neuroscience. 2013;14:472–487. doi: 10.1038/nrn3526. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, et al. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology. 2014;39:311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C. Animal models of Tourette syndrome and obsessive-compulsive disorder. In: LeDoux M, editor. Animal Models of Movement Disorders. San Deigo: Elsevier: Academic Press; 2014. pp. 748–766. [Google Scholar]
- Pittenger C. The neurobiology of tic disorders and obsessive-compulsive disorder: human and animal studies. In: Nestler E, Buxbaum J, Sklar P, Charney DS, editors. Charney and Nestler’s Neurobiology of Mental Illness. New York: Oxford University Press; 2017. [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacology & therapeutics. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Dulawa S, Thompson SL. Animal models of OCD: a conceptual framework. In: Pittenger C, editor. Obsessive-Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. New York: Oxford University Press; 2017. [Google Scholar]
- Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal inhibition. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Rapanelli M, Frick L, Pogorelov V, Ohtsu H, Bito H, Pittenger C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Translational Psychiatry in press. doi: 10.1038/tp.2016.290. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Horn KD, Schwarcz RC, Pogorelov V, Nairn AC, Pittenger C. The Histamine H3 Receptor Differentially Modulates Mitogen-activated Protein Kinase (MAPK) and Akt Signaling in Striatonigral and Striatopallidal Neurons. J Biol Chem. 2016;291:21042–21052. doi: 10.1074/jbc.M116.731406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Pogorelov V, Ota KT, Abbasi E, Ohtsu H, Pittenger C. Dysregulated intracellular signaling in the striatum in a pathophysiologically grounded model of Tourette syndrome. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:1896–1906. doi: 10.1016/j.euroneuro.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Pittenger C. Histamine and histamine receptors in Tourette syndrome and other neuropsychiatric conditions. Neuropharmacology. 2016;106:85–90. doi: 10.1016/j.neuropharm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Eapen V, Cavanna AE. The international prevalence, epidemiology, and clinical phenomenology of Tourette syndrome: a cross-cultural perspective. Journal of psychosomatic research. 2009;67:475–483. doi: 10.1016/j.jpsychores.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF, Matuskey D, Lee JY, O’Connor KC, Huang Y, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A. 2015;112:12468–12473. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Tanner C, Dure L. The epidemiology of tics and Tourette syndrome in children and adolescents. Advances in neurology. 2001;85:261–271. [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Mathews CA, Ben-Shlomo Y. Prevalence of Tourette syndrome and chronic tics in the population-based Avon longitudinal study of parents and children cohort. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:192–201. doi: 10.1016/j.jaac.2011.11.004. e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, Evans P, Gamazon E, Edlund CK, Service SK, et al. Genome-wide association study of Tourette’s syndrome. Molecular psychiatry. 2013;18:721–728. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Malinowska B, Kathmann M, Gothert M. Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fundam Clin Pharmacol. 1994;8:128–137. doi: 10.1111/j.1472-8206.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Neumann D, Seifert R. Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R–deficient mice. Neurosci Biobehav Rev. 2014;47:101–121. doi: 10.1016/j.neubiorev.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Seifert R. The histamine H4-receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology. 2016;106:116–128. doi: 10.1016/j.neuropharm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, Shmelkov E, Kushner JS, Baljevic M, Dincheva I, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Wong DF, Brown JE, Brandt J, Krafft L, Shaya E, Dannals RF, Wagner HN., Jr Positron emission tomography evaluation of dopamine D-2 receptors in adults with Tourette syndrome. Adv Neurol. 1992;58:233–239. [PubMed] [Google Scholar]
- Stanwood GD, Lucki I, McGonigle P. Differential regulation of dopamine D2 and D3 receptors by chronic drug treatments. J Pharmacol Exp Ther. 2000;295:1232–1240. [PubMed] [Google Scholar]
- Stillman AA, Krsnik Z, Sun J, Rasin MR, State MW, Sestan N, Louvi A. Developmentally regulated and evolutionarily conserved expression of SLITRK1 in brain circuits implicated in Tourette syndrome. The Journal of comparative neurology. 2009;513:21–37. doi: 10.1002/cne.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Nomura M, Misawa M, Onodera K. Evaluation of the histamine H1-antagonist-induced place preference in rats. Jpn J Pharmacol. 1999;81:332–338. doi: 10.1254/jjp.81.332. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biological psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KA, Swedo SE. Post-infectious autoimmune disorders: Sydenham’s chorea, PANDAS and beyond. Brain Res. 2015;1617:144–154. doi: 10.1016/j.brainres.2014.09.071. [DOI] [PubMed] [Google Scholar]
- Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, Duman RS, Vaccarino FM, DiLeone RJ, Pittenger C. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci U S A. 2015a;112:893–898. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neurosci Lett. 2015b;595:50–53. doi: 10.1016/j.neulet.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking neurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Privou C, Huston JP. Differential sensitivity of the caudal and rostral nucleus accumbens to the rewarding effects of a H1-histaminergic receptor blocker as measured with place-preference and self-stimulation behavior. Neuroscience. 1999;94:93–103. doi: 10.1016/s0306-4522(99)00309-7. [DOI] [PubMed] [Google Scholar]