Abstract
Auto-antibodies to cancer antigens hold great promise as sensitive amplified biomarkers for early detection of cancer. Most high through-put strategies to discover such auto-antibodies largely fail to allow identification of antibodies specific for cancer-associated posttranslational modified variants of normal proteins. We hypothesized that aberrant processed proteins are likely auto-antibody targets. MUC1 is over-expressed and aberrantly glycosylated in many cancers and we sought to evaluate the potential of natural cancer-induced auto-antibodies to such aberrant O-glycoforms of MUC1 as sensitive diagnostic biomarkers of disease. We first demonstrated, using an antibody-based glycoprofiling ELISA, that circulating mucins in cancer patients exclude truncated aberrant cancer-associated glycoforms. We then developed an O-glycopeptide microarray and used this to demonstrate detection of IgG antibodies to MUC1 aberrant O-glycopeptide epitopes in patients vaccinated with 25Tn-MUC1-106-mer conjugated to KLH. Finally, screening of sera from breast, ovarian and prostate cancer patients led to identification of three distinct aberrant MUC1 O-glycopeptide epitopes that are targeted by cancer-associated IgG auto-antibodies. The results suggest that auto-antibodies to aberrant O-glycopeptide epitopes may represent a fruitful and previously unaddressed source of sensitive biomarkers for early detection of cancer. The methods developed for chemoenzymatic synthesis of O-glycopeptides in combination with microarrays allow for broader data-mining of the entire cancer O-glycopeptidome.
Keywords: Auto-antibodies, mucin, O-glycosylation, O-glycopeptide, array
Introduction
Cancer-associated auto-antibodies represent appealing biomarkers. Auto-antibodies may develop early in carcinogenesis when tumor-associated antigens appear on premalignant or malignant lesions. Antibody responses can produce relatively high concentrations in circulation with a long circulation time, and they can be detected with sensitive and specific methods (1;2). In contrast, antigens produced by small premalignant or malignant lesions are generally produced in vanishingly small levels that due to dilution and clearance from blood may not be detectable. Discovery of specific auto-antibodies to cancer antigens have been undertaken by different approaches. Classical studies identified auto-antibodies reactive with tumor cells, tissues, or isolated proteins. Analysis of human monoclonal antibodies with cancer selective reactivity has generally identified IgM antibodies reactive with carbohydrate epitopes (3) or glycosylation dependent epitopes (4). Proteome-wide screening techniques include expressed cDNA libraries (SEREX) (5), protein and peptide arrays (6;7), random or designed phage displays (8), and more recently self-assembling protein arrays (9;10). Cancer-associated auto-antibodies characterized to date have been found to bind intracellular proteins with functions important in cell cycle regulation, such as GPR78 (8), p53 (11), NY-ESO-1 (12), and CDC25 (13), but also some cell membrane glycoproteins such as MUC1 (14), HER2 (15) and Mesothelin (16). In the case of p53, the induction of auto-antibodies is thought to be induced by increased expression and presentation caused by somatic mutations, although these mutations are often not included in the epitopes bound by antibodies (17). Auto-antibodies to cell membrane and secreted proteins appear to be underrepresented, which may be due to the low frequency of somatic mutations in these genes (18) combined with the need for immunological tolerance to exposed self-proteins. Another contributing factor may be related to technical and experimental limitations, as most broad proteomic screens conducted to date were designed to interrogate the proteome in the absence of posttranslational modifications (PTMs), and in particular without unique PTMs associated with cancer. Although the latter problem is widely recognized by investigators in the field, there have been few solutions brought forward.
Malignant transformation of cells is always accompanied by alterations in PTMs of proteins, and a well documented example hereof is the abundant mucin-type O-glycosylation found on mucins and other O-glycoproteins (19). Tumor-associated changes in the types and levels of mucins expressed as well as their aberrant glycosylation, create a diverse set of unusual molecular structures found on the surface of cancer cells and in secretions. These molecular structures generally represent glycoproteins with truncated immature O-glycans, which may only exist as brief biosynthetic intermediates in the early Golgi apparatus of normal cells. The immune-system may thus not be exposed to these structures, and lack immunological tolerance may provoke both auto-antibodies and cell mediated immunity when expressed by cancer cells. We therefore hypothesized that auto-immunity to cancer antigens would be directed to cancer-specific epitopes generated by the combination of the normal protein backbone and aberrant O-glycosylation. The MUC1 mucin is heavily O-glycosylated in a large 20 amino acid tandem repeated region, which is aberrantly glycosylated in cancer (20) and can be detected in serum of late stage cancer patients (21;22). Man is in general immunologically tolerant to the MUC1 tandem repeat protein core and its normal glycosylated forms (20;23), although, several studies have shown that up to 10–15% of healthy controls and cancer patients have antibody reactivity to MUC1 peptides as detected by ELISA assays; however, the detected levels are very low and have led to contradictory results by different investigators (24–27). We recently identified an immunodominant O-glycopeptide epitope (Tn/STn-MUC1) that results from aberrant glycosylation and for which there does not appear to be immunological tolerance (28–30) (See Supplementary Fig. S1A. for structures of O-glycans). We have also shown that the cancer-associated Tn-glycoform of MUC1 is selectively taken up by dendritic cells and delivered to the MHC class I and II pathways (31), and the Tn–MUC1 glycoform induces potent IgG responses in MUC1 transgenic mice (28) and cancer patients (30). Importantly, the elicited immune response is specifically directed to the combined glycopeptide epitope with little or no antibody specificity for the Tn carbohydrate hapten. The Tn/STn-MUC1 glycopeptide epitope is broadly expressed in essentially all breast cancers as well as many other adenocarcinomas (29). Cancer-associated O-glycopeptide epitopes from MUC1 therefore represent likely candidates for natural auto-antibodies induced in cancer patients.
In this report we present a versatile chemoenzymatic approach to produce libraries of cancer-associated O-glycopeptides that combined with a microarray platform allows high through-put detection of auto-antibodies to the O-glycopeptidome. Analysis of such an array with a glycopeptide library derived from MUC1 demonstrated sensitive detection of cancer-associated auto-antibodies to distinct O-glycopeptide epitopes.
Materials and Methods
Human sera
Human sera were obtained from the following sources: Preimmune and post-vaccination (5 sc injections biweekly of 2–4 μg 25Tn-106mer-MUC1-KLH conjugate) sera from n=20 breast cancer patients (stage III/IV after treatment and disease free) enrolled in a phase I study after written informed consent (Protocol approved by Memorial Sloan-Kettering Institutional Review Board and FDA). Sera from breast cancer (n = 28), ovarian cancer (n = 20), and prostate cancer (n = 10) patients collected close to time of first diagnosis of cancer and prior to treatment including surgery were obtained from Asterand Inc. (six ovarian sera were from The Cooperative Human Tissue Network (CHTN)) (Supplementary Table 1S). Age and sex matched healthy control sera (n=39) were obtained from Asterand, Inc. All sera from Asterand were collected and stored following the same standard operating procedure which involved clotting for 30 min and freezing within 60 min of collection. A second set of healthy control sera (n=33) were obtained from blood donors.
Chemoenzymatic Synthesis of O-glycopeptides
Peptides were synthesized and O-glycosylated in vitro using recombinant glycosyltransferases as previously described (28). Briefly, different polypeptide GalNAc-transferase isoforms were used to direct GalNAc O-glycan occupancies on peptides and the core1, β3GalT, core 3 β3GlcNAc-T and ST6GalNAc-I were used to produce T, core 3 and STn glycoforms. See supplementary Fig. S1 for structures of glycopeptides. All glycopeptides were purified by HPLC and characterized by MALDI-TOF. The MUC1 glycopeptides were essentially homogenous compounds with 6, 9 or 15 O-glycans attached except that the 15 O-glycan MUC1 glycopeptide contained a mixture of 14–15 O-glycans as reported previously (28).
O-glycopeptide array print and analysis
(Glyco)peptides and control structures were printed on Schott Nexterion® Slide H or Schott Nexterion® Slide H MPX 16 (Schott AG, Mainz, Germany). Quadruplicates of all compounds were printed at 20, 5, and 1 μM in 150 mM sodium phosphate pH 8.5 with 0.005% CHAPS and printed on a BioRobotics MicroGrid II spotter (Genomics Solution) with a 0.21 mm pitch using Stealth 3B Micro Spotting Pins (Telechem International ArrayIt Division). Ovine submaxillary mucin (OSM) (Isosep) and its desialylated counterpart asialo-OSM (AOSM) were printed as controls at 10, 2.5, and 0.5 μM. After printing, slides were incubated for 1 h in a humidified hybridization chamber with 70–100% relative humidity and stored until use at 4°C. Prior to use unspotted slide areas were blocked for 1 h with 25 mM ethanolamine in 100 mM sodium borate pH 8.5. Human sera serially diluted from 1:25–1:400 or monoclonal antibodies (1 μg/ml or hybridoma supernatants) were incubated in a closed container with gentle agitation for 1 h, washed three times in PBS with 0.05% Tween-20 (PBS-T), followed by 1 h incubation with appropriate secondary antibodies. Human IgM and IgG antibodies were detected with Cy3-conjugated goat anti-human IgG (Fc specific) and goat anti-human IgM (Sigma) diluted 1:5000 in PBS-T. Murine monoclonal antibodies were detected with Cy3-conjugated goat anti-mouse IgM (μ chain specific) and goat anti-mouse IgG (H+L) (Jackson Laboratories) diluted 1:5000. After washing slides were rinses shortly in H2O, dried by centrifugation (200×g) and scanned in a ProScanArray HT Microarray Scanner (PerkinElmer) followed by image analysis with ProScanArray Express 4.0 software (PerkinElmer). For comparison, slides were scanned with identical scanning parameters calculated by automatic sensitivity calibration. Data were analyzed and plotted using Microsoft Excel or GraphPad Prism software.
Results
A prerequisite for the existence of circulating auto-antibodies would presumably be that the corresponding circulating antigen would be found in immune complexes and/or removed from circulation. We therefore developed and tested highly sensitive ELISA glycoprofiling assays for the two well-known cancer-associated mucins, MUC1 detected in the CA15-3 serum assay and MUC16 detected in the CA125 assay (Supplementary Data). We were essentially unable to detect the short truncated cancer glycoforms of either of these mucins in circulation, and the detectable glycoforms included only sialylated core 1 and complex type O-glycans (Supplementary Fig. S1 and S2). This is in agreement with recent studies showing that cancer mucins without sialic acids are removed by scavenger lectin receptors on liver macrophages (32). The glycoprofiling results using monoclonal antibodies with well defined specificities (33), demonstrated that circulating MUC1 does not include the aberrant Tn, STn and T glycoforms and opens for the possibility that circulating auto-antibodies directed to such glycoforms participate in their removal in addition to lectin receptors.
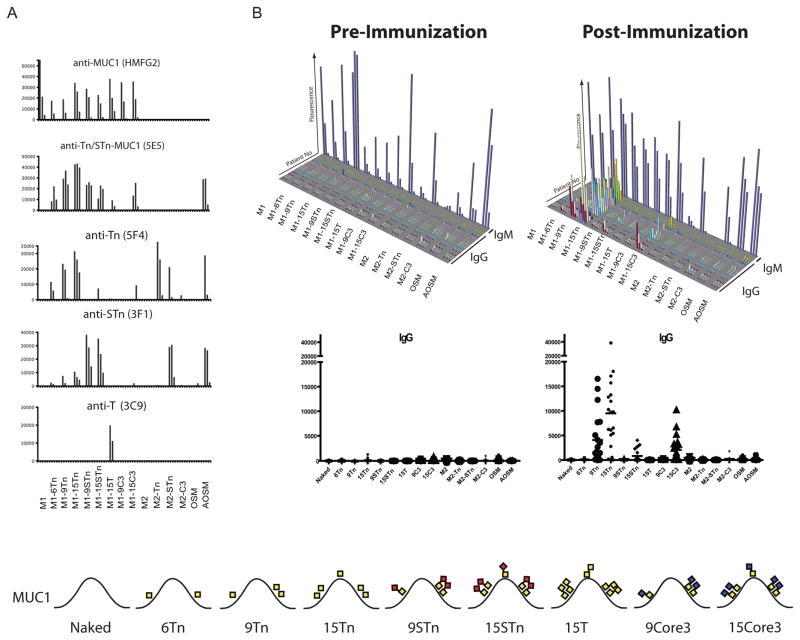
The primary aim of this study was therefore to develop a discovery platform for selective detection of cancer-associated auto-antibodies directed to aberrant O-glycopeptide epitopes without interference by anti-carbohydrate hapten antibodies. The size of the potential O-glycopeptidome is vast with thousands of distinct O-glycoproteins combined with numerous distinct aberrant O-glycan structures. We have for that reason developed a toolbox of recombinant glycosyltransferase enzymes that allow enzymatic synthesis of diverse O-glycan structures with some degree of control of sites of attachment of O-glycans at serine and threonine residues in peptides (28). The first step involves site-directed synthesis of GalNAc-glycopeptides using a panel of human polypeptide GalNAc-transferases with different peptide substrate specificities by which a high degree of control of sites of O-glycan attachment to peptides can be achieved. Subsequent steps utilize a panel of galactosyltransferases, GlcNAc-transferases and sialyltransferases to produce truncated O-glycan structures associated with cancer. These reagents were used to synthesize a library of MUC1 cancer-associated glycopeptides in solution using a synthetic 60-mer tandem repeat peptide as substrate (Fig. 1) (28). In order to accommodate large glycopeptide libraries we chose to use a slide microarray format on NHS-activated hydrogel slides, as Blixt et al. (34;35) have demonstrated that these provide a remarkably low background for detection of human serum antibodies. We based our initial microarray on MUC1 and control peptides given the evidence that aberrant glycosylation of MUC1 is associated with the progression of a number of different adenocarcinomas, but the available toolbox could be applied to peptides from any O-glycoprotein. The conditions for the array assay were standardized by using a panel of monoclonal antibodies with peptide, carbohydrate hapten and O-glycopeptide specificities, which confirmed quality and specificity of the array assay (Fig. 1A).
Fig. 1. Microarray platform for detection of MUC1 O-glycopeptide specific antibodies. Immunization of cancer patients with GalNAc-MUC1 break tolerance eliciting glycopeptide specific antibodies.
A, GalNAc glycosylated variants of MUC1 (6Tn, 9Tn, and 15Tn) were synthesized chemoenzymatically using recombinant polypeptide GalNAc-transferases (GalNAc-T2, -T4, and -T11) (yellow boxes indicate the position of GalNAc in each of the three MUC1 repeats in the produced 60-mer MUC1 (glyco)peptides). Further elongation was carried out by β3GnT6, c1Gal-T, or ST6GalNAc-T1 creating truncated core 3, core 1, and STn structures respectively. MUC2 glycopeptides were synthesized by similar methods. Glycopeptides and control structures were printed on NHS-activated hydrogel slides in quadruplicates at three different concentrations. The glycopeptide array was validated by incubation with glycan and glycopeptide specific MAbs detected by Cy3-conjugated secondary antibodies and expressed as fluorescence intensity visualized in column diagram. Anti-MUC1 peptide MAb HMFG2 recognized both non-glycosylated (designated M1) and all glycoforms of MUC1 confirming efficient printing of all MUC1 compounds. The glycopeptide specific MAb 5E5 recognizes the immunodominant Tn/STn-MUC1 epitope -GSTAP- with one GalNAc residue in the threonine (6Tn-MUC1) or both the serine and threonine (9/15Tn-MUC1). In contrast the carbohydrate hapten antibodies recognizing Tn (GalNAcα1-O-Ser/Thr) MAb (5F4), STn (NeuAcα2-6GalNAcα1-O-Ser/Thr) MAb (3F1) react regardless of peptide backbone as shown by reactivity with the corresponding glycoforms of MUC2 (designated M2) and the Tn and STn glycosylated mucins AOSM and OSM. Finally the anti-T (Galβ1-3GalNAcα1-O-Ser/Thr) MAb reacts specifically with T-MUC1. B, 3D-column diagram (above) and dot-plot presentation (below) showing results of analysis of human sera from a clinical trial of a MUC1 glycopeptide vaccine in breast cancer patients (n=20) immunized with 106-mer MUC1 tandem repeat peptide with 25 Tn O-glycans conjugated to KLH. The immunogen corresponds to the 15Tn MUC1 60-mer glycopeptide used in this study with Tn residues at all five potential O-glycosylation sites in the 20-mer tandem repeated peptide. The array was incubated with diluted (1:25) serum followed by incubation with IgG and IgM specific secondary Cy3 conjugated antibodies. Results from all twenty subjects analyzed for IgG shown in rows 1–20 with glycopeptides as indicated. Pre-vaccination sera showed essentially no IgG antibodies, while vaccination with the 25Tn MUC1 vaccine induced IgG antibodies specifically reactive with the 9Tn and 15Tn MUC1 glycopeptides but not unglycosylated MUC1. Results from one subject analyzed for IgM is shown in last row. Pre-immune and vaccinated patients have abundant IgM antibodies to Tn, STn, and T carbohydrate haptens. This underscores the importance of selective detection of IgG responses in order to identify true combined glycopeptide epitopes. C, Summary of the results with indication of the number of patients tested positive for each target out of the total number of tested patients. Values above 3 times the SD of the average values obtained with sera before vaccination were considered positive. A total of 18 out of the 20 subjects vaccinated showed induction of IgG auto-antibodies to 9Tn and 15Tn MUC1 glycopeptides. These glycopeptides have in common two O-glycans in the immunodominant -GSTAP- epitope suggesting that this is the reactive epitope. Partial reactivity was seen with STn-MUC1 and core 3 MUC1 in 5 and 7 immunized patients, respectively. No reactivity was seen with Tn and STn MUC2 glycopeptides or AOSM and OSM. The graphic presentation of MUC1 glycoforms shows one 20 amino acid MUC1 tandem repeat sequence (HGVTSAPDTRPAPGSTAPPA) with glycans using symbols as in legend to Supplementary Fig. S1.
Subsequently, the utility of the assay was tested by analysis of human sera derived from a pilot clinical trial in which a MUC1 Tn-glycopeptide vaccine was administered to twenty breast cancer patients who had undergone radical treatment and were classified as disease free. Patients received up to five injections of a 106-mer MUC1 tandem repeat peptide with 25 Tn O-glycans conjugated to Keyhole Limpet Hemocyanin (KLH) with trial design and protocol essentially as previously reported (30). Results of ELISA assays demonstrated that patients had no preexisting IgG antibodies and all developed detectable IgG Tn-MUC1 glycopeptide antibodies after vaccination (30). Analysis of these sera with the O-glycopeptide array confirmed these results, and demonstrated that there was induction of IgG antibodies reactive with only the Tn-MUC1 glycopeptides with at least two O-glycans in the immunodominant -GSTAP- epitope, i.e. the 9Tn and 15Tn-MUC1 and not the 6Tn-MUC1 glycoforms (Fig. 1C). In five subjects antibodies to STn-MUC1 were also detected. The induced antibodies did not cross-react with Tn or STn haptens as evidenced by lack of reactivity with other Tn and STn-glycopeptides. This reactivity pattern is similar to that of the cancer-specific 5E5 antibody except that this also react with a single O-glycan in the -GSTAP- epitope (28). Some reactivity to the truncated core 3 MUC1 glycoform (GlcNAcβ1-3GalNAcα1-O-Ser/Thr) was also observed and these may have resulted from “epitope spreading”. The analysis of IgM antibodies revealed as expected broad reactivity with all glycoforms of the glycopeptides, which makes it impossible to discern potential O-glycopeptide specific antibodies. Carbohydrate-hapten specific antibodies are generally of the IgM isotype, although examples of anti-STn IgG are found (36). As shown in Fig. 1B sera from pre-immune and vaccinated patients have abundant IgM antibodies to Tn and STn carbohydrate haptens, which exhibited no apparent preference for the peptide backbone. The levels of IgM antibodies to Tn and STn structures were enhanced by the Tn-MUC1 vaccination, but no IgG antibodies to Tn and STn hapten were detected (indicated by lack of reactivity with MUC2 glycopeptides and AOSM/OSM) in agreement with previous ELISA analyses (30). Some IgM antibodies reactive with the unglycosylated MUC1 as well as MUC2 peptides were also observed. Thus the IgM carbohydrate hapten antibodies made it impossible to discern antibodies with distinct O-glycopeptide specificity. These results stress the importance of using assay methods that allow selective detection of combined glycopeptide epitopes.
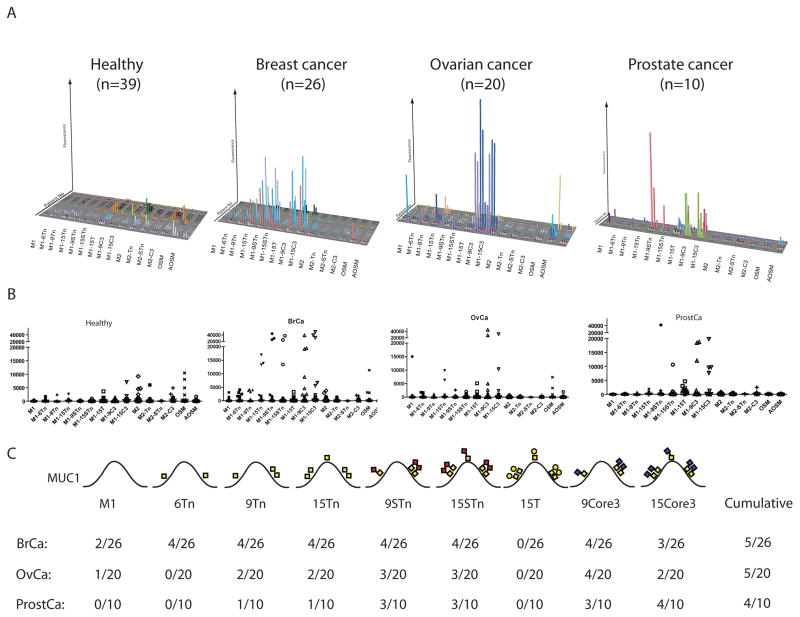
The O-glycopeptide array was then used to evaluate the existence of natural auto-antibodies in healthy controls and newly diagnosed patients with breast, ovarian and prostate cancer (Fig. 2). Analysis of IgM antibodies yielded broad carbohydrate reactivity without discernable O-glycopeptide specificities in both healthy controls and cancer patients similar to results in Fig. 1. In striking contrast, IgG antibodies from healthy control sera were essentially non-reactive with the glycopeptides, while specific IgG antibodies to Tn-MUC1, STn-MUC1, and truncated core 3 O-glycopeptides were identified in sera from newly diagnosed breast, ovarian and prostate cancer patients (Fig. 2A lower panels). These cancer-associated IgG antibodies are directed to combination O-glycopeptide epitopes dependent both on the glycoform and the peptide sequence, since they did not react with other glycopeptides with the same glycoforms. Three distinct MUC1 O-glycopeptide epitopes with different O-glycan structures (Tn, STn, and core 3) reactive with IgG were identified, and there were different but partially overlapping distributions of these specificities in patients. Cross-inhibition studies confirmed that these were at least partially distinct epitopes in the different patients (not shown). A selection of sera were tested in multiple dilutions (1:25, 1:100, 1:200, 1:1,000, 1:3,000) demonstrating quite variable titers of antibodies from 1:100 to more than 1:3,000. In most sera the auto-antibody reactivity declined with dilutions over 1:200, while a few sera could be diluted 1:3,000 without substantial change in reactivity (not shown). The array analysis did not reveal substantial auto-antibodies to unglycosylated MUC1, which is in contrast to some previous reports using ELISA assays (24–27).
Fig. 2. Detection of cancer-induced auto-antibodies to MUC1 O-glycopeptide epitopes by microarray analysis.
A, 3D-column diagram of IgG antibody reactivity in sera from healthy controls (n=39) and newly diagnosed patients with breast (n=26), ovarian (n=20) and prostate (n=10) cancer (Supplementary Table S1). Specific IgG responses were detected in identified breast, ovarian and prostate cancer patients towards Tn-MUC1, STn-MUC1, T-MUC1 or truncated core 3 O-glycopeptide epitopes. Only very few healthy subjects and cancer patients had IgG antibodies to AOSM and OSM, which are considered Tn and STn hapten antibodies. B, Dot-plot analysis of the antibody responses for each glycopeptide target. C, Summary of results for each MUC1 glycopeptide with indication of glycan structures and positions within the MUC1 tandem repeat. Values were considered positive if above 3 times the SD of the average value obtained with sera from healthy individuals. A total of five out of the 26 breast cancer patients showed induction of IgG auto-antibodies to either Tn, STn, T or core 3 MUC1 glycopeptides. The same reactivity pattern was seen in ovarian cancer patients with five out of 20 patients demonstrating IgG auto-antibodies to one or more of the MUC1 glycopeptides. In contrast 4 out of 10 prostate cancer patients showed induction of auto-antibodies to STn, T and, core 3 MUC1 glycopeptides. In the majority of individuals no reactivity was seen with Tn and STn MUC2 glycopeptides or AOSM and OSM.
Discussion
The present study tested and confirmed the hypothesis that auto-antibodies to aberrant O-glycopeptide epitopes represent a fruitful source of sensitive biomarkers for early detection of cancer. Cancer-associated IgG auto-antibodies to several O-glycopeptide epitopes were identified in MUC1 while IgG antibodies to peptide epitopes were notdetected. The study therefore clearly supports that auto-antibody biomarker discovery strategies should include aberrant PTMs for greatest success. Chemoenzymatic synthesis of cancer-associated O-glycopeptides in combination with a microarray platform was demonstrated to be a feasible strategy for broader analysis of the entire cancer O-glycopeptidome.
Initially we found absence of immature non-sialylated MUC1 and MUC16 glycoforms in serum of cancer patients with elevated mucin levels suggesting that these glycoforms are removed by immune cells or scavenger receptors recognizing immature uncapped glycans. This is in agreement with Varki and coworkers (32) “Tip of the iceberg” theory proposing that circulating tumor glycoprotein antigens, including cancer mucins without sialic acids, are removed by scavenger receptors and hence inherently represent insensitive biomarkers. This selective clearance provides a plausible mechanism by which aberrant glycoforms of mucins and O-glycoproteins are presented to and stimulate the immune system (31). MUC1 is also considered a prime candidate for immunotherapies and antibody based therapies may take advantage of targeting the cancer-associated glycoforms of MUC1 as there will be limited inhibition of targeting by circulating antigens.
Detection of auto-antibodies to O-glycopeptide combination epitopes, which include both the peptide backbone as well as cancer-associated PTM hapten structures, is complicated by the presence of rather ubiquitous natural antibodies to the carbohydrate haptens. It is well established that non-self carbohydrate structures are immunogenic in man, and natural antibodies to the cancer-associated truncated carbohydrate haptens T, Tn and STn are among the most widely studied (37). While titers of antibodies to Tn and STn appear to be increased in cancer patients, essentially all individuals have IgM antibodies with some specificity to these structures. Recent studies of the specificity of antibodies to the Globo-H carbohydrate hapten using a novel glycan array show similar results (38). It has been shown that some anti-carbohydrate antibodies result from exposure to related structures found in gut microflora (39); however, expression of aberrant glycosylated glycoproteins in cancer are believed to contribute as well (37). The clinical significance of these antibodies in relation to cancer is largely unknown, but they have not emerged as truly useful biomarkers of cancers. In contrast, antibodies directed to combined O-glycopeptide epitopes, which would have specificity for distinct proteins carrying aberrant cancer O-glycans, are expected to have high affinity, be class switched to IgG, and absent in healthy individuals (28;29). We have previously demonstrated that several different Tn-glycopeptides generate glycopeptide specific IgG antibodies and not carbohydrate hapten antibodies (40). More recently, a protective cancer specific auto-antibody in a spontaneous mouse cancer model was shown to be directed to a Tn-glycopeptide epitope of the mouse ortholog of human podoplanin, OTS8 (41). The current results clearly demonstrate that it is possible to detect auto-antibodies to O-glycopeptide epitopes on glycopeptides arrays by limiting the detection to IgG subclass without interference by carbohydrate hapten antibodies.
The discovery platform for human auto-antibodies was developed on microarray hydrogel slides for high through-put analysis, minimum consumption of compounds, and because hydrogel slides provide a remarkably low background for detection of human serum antibodies (42). The array was qualified with a panel of monoclonal antibodies and lectins as well as by demonstration of sensitive detection of vaccine induced IgG antibodies to specific for Tn/STn-MUC1. Application of the array on sera with newly diagnosed cancers revealed IgG auto-antibodies directed to three distinct O-glycopeptide epitopes, Tn-MUC1, STn-MUC1, and truncated core 3-MUC1, which were not found in two sets of healthy control sera (Fig. 2A lower panel). Future studies are needed to address occurrence of these auto-antibodies in benign diseases of differential diagnostic interest. We have performed a larger study of colorectal cancer, where comparative sera from inflammatory disease controls were available, and the identified auto-antibodies were not found in the benign conditions except that antibodies to the truncated core 3 MUC1 glycopeptide epitope was found at lower incidence and lower intensity (Pedersen et al. manuscript in preparation).
Induction of auto-antibodies directed to Tn and STn-MUC1 in both breast, ovarian and prostate cancers are in agreement with our understanding of the expression pattern of MUC1 and the cancer-associated glycoforms Tn and STn. The genetic and biosynthetic basis for expression of these glycans may relate to somatic mutations in Cosmc, a chaperone for the enzyme C1β3Gal-T enzyme that controls synthesis of the common core 1 O-glycosylation pathway (See Fig. S1), but over-expression of the ST6GalNAc-I sialyltransferase responsible for STn synthesis can also plays a role (43;44). More intriguing is the finding of antibodies to truncated core 3 MUC1 in prostate, breast and ovarian cancer patients. The core 3 glycosylation pathway is generally believed to be limited to the mucosa of the digestive tract and truncated core 3 O-glycans have been found in colon cancer (45) in accordance with the expression pattern for the controlling enzyme β3GnT6 (46). While this enzyme is down-regulated in colon cancer, little is known about its expression in other types of cancer. Structural studies of O-glycoproteins from breast cancer have not identified core 3 related O-glycans, however, a recent report have shown the existence of cancer-associated truncated glycans with terminal βGlcNAc on glycoproteins and glycolipids in many cancer types including ductal breast carcinoma (47). Interestingly, overexpression of β3GnT6 in cancer cells suppresses the metastatic phenotype and mice deficient in this enzyme spontaneously develop colon cancer (48).
In contrast to some previous reports using ELISA assays (24–27), only minimal reactivity was seen against non-glycosylated MUC1. The reason for this discrepancy is not clear at present, but the array analysis does show very weak signals under the cut-off for the analysis in several sera in addition to the one strongly positive ovarian cancer patient. Regardless, the identified MUC1 glycopeptide auto-antibody targets produce much more robust signals with high cancer specificity. It is clearly of importance to understand the timing and dynamics of such auto-antibodies, as stressed by the lack of MUC1 auto-antibodies in serum obtained before vaccination in the twenty treated disease-free breast cancer patients analyzed in Fig. 1. This finding may suggest that the MUC1 glycoform antibodies disappear as the disease progresses and/or after treatment. This would be in agreement with studies of p53 auto-antibodies that may reappear at relapse (49). Other studies of auto-antibodies to p53 have shown that these may occur prior to diagnosis of cancer (50), which provide support for using auto-antibodies as sensitive and early biomarkers.
In conclusion, our study demonstrates that the aberrantly glycosylated O-glycopeptidome has great potential as a source of targets for cancer induced auto-antibodies. We present to our knowledge the first evidence of cancer-associated auto-antibodies to defined O-glycopeptide epitopes, which should provide bases for further exploration of similar targets. Our proof-of-concept study using MUC1 as a model identified three distinct cancer-associated IgG auto-antibodies directed to distinct O-glycopeptide epitopes. The prevalence of the combination of these MUC1 biomarkers in cancer were similar or higher than those found for other identified auto-antibodies to protein epitopes and all have very low prevalence in healthy controls. None of these biomarker targets individually approach levels of sensitivity for clinically useful biomarker assays; however, we envision that these combined with similar targets from other O-glycoproteins altered in cancer will eventually reach acceptable levels of sensitivity. The O-glycopeptidome of man is currently poorly understood due to lack of predictive consensus sequence motifs for protein O-glycosylation, but there are hundreds if not thousand O-glycoproteins in the human proteome. Sites of O-glycosylation is directed by up to 20 human polypeptide GalNAc-transferases with different substrate specificities, which allow for high control of glycosylation patterns of proteins in cells and at the same time high probability for altered patterns of O-glycans decorations in cancer cells. Our strategy for discovery of further cancer-associated O-glycopeptide epitopes recognized by auto-antibodies will be to produce large glycopeptide arrays decorated with O-glycans produced by in vitro O-glycosylation with different polypeptide GalNAc-transferases in combination with other glycosyltransferases. This should enable data-mining this large and unexplored field of potential biomarkers. While MUC1 is expressed in many common adenocarcinomas, which correlates with our finding of MUC1 auto-antibodies in several types of cancer, we envision that other glycoproteins with more restricted organ and cancer-type expression patterns may eventually aid in developing disease and organ specific auto-antibody signatures with high sensitivity and specificity. The concept of auto-antibody signatures can be extended to many other posttranslational protein modifications that may be altered in disease and serve as biomarkers.
Supplementary Material
Acknowledgments
Grant support: Carlsberg Foundation, Benzon Foundation, Danish Research Council, Center of Excellence Copenhagen University (HC); Breast Cancer Research Foundation (JB); NIH (PO1 CA052477 1U01CA128437-01) EU-FP7-HEALTH-2007-A 201381 (MAH, JB, JT-P, HC).
Susanne Johannesen for excellent technical assistance.
Footnotes
Disclosure of potential conflict of interest: M.A. Tarp, U. Mandel, J. Taylor-Papadimitriou, Joy Burchell, and H. Clausen are inventors of a patent application.
References
- 1.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–94. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KS, Labaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J ProteomeRes. 2005;4:1123–33. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H, Hirohashi S, Shimosato Y, Enjoji M, Clausen H, Hakomori S. Establishment of an anti-A human monoclonal antibody from a blood group A lung cancer patient: evidence for the occurrence of autoimmune response to difucosylated type-2 chain A. Eur J Immunol. 1989;19:2197–203. doi: 10.1002/eji.1830191204. [DOI] [PubMed] [Google Scholar]
- 4.Rauschert N, Brandlein S, Holzinger E, Hensel F, Muller-Hermelink HK, Vollmers HP. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest. 2008;88:375–86. doi: 10.1038/labinvest.2008.2. [DOI] [PubMed] [Google Scholar]
- 5.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira-Faca SR, Kuick R, Puravs E, Zhang Q, Krasnoselsky AL, Phanstiel D, et al. Identification of 14-3-3 theta as an antigen that induces a humoral response in lung cancer. Cancer Res. 2007;67:12000–6. doi: 10.1158/0008-5472.CAN-07-2913. [DOI] [PubMed] [Google Scholar]
- 8.Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran N, Raphael JV, Hainsworth E, Demirkan G, Fuentes MG, Rolfs A, et al. Next-generation high-density self-assembling functional protein arrays. Nat Methods. 2008;5:535–8. doi: 10.1038/nmeth.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KS, Ramachandran N, Wong J, Raphael JV, Hainsworth E, Demirkan G, et al. Application of protein microarrays for multiplexed detection of antibodies to tumor antigens in breast cancer. J Proteome Res. 2008;7:1490–9. doi: 10.1021/pr700804c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubin R, Schlichtholz B, Bengoufa D, Zalcman G, Tredaniel J, Hirsch A, et al. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53:5872–6. [PubMed] [Google Scholar]
- 12.Kawabata R, Wada H, Isobe M, Saika T, Sato S, Uenaka A, et al. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int J Cancer. 2007;120:2178–84. doi: 10.1002/ijc.22583. [DOI] [PubMed] [Google Scholar]
- 13.Liu WL, Zhang G, Wang JY, Cao JY, Guo XZ, Xu LH, et al. Proteomics-based identification of autoantibody against CDC25B as a novel serum marker in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2008;375:440–5. doi: 10.1016/j.bbrc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Snijdewint FGM, Mensdorff-Pouilly S, Karuntu-Wanamarta AH, Verstraeten AA, Zanten-Przybysz I, Hummel P, et al. Cellular and humoral immune responses to MUC1 mucin and tandem-repeat peptides in ovarian cancer patients and controls. Cancer Immunol Immunother. 1999;48:47–55. doi: 10.1007/s002620050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–73. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrom I, Friedman E, Verch T, Yang Y, Korach J, Jaffar J, et al. Anti-mesothelin antibodies and circulating mesothelin relate to the clinical state in ovarian cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17:1520–6. [Google Scholar]
- 17.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–74. [PubMed] [Google Scholar]
- 18.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 19.Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780:546–63. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, et al. MUC1 and the immunobiology of cancer. Journal of Mammary Gland Biology and Neoplasia. 2002;7:209–21. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 21.Burchell J, Wang D, Taylor-Papadimitriou J. Detection of the tumour-associated antigens recognized by the monoclonal antibodies HMFG-1 and 2 in serum from patients with breast cancer. Int J Cancer. 1984;34:763–8. doi: 10.1002/ijc.2910340605. [DOI] [PubMed] [Google Scholar]
- 22.Bon GG, van Kamp GJ, Verstraeten RA, Mensdorff-Pouilly S, Hilgers J, Kenemans P. Quantification of MUC1 in breast cancer patients - A method comparison study. Eur J Obst Gynecol Reprod Biol. 1999;83:67–75. doi: 10.1016/s0301-2115(98)00302-9. [DOI] [PubMed] [Google Scholar]
- 23.Rughetti A, Pellicciotta I, Biffoni M, Backstrom M, Link T, Bennet EP, et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J Immunol. 2005;174:7764–72. doi: 10.4049/jimmunol.174.12.7764. [DOI] [PubMed] [Google Scholar]
- 24.Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FGM, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides. Int J Cancer. 2000;86:702–12. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:30–5. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 26.Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Drakaki H, Loveland BE, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1. Breast Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FGM, Kok A, van Kamp GJ, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–83. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 28.Tarp MA, Sorensen AL, Mandel U, Paulsen H, Burchell J, Taylor-Papadimitriou J, et al. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17:197–209. doi: 10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 30.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 31.Napoletano C, Rughetti A, Tarp MA, Coleman J, Bennet EP, Picco G, et al. Tumor Associated Tn-MUC1 Glycoform is Internalized through the Macrophage Galactose-type C-type Lectin and delivered to the HLA Class I and II Compartments in Dendritic Cells. Cancer Res. 2007 doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- 32.Wahrenbrock MG, Varki A. Multiple hepatic receptors cooperate to eliminate secretory mucins aberrantly entering the bloodstream: are circulating cancer mucins the “tip of the iceberg”? Cancer Res. 2006;66:2433–41. doi: 10.1158/0008-5472.CAN-05-3851. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, et al. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nat Methods. 2007;4:437–44. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez RA, Blixt O, Razi N, Lee A, Johnson TK, Fleshman A, et al. High throughput screening of multiple glycan binding proteins and antibodies using a solid phase glycan array - Deciphering the carbohydrate code. Glycobiology. 2004;14:1106. [Google Scholar]
- 35.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjeldsen T, Hakomori S, Springer GF, Desai P, Harris T, Clausen H. Coexpression of sialosyl-Tn (NeuAcα2–6GalNAcα1-O-Ser/Thr) and Tn (GalNAcα1-O-Ser/Thr) blood group antigens on Tn erythrocytes. Vox Sang. 1989;57:81–7. doi: 10.1111/j.1423-0410.1989.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 37.Springer GF. T and Tn, General Carcinoma Auto-Antigens. Science. 1984;224:1198–206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 38.Wang CC, Huang YL, Ren CT, Lin CW, Hung JT, Yu JC, et al. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci USA. 2008;105:11661–6. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Springer GF, Horton RE, Forbes M. Origin of antihuman blood group B agglutinins in germfree chicks. Ann N Y Acad Sci. 1959;78:272–5. doi: 10.1111/j.1749-6632.1959.tb53110.x. [DOI] [PubMed] [Google Scholar]
- 40.Reis CA, Sorensen T, Mandel U, David L, Mirgorodskaya E, Roepstorff P, et al. Development and characterization of an antibody directed to an alpha-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J. 1998;15:51–62. doi: 10.1023/a:1006939432665. [DOI] [PubMed] [Google Scholar]
- 41.Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 2006;314:304–8. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 42.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcos NT, Pinho S, Grandela C, Cruz A, Samyn-Petit B, Harduin-Lepers A, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 44.Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–94. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 45.Robbe-Masselot C, Herrmann A, Maes E, Carlstedt I, Michalski JC, Capon C. Expression of a Core 3 Disialyl-Le(x) Hexasaccharide in Human Colorectal Cancers: A Potential Marker of Malignant Transformation in Colon. J Proteome Res. 2009 doi: 10.1021/pr800740j. [DOI] [PubMed] [Google Scholar]
- 46.Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide β1,3-N-acetylglucosaminyltransferase (β3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277:12802–9. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 47.Satomaa T, Heiskanen A, Leonardsson I, Angstrom J, Olonen A, Blomqvist M, et al. Analysis of the human cancer glycome identifies a novel group of tumor-associated N-acetylglucosamine glycan antigens. Cancer Res. 2009;69:5811–9. doi: 10.1158/0008-5472.CAN-08-0289. [DOI] [PubMed] [Google Scholar]
- 48.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–29. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Regele S, Vogl FD, Kohler T, Kreienberg R, Runnebaum IB. p53 autoantibodies can be indicative of the development of breast cancer relapse. Anticancer Res. 2003;23:761–4. [PubMed] [Google Scholar]
- 50.Trivers GE, De Benedetti VM, Cawley HL, Caron G, Harrington AM, Bennett WP, et al. Anti-p53 antibodies in sera from patients with chronic obstructive pulmonary disease can predate a diagnosis of cancer. Clin Cancer Res. 1996;2:1767–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.