Abstract
Human prion diseases are infectious and invariably fatal neurodegenerative diseases. They include sporadic Creutzfeldt-Jakob disease (sCJD), the most common form, and variant CJD (vCJD), which is caused by interspecies transmission of prions from cattle infected by bovine spongiform encephalopathy. Development of a biochemical assay for the sensitive, specific, early, and noninvasive detection of prions (PrPSc) in the blood of patients affected by prion disease is a top medical priority to increase the safety of the blood supply. vCJD has already been transmitted from human to human by blood transfusion, and the number of asymptomatic carriers of vCJD in the U.K. alone is estimated to be 1 in 2000 people. We used the protein misfolding cyclic amplification (PMCA) technique to analyze blood samples from 14 cases of vCJD and 153 controls, including patients affected by sCJD and other neurodegenerative or neurological disorders as well as healthy subjects. Our results showed that PrPSc could be detected with 100% sensitivity and specificity in blood samples from vCJD patients. Detection was possible in any of the blood fractions analyzed and could be done with as little as a few microliters of sample volume. The PrPSc concentration in blood was estimated to be ~0.5 pg/ml. Our findings suggest that PMCA may be useful for premortem noninvasive diagnosis of vCJD and to identify prion contamination of the blood supply. Further studies are needed to fully validate the technology.
INTRODUCTION
Human prion diseases are infectious and invariably fatal neuro-degenerative diseases. They include sporadic Creutzfeldt-Jakob disease (sCJD), the most common form, and variant CJD (vCJD), which is associated with the consumption of cattle infected with bovine spongiform encephalopathy (BSE) (1, 2). Currently, there is not a regulatory-approved assay for sensitive, objective, and noninvasive biochemical diagnosis of these diseases. This is a major problem for public health, because prion diseases are transmitted iatrogenically from human to human and because asymptomatic carriers may far outnumber clinically affected individuals (3).
The infectious agent responsible for these diseases, termed prion, appears to be composed exclusively of a conformationally altered form (PrPSc) of a naturally occurring protein (PrPC), which has the exceptional ability to infect individuals and propagate in the body without the need for genetic material (4). PrPSc is not only the main component of the infectious agent and the likely culprit of neurodegeneration but also the best surrogate marker for the disease. A major challenge for early diagnosis based on PrPSc detection is that this marker is present at high levels only in the central nervous system at late stages of the disease. However, several lines of evidence indicate that prions are also present in small quantities in peripheral tissues and biological fluids, such as lymphoid organs, cerebrospinal fluid, and blood (5, 6). Detection of PrPSc in blood is very challenging because little is known about its quantity, nature, and distribution in this fluid. On the basis of animal infectivity studies, it is estimated that in rodent plasma and buffy coat fractions, there is as little as 1 to 10 median lethal dose (LD50) infectious units in 1 ml of whole blood, which translates into the equivalent of 10 to 1000 million–fold less than the amount of infectious material present in diseased brain tissue (7). Moreover, the high amounts of normal PrPC found in blood as well as uncertainty about the biochemical and structural properties of blood-derived PrPSc (8–12) make it very difficult to develop a diagnostic test, relying on existing biochemical and immunological methods for detecting PrPSc. In the case of CJD, it is also imperative that an effective test not only have high sensitivity but also be highly specific. Considering that no treatment is available for this disease, it is not ethically acceptable to have a test with a high frequency of false positives.
Our strategy to achieve sensitive and specific detection of PrPSc was to use an amplification technology that reproduces PrPSc replication in vitro (13). This system is called protein misfolding cyclic amplification (PMCA) and consists of cycles of accelerated prion replication, combining phases of PrPSc growth with fragmentation of the polymers to increase the number of seeding-competent units. The cyclic nature of the system permits using as many cycles as required to reach the amplification state needed for the detection of PrPSc in a particular sample (13, 14). We have previously reported proof-of-concept experiments in which the technology was applied to replicate the misfolded protein from diverse species (15). The technology has been automated, leading to a marked increase in the efficiency of amplification. In its most current form used for vCJD samples (16), one round of 96 PMCA cycles (2 days) results in the ability to detect PrPSc in up to a 100 million–fold (108) dilution of brain tissue; after two rounds, we reached the highest detectability possible for PrPSc, which is about 10 billion–fold (1010) dilution. Moreover, our results demonstrated that PMCA is capable of detecting as little as about 26 monomers of PrP (17, 18), which, according to recent data on the minimal size of the infectious particle (19), would correspond to a single particle of oligomeric infectious PrPSc. These data demonstrate that PMCA has a similar power of amplification as polymerase chain reaction techniques used to amplify DNA and opens up possibilities for development of an assay for the highly sensitive detection of PrPSc. We have demonstrated that after amplification, we can detect PrPSc in blood of hamsters experimentally infected with scrapie during both the symptomatic (20) and presymptomatic phases of the disease (21), as well as in urine of human patients affected by vCJD (16). A recent report showed PrPSc detection by PMCA in white blood cells of three patients affected by vCJD (22). Using an ovine PrPC substrate for the PMCA reaction, this study showed a limit of detection equivalent to 10−7 brain dilution and positive PrPSc detection in three of the four vCJD samples tested, suggesting the possibility of the absence of prionemia in certain patients (22).
The major goal of our study was to develop a more sensitive PMCA assay for PrPSc detection in vCJD blood samples and then use it to evaluate the presence of prions in blood from patients affected by this disease and estimate sensitivity and specificity as well as the approximate quantity of PrPSc present in vCJD blood.
RESULTS
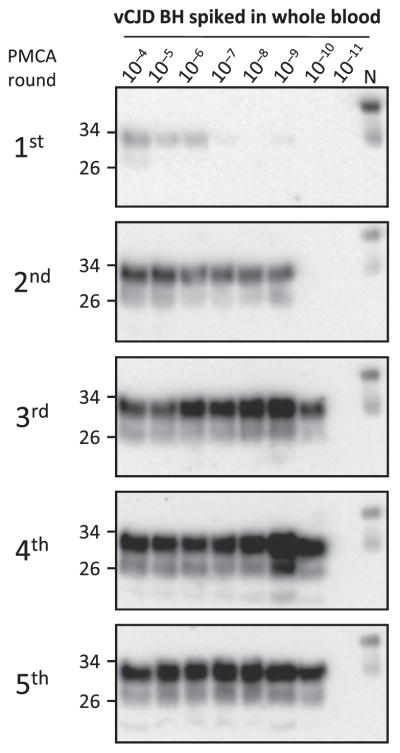
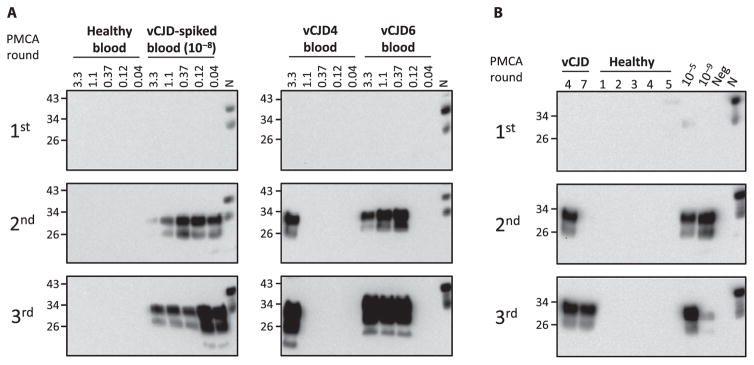
To investigate detection of vCJD PrPSc in blood by PMCA, we first performed spiking experiments diluting vCJD brain homogenate into healthy whole blood to optimize conditions and evaluate the limit of sensitivity. As observed in our previous experiments to detect PrPSc in animal blood, human blood inhibited the PMCA reaction (20, 21). For this reason, it was necessary to process the blood samples to enrich in PrPSc and remove other blood components that interfered with PMCA. The process consisted of a centrifugation in the presence of sarkosyl, followed by washing in phosphate-buffered saline (PBS) (Fig. 1). The resulting material was subjected to sequential rounds of PMCA using as a substrate brain homogenates from transgenic mice expressing human PrP with the Met/Met genotype at codon 129 [TgHuPrP(129MM)]. After the first round of PMCA, we detected up to a 10−6 dilution of vCJD brain homogenate spiked in whole blood (Fig. 2). This level of detection was clearly lower than the one observed when vCJD brain homogenate was diluted directly in conversion buffer (16), suggesting that despite the cleaning procedure, there was still some interference from blood components. After two rounds of PMCA, we detected PrPSc up to a 10−9 dilution, and after three rounds, we reached the maximum level of detection, equivalent to a 10−10 dilution of the vCJD brain homogenate (Fig. 2).
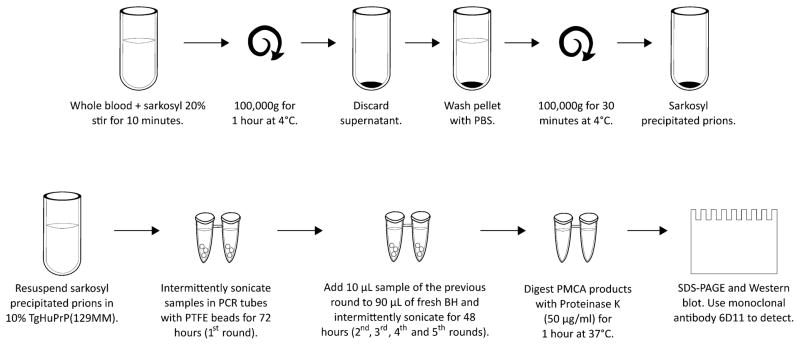
Fig. 1. Schematic representation of the processing of blood samples and the PMCA procedure.
To remove inhibitors of the PMCA reaction, samples of whole blood (or separated blood components) were incubated with 1 volume of 20% sarkosyl. After centrifugation, the pellet was washed in PBS and centrifuged again. The new pellet was resuspended directly in 10% brain homogenate (BH) from TgHuPrP(129MM) transgenic mice and was placed in a 0.2-ml tube with three polytetrafluoroethylene (PTFE) beads. Samples were subjected to a first round of 144 PMCA cycles, followed by subsequent rounds of 96 PMCA cycles. The PrPSc signal was detected by Western blotting after proteinase K digestion. SDS-PAGE, SDS–polyacrylamide gel electrophoresis.
Fig. 2. Optimization and limit of detection of vCJD PrPSc in blood.
To optimize PrPSc detection by PMCA and determine the limit of detection, whole blood from a healthy person was spiked with vCJD brain homogenate at different dilutions (10−4 to 10−11). After processing by centrifugation in the presence of sarkosyl (as described in Fig. 1), samples were subjected to various rounds of PMCA (the first round consisted of 144 cycles, and subsequent rounds consisted of 96 cycles). The PrPSc signal was assessed by Western blot analysis after proteinase K digestion. N refers to the normal (healthy) brain homogenate used as a migration control marker. Numbers on the left indicate the position of molecular weight markers.
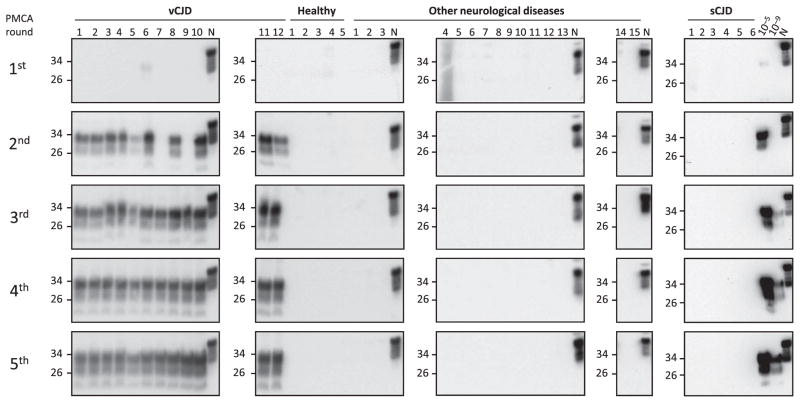
We then analyzed blood samples from 14 patients suffering from vCJD and compared them to samples from people affected by other neurodegenerative (60 samples) or nondegenerative neurological disorders (26 samples) and healthy individuals (49 samples) (Table 1). Our results showed that most of the vCJD blood samples analyzed were positive after two rounds of PMCA, and all were positive after three rounds, whereas none of the control samples gave any signal, even after five rounds of PMCA (Fig. 3 and Table 1). These data indicated that the PMCA technology had a 100% sensitivity (95% confidence interval, 76.8 to 100%) and specificity (95% confidence interval, 97.6 to 100%) for detection of PrPSc in blood of vCJD patients. To analyze whether detection of PrPSc in blood was specific for vCJD compared to other forms of human prion diseases, we studied samples from sCJD patients. The results showed that none of the six sCJD whole-blood samples tested was positive even after five rounds of PMCA (Fig. 3). These results do not necessarily mean that there was no PrPSc in the sCJD blood samples but that with the set of PMCA conditions used, it could not be detected. In a spiking experiment where healthy blood contained different dilutions of sCJD brain homogenate, there was no detection of PrPSc even at the lowest dilutions tested (fig. S1). We are currently optimizing a different set of PMCA conditions to efficiently detect sCJD prions.
Table 1.
Blood samples and PrPSc detection by PMCA.
| Clinical diagnosis | Total patients | PrPSc detected in blood |
|---|---|---|
| vCJD | 14 | 14/14 |
| sCJD* | 16 | 0/16 |
| Other neurodegenerative diseases† | 62 | 0/62 |
| Other neurological diseases‡ | 26 | 0/26 |
| Healthy controls | 49 | 0/49 |
Of these 16 sCJD samples analyzed, 6 were whole blood, 5 were plasma, and 5 were white blood cells from distinct sCJD patients.
Include samples from patients with Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, and frontotemporal dementia.
Include samples from patients with vascular dementia, seizures, epilepsy, psychiatric diseases, traumatic brain injury, mild cognitive impairment, demyelinating disease, and encephalitis.
Fig. 3. PrPSc detection in blood samples using PMCA.
Representative samples of whole blood (250 μl) from 12 patients affected by vCJD, 5 healthy controls, 15 patients affected by other neurodegenerative and neurological disorders, and 6 sCJD patients were subjected to five rounds of PMCA. PrPSc signal was detected by Western blot after proteinase K treatment. N refers to normal brain homogenate from TgHuPrP(129MM) transgenic mice without proteinase K treatment, which was used as a migration control. Numbers on the left indicate the position of molecular weight markers.
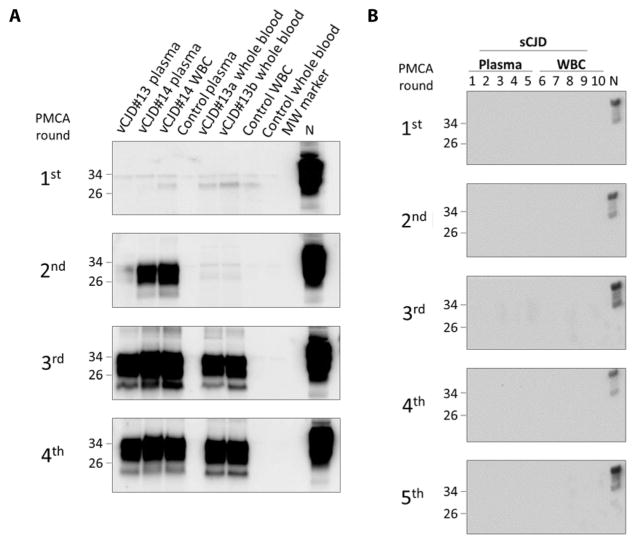
To analyze the blood fraction that carries PrPSc, we took blood samples from two cases of vCJD and separated plasma and white blood cells. PrPSc was detectable in both of these fractions after a similar number of amplification cycles (Fig. 4), indicating that the amount of prions in plasma and white blood cells was similar. However, we cannot rule out substantial differences in the quantity of PrPSc in different blood fractions that were masked by the very high efficiency of the assay. Also, PrPSc was detectable in whole blood from a patient collected at two different times during symptomatic disease (Fig. 4A, lanes 5 and 6). We analyzed similar fractions of sCJD blood, confirming the absence of prion detection in the setting used to amplify vCJD prions (Fig. 4B). We also analyzed the molecular typing of prions amplified from blood by PMCA (fig. S2). As expected, the material amplified from blood had the same type 2B pattern (that is, 19 kDa, enriched in the diglycosylated form) displayed by prions from vCJD brain homogenates, in contrast to the type 1 and 2 patterns of classical sCJD.
Fig. 4. Detection of PrPSc in different blood fractions and at different times during clinical disease.
(A) Samples of whole blood, plasma, and white blood cells (WBCs) from two patients (#13 and #14) affected by vCJD and one healthy control were analyzed for the presence of PrPSc in different blood fractions. In addition, whole blood from one patient was collected and tested at two different time points during the clinical phase of the disease. Samples were processed and subjected to four rounds of PMCA (first round consisted of 144 cycles and subsequent rounds of 96 cycles). Lane 1, vCJD patient #13 plasma; lane 2, vCJD patient #14 plasma; lane 3, vCJD patient #14 WBC; lane 4, healthy control plasma; lane 5, vCJD patient #13 whole blood “a” (collected on September 2002); lane 6, vCJD patient #13 whole blood “b” (collected on November 2002); lane 7, healthy control WBC; lane 8, healthy control whole blood; lane 9, molecular weight standard (MW). N refers to normal brain homogenate from TgHuPrP(129MM) transgenic mice without proteinase K treatment, which was used as a migration control. Numbers on the left indicate the position of molecular weight markers. (B) Samples of plasma or WBCs from 10 different patients affected by sCJD were tested under the same conditions as in (A). All samples were negative after five sequential rounds of PMCA.
To estimate the minimum volume of blood required for our assay, we tested different quantities of whole blood from two different vCJD patients. Our results showed that as little as 3.3 or even 0.37 μl of blood was sufficient to readily detect PrPSc in human vCJD blood after PMCA amplification (Fig. 5A). The differences in the minimum volume of blood needed to detect the signal for the two distinct vCJD samples tested likely reflected the dissimilar amounts of prions present in different blood samples. However, we cannot rule out that some samples may have different amounts of blood components that interfere with the PMCA reaction. Reduction of the volume of material used also avoided the need for a precleaning step, because the concentration of PMCA inhibitors present in blood was reduced to a level that did not interfere with the reaction. In samples of healthy blood spiked with a 10−8 dilution of vCJD brain homogenate, the signal in the second PMCA round was higher when the volume of sample was lower (Fig. 5A). Elimination of the precleaning step is important for practical and routine use of the technology in blood detection because this step is time-consuming and labor-intensive. The small minimum volume of blood needed for PrPSc detection together with the known ability of PrPSc to bind to a variety of surfaces (23–25) led us to hypothesize that just placing a blood sample in the PMCA tube may enable enough prions to bind to the tube to allow prion replication in a PMCA reaction. To investigate this possibility, 100 μl of whole blood from two different patients affected by vCJD, as well as from five healthy controls, and normal blood spiked with a 10−5 and a 10−9 dilution of vCJD brain homogenate were incubated in PMCA tubes for 1 hour at room temperature. Thereafter, the entire volume of the samples was removed, and 100 μl of PMCA substrate [10% TgHuPrP(129MM)] was added to the same tube. After serial rounds of PMCA, the presence of PrPSc was detected by Western blot after proteinase K digestion. The results showed that a PrPSc signal was detected in both vCJD blood samples, as well as in the positive controls spiked with vCJD brain homogenate (Fig. 5B). The PMCA rounds for detection in vCJD blood samples after binding of the agent to the tubes were the same as that when using the standard protocol of centrifugation in detergent. Sample vCJD4 was detectable in both cases in round 2, and sample vCJD7 was detectable after three rounds of PMCA (compare Figs. 3 and 5B). No signal was detectable in any of the healthy controls. These results may enable the implementation of a simpler procedure for PrPSc detection in blood, but the robustness of the assay needs to be confirmed by analyzing larger numbers of vCJD blood samples.
Fig. 5. Detection of PrPSc in small volumes of blood.
(A) To estimate the minimum volume of whole blood needed for PrPSc detection, different volumes of whole blood from two vCJD patients (samples #4 and #6) were directly added to a 10% brain homogenate from TgHuPrP(129MM) transgenic mice. We used blood from a healthy individual spiked with a 10−8 dilution of vCJD brain homogenate as a positive control. Samples were subjected to three sequential rounds of PMCA, and PrPSc was detected by Western blot. (B) Samples of whole blood (100 μl) from two vCJD patients (#4 and #7) and five healthy controls and two blood samples from controls spiked with 10−5 or 10−9 dilutions of vCJD brain homogenate were incubated with PMCA tubes containing three PTFE beads for 1 hour at room temperature in an end-over-end mixer. The blood samples were removed, and 100 μl of PMCA substrate was added to the tube. Samples were subjected to various rounds of PMCA (first round of 144 cycles and subsequent rounds of 96 cycles), and PrPSc was detected by Western blot after proteinase K digestion. In both panels, N refers to normal brain homogenate from TgHuPrP(129MM) transgenic mice without proteinase K treatment, which was used as a migration control. Numbers on the left indicate the position of molecular weight markers.
Finally, to estimate the quantity of PrPSc present in vCJD blood, we used the quantitative PMCA technology, which compared the number of cycles required to detect the signal with that for blood spiked with known concentrations of PrPSc (18). Our data showed that in 11 of the 14 vCJD samples analyzed, PrPSc was detectable after two PMCA rounds (Figs. 3 and 4). By comparison with the spiked samples, we estimated this to be equivalent to the amount of PrPSc present in a 10−9 dilution of the brain homogenate, which extrapolated to about 5 × 10−13 g/ml. This concentration is similar to previous estimations using bioassays that indicated that the quantity of prions in blood is on the order of 1 to 10 LD50 per milliliter of blood (7). Considering our previous estimation of the concentration of PrPSc excreted in urine of vCJD patients (16), we conclude that the concentration of PrPSc in blood is between two and three orders of magnitude higher than in urine.
DISCUSSION
So far, 231 cases of vCJD have been reported mostly in the U.K. and France (with 4 cases in the United States) (26), and the future of this epidemic remains unknown. Fortunately, the spread of classical BSE has been largely controlled, thanks to the implementation of feeding restrictions and surveillance (27–29). However, the appearance of atypical and genetic forms of BSE is an additional concern because the characteristics of transmission of these new forms may be different from that of traditional BSE (30–33). Nevertheless, it is estimated that millions of people have been exposed to BSE prions, and it is currently unclear how many people may silently carry infectious material. Studies in transgenic mouse models of human prion disease showed that infection with BSE prions frequently produces subclinical or carrier states, which upon a secondary infection can produce full-blown disease (3, 34, 35). Strikingly, a recent study searching for PrPSc immunoreactivity in archived surgically resected appendix samples in the U.K. estimated that 30,000 people in this country are asymptomatic carriers of vCJD infection (36). It is probable that this number is underestimated, because the methods used to detect prions in lymphoreticular specimens are unlikely to have 100% sensitivity. The possible existence of a large number of carriers of vCJD prions represents a significant risk for iatrogenic transmission of vCJD from human to human, a pathway that has already been shown to happen in other human prion diseases, such as kuru and iatrogenic CJD (37). Iatrogenic transmission of vCJD through blood transfusion is a major concern. Unfortunately, this route has already proven to be a problem because several cases have been linked to transfusion of blood donated by infected individuals in the preclinical stage of the disease (38–40).
Currently, there is no premortem biochemical diagnosis for vCJD or any validated procedure to detect prions in blood or other human-derived tissues that might represent a concern for iatrogenic transmission of vCJD. People affected by vCJD die, on average, 2 years after the first clinical signs appear. The disease normally starts with psychiatric alterations (depression, anxiety, and hallucinations), which are common in other diseases, and only begins to show more typical signs of a neurodegenerative condition (ataxia, myoclonus, and dementia) several months after the first symptoms have occurred. Even then, the diagnosis of vCJD is uncertain and is only confirmed by postmortem examination of the brain for the presence of prions with the vCJD signature. A definitive diagnosis of vCJD in the early stages of clinical disease will be crucial for differential diagnosis from other neurological disorders that share similar clinical abnormalities. Furthermore, early diagnosis of vCJD would allow any potential therapy to be given before substantial brain damage has occurred. It would also allow any public health measures to be implemented rapidly, for example, tracing recipients of blood donated by individuals with vCJD.
Sensitive detection of prions in biological fluids has been reported using prion amplification techniques, including PMCA and RT-QuIC (real-time quaking-induced conversion) (41, 42). Both PMCA and RT-QuIC take advantage of the seeding of protein misfolding by prions to substantially amplify the signal and detect small amounts of PrPSc. Detection of PrPSc by RT-QuIC in cerebrospinal fluid (43, 44) and nasal mucosa (45) is currently being used in the clinical diagnosis of sCJD. There has been less work on the application of RT-QuIC to vCJD samples, and this technology appears less efficient for blood samples. Our current study demonstrates that small quantities of PrPSc were present in the blood (both in plasma and associated with white cells) of the 14 vCJD patients analyzed. PrPSc was detectable by PMCA with 100% sensitivity and specificity. Similar results are reported by Bougard et al. in this issue using PMCA after capturing PrPSc from blood using plasminogen-coated beads (46). Strikingly, Bougard et al. also showed positive detection of PrPSc in two blood samples taken at the preclinical stage of the disease from donors that years later developed vCJD. Although both our study and that by Bougard et al. show 100% sensitivity and specificity, it is important to highlight the caveat that these estimations are based on a very small number of vCJD blood samples (14 cases in our study and 18 cases in the study by Bougard et al.). Future double-blind studies are needed with a larger number of blood samples to allow statistical validation of this assay. In addition to the small number of samples analyzed, other limitations of our study include the fact that all samples came from individuals at later stages of clinical disease and blood samples from presymptomatic cases were not analyzed. Finally, it is important to highlight that because PMCA can detect subinfectious amounts of prions, a positive signal by PMCA does not necessarily indicate that the carrier will eventually develop the clinical disease.
There is evidence that at least four cases of vCJD were acquired by transfusion with blood taken from donors at the preclinical stage of the disease (38–40). Given that PMCA can detect the equivalent of a single particle of PrPSc (17, 18), our findings suggest that PMCA may be useful for noninvasive diagnosis of this disease in presymptomatic individuals, although this possibility needs to be tested. We have previously shown preclinical detection of prions in blood samples from animals experimentally infected with prion diseases (21). Further studies are needed to demonstrate the usefulness of PMCA for presymptomatic detection of vCJD prions in biological fluids and to investigate the earliest time point during the preclinical phase of vCJD at which PrPSc can be detected in blood.
MATERIALS AND METHODS
Study design
We analyzed blood samples from 14 vCJD patients and 137 controls (either healthy or with different neurological or neurodegenerative disorders). We also analyzed 16 samples from individuals affected by sCJD. Samples were obtained randomly without any exclusion or inclusion criteria other than the respective clinical diagnosis. Data reported included all samples analyzed. No samples or data were excluded after analysis. Although this study was not done with blinded samples, we analyzed vCJD samples and controls at random from initial centrifugation until Western blot. The identity of the samples was seen only after the results were obtained.
Patient samples
Samples of frozen whole blood were collected at different stages of clinical vCJD from 14 different patients. Twelve of the samples were collected in the U.K., and two were collected in Italy. The disease was confirmed postmortem by neuropathological and biochemical analyses. As controls, we used four groups of whole-blood samples, including 62 patients affected by other neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, and Lewy body dementia), 26 patients affected by nondegenerative neurological disorders (vascular dementia, traumatic brain injury, stroke, epilepsy, encephalitis, and mood disorders), and 49 healthy individuals (Table 1). Additionally, we analyzed five samples of plasma and five samples of white blood cells from sCJD patients as well as six whole-blood samples. The diagnosis of sCJD was also confirmed postmortem by Western blot and neuropathological analysis, whereas the diagnosis of other neurological diseases was determined clinically with the help of imaging and biochemical assays when available. Blood collection was approved by the respective institutional review boards at the authors’ institutions.
Processing of blood samples
Samples of whole blood, and in some cases separated plasma or white blood cell fractions, were processed as previously described to remove the bulk of proteins and other components that interfered with the PMCA reaction (Fig. 1). Briefly, 250 μl of a sample was mixed and incubated with 1 volume of 20% sarkosyl for 10 min at room temperature. Thereafter, samples were centrifuged at 100,000g for 1 hour at 4°C, the supernatant was discarded, and the pellet was washed in 500 μl of PBS. Tubes were centrifuged again at 100,000g for 30 min at 4°C. The pellet was resuspended directly in 10% brain homogenate from TgHuPrP(129MM) transgenic mice, supplemented with 0.05% digitonin and 12 mM EDTA (see below).
PMCA procedure
The PMCA reaction was carried out as previously described (16, 47) using as a substrate brain homogenate from transgenic mice expressing human PrP with Met/Met genotype at position 129 [TgHuPrP(129MM)]. Brain substrate was prepared at a concentration of 10% (w/v) in conversion buffer (PBS supplemented with 150 mM NaCl and 1% Triton X-100) with protease inhibitors (cOmplete, EDTA-free; Roche). Debris was removed by a low-speed centrifugation (800g, 1 min, 4°C), and brain homogenates were stored frozen at −80°C until further use.
For PMCA, samples were subjected to a first round of 144 cycles of PMCA in 0.2-ml tubes (Eppendorf, catalog no. 951010022) containing three PTFE beads (Hoover Precision Products). Each cycle consisted of 29 min and 30 s of incubation at 37/40°C, followed by a 30-s pulse of sonication set at an amplitude of 30, using the Qsonica micro-plate horn sonicator (Model Q700) equipped with a titanium horn. Subsequent rounds of 96 PMCA cycles were performed by taking an aliquot of the amplified material that was diluted 10-fold into fresh TgHuPrP129MM brain homogenate. No multichannel pipettes were used to reduce the risk of cross-contamination. After PMCA, samples were taken for detection of PrPSc using Western blot after digestion with proteinase K (50 μg/ml) for 1 hour at 37°C (16, 47).
Supplementary Material
Fig. S1. Spiking of sCJD brain homogenates in blood.
Fig. S2. Molecular typing of vCJD prions amplified by PMCA from blood.
Acknowledgments
We thank E. Bistaffa (Istituto Neurologico Carlo Besta) for technical help and R. Will (University of Edinburgh) for providing many of the blood samples used in this study. We are also grateful to G. Telling (Colorado State University) for providing a colony of transgenic mice expressing human PrP.
Funding: This study was supported in part by grants from the NIH (P01AI106705, R01NS049173, and R42NS079060) to C.S., the Italian Ministry of Health and Associazione Italiana Encefalopatie da Prioni (AIEnP) to F.T., and La Fondation pour la Recherche Médicale (FABS201402) to F.M. The U.K. National CJD Research and Surveillance Unit is funded by the Department of Health and the Scottish Government. The funders had no role in the study design, the data collection and analysis, or the preparation of the manuscript.
Footnotes
Author contributions: L.C.-M. designed the studies, carried out most of the experiments, analyzed the results, and prepared the figures. F.M. and S.P. performed PMCA analysis of some of the patient blood samples. J.W.I., F.T., and P.E.S. provided blood samples and critically reviewed the manuscript. C.S. is the principal investigator of the project and was responsible for coordinating research activity, analyzing the data, writing the manuscript, and producing the final version of the article.
Competing interests: C.S. is the inventor on several patents related to the PMCA technology: US7351526/WO02/04954, “Early diagnosis of conformational disorders”; US20110311997, “Methods for estimating prion concentration in tissues and biological fluids by quantitative PMCA”; and US7598046/WO2004/111652, “Use of prion conversion modulating agents.” C.S. is the founder, chief scientific officer, and vice-president of Amprion Inc., a biotech company focusing on the commercial utilization of PMCA for prion diagnosis. The other authors declare no competing interests.
Data and materials availability: All requests for materials should be directed to C.S. and will be made available subject to a material transfer agreement.
REFERENCES AND NOTES
- 1.Aguzzi A, Calella AM. Prions: Protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 2.Collinge J. Prion diseases of humans and animals: Their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, Tuzi NL, Head MW, Ironside JW, Will RG, Manson JC. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguzzi A. Prion diseases, blood and the immune system: Concerns and reality. Haematologica. 2000;85:3–10. [PubMed] [Google Scholar]
- 6.Wadsworth JDF, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown P. Creutzfeldt-Jakob disease: Blood infectivity and screening tests. Semin Hematol. 2001;38(4 Suppl 9):2–6. doi: 10.1016/s0037-1963(01)90130-1. [DOI] [PubMed] [Google Scholar]
- 8.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 9.Kuczius T, Groschup MH. Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol Med. 1999;5:406–418. [PMC free article] [PubMed] [Google Scholar]
- 10.Thackray AM, Hopkins L, Bujdoso R. Proteinase K-sensitive disease-associated ovine prion protein revealed by conformation-dependent immunoassay. Biochem J. 2007;401:475–483. doi: 10.1042/BJ20061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronier S, Gros N, Tattum MH, Jackson GS, Clarke AR, Collinge J, Wadsworth JDF. Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J. 2008;416:297–305. doi: 10.1042/BJ20081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H, Serban A, Vey M, Baron H, Giles K, Miller BL, DeArmond SJ, Prusiner SB. Diagnosis of human prion disease. Proc Natl Acad Sci USA. 2005;102:3501–3506. doi: 10.1073/pnas.0409651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 14.Soto C, Saborio GP, Anderes L. Cyclic amplification of protein misfolding: Application to prion-related disorders and beyond. Trends Neurosci. 2002;25:390–394. doi: 10.1016/s0166-2236(02)02195-1. [DOI] [PubMed] [Google Scholar]
- 15.Soto C, Anderes L, Suardi S, Cardone F, Castilla J, Frossard MJ, Peano S, Saa P, Limido L, Carbonatto M, Ironside J, Torres JM, Pocchiari M, Tagliavini F. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 2005;579:638–642. doi: 10.1016/j.febslet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Moda F, Gambetti P, Notari S, Concha-Marambio L, Catania M, Park KW, Maderna E, Suardi S, Haïk S, Brandel JP, Ironside J, Knight R, Tagliavini F, Soto C. Prions in the urine of patients with variant Creutzfeldt–Jakob disease. N Engl J Med. 2014;371:530–539. doi: 10.1056/NEJMoa1404401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saá P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat Methods. 2010;7:519–520. doi: 10.1038/nmeth.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 21.Saá P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 22.Lacroux C, Comoy E, Moudjou M, Perret-Liaudet A, Lugan S, Litaise C, Simmons H, Jas-Duval C, Lantier I, Béringue V, Groschup M, Fichet G, Costes P, Streichenberger N, Lantier F, Deslys JP, Vilette D, Andréoletti O. Preclinical detection of variant CJD and BSE prions in blood. PLOS Pathog. 2014;10:e1004202. doi: 10.1371/journal.ppat.1004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med. 1999;5:240–243. [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLOS Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep. 2015;11:1168–1175. doi: 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maheshwari A, Fischer M, Gambetti P, Parker A, Ram A, Soto C, Concha-Marambio L, Cohen Y, Belay ED, Maddox RA, Mead S, Goodman C, Kass JS, Schonberger LB, Hussein HM. Recent US case of variant Creutzfeldt-Jakob Disease—Global implications. Emerg Infect Dis. 2015;21:750–759. doi: 10.3201/eid2105.142017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueston WD. BSE and variant CJD: Emerging science, public pressure and the vagaries of policy-making. Prev Vet Med. 2013;109:179–184. doi: 10.1016/j.prevetmed.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Matthews D, Adkin A. Bovine spongiform encephalopathy: Is it time to relax BSE-related measures in the context of international trade? Rev Sci Tech. 2011;30:107–117. doi: 10.20506/rst.30.1.2019. [DOI] [PubMed] [Google Scholar]
- 29.Ducrot C, Arnold M, de Koeijer A, Heim D, Calavas D. Review on the epidemiology and dynamics of BSE epidemics. Vet Res. 2008;39:15. doi: 10.1051/vetres:2007053. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson-Smith MA, Richt JA. Rare BSE mutation raises concerns over risks to public health. Nature. 2009;457:1079. doi: 10.1038/4571079b. [DOI] [PubMed] [Google Scholar]
- 31.Béringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL, Laude H. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis. 2008;14:1898–1901. doi: 10.3201/eid1412.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkema-Buschmann A, Fast C, Kaatz M, Eiden M, Ziegler U, McIntyre L, Keller M, Hills B, Groschup MH. Pathogenesis of classical and atypical BSE in cattle. Prev Vet Med. 2011;102:112–117. doi: 10.1016/j.prevetmed.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, Catania M, Rossi G, Di Fede G, Giaccone G, Bruzzone MG, Minati L, Corona C, Acutis P, Gelmetti D, Lombardi G, Groschup MH, Buschmann A, Zanusso G, Monaco S, Caramelli M, Tagliavini F. Conversion of the BASE prion strain into the BSE strain: The origin of BSE? PLOS Pathog. 2007;3:e31. doi: 10.1371/journal.ppat.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill AF, Collinge J. Subclinical prion infection in humans and animals. Br Med Bull. 2003;66:161–170. doi: 10.1093/bmb/66.1.161. [DOI] [PubMed] [Google Scholar]
- 35.Lasmézas CI, Fournier JG, Nouvel V, Boe H, Marcé D, Lamoury F, Kopp N, Hauw JJ, Ironside J, Bruce M, Dormont D, Deslys JP. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt–Jakob disease: Implications for human health. Proc Natl Acad Sci USA. 2001;98:4142–4147. doi: 10.1073/pnas.041490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, Linehan J, Simmons M, Webb P, Bellerby P, Andrews N, Hilton DA, Ironside JW, Beck J, Poulter M, Mead S, Brandner S. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: Large scale survey. BMJ. 2013;347:f5675. doi: 10.1136/bmj.f5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown P, Preece MA, Brandel J-P, Sato T, McShane L, Zerr I, Fletcher A, Will RG, Pocchiari M, Cashman NR, Huillard d’Aignaux J, Cervenáková L, Fradkin J, Schonberger LB, Collins SJ. Iatrogenic Creutzfeldt-Jakob disease at the millenium. Neurology. 2000;55:1075–1081. doi: 10.1212/wnl.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 38.Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 39.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 40.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, Linehan JM, Brandner S, Wadsworth JDF, Hewitt P, Collinge J. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: A case report. Lancet. 2006;368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 41.Orru CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. New generation QuIC assays for prion seeding activity. Prion. 2012;6:147–152. doi: 10.4161/pri.19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saá P, Cervenakova L. Protein misfolding cyclic amplification (PMCA): Current status and future directions. Virus Res. 2015;207:47–61. doi: 10.1016/j.virusres.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17:175–178. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- 44.Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:e02451–e14. doi: 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, Fiorini M, Pocchiari M, Monaco S, Caughey B, Zanusso G. A test for Creutzfeldt–Jakob disease using nasal brushings. N Engl J Med. 2014;371:519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bougard D, Brandel JP, Bélondrade M, Béringue V, Segarra C, Fleury H, Laplanche JL, Mayran C, Nicot S, Green A, Welaratne A, Narbey D, Fournier-Wirth C, Knight R, Will R, Tiberghien P, Haïk S, Coste J. Detection of prions in the plasma of pre-symptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci Transl Med. 2016;8:370ra182. doi: 10.1126/scitranslmed.aag1257. [DOI] [PubMed] [Google Scholar]
- 47.Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C. Protein misfolding cyclic amplification of infectious prions. Nat Protoc. 2012;7:1397–1409. doi: 10.1038/nprot.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Spiking of sCJD brain homogenates in blood.
Fig. S2. Molecular typing of vCJD prions amplified by PMCA from blood.