Abstract
Semaphorins are extracellular signaling proteins that are essential for the development and maintenance of many organs and tissues. The more than 20-member semaphorin protein family includes secreted, transmembrane and cell surface-attached proteins with diverse structures, each characterized by a single cysteine-rich extracellular sema domain, the defining feature of the family. Early studies revealed that semaphorins function as axon guidance molecules, but it is now understood that semaphorins are key regulators of morphology and motility in many different cell types including those that make up the nervous, cardiovascular, immune, endocrine, hepatic, renal, reproductive, respiratory and musculoskeletal systems, as well as in cancer cells. Semaphorin signaling occurs predominantly through Plexin receptors and results in changes to the cytoskeletal and adhesive machinery that regulate cellular morphology. While much remains to be learned about the mechanisms underlying the effects of semaphorins, exciting work has begun to reveal how semaphorin signaling is fine-tuned through different receptor complexes and other mechanisms to achieve specific outcomes in various cellular contexts and physiological systems. These and future studies will lead to a more complete understanding of semaphorin-mediated development and to a greater understanding of how these proteins function in human disease.
Keywords: Semaphorin, Plexin, Neuropilin, Cellular Guidance, Axon Guidance, Cell Morphology, Signaling to the Cytoskeleton, Navigation, Motility, Inhibition, Repulsive Signaling
The semaphorins (Semas) are a large and diverse family of proteins with essential roles in the development and function of many different physiological systems. As a testament to their broad importance in biology, Semas have been discovered in worms, flies, chick, mammals, and viruses and are expressed in most tissues (1). The Sema family includes proteins that are secreted, cell surface-attached and membrane-bound. The hallmark of the Sema protein family is the sema domain, an approximately 500 amino acid extracellular domain (2). Apart from the conserved sema domain, the overall protein domain organization of Sema family members are quite different, and Semas participate in a wide variety of processes from embryogenesis to adult organ homeostasis. In general, Semas act as signaling ligands that regulate the shape and motility of cells during the development and operation of the nervous, cardiovascular, immune, endocrine, hepatic, renal, reproductive, respiratory and musculoskeletal systems. In addition, Sema signaling has been linked to diseases affecting these systems and to cancer progression. In this chapter, we introduce the Sema protein family, highlight the cell biological effects of Sema signaling, and provide an overview of Sema function in several well-studied contexts.
History
While multiple early investigations identified Semas in several systems (reviewed in (1)), they were first characterized in the early 1990s for their ability to affect axon growth and guidance. Kolodkin and colleagues identified a protein they called “Fasciclin IV,” which was involved in axon guidance during grasshopper embryonic development (3). At the same time, Raper and colleagues isolated a biochemical fraction from embryonic chick brain that induced the collapse of neuronal growth cones in culture (4); findings that led them to identify the “collapsing factor” in this fraction as a protein, which they named Collapsin (5). Interestingly, sequence comparison revealed that the sema domain was a distinctive protein domain and Collapsin and Fasciclin IV, now known as Sema3A and Sema-1a, respectively, were the first identified members of the Sema family of proteins (2). These early studies established the importance of Semas as axon guidance signals and today Semas are considered one of the 4 classes of canonical axon guidance molecules (along with netrins, slits, and ephrins) (6). Although Semas are now known to be involved in a variety of events outside of their role in axon guidance, these initial studies highlighted what turned out to be an important theme of Sema signaling: that they direct axon guidance (and the movement of other cells) by altering the cytoskeletal and adhesive elements that are necessary for specifying cell morphology.
Protein Organization and Structure
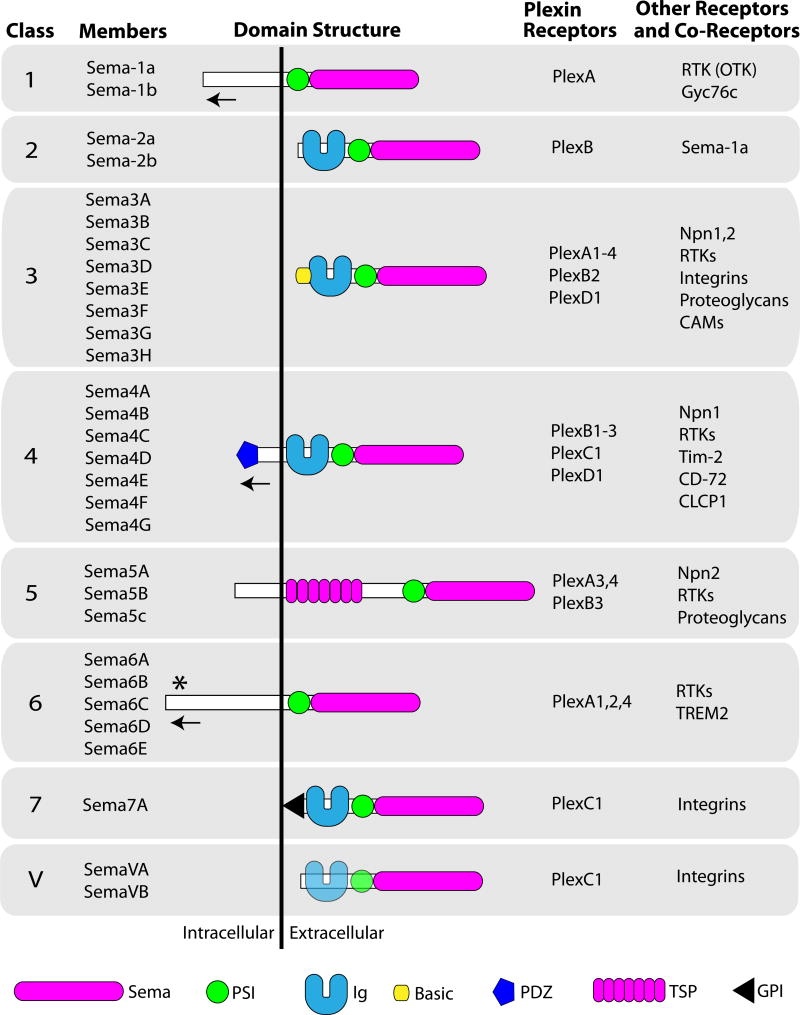
Since the discovery of the founding members, the Sema family has grown to include 30 proteins that are divided into 8 classes based on structural features and distribution among different phyla (Figure 1; (7)). Class 1 and 2 Semas are found only in invertebrates, while class 3–7 are found only in vertebrates (with the one exception being a Class 5 member, Sema-5c, which is also found in invertebrates). Class V members are found in viruses. Class 1, 4, 5, and 6 members are transmembrane, class 2, 3 and V members are secreted, and class 7 members are glycosylphosphatidylinositol (GPI)-linked. In addition, class 4, 5 and 7 members, and possibly others, are cleaved and released extracellularly (e.g., (8–10)).
Figure 1. The Semaphorin Protein Family.
Semaphorin protein family members are grouped into 8 classes based on their domain structure. Class 1 and 2 Semas and Sema5c are found in invertebrates, Class 3–7 Semas are found in vertebrates and Class V Semas are found in viruses. Plexin receptors, the predominant receptors for Semas, are grouped into 4 classes (A–D) and each plexin receptor class interacts with a particular Sema class or classes to mediate signaling. A number of other membrane-associated receptors and co-receptors are also important for Sema signal transduction. These proteins directly bind Semas and initiate signaling (e.g., integrins), act as ligand binding co-receptors (e.g., Npn1,2), and/or work as part of multimeric receptor complexes (e.g., RTKs). Proteins functioning downstream of receptor complexes to mediate Sema signaling are not shown (see text). At least some transmembrane Semas also function as receptors in reverse signaling (e.g., leftward arrows) and participate in cis (within the same cell) interactions with plexin receptors (e.g., asterisk). Semi-transparency of Class V semaphorin Ig and PSI domains indicates that these domains are present in some, but not all, viral semaphorins. Sema (semaphorin domain), PSI (plexin-semaphorin-integrin domain), Ig (immunoglobulin domain), Basic (basic domain), PDZ (PDZ domain), TSP (Thrombospondin domain), GPI (glycosylphosphatidylinositol linkage), RTK (receptor tyrosine kinase), Npn1 (Neuropilin 1), Npn2 (Neuropilin 2), CAM (cell adhesion molecule) Tim-2 (T-cell Ig and mucin domain containing protein 2), CD72 (B cell differentiation antigen CD72), CLCP1 (CUB, LCCL-homology, Coagulation factor V/VIII homology domains protein 1), TREM2 (Triggering Receptor Expressed on Myeloid Cells 2), DAP12 (DNAX activating protein of 12 kDa)((1,12,23,46,232,233) and references therein.
The defining feature of Semas, the sema domain, is present as a single copy located at the N-terminus of Sema proteins and is essential for Sema signaling (Figure 1; reviewed in (11–14)). Interestingly, sema domains are also found in plexin (Plex) family proteins and in several receptor tyrosine kinases, and these proteins are included in the Sema superfamily (14). At least six independent crystal structures of sema domains have now been published (15–20), revealing a 7-blade beta propeller fold structure for all Semas characterized to date. These structural studies have also indicated that the Sema domain mediates homophilic dimerization between Semas, which is consistent with functional studies suggesting that dimerization is important for Sema function (e.g., (21,22)). Positioned carboxy-terminal (C-terminal) to the sema domain, almost all Semas (except some viral family members) have a cysteine-rich region that is known as a plexin-semaphorin-integrin (PSI) domain because it is homologous to the beta chain of integrins and is also found in plexin family members. The PSI domain folds as a cysteine knot and is found structurally close to the sema domain (14). Class 2, 3, 4, 7 and V Semas also contain Ig-like domains C-terminal to their PSI domains. Other protein domains represented among Sema family members include a basic domain in class 3 Semas, thrombospondin repeats in class 5 Semas and a GPI linkage domain for class 7 Semas (Figure 1).
Receptors
Several different protein families are known to directly bind to Semas and function as receptors (Figure 1). In addition, a number of co-receptors also associate with Sema receptors and become activated to expand the signaling response to Sema binding (see (12) for a recent list of Sema receptors and their expression patterns and (1) for associated signaling proteins). Most Sema signaling is mediated by plexin receptors and members of all classes of Semas have been found to interact with plexins (23). The first plexin receptor was identified as a cell adhesion molecule expressed in neurons (24) and the connection between Semas and plexins was revealed by the discovery that a viral Sema associated with a receptor on monocytes (now known as PlexC1; (25)). Plexins are large transmembrane receptors containing an extracellular sema domain, which mediates the interaction with Semas, several other known extracellular protein domains, and a highly conserved intracellular domain containing a GTPase activating protein (GAP) homology domain (reviewed in (12)). The plexin family includes 2 classes in invertebrates (A and B) and 4 classes in vertebrates (A–D) (23).
Neuropilins (Npn) are also well-characterized Sema receptors, best known for their roles as binding proteins for class 3 secreted Semas ((26–28); reviewed in (29)). With the exception of Sema3E, which directly binds PlexD1 (30), class 3 Semas require Npns as co-receptors to mediate signaling. Npn receptors have very short intracellular domains that are not required in some contexts for transduction of Sema signaling (e.g., (31,32); but see (33)). Instead, Npns work with various signal transducing receptors, including plexins and cell adhesion molecules (CAMs), and may act to stabilize Sema/receptor interactions (e.g., (16), see (34) for receptor components utilized for class 3 Sema signaling through Npns). Additional receptors that directly bind Semas include CD72 (35), Tim2 (36), integrins (37) and proteoglycans (38–40).
Co-receptors that associate with Sema binding receptors have profound effects on the signaling outcome of Sema-receptor interactions. As mentioned above, cell adhesion molecules, such as Nr-CAM and L1 CAM that associate with Npn receptors, can be required for mediating Sema effects and for transducing class 3 Sema signals (reviewed in (34)). In addition, a number of receptor tyrosine kinases (RTKs), such as vascular endothelial growth factor receptor 2 (VEGFR2), Met, ErbB2, and off-track (OTK) associate with plexins and Npns and become transactivated upon Sema binding (41,42), dramatically altering the outcome of signaling through particular plexins (e.g., (43,44)). In addition to acting as signaling ligands, transmembrane Semas can also act as receptors, a phenomenon known as reverse signaling (Figure 1; reviewed in (45,46)).
Cell Biological Effects and Signaling
The molecular mechanisms responsible for the wide-ranging effects of Semas are still far from clear, but a great deal of progress has been made toward characterizing signaling pathways that contribute to Sema function in particular contexts (reviewed in (12,45–51)). Early studies in neurons demonstrated that Semas cause dramatic changes to cell morphology including the rapid and dramatic collapse of cell processes (e.g., (4,52)). Subsequent work has shown that these cell morphological changes occur in many different cell types and are the result of changes to both the cytoskeleton and cell adhesion that are mediated by Sema signaling through plexin receptors (reviewed in (12,53)). Specifically, plexin activity initiates signaling pathways that negatively regulate the actin and microtubule cytoskeletons and reduce cell adhesion. Here we briefly discuss some of the best-characterized processes underlying the cellular effects of Semas and present some of the mechanisms that allow the fine-tuning of responses to Sema signaling in different cellular contexts.
Many proteins work to mediate Sema/plexin signaling (reviewed in (12)), but among these, small GTPases play a particularly important role (54). Small GTPases are well known as regulators of the cytoskeleton and cellular adhesion. These proteins are “turned on” by guanine nucleotide exchange proteins (GEFs) and “turned off” by GAPs (reviewed in (55)). All plexins contain a highly conserved intracellular GAP homology domain that directly activates the GTPase activity of Ras and Rap family GTPases ((56,57); reviewed in (58)). Ras and Rap family GTPases promote integrin function to control cell adhesion and also have effects on the actin cytoskeleton (59,60). For example, upon Sema binding, plexin GAP activity toward R-Ras reduces levels of the active GTP-bound form of the GTPase, leading to reduced integrin activation (reviewed in (12)).
In addition to plexin GAP activity, plexins have a Rho binding domain (RBD) that interacts with Rho family GTPases (12), key players in actin cytoskeletal arrangements that control cell shape and movement (reviewed in (61)). The effect of plexin binding to Rho proteins is not completely understood and will probably differ for individual GTPases and plexin types (12). One idea is that Rho binding might result in reduced GTPase activity through sequestration of an otherwise active protein to result in changes to downstream effector function. For example, binding of Rac1 (a Rho family GTPase) by plexin A or B family members could sequester Rac1 away from p21-activated kinase (PAK) with downstream effects on actin dynamics through LIMK and cofilin (reviewed in (53)). Rnd1, a constitutively active GTPase (62), may also be sequestered by binding to plexins, with subsequent effects on downstream signaling through this GTPase (reviewed in (12)). Another possibility is that binding of Rho family proteins, such as Rnd1, to the plexin RBD is important for activation of plexin GAP activity in combination with Sema binding (reviewed in (12,58,63)). Cellular events that interfere with Rho GTPase binding to the RBD could interfere with Sema-mediated activation of plexin, providing an additional level of control for plexin signaling. Interestingly, different plexins appear to have different affinities for specific Rho family GTPases (and their regulatory proteins such as GAPs and GEFs; reviewed in (12)), but such differences and their functional consequences are not well understood.
While roles for small GTPases in Sema signaling have become clear over the past 15 years, what had been missing was a means by which Semas/plexins could directly influence the cytoskeleton. Several models had been put forth to explain how Semas dramatically collapse the actin cytoskeleton (reviewed in (53)), but new insights were gained when it was discovered that the multidomain oxidoreductase (Redox) enzyme, Mical, which associates with PlexA, is an F-actin disassembly factor. The MICALs, which include one Drosophila Mical and three mammalian MICALs, bind plexin receptors and directly induce actin cytoskeletal changes downstream of plexin receptors (reviewed in (53)). Biochemical analyses have demonstrated that Mical enzymatically modifies actin by oxidizing a methionine residue that is critical for actin polymerization, leading to actin filament severing and decreased polymerization (48,64). The enzyme SelR/MsrB reverses the effect of Mical, restoring actin polymerization (65,66). Together, Mical and SelR comprise an actin regulatory system that acts directly downstream of Semas/plexins (64) to affect cellular morphology and axon guidance.
In addition to adhesion and actin-related effects, there is also evidence that Sema/plexin signaling affects microtubule function through the collapsin response mediator protein (CRMP) microtubule regulatory proteins (reviewed in (51,67,68)). Another emerging hypothesis is that extracellular guidance cues, including Semas, have the ability to modify membrane dynamics, such as endo- and exocytosis. These effects appear to be central to their function as guidance cues (reviewed in (69)). For example, Sema3A induces assymetric endocytosis that is necessary for its repulsive effects on axonal growth cones (70). Mechanisms underlying these effects are just beginning to be elucidated.
The general mechanisms described above account for many of the cellular effects of Semas and plexins as a group, but how do Semas regulate such a wide variety of processes in so many different biological contexts? Several aspects of Sema/plexin biology make this possible (reviewed in (46)). Perhaps most importantly, a particular Sema binds to different types of plexin receptors, as well as other types of receptors (listed in the Receptors section), and the effects of this binding are modified by co-receptors (e.g., (45)). Thus, the receptor complexes utilized by a particular Sema appear quite variable and each complex has the potential to generate distinct, even opposite, signaling outcomes (e.g., (34)). There are numerous examples of cell populations that generate alternate responses to the same Sema based on different expression of particular co-receptors, such as Npns (71,72) and receptor tyrosine kinases (reviewed in (41)). The nature of how different co-receptors change Sema/plexin signaling outputs is not clear and will likely differ for each co-receptor. Mechanisms may include crosstalk between co-receptor and plexin signaling pathways, changes in ligand presentation or modification of the plexin receptor itself, for example through transphosphorylation (41,46).
The outcome of Sema/plexin engagement also involves a number of intracellular proteins and second messengers. For example, a number of intracellular tyrosine kinases, including Pyk2, Syk, FAK, Fer/Fes, Fyn and Src family members have been implicated in Sema/plexin signaling (reviewed in (12)). Although still incompletely understood, results indicate that these kinases initiate signaling through well-known intracellular signaling cascades such as PI3K/AKT and MAPK/ERK and are known to regulate plexin interacting proteins. Different serine/threonine kinases, such as Raf, GSK3-β and Rho kinases, are also involved in plexin signaling, and results indicate that they are regulated both as a consequence of plexin GAP activity, and/or through tyrosine kinase initiated signaling cascades (reviewed in (12)).
Second messengers also play a prominent role in Sema signaling. Early studies documented that repulsive Sema signaling in neurons is blocked by elevation of the intracellular second messenger cAMP (73,74). It is now appreciated that navigating growth cones use intracellular Ca2+ influx and release patterns that are controlled by opposing levels of cAMP and cGMP to integrate multiple extracellular signals during guidance (reviewed in (69)). The interplay between Semas and cyclic nucleotides is still incompletely understood, but it has recently been demonstrated that the cAMP-dependent Protein Kinase (PKA) phosphorylates the PlexA GAP domain (75). This phosphorylation recruits the protein 14-3-3ε, which binds to PlexA and suppresses PlexA GAP activity toward Ras family GTPases (75). These events antagonize Sema/PlexA repulsive axon guidance by maintaining integrin adhesion and provide a mechanistic link between cAMP, PKA and plexin (58). Collectively, these data indicate that signaling via intracellular kinases provides another means through which Sema/plexin signaling is regulated according to cellular context.
Sema/plexin signaling is further complicated by several other mechanisms. First, competition between different Sema ligands for the same receptor and cis interactions between Semas and plexins alter the availability of both proteins for trans signaling (reviewed in (46)). For example, cis (on the same cell) binding between semaphorins and plexins inhibits the binding of Semas/plexins in trans (on different cells), thereby suppressing intercellular signaling (76–78). Cis binding also appears to activate plexin signaling in cis (79). Second, transmembrane Semas can act as receptors (“reverse signaling”; reviewed in (45,46)). For example, the cytoplasmic domain of Sema-1a interacts with regulators of Rho GTPases during axon pathfinding and target recognition (80). In addition, there is evidence that Semas act as receptors for themselves (81). Finally, emerging data has shown that mechanisms involving endocytosis and trafficking of receptor complexes can diversify Sema signaling responses: Cells expressing the same Sema receptor complexes can display altered sensitivity to Sema ligands based on the availability of distinct endocytic pathway components that control internalization and trafficking of receptor proteins (82–84). Together, these mechanisms and others create intricate layers of control that allow Semas to exert pleiotropic effects in many different cellular contexts.
Physiological Functions
Due to the large number and diversity of Semas, and their ability to bind and activate various receptors and co-receptors, it is perhaps not surprising that Sema signaling has been implicated in a continuously growing list of physiological processes. Semas have been widely studied in the nervous system, the circulatory system, and the immune system and are also being actively explored for their role in bone, kidney, lung, and more. In addition, recent work has continued to indicate that Semas have a major impact on cancer progression. The central function of Semas in each of these contexts is to initiate signaling networks that modulate cellular adhesion and the underlying cytoskeleton and to affect cell shape, differentiation, motility and survival. Below, we provide an overview of some of the major functions of Semas in several widely studied systems.
Nervous System
Since the earliest breakthroughs that identified Semas as cues for growing axons, these guidance molecules have been shown to be involved in many processes that shape the nervous system during development and beyond. Semas regulate neuronal proliferation and migration, help determine neuronal polarity, act as repulsive and attractive cues for axons and dendrites, regulate synapse formation and function, and affect dendrite morphology (reviewed in (51,85,86) and others, see below). Sema signaling is important for some of the earliest cell migration events that shape the nervous system. For example, during vertebrate embryogenesis, molecular gradients of Semas direct the migration and segregation of neural crest cells, placing them in position to form the peripheral nervous system (PNS, reviewed in (87)). During development of the central nervous system (CNS), Semas control migration of a number of neuronal types including GABA-ergic interneurons (88,89), cortical neurons (90), and cerebellar granule neurons (91,92), and help to establish the boundary between the CNS and the PNS by blocking the migration of CNS neurons out of the spinal cord (93–95).
Semas are best known for establishing nervous system patterning through axon guidance, in particular by acting as repulsive cues for developing axons. A classic example occurs during mouse embryonic development. Sema3A and Sema3F are expressed in regions around peripheral sensory and motor projections from the CNS and prevent the incorrect sprouting of developing neurons. In contrast, semaphorin mutants exhibit ectopic sprouting due to loss of inhibitory Sema signaling (reviewed in (85,86,96)). Semas also direct CNS axon pathfinding in vertebrates by guiding commissural (97) and retinal axons (98) across the midline, directing corticospinal tract (99,100) and corpus callosum formation (101), and positioning thalamic inputs to the cortex (102). When the expression of Semas or their receptors are altered, these pathfinding events occur abnormally, resulting in aberrant axon projections. Furthermore, a number of the axon guidance events mediated by Semas involve axon-axon signaling such that Sema signaling controls axonal fasciculation via repulsive signaling between axons (reviewed in (85)). For example, motor axons in Drosophila utilize the transmembrane repellent Sema-1a and its receptor PlexA to stimulate the defasciculation of other motor axons at important choice points (103,104). Likewise, although utilizing a different mechanism, Sema signaling between axons sorts mouse olfactory axons within incoming nerve bundles based on the levels of expression of Npn or plexin receptors to organize target innervation (Reviewed (86,105,106)). Finally, recent studies have revealed that Sema signaling helps establish the initial identity of a growing neuronal cell process as either an axon or a dendrite, indicating that Sema signaling influences cell polarity (107,108).
Semas are also important for precise synaptic targeting. For example, Sema6C and Sema6D expression in the dorsal horn of the vertebrate spinal cord repels sensory axons that express PlexA1, but not other sensory neuron populations, to allow proper organization of sensory neuron inputs to the spinal cord (109). Semas also control retinotectal mapping in Xenopus (110) and zebrafish (111), and formation of neuronal lamina in the vertebrate retina (reviewed in (112)) and hippocampus (78,113). In addition, Semas are involved in several processes surrounding synapse formation, including specific synaptic partner choice decisions between neurons, synapse development, axon pruning and regulation of dendrite development (reviewed in (51,86,114–116)). Recent work has also shown that a single Sema influences multiple developmental processes in the same neuron at different stages (e.g., (117,118)), indicating that responses to Semas are finely tuned depending on the signaling context within the cell. It is also becoming increasingly clear that in addition to their essential developmental functions, many guidance molecules, including Semas, function in the adult nervous system to affect synaptic physiology and plasticity (reviewed in (51,115)).
Perhaps not surprisingly, given their many roles in development and function, Semas and their related receptors have been implicated in developmental and adult onset nervous system diseases (reviewed in (12,51,119,120)), including CHARGE syndrome (121), epilepsy (122,123) schizophrenia and anxiety disorders (124–127), autism and impaired verbal performance (128), Alzheimer’s disease (AD) (129,130), Parkinson’s disease (PD) (131,132), Amyotrophic Lateral Sclerosis (ALS) (reviewed in (133)) and multiple sclerosis (MS) (reviewed (134)). While each of these diseases/disorders has a distinct etiology, it is possible that abnormal Sema expression or function could contribute to pathological changes in neuronal connectivity that are characteristic of disease including synaptic reorganization, loss of synapses or altered synaptic function. Semas are also important molecular players after nervous system injury (51,135,136). In CNS injury, Semas and other guidance molecules are upregulated near injury sites and have the ability to act as molecular repellants for adult axons. These inhibitors are thought to be a major factor contributing to the inability of CNS axons to regenerate after an injury. In addition, Semas play a role in oligodendrocyte migration and differentiation (e.g., (137–142); reviewed in (139)). In demyelinating disease, such as MS, and after CNS injury, oligodendrocytes fail to remyelinate axons, leaving neurons dysfunctional. Accumulating data is suggesting that Semas contribute to remyelination failure due to their effects on oligodendrocytes (e.g., (143); reviewed in (134)).
Endocrine System
As a function of their role in neuronal guidance, Semas are involved in the development of the neuroendocrine system through their effects on the migration of gonadotropin releasing hormone (GnRH) neurons (reviewed in (144,145)). Hypothalamic GnRH neurons secrete GnRH to stimulate the release of key reproductive hormones from the anterior pituitary to control puberty onset, gametogenesis and estrous cycling (146). In mouse models, Semas are expressed along the migratory path of GnRH neurons and loss of several Semas or their receptors results in abnormal migration of GnRH neurons and reproductive abnormalities, (147–150). Interestingly, recent work has shown that Sema7A controls the periodic neuroglial remodeling that takes place in the hypothalamus during the adult ovarian cycle (151). Outside the CNS, Sema4D is expressed during development of follicles in the mouse ovary and increases follicular production of steroid hormones (152), while loss of Sema4D results in abnormal reproduction (153).
Circulatory System
Sema signaling is involved in vasculogenesis (the formation of primordial blood vessels through differentiation and assembly of endothelial cells), angiogenesis (the formation of new blood vessels from existing vessels), heart formation and lymphatic development. Blood vessel and neuronal networks are often patterned in similar ways and it has become evident that Semas and other classical “neuronal guidance cues” organize both systems (reviewed in (154,155)). During blood vessel growth, extending vessels are guided by endothelial tip cells. Tip cells are somewhat similar to neuronal growth cones, the growing tips of neurons, and express many of the same receptors (156,157). For example, PlexD1 receptors are expressed by developing blood vessel endothelial cells (158) and genetic experiments in mouse and zebrafish have established that class 3 Semas signal through these plexin receptors to direct the growth of intersomitic blood vessels and formation of the dorsal aorta of the heart (reviewed in (85,156)). Loss of Sema signaling due to genetic manipulation of either Sema or PlexD1 indicates that Semas inhibit/repel PlexD1-expressing blood vessel cells. Similar inhibitory/repulsive signaling between Sema3E and PlexD1 positions the vasculature in the retina (114).
Semas are also involved in development of the heart. Class 3 Sema mutants (159,160) and plexin mutants (161) exhibit heart morphological defects and Sema signaling has been implicated in several key cell migration events related to heart morphogenesis, such as neural crest cell migration (reviewed in (87)) and migration of cardiac endothelial cells and myocardial cells (162). In addition, Sema3A mutant mice display heart innervation defects consistent with abnormal sympathetic neuron innervation, suggesting a role for Sema signaling in cardiac innervation (reviewed in (163)). Finally, Semas may play a role in formation of the lymphatic system (164,165).
In the adult circulatory system, Semas are known to affect vascular permeability (reviewed in (166,167)) and the response to vascular injury (reviewed in (168)). Both of these aspects of vascular biology are related to the generation and maintenance of cell-cell contacts and in both cases Sema signaling regulates adherence between cells. In the case of vascular permeability, Sema3A and Sema7A expression weakens the junctions between endothelial cells to increase vascular permeability while Sema3F signaling strengthens these junctions to reduce permeability (reviewed in (167)). In the case of vascular injury, Sema4D, and potentially other Semas, appear to play an important part in establishing interactions between platelets that allow thrombus formation during hemostasis (168).
Interestingly, Semas antagonize the effects of vascular endothelial growth factors (VEGFs), one of the major classes of molecules that promote vascular growth and permeability, while VEGF influences plexin expression patterns (reviewed in (114,169,170)). In addition, the presence of VEGF receptors alters intracellular signaling pathways activated by Sema binding to plexin receptors (44) and VEGF receptors may also serve as signal transducing receptors for Semas (43). Thus, there is a close interplay between VEGF and Sema signaling pathways that is linked to Sema function in the cardiovascular system.
Immune System
Sema4D (CD100) was the first Sema described in the immune system when it was identified as an antigen on T lymphocytes (171,172). Now, immune functions have been described for other Class 4 Semas, as well as Class 3, 6 and 7 Semas, and for Npn and plexin receptors (reviewed in (173)). Both increased and decreased Sema signaling has been linked to immune system diseases including MS, rheumatoid arthritis, systemic lupus erythematosus, allergic diseases and graft-versus-host disease (reviewed in (173)). In addition, because the immune system is an integral part of the body’s response to disease and injury, Semas may function in a number of different pathological situations through their ability to regulate immunity.
Similar to the nervous and vascular systems, one function of Semas in immunity is to control cell movements. Perturbations of Sema signaling lead to abnormal immune cell migration in vitro and Sema knockout mice exhibit defective immune cell migration and function (reviewed in (13,174,175)). Recent studies have begun to uncover how immune cell migration events are mediated by Semas. For example, several Semas and their receptors (Npns and plexins) are expressed in the thymus and by T-lymphocytes (thymocytes) that migrate through the thymus to become mature T-cells (reviewed in (176)). Secreted Semas act as chemorepellants for migrating thymocytes (177,178) and loss of Sema or plexins leads to improper migration (e.g., (179)). As another example, Sema3A is involved in trafficking of dendritic cells from peripheral tissues to the lymphatic system in response to immune challenge, a process that requires Npn and plexin expression by dendritic cells (180).
In addition to immune cell migration events, transmembrane and GPI-linked Semas are involved in cell-cell communication between different types of immune cells to regulate their function (reviewed in (173,175)). These interactions involve several unique Sema receptors, including CD72, Tim-2, alpha-beta integrins and the co-receptors TREM2 and DAP12. For example, Semas are involved in the complex bidirectional signaling that occurs between dendritic cells and T-cells to initiate T-cell mediated antibody responses (36,181–186), and in B cell-B cell and B cell-T cell interactions that mediate B cell function (35). Semas also enhance interactions between basophils and T cells that regulate basophil function (187) and regulate macrophage activation by antigen-specific T cells at sites of inflammation (188,189).
Musculoskeletal System
A role for Semas in bone function was first discovered when Sema3A knock out mice were observed to have reduced bone formation (159). Recent studies have provided clues to how Sema3A and other Semas are involved in bone homeostasis and bone disease (reviewed in (173,190,191)). Bone is a dynamic organ that is continually remodeled by “bone building” osteoblast cells and “bone destroying” (or absorbing) osteoclast cells, a process that is tightly controlled by cell-cell communication between multiple cell types (192,193). Both osteoblasts and osteoclasts express Sema family proteins and several studies point to the idea that Sema signaling controls bone homeostasis by regulating the activity, differentiation and migration of these cell types (191). For example, Sema3A signaling through Npn1 inhibits differentiation of osteoclasts while simultaneously stimulating signaling pathways that promote differentiation of osteoblasts, suggesting a net “bone promoting” role for Sema3A (194). Interestingly, these effects appear to be mediated by innervating sensory neurons (195). In contrast, genetic experiments in mice have indicated that Sema4D/PlexB1 signaling promotes osteoclast differentiation and inhibits osteoblast function and formation, suggesting a net bone-reducing role for this Sema (186,196–198). Several other Semas and Sema receptors have also been implicated in bone function. For example, polymorphisms in human SEMA7A and PLEXA2 genes are associated with reduced bone density (199,200) and both PlexD1 (30,161) and Sema3B (201) deficiency in mice cause abnormal bone phenotypes.
Other Systems
Semas are ubiquitously expressed, perhaps in all tissues of the body (1), and the role of Semas during development and function of many of these tissues is just beginning to be explored. Emerging data points to important functions for Semas in development of the kidney, lungs, eye, muscle and other organs. Interestingly, both kidney and lung development are characterized by epithelial branching morphogenesis, the process in which an initial epithelial tube expands into a complex structure by repetitive branching (202). This is the same process that shapes the developing vasculature and in which Semas are involved. For example, kidney development involves formation of a ureteric bud, which branches to become the collecting duct system of the kidney (202). Semas and their receptors, including plexins, Npns and VEGFR2, are dynamically regulated during patterning of the kidney’s duct system and Sema mutant mice show kidney morphological defects, including abnormal ureteric bud branching (reviewed in (203)). Gain and loss of function studies in mouse kidney have revealed that Sema3A negatively regulates ureteric bud branching and endothelial cell migration while Sema3C promotes bud branching, endothelial cell proliferation, survival and adhesion during kidney development (203). Additionally, Semas may play a role in adult kidney function and disease (204,205). Moreover, in organ culture experiments modeling lung development, different Semas regulate branching and proliferation of epithelial cells to alter the number of pulmonary buds formed during epithelial branching morphogenesis (206). In development of these organs and others, such as the eye, Semas are expressed in epithelial tissues and may act to control epithelial barrier formation and function (reviewed in (167)). Epithelial barriers are established through cell-cell interactions between adjacent epithelial cells. Thus, the ability of Semas to act as cell-cell signaling conduits and to affect cellular adhesion may provide clues to their function in this context (167).
Cancer
Among the earliest studies linking Semas with cancer identified the SEMA3A gene within a region of human chromosome 3 that is deleted in certain types of lung cancer ((207–209); reviewed in (210)). Subsequently, many studies have revealed that Sema expression is altered in cancer cells and cells of the tumor microenvironment and that these changes affect tumor progression in several ways (reviewed in (211–214)). First, Semas act directly on cancer cells, which express Semas and their receptors, to affect tumor cell growth, motility and metastasis. For example, Sema3A inhibits the motility of breast cancer (215,216) and prostate cancer cells (217) and Sema3B and 3F inhibit growth of lung cancer cells (218,219). On the other hand, Sema6D binding to PlexA1 in complex with VEGFR2 results in growth of malignant mesothelioma cells (220). Second, Semas have been found to both promote and inhibit tumor angiogenesis through their known effects on endothelial cells (reviewed in (213)). During cancer progression the normal balance of pro vs. anti-angiogenic signals in tissue, including expression of Sema proteins and their receptors, is distorted to promote angiogenesis (41). For instance, Sema4D is upregulated in cancer and promotes tumor angiogenesis (e.g., (221–224)) while several class 3 Semas, with anti-angiogenic effects (213) are downregulated in cancer (e.g., (224,225)). Thus, the use of anti-angiogenic Semas to reduce vascularization of tumors is a possible therapeutic application (reviewed in (212)). Finally, Semas modulate the behavior of other cells, such as fibroblasts and inflammatory cells, in the tumor microenvironment (reviewed in (41,211,212)). For example, several Semas have a pro-tumor role due to their ability to recruit specific populations of tumor associated macrophages and monocytes and to regulate production of pro-angiogenic and pro-inflammatory molecules by these cells (reviewed in (226)).
Understanding the role of Semas in cancer biology is complicated by the fact that although particular classes of Semas can be generally classified as either tumor promoting or anti-tumorigenic, a particular Sema has very different effects on tumor progression depending on cellular context (reviewed in (213)). This is likely due to the presence of different Sema receptors, especially receptor tyrosine kinases, that complex with plexin and Npn receptors in different cell types or under different conditions. These co-receptors and the signaling pathways they activate have a major influence on the outcome of Sema signaling in particular cancers (41,42). For example, during Sema4D/PlexB1 signaling in breast cancer cells, the presence of ErbB2 as part of the plexin receptor complex increases cell migration and invasiveness. On the other hand, the presence of the Met receptor tyrosine kinase reduces cell migration and invasion (227–231). Elucidating how Sema signaling is altered in different cancer contexts may lead to treatment strategies that mitigate the tumorigenic properties of Semas while harnessing their cancer fighting functions.
Concluding Remarks
Over the past several decades, intense research has shown that Semas are involved in an array of biological events that underlie the development and homeostasis of a range of essential organ systems. In addition, Semas contribute/have been linked to the pathology of multiple debilitating diseases, including cancer, neurodegenerative disease, and immune disease. Considerable progress has been made towards understanding this important family of signaling ligands, including elucidation of their structural details, identification of their various receptors and co-receptors, and characterization of a number of their downstream signaling molecules. In addition, mechanisms that account for the diversity of Sema signaling responses in different cellular contexts have begun to be uncovered. Despite these important findings, our understanding of Sema biology is still limited and many questions and areas for future research remain. These include: 1) understanding how the structural diversity of Semas contributes to their ability to activate distinct cellular signaling pathways and function in different contexts, 2) further identifying/characterizing specific co-factors, intracellular proteins and receptor mechanisms that are responsible for modifying the cellular response to Sema signaling, 3) piecing together how Sema signaling networks interact with other cellular signaling networks to result in biological outcomes and 4) identifying which Sema-activated signaling pathways are most important for a given developmental process or in a certain disease state and which (if any) are universally activated. These insights, along with a more comprehensive understanding of the mechanisms responsible for the effects of Semas on the cytoskeleton and adhesion, will greatly inform our understanding of Sema function in development and pathology.
Acknowledgments
We thank Jeroen Pasterkamp for helpful comments on the manuscript. This work was supported by NIH (NS073968) and Welch Foundation (I-1749) grants to J.R.T.
References
- 1.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin AL, Matthes DJ, O’Connor TP, et al. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- 4.Raper JA, Kapfhammer JP. The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron. 1990;4:21–29. doi: 10.1016/0896-6273(90)90440-q. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 6.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 7.Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- 8.Browne K, Wang W, Liu RQ, et al. Transmembrane semaphorin5B is proteolytically processed into a repulsive neural guidance cue. Journal of neurochemistry. 2012;123:135–146. doi: 10.1111/j.1471-4159.2012.07885.x. [DOI] [PubMed] [Google Scholar]
- 9.Elhabazi A, Delaire S, Bensussan A, et al. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- 10.Holmes S, Downs AM, Fosberry A, et al. Sema7A is a potent monocyte stimulator. Scandinavian journal of immunology. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 11.Gherardi E, Love CA, Esnouf RM, et al. The sema domain. Current opinion in structural biology. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends in immunology. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Siebold C, Jones EY. Structural insights into semaphorins and their receptors. Seminars in cell & developmental biology. 2013;24:139–145. doi: 10.1016/j.semcdb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Antipenko A, Himanen JP, van Leyen K, et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 16.Janssen BJ, Malinauskas T, Weir GA, et al. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nature structural & molecular biology. 2012;19:1293–1299. doi: 10.1038/nsmb.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen BJ, Robinson RA, Perez-Branguli F, et al. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Juo ZS, Shim AH, et al. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love CA, Harlos K, Mavaddat N, et al. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nature structural biology. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 20.Nogi T, Yasui N, Mihara E, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- 21.Klostermann A, Lohrum M, Adams RH, et al. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- 22.Koppel AM, Raper JA. Collapsin-1 covalently dimerizes, and dimerization is necessary for collapsing activity. J Biol Chem. 1998;273:15708–15713. doi: 10.1074/jbc.273.25.15708. [DOI] [PubMed] [Google Scholar]
- 23.Perala N, Sariola H, Immonen T. More than nervous: the emerging roles of plexins. Differentiation; research in biological diversity. 2012;83:77–91. doi: 10.1016/j.diff.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Ohta K, Mizutani A, Kawakami A, et al. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14:1189–1199. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 25.Comeau MR, Johnson R, DuBose RF, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–482. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Chedotal A, He Z, et al. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 27.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 28.Kolodkin AL, Levengood DV, Rowe EG, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 29.Raimondi C, Ruhrberg C. Neuropilin signalling in vessels, neurons and tumours. Seminars in cell & developmental biology. 2013;24:172–178. doi: 10.1016/j.semcdb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Gu C, Yoshida Y, Livet J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura F, Tanaka M, Takahashi T, et al. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 32.Renzi MJ, Feiner L, Koppel AM, et al. A dominant negative receptor for specific secreted semaphorins is generated by deleting an extracellular domain from neuropilin-1. J Neurosci. 1999;19:7870–7880. doi: 10.1523/JNEUROSCI.19-18-07870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran TS, Rubio ME, Clem RL, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Frontiers in cellular neuroscience. 2012;6:28. doi: 10.3389/fncel.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 36.Kumanogoh A, Marukawa S, Suzuki K, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 37.Pasterkamp RJ, Peschon JJ, Spriggs MK, et al. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 38.Cho JY, Chak K, Andreone BJ, et al. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes & development. 2012;26:2222–2235. doi: 10.1101/gad.193136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Wit J, De Winter F, Klooster J, et al. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Kantor DB, Chivatakarn O, Peer KL, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Cagnoni G, Tamagnone L. Semaphorin receptors meet receptor tyrosine kinases on the way of tumor progression. Oncogene. 2013 doi: 10.1038/onc.2013.474. [DOI] [PubMed] [Google Scholar]
- 42.Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO reports. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellon A, Luchino J, Haigh K, et al. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66:205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Toyofuku T, Zhang H, Kumanogoh A, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes & development. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development. Development. 2014;141:3292–3297. doi: 10.1242/dev.105544. [DOI] [PubMed] [Google Scholar]
- 47.Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol. 2007;600:90–108. doi: 10.1007/978-0-387-70956-7_8. [DOI] [PubMed] [Google Scholar]
- 48.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334:1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 51.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan J, Mansfield SG, Redmond T, et al. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung RJ, Terman JR. Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly. Cytoskeleton (Hoboken) 2011;68:415–433. doi: 10.1002/cm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- 55.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, He H, Srivastava N, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oinuma I, Ishikawa Y, Katoh H, et al. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 58.Yang T, Terman JR. Regulating small G protein signaling to coordinate axon adhesion and repulsion. Small GTPases. 2013;4:34–41. doi: 10.4161/sgtp.22765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinbara K, Goldfinger LE, Hansen M, et al. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- 61.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 62.Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- 63.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hung RJ, Yazdani U, Yoon J, et al. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hung RJ, Spaeth CS, Yesilyurt HG, et al. SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics. Nat Cell Biol. 2013;15:1445–1454. doi: 10.1038/ncb2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee BC, Peterfi Z, Hoffmann FW, et al. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Molecular cell. 2013;51:397–404. doi: 10.1016/j.molcel.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasterkamp RJ. R-Ras fills another GAP in semaphorin signalling. Trends Cell Biol. 2005;15:61–64. doi: 10.1016/j.tcb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tojima T, Hines JH, Henley JR, et al. Second messengers and membrane trafficking direct and organize growth cone steering. Nature reviews Neuroscience. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Wolman MA, Liu Y, Tawarayama H, et al. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci. 2004;24:8428–8435. doi: 10.1523/JNEUROSCI.2349-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chauvet S, Cohen S, Yoshida Y, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 74.Song H, Ming G, He Z, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 75.Yang T, Terman JR. 14-3-3epsilon couples protein kinase A to semaphorin signaling and silences plexin RasGAP-mediated axonal repulsion. Neuron. 2012;74:108–121. doi: 10.1016/j.neuron.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haklai-Topper L, Mlechkovich G, Savariego D, et al. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J. 2010;29:2635–2645. doi: 10.1038/emboj.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun LO, Jiang Z, Rivlin-Etzion M, et al. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241974. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suto F, Tsuboi M, Kamiya H, et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 79.Mizumoto K, Shen K. Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron. 2013;77:655–666. doi: 10.1016/j.neuron.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeong S, Juhaszova K, Kolodkin AL. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron. 2012;76:721–734. doi: 10.1016/j.neuron.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sweeney LB, Chou YH, Wu Z, et al. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011;72:734–747. doi: 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Law CO, Kirby RJ, Aghamohammadzadeh S, et al. The neural adhesion molecule TAG-1 modulates responses of sensory axons to diffusible guidance signals. Development. 2008;135:2361–2371. doi: 10.1242/dev.009019. [DOI] [PubMed] [Google Scholar]
- 83.Dang P, Smythe E, Furley AJ. TAG1 regulates the endocytic trafficking and signaling of the semaphorin3A receptor complex. J Neurosci. 2012;32:10370–10382. doi: 10.1523/JNEUROSCI.5874-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carcea I, Ma’ayan A, Mesias R, et al. Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J Neurosci. 2010;30:15317–15329. doi: 10.1523/JNEUROSCI.1821-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annual review of cell and developmental biology. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida Y. Semaphorin signaling in vertebrate neural circuit assembly. Frontiers in molecular neuroscience. 2012;5:71. doi: 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruhrberg C, Schwarz Q. In the beginning: Generating neural crest cell diversity. Cell adhesion & migration. 2010;4:622–630. doi: 10.4161/cam.4.4.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 89.Marin O, Yaron A, Bagri A, et al. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 90.Chen G, Sima J, Jin M, et al. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 91.Kerjan G, Dolan J, Haumaitre C, et al. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- 92.Renaud J, Kerjan G, Sumita I, et al. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci. 2008;11:440–449. doi: 10.1038/nn2064. [DOI] [PubMed] [Google Scholar]
- 93.Bron R, Vermeren M, Kokot N, et al. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural development. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mauti O, Domanitskaya E, Andermatt I, et al. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural development. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vermeren M, Maro GS, Bron R, et al. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- 96.Wang F, Julien DP, Sagasti A. Journey to the skin: Somatosensory peripheral axon guidance and morphogenesis. Cell adhesion & migration. 2013;7:388–394. doi: 10.4161/cam.25000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou Y, Stoeckli E, Chen H, et al. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 98.Kuwajima T, Yoshida Y, Takegahara N, et al. Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron. 2012;74:676–690. doi: 10.1016/j.neuron.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faulkner RL, Low LK, Liu XB, et al. Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural development. 2008;3:21. doi: 10.1186/1749-8104-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Runker AE, Little GE, Suto F, et al. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural development. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niquille M, Garel S, Mann F, et al. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS biology. 2009;7:e1000230. doi: 10.1371/journal.pbio.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leighton PA, Mitchell KJ, Goodrich LV, et al. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- 103.Winberg ML, Noordermeer JN, Tamagnone L, et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- 104.Yu HH, Araj HH, Ralls SA, et al. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 105.Bashaw GJ. Semaphorin directs axon traffic in the fly olfactory system. Neuron. 2007;53:157–159. doi: 10.1016/j.neuron.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Imai T. Positional information in neural map development: lessons from the olfactory system. Development, growth & differentiation. 2012;54:358–365. doi: 10.1111/j.1440-169X.2012.01334.x. [DOI] [PubMed] [Google Scholar]
- 107.Nishiyama M, Togashi K, von Schimmelmann MJ, et al. Semaphorin 3A induces CaV2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol. 2011;13:676–685. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shelly M, Cancedda L, Lim BK, et al. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoshida Y, Han B, Mendelsohn M, et al. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Campbell DS, Regan AG, Lopez JS, et al. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Berndt J, Su F, et al. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci. 2004;24:310–318. doi: 10.1523/JNEUROSCI.4287-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baier H. Synaptic laminae in the visual system: molecular mechanisms forming layers of perception. Annual review of cell and developmental biology. 2013;29:385–416. doi: 10.1146/annurev-cellbio-101011-155748. [DOI] [PubMed] [Google Scholar]
- 113.Tawarayama H, Yoshida Y, Suto F, et al. Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. J Neurosci. 2010;30:7049–7060. doi: 10.1523/JNEUROSCI.0073-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oh WJ, Gu C. The role and mechanism-of-action of Sema3E and Plexin-D1 in vascular and neural development. Seminars in cell & developmental biology. 2013;24:156–162. doi: 10.1016/j.semcdb.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tillo M, Ruhrberg C, Mackenzie F. Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell adhesion & migration. 2012;6:541–546. doi: 10.4161/cam.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Joo WJ, Sweeney LB, Liang L, et al. Linking cell fate, trajectory choice, and target selection: genetic analysis of Sema-2b in olfactory axon targeting. Neuron. 2013;78:673–686. doi: 10.1016/j.neuron.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riccomagno MM, Hurtado A, Wang H, et al. The RacGAP beta2-Chimaerin selectively mediates axonal pruning in the hippocampus. Cell. 2012;149:1594–1606. doi: 10.1016/j.cell.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Progress in neurobiology. 2007;82:57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 120.Yaron A, Zheng B. Navigating their way to the clinic: emerging roles for axon guidance molecules in neurological disorders and injury. Developmental neurobiology. 2007;67:1216–1231. doi: 10.1002/dneu.20512. [DOI] [PubMed] [Google Scholar]
- 121.Lalani SR, Safiullah AM, Molinari LM, et al. SEMA3E mutation in a patient with CHARGE syndrome. Journal of medical genetics. 2004;41:e94. doi: 10.1136/jmg.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gant JC, Thibault O, Blalock EM, et al. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–645. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang J, Houk B, Shah J, et al. Genetic background regulates semaphorin gene expression and epileptogenesis in mouse brain after kainic acid status epilepticus. Neuroscience. 2005;131:853–869. doi: 10.1016/j.neuroscience.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 124.Fujii T, Uchiyama H, Yamamoto N, et al. Possible association of the semaphorin 3D gene (SEMA3D) with schizophrenia. Journal of psychiatric research. 2011;45:47–53. doi: 10.1016/j.jpsychires.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Mah S, Nelson MR, Delisi LE, et al. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Molecular psychiatry. 2006;11:471–478. doi: 10.1038/sj.mp.4001785. [DOI] [PubMed] [Google Scholar]
- 126.Runker AE, O’Tuathaigh C, Dunleavy M, et al. Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology. PloS one. 2011;6:e26488. doi: 10.1371/journal.pone.0026488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wray NR, James MR, Mah SP, et al. Anxiety and comorbid measures associated with PLXNA2. Archives of general psychiatry. 2007;64:318–326. doi: 10.1001/archpsyc.64.3.318. [DOI] [PubMed] [Google Scholar]
- 128.Rujescu D, Meisenzahl EM, Krejcova S, et al. Plexin B3 is genetically associated with verbal performance and white matter volume in human brain. Molecular psychiatry. 2007;12:190–194. 115. doi: 10.1038/sj.mp.4001903. [DOI] [PubMed] [Google Scholar]
- 129.Good PF, Alapat D, Hsu A, et al. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. Journal of neurochemistry. 2004;91:716–736. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- 130.Uchida Y, Ohshima T, Sasaki Y, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes to cells : devoted to molecular & cellular mechanisms. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 131.Clarimon J, Scholz S, Fung HC, et al. Conflicting results regarding the semaphorin gene (SEMA5A) and the risk for Parkinson disease. American journal of human genetics. 2006;78:1082–1084. doi: 10.1086/504727. author reply 1092-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maraganore DM, de Andrade M, Lesnick TG, et al. High-resolution whole-genome association study of Parkinson disease. American journal of human genetics. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Battum EY, Brignani S, Pasterkamp RJ. Axon guidance proteins in neurological disorders. The Lancet Neurology. 2015;14:532–546. doi: 10.1016/S1474-4422(14)70257-1. [DOI] [PubMed] [Google Scholar]
- 134.Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain : a journal of neurology. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 135.Fawcett JW, Schwab ME, Montani L, et al. Defeating inhibition of regeneration by scar and myelin components. Handbook of clinical neurology. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- 136.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Armendariz BG, Bribian A, Perez-Martinez E, et al. Expression of Semaphorin 4F in neurons and brain oligodendrocytes and the regulation of oligodendrocyte precursor migration in the optic nerve. Mol Cell Neurosci. 2012;49:54–67. doi: 10.1016/j.mcn.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Bernard F, Moreau-Fauvarque C, Heitz-Marchaland C, et al. Role of transmembrane semaphorin Sema6A in oligodendrocyte differentiation and myelination. Glia. 2012;60:1590–1604. doi: 10.1002/glia.22378. [DOI] [PubMed] [Google Scholar]
- 139.Cohen RI. Exploring oligodendrocyte guidance: ‘to boldly go where no cell has gone before’. Cell Mol Life Sci. 2005;62:505–510. doi: 10.1007/s00018-004-4485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leslie JR, Imai F, Fukuhara K, et al. Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development. 2011;138:4085–4095. doi: 10.1242/dev.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiang X, Zhang X, Huang QL. Plexin A3 is involved in semaphorin 3F–mediated oligodendrocyte precursor cell migration. Neuroscience letters. 2012;530:127–132. doi: 10.1016/j.neulet.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 142.Yamaguchi W, Tamai R, Kageura M, et al. Sema4D as an inhibitory regulator in oligodendrocyte development. Mol Cell Neurosci. 2012;49:290–299. doi: 10.1016/j.mcn.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 143.Syed YA, Hand E, Mobius W, et al. Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci. 2011;31:3719–3728. doi: 10.1523/JNEUROSCI.4930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giacobini P, Prevot V. Semaphorins in the development, homeostasis and disease of hormone systems. Seminars in cell & developmental biology. 2013;24:190–198. doi: 10.1016/j.semcdb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 145.Messina A, Giacobini P. Semaphorin signaling in the development and function of the gonadotropin hormone-releasing hormone system. Frontiers in endocrinology. 2013;4:133. doi: 10.3389/fendo.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ojeda SRSM. Physiology of the gonadotropin-releasing hormone neuronal network. Raven Press; New York: 2006. Physiology of Reproduction. [Google Scholar]
- 147.Giacobini P, Messina A, Morello F, et al. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol. 2008;183:555–566. doi: 10.1083/jcb.200806160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Messina A, Ferraris N, Wray S, et al. Dysregulation of Semaphorin7A/beta1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Human molecular genetics. 2011;20:4759–4774. doi: 10.1093/hmg/ddr403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cariboni A, Hickok J, Rakic S, et al. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:2387–2395. doi: 10.1523/JNEUROSCI.5075-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cariboni A, Davidson K, Rakic S, et al. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Human molecular genetics. 2011;20:336–344. doi: 10.1093/hmg/ddq468. [DOI] [PubMed] [Google Scholar]
- 151.Parkash J, Messina A, Langlet F, et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nature communications. 2015;6:6385. doi: 10.1038/ncomms7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Regev A, Goldman S, Shalev E. Semaphorin-4D (Sema-4D), the Plexin-B1 ligand, is involved in mouse ovary follicular development. Reproductive biology and endocrinology : RB&E. 2007;5:12. doi: 10.1186/1477-7827-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dacquin R, Domenget C, Kumanogoh A, et al. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PloS one. 2011;6:e26627. doi: 10.1371/journal.pone.0026627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 155.Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tamagnone L, Mazzone M. Semaphorin signals on the road of endothelial tip cells. Dev Cell. 2011;21:189–190. doi: 10.1016/j.devcel.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 158.van der Zwaag B, Hellemons AJ, Leenders WP, et al. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- 159.Behar O, Golden JA, Mashimo H, et al. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 160.Feiner L, Webber AL, Brown CB, et al. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 161.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 162.Toyofuku T, Kikutani H. Semaphorin signaling during cardiac development. Adv Exp Med Biol. 2007;600:109–117. doi: 10.1007/978-0-387-70956-7_9. [DOI] [PubMed] [Google Scholar]
- 163.Ieda M, Fukuda K. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: the regulatory mechanisms of cardiac innervation and their critical roles in cardiac performance. Journal of pharmacological sciences. 2009;109:348–353. doi: 10.1254/jphs.08r25fm. [DOI] [PubMed] [Google Scholar]
- 164.Bouvree K, Brunet I, Del Toro R, et al. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ Res. 2012;111:437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jurisic G, Maby-El Hajjami H, Karaman S, et al. An unexpected role of semaphorin3a–neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res. 2012;111:426–436. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Frontiers in oncology. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Treps L, Le Guelte A, Gavard J. Emerging roles of Semaphorins in the regulation of epithelial and endothelial junctions. Tissue barriers. 2013;1:e23272. doi: 10.4161/tisb.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wannemacher KM, Wang L, Zhu L, et al. The role of semaphorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22:461–465. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chauvet S, Burk K, Mann F. Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues. Cell Mol Life Sci. 2013;70:1685–1703. doi: 10.1007/s00018-013-1278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor perspectives in medicine. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bougeret C, Mansur IG, Dastot H, et al. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148:318–323. [PubMed] [Google Scholar]
- 172.Delaire S, Elhabazi A, Bensussan A, et al. CD100 is a leukocyte semaphorin. Cell Mol Life Sci. 1998;54:1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumanogoh A, Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nature reviews Immunology. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 174.Roney K, Holl E, Ting J. Immune plexins and semaphorins: old proteins, new immune functions. Protein & cell. 2013;4:17–26. doi: 10.1007/s13238-012-2108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nature immunology. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 176.Mendes-da-Cruz DA, Stimamiglio MA, Munoz JJ, et al. Developing T-cell migration: role of semaphorins and ephrins. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:4390–4399. doi: 10.1096/fj.11-202952. [DOI] [PubMed] [Google Scholar]
- 177.Garcia F, Lepelletier Y, Smaniotto S, et al. Inhibitory effect of semaphorin-3A, a known axon guidance molecule, in the human thymocyte migration induced by CXCL12. Journal of leukocyte biology. 2012;91:7–13. doi: 10.1189/jlb.0111031. [DOI] [PubMed] [Google Scholar]
- 178.Lepelletier Y, Smaniotto S, Hadj-Slimane R, et al. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A–mediated interactions. Proc Natl Acad Sci U S A. 2007;104:5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi YI, Duke-Cohan JS, Ahmed WB, et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takamatsu H, Takegahara N, Nakagawa Y, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nature immunology. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kumanogoh A, Shikina T, Suzuki K, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A–deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 182.Kumanogoh A, Suzuki K, Ch’ng E, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169:1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- 183.Nakatsuji Y, Okuno T, Moriya M, et al. Elevation of Sema4A implicates Th cell skewing and the efficacy of IFN-beta therapy in multiple sclerosis. J Immunol. 2012;188:4858–4865. doi: 10.4049/jimmunol.1102023. [DOI] [PubMed] [Google Scholar]
- 184.Okuno T, Nakatsuji Y, Moriya M, et al. Roles of Sema4D–plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:1499–1506. doi: 10.4049/jimmunol.0903302. [DOI] [PubMed] [Google Scholar]
- 185.Shi W, Kumanogoh A, Watanabe C, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 186.Takegahara N, Takamatsu H, Toyofuku T, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 187.Nakagawa Y, Takamatsu H, Okuno T, et al. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J Immunol. 2011;186:2881–2888. doi: 10.4049/jimmunol.1003485. [DOI] [PubMed] [Google Scholar]
- 188.Kang S, Okuno T, Takegahara N, et al. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin. J Immunol. 2012;188:1108–1116. doi: 10.4049/jimmunol.1102084. [DOI] [PubMed] [Google Scholar]
- 189.Suzuki K, Okuno T, Yamamoto M, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 190.Harre U, Schett G. Bone research in 2012: the ups and downs of bone in health and rheumatic disease. Nature reviews Rheumatology. 2013;9:67–68. doi: 10.1038/nrrheum.2012.219. [DOI] [PubMed] [Google Scholar]
- 191.Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Seminars in cell & developmental biology. 2013;24:163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 192.Del Fattore A, Teti A, Rucci N. Bone cells and the mechanisms of bone remodelling. Front Biosci (Elite Ed) 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 193.Nakahama K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67:4001–4009. doi: 10.1007/s00018-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hayashi M, Nakashima T, Taniguchi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 195.Fukuda T, Takeda S, Xu R, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 196.Kaifu T, Nakahara J, Inui M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. The Journal of clinical investigation. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 198.Negishi-Koga T, Shinohara M, Komatsu N, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nature medicine. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 199.Hwang JY, Lee JY, Park MH, et al. Association of PLXNA2 polymorphisms with vertebral fracture risk and bone mineral density in postmenopausal Korean population. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17:1592–1601. doi: 10.1007/s00198-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 200.Koh JM, Oh B, Lee JY, et al. Association study of semaphorin 7a (sema7a) polymorphisms with bone mineral density and fracture risk in postmenopausal Korean women. Journal of human genetics. 2006;51:112–117. doi: 10.1007/s10038-005-0331-z. [DOI] [PubMed] [Google Scholar]
- 201.Sutton AL, Zhang X, Dowd DR, et al. Semaphorin 3B is a 1,25-Dihydroxyvitamin D3-induced gene in osteoblasts that promotes osteoclastogenesis and induces osteopenia in mice. Mol Endocrinol. 2008;22:1370–1381. doi: 10.1210/me.2007-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev. 2009;19:484–490. doi: 10.1016/j.gde.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reidy K, Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tapia R, Guan F, Gershin I, et al. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney international. 2008;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 205.Veron D, Reidy KJ, Bertuccio C, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney international. 2010;77:989–999. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 206.Kagoshima M, Ito T. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes to cells : devoted to molecular & cellular mechanisms. 2001;6:559–571. doi: 10.1046/j.1365-2443.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 207.Roche J, Boldog F, Robinson M, et al. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- 208.Sekido Y, Bader S, Latif F, et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xiang RH, Hensel CH, Garcia DK, et al. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 210.Potiron VA, Roche J, Drabkin HA. Semaphorins and their receptors in lung cancer. Cancer letters. 2009;273:1–14. doi: 10.1016/j.canlet.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gu C, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Experimental cell research. 2013;319:1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer cell. 2012;22:145–152. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 213.Neufeld G, Sabag AD, Rabinovicz N, et al. Semaphorins in angiogenesis and tumor progression. Cold Spring Harbor perspectives in medicine. 2012;2:a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thirant C, Gavard J, Junier MP, et al. Critical multiple angiogenic factors secreted by glioblastoma stem-like cells underline the need for combinatorial anti-angiogenic therapeutic strategies. Proteomics Clinical applications. 2013;7:79–90. doi: 10.1002/prca.201200102. [DOI] [PubMed] [Google Scholar]
- 215.Bachelder RE, Lipscomb EA, Lin X, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer research. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 216.Pan H, Bachelder RE. Autocrine Semaphorin3A stimulates eukaryotic initiation factor 4E–dependent RhoA translation in breast tumor cells. Experimental cell research. 2010;316:2825–2832. doi: 10.1016/j.yexcr.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. International journal of oncology. 2007;30:1231–1238. [PubMed] [Google Scholar]
- 218.Tomizawa Y, Sekido Y, Kondo M, et al. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci U S A. 2001;98:13954–13959. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xiang R, Davalos AR, Hensel CH, et al. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer research. 2002;62:2637–2643. [PubMed] [Google Scholar]
- 220.Catalano A, Lazzarini R, Di Nuzzo S, et al. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer research. 2009;69:1485–1493. doi: 10.1158/0008-5472.CAN-08-3659. [DOI] [PubMed] [Google Scholar]
- 221.Basile JR, Barac A, Zhu T, et al. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer research. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 222.Basile JR, Castilho RM, Williams VP, et al. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. The Journal of experimental medicine. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bielenberg DR, Hida Y, Shimizu A, et al. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. The Journal of clinical investigation. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Maione F, Molla F, Meda C, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. The Journal of clinical investigation. 2009;119:3356–3372. doi: 10.1172/JCI36308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Molecular aspects of medicine. 2011;32:123–145. doi: 10.1016/j.mam.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 227.Barberis D, Casazza A, Sordella R, et al. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 228.Sun T, Krishnan R, Swiercz JM. Grb2 mediates semaphorin-4D–dependent RhoA inactivation. J Cell Sci. 2012;125:3557–3567. doi: 10.1242/jcs.101063. [DOI] [PubMed] [Google Scholar]
- 229.Swiercz JM, Kuner R, Behrens J, et al. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 230.Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 232.Chak K, Kolodkin AL. Function of the Drosophila receptor guanylyl cyclase Gyc76C in PlexA-mediated motor axon guidance. Development. 2014;141:136–147. doi: 10.1242/dev.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nagai H, Sugito N, Matsubara H, et al. CLCP1 interacts with semaphorin 4B and regulates motility of lung cancer cells. Oncogene. 2007;26:4025–4031. doi: 10.1038/sj.onc.1210183. [DOI] [PubMed] [Google Scholar]