Abstract
Alternative mating strategies are common in nature and are generally thought to increase the intensity of sexual selection. However, cuckoldry can theoretically decrease the opportunity for sexual selection, particularly in highly polygamous species. We address here the influence of sneaking (fertilization thievery) on the opportunity for sexual selection in the sand goby Pomatoschistus minutus, a marine fish species in which males build and defend nests. Our microsatellite-based analysis of the mating system in a natural sand goby population shows high rates of sneaking and multiple mating by males. Sneaker males had fertilized eggs in ≈50% of the assayed nests, and multiple sneakers sometimes fertilized eggs from a single female. Successful males had received eggs from 2 to 6 females per nest (mean = 3.4). We developed a simple mathematical model showing that sneaking in this polygynous sand goby population almost certainly decreases the opportunity for sexual selection, an outcome that contrasts with the usual effects of cuckoldry in socially monogamous animals. These results highlight a more complex and interesting relationship between cuckoldry rates and the intensity of sexual selection than previously assumed in much of the literature on animal mating systems.
Keywords: microsatellites, mating systems, paternity, polygyny
Molecular-based investigations of parentage in natural populations have led to the understanding that the genetic mating system is more relevant to the process of sexual selection than the social mating system (1). Myriad studies of parentage, particularly in birds, have demonstrated that extra-pair fertilizations (EPFs) and other alternative mating tactics are common in many species (2, 3); thus, a major research goal has been to deduce the causes and consequences of such strategies in nature. One common belief, supported by numerous studies of avian parentage, seems to be that EPFs increase the opportunity for sexual selection (4–10).
However, the effect of EPFs on the strength of sexual selection depends on both the form of the mating system in the absence of EPFs and on the details of the mating success of cuckolding males. Thus, in theory, EPFs may not always increase the opportunity for sexual selection (11, 12). This consideration is important for sexual selection theory. For example, Møller and Birkhead (7) showed a positive relationship between EPFs and plumage brightness in birds, which they interpreted as evidence of intersexual selection on male coloration. This interpretation rests on the assumptions, among others, that “extra-pair paternity results in an increase in the variation in male mating success,” (7) and that variance in mating success is related to the intensity of sexual selection (13). If, for example, EPFs were to decrease the variance in male mating success, then sexual selection would not be a viable explanation for the pattern observed by Møller and Birkhead (7). Fortunately for their argument, most birds are socially monogamous, and, in most socially monogamous species, EPFs probably result in an increase in the variance in male mating success (8, 14). However, the situation becomes more complex for nonmonogamous mating systems, where there is no a priori reason to expect that EPFs increase the opportunity for sexual selection. Thus, our goal in the present study was to investigate the effect of cuckoldry on the opportunity for sexual selection in a species with a polygamous social mating system.
We focus on a measure of the opportunity for sexual selection based on the variance in mating success (13), simply because this metric is used most commonly. It does have some flaws (15), and other ways of characterizing the mating system with respect to sexual selection may be more appropriate in some cases (15–18). Nevertheless, variance-based measures of the mating system permit easy comparisons among studies, and under most circumstances should be related to the intensity of sexual selection.
The sand goby, Pomatoschistus minutus, provides an excellent model system for investigating alternative mating strategies and sexual selection. The sand goby mating system is similar to many avian mating systems in that the male provides prolonged care to offspring. However, sand gobies are highly polygynous by bird standards (19) and do not exhibit any maternal care of offspring. They reproduce in shallow sandy areas where the males build nests under mussel shells. Females attach eggs in a single layer to the ceiling of the shell, where they are fertilized. The female leaves and the male remains to fan and defend the eggs until they hatch 1–3 weeks later. Despite prolonged paternal care, sexual selection acts most strongly on males of this species (20–23). Furthermore, apparent sneaking behavior has been observed visually in this species (24), but has not been confirmed genetically.
Here we use microsatellites to investigate the genetic mating system of sand gobies to evaluate the hypothesis that cuckoldry might in some cases decrease (rather than increase) the opportunity for sexual selection. Specifically, we have (i) documented the occurrence of successful sneaking in nature, (ii) characterized the mating success (defined here as the number of females with whom a male sires offspring) and reproductive success (the total number of offspring sired) of nest-holding males, and (iii) developed a simple mathematical model to relate the observed patterns of mating success to the opportunity for sexual selection.
Methods and Analyses
Sampling of Males and Nests.
Sand gobies were collected from shallow waters (0.5–1.0 m) near Klubban Biological Station on the West Coast of Sweden (58° 15′N, 11° 28′E) from May 11–13, 1998, during the peak of sand goby breeding. Nests and guardian males were located by snorkeling and returned live to the lab. For each nest, a fin clip of the male and 20–100 progeny were preserved in a DMSO buffer (20% DMSO/250 mM EDTA, pH 7.5/saturated NaCl) for genetic analysis. The area covered by eggs on the shell's surface was determined by tracing the outline of the egg mass onto paper, trimming the edges, and weighing this paper relative to a reference piece of known area. An additional sample of males was collected by hand trawl.
Microsatellite Development and Analysis.
Microsatellite markers were cloned from a single P. minutus adult by following procedures described elsewhere (25). The clones were screened, positives were identified and sequenced, and primers flanking the microsatellites were designed. Three microsatellite loci were used (Table 1).
Table 1.
Summary of information for the three sand goby microsatellite loci
| Locus | Primer sequences (5′ → 3′) | Cloned repeat | No. of alleles | n | Heterozygosity
|
Excl. Prob. | |
|---|---|---|---|---|---|---|---|
| Obs. | Exp. | ||||||
| Pmin05 | TTTCCCCCGAACAACACAAC | [GT]31 | 56 | 62 | 0.968 | 0.979 | 0.942 |
| TTCCCATGCCTCCTTTTGTC | |||||||
| Pmin01 | CACAAAGTCAATCCTAAATA | [GT]41 | 63 | 62 | 0.968 | 0.984 | 0.952 |
| CCAAACTGTTTAGCACTG | |||||||
| Pmin10 | AACCGCCCAATCCACAAC | [GT]25 | 17 | 16 | 0.938 | 0.956 | 0.850 |
| GAATGTCCCGAGAAACTGGAG | |||||||
Shown are primer sequences, the sequence of the original cloned microsatellite, and the number of alleles per locus in an adult sample of n individuals. Also shown are expected and observed heterozygosities as well as expected exclusion probabilities (given a known mother–offspring pair; ref. 28).
A standard phenol–chloroform protocol was used to extract DNA from adult fin clips. Embryos were prepared for the PCR by using a simple single-tube extraction protocol (26, 27). Each 10-μl PCR contained 1X Promega Taq buffer, 0.5 units Promega Taq polymerase, 1.3–1.5 mM MgCl2, 0.15 μM each primer, and 0.1 mM each dNTP. Cycling consisted of an initial denaturation of 2 min at 95°C, followed by 32 cycles of 1 min at 95°C, 1 min at an optimal annealing temperature (52°C for Pmin01, 60°C for Pmin05 and Pmin10), and 2 min at 72°C. The cycling was concluded by a final 3-min extension at 72°C.
One primer from each locus was dye-labeled during its commercial synthesis. After PCR, 0.7 μl of each product was combined with 2 μl of deionized formamide, 0.4 μl of GeneScan-500 ROX size standard (Perkin–Elmer), and 0.5 μl of loading buffer. The PCR fragments then were resolved by electrophoresis through a 4.2% acrylamide gel, using an ABI377 automated sequencer. Sizing of fragments was aided by the use of GENOTYPER 2.5 (Perkin–Elmer). Numerous individuals from different nests were assayed side by side on gels to accurately assess relative allele sizes, an important concern given the large number of alleles per locus. Allele frequencies were determined from the genotypes of nest-holding males combined with those maternal genotypes (inferred from progeny arrays) that could be obtained unambiguously from 10 or more full-sibling progeny.
Parentage Assessment and Genotype Reconstruction.
A total of 24 nests with attendant males were collected. All 24 males and a sample of 20–51 progeny from each nest were assayed for the two most polymorphic loci (Pmin05 and Pmin01). The third locus (Pmin10) was used to confirm exclusions and resolve ambiguities (e.g., in cases in which an embryo could not be assigned to an inferred mother on the basis of Pmin05 and Pmin01). Our initial goal was to seek evidence of sneaking, which typically (depending on allele frequencies) should result in embryos that at one or more loci share no alleles with the guardian male. All except one of our single-locus exclusions were verified with data from at least one additional locus. The one exception we provisionally attribute to a de novo paternal germline mutation. Thus, in general, de novo mutations in the paternal lines seemed not to be an important source of error in this study.
For comparison with other marker systems, we calculated the average exclusion probability for each locus given a known mother–offspring pair (28). We also calculated the probability of exclusion associated with each male, when neither parent was known with certainty (29). This exclusion probability reflects the proportion of unrelated progeny in the population that we would expect to share no alleles with the nest holder. To determine the minimum number of females contributing to each nest, we first removed the sneaked progeny from consideration. Then, from simultaneous inspection of the maternal alleles at both loci, we determined the minimum number of females that could explain the progeny array and reconstructed maternal genotypes (for details, see ref. 30). In three instances, a single embryo had a genotype at one locus that could not be explained without the addition of another female. We interpreted these embryos as carrying single-locus de novo mutations in the respective maternal lines, without invoking the presence of an additional dam for the nest.
The Model Relating Cuckoldry to the Opportunity for Sexual Selection.
Because this study did not involve a closed population, not all parameters relevant to the opportunity for sexual selection were accessible. Thus, we developed a model that used some simplifying assumptions. The main parameters of interest were (i) the distribution of male mating success (i.e., the number of females with whom a male sired offspring) and (ii) the distribution of male reproductive success (i.e., the total number of offspring sired by a male). Assuming that Nn represents the proportion of males with eggs from n females in his nest (in the absence of sneaking), and that Mn represents the proportion of males with mating success n after all cuckoldry takes place, then,
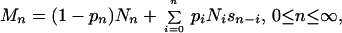 |
1 |
where pn is the probability that a male with eggs from n females in his nest attempts to sneak, and sn is the proportion of sneaker males that successfully fertilize eggs from n females as a consequence of sneaking. In our model, each sn is given by a Poisson distribution with mean s̄, such that
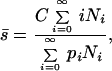 |
2 |
where C is the proportion of clutches containing cuckolded offspring in the population. The expected distribution of mating success under various patterns of sneaking can be calculated exactly by using the above equations. However, calculation of the variance in reproductive success is slightly more complex, hence we created a computer program to randomly generate populations (each containing 100,000 males) by using the above parameters. We used the observed distribution (determined from our genetic data) of the number of offspring (measured as egg area) sired per successful nest-holder mating event and per cuckoldry event to randomly assign reproductive success for each male in the simulation. The variances in mating success and reproductive success were calculated from each randomly generated population.
Other key parameters of this model were estimated from our genetic data as well. For example, N1 through N∞ are given by the number of females that mated with each successful nest-guarding male, and C can be determined by the proportion of the total clutches that experienced cuckoldry. We did not collect unsuccessful males during this study, but in a previous study of the same population, Forsgren et al. (31) found that 42% of nests had no eggs. Thus, initially we assumed the number of unsuccessful nesting males to be 17, which, when combined with our 23 observed successful nest holders, yields N0 = 0.425. We also repeated the analysis assuming that three times as many unsuccessful males were present in the population, resulting in a value of 0.689 for N0. We assumed that the probability that a nest-holding male with eggs in his nest would attempt to sneak (pnh) was independent of the number of females from which he had received eggs (i.e., pnh = p1 = p2 = … = pn).
Results
The Microsatellite Markers.
The GT-rich microsatellite markers cloned from P. minutus proved highly polymorphic, with 17–63 alleles per locus and heterozygosities ranging from 0.94 to 0.97 (Table 1). The combined expected exclusion probability (given a known parent–offspring pair) for the three loci was 0.9996. We detected no departures from the Hardy–Weinberg equilibrium and no evidence for genotypic disequilibrium at the population level (exact tests in genepop; ref. 32). At the within-family level, however, the loci Pmin01 and Pmin10 seemed to be linked, but Pmin10 was used sparingly, and this linkage did not affect our results in any way. We found no evidence for null (nonamplifying) alleles, even though they are easily detected by the unexpected patterns of segregation that they produce in progeny arrays.
Every adult individual assayed in this study had a unique two-locus genotype (Pmin05 and Pmin01), including the females and sneakers whose genotypes were reconstructed from progeny arrays. Thus, our power to detect multiple mating and sneaking was high. Simulations run by using the published program GERUDSIM1.0 (33) indicated that, based on our sampling scheme and observed allele frequencies, our probability of correctly recovering the number of females contributing eggs to a male's nest was greater than 0.99, assuming that between 1 and 5 females contributed equally to a nest containing thousands of eggs. In addition, exclusion probabilities (with neither parent known) ranged from 0.970 to 0.998, indicating that these two loci should be sufficient to identify nearly every embryo in our sample that was the result of sneaked fertilizations. Thus, our minimum estimates of sneaking frequency and multiple mating seem to be very close to the true values, and we would not expect a small number of undetected events to affect our conclusions in any way.
The Genetic Mating System of Sand Gobies.
Our results unambiguously show a high rate of successful sneaking in this population of sand gobies. In one case, a male (KB16, Table 2) was excluded as the father of every embryo in his nest. The progeny array was consistent with the nest having had three dams and a single sire; thus, the most likely explanation is that KB16 was not the original nest owner. This nest, therefore, has been excluded from further analysis.
Table 2.
Summary of data for sand goby males
| Male | Dried body weight, mg | Egg area, cm2 | No. of embryos assayed | No. of females mated | No. of sneaker males | No. of eggs sneaked |
|---|---|---|---|---|---|---|
| KB01 | 226 | 35.6 | 51 | 5 | 3 | 11 |
| KB02 | 451 | 31.4 | 40 | 5 | 0 | 0 |
| KB03 | 150 | 21.7 | 45 | 4 | 3 | 32 |
| KB04 | 447 | 26.8 | 44 | 3 | 0 | 0 |
| KB05 | 269 | 38.6 | 20 | 3 | 0 | 0 |
| KB06 | 212 | 16.0 | 32 | 3 | 1 | 1 |
| KB07 | — | 28.4 | 38 | 5 | 1 | 1 |
| KB08 | 389 | 36.6 | 40 | 4 | 2 | 6 |
| KB09 | 251 | 27.7 | 34 | 2 | 3 | 4 |
| KB10 | 291 | 33.7 | 44 | 2 | 0 | 0 |
| KB11 | 379 | 32.2 | 39 | 3 | 0 | 0 |
| KB12 | 187 | 14.8 | 44 | 2 | 2 | 15 |
| KB13 | 305 | 28.3 | 43 | 5 | 5 | 12 |
| KB14 | 205 | 28.8 | 40 | 6 | 0 | 0 |
| KB16 | 125 | 26.8 | 40 | 0 | n/a | n/a |
| KB17 | 222 | 21.5 | 46 | 2 | 0 | 0 |
| KB18 | 137 | 14.3 | 45 | 3 | 1 | 2 |
| KB20 | 279 | 17.4 | 43 | 3 | 0 | 0 |
| KB22 | 123 | 15.8 | 44 | 3 | 2 | 2 |
| KB25 | 234 | 24.3 | 47 | 4 | 0 | 0 |
| KB26 | 196 | 14.2 | 46 | 4 | 0 | 0 |
| KB28 | 184 | 7.9 | 42 | 2 | 0 | 0 |
| KB29 | 222 | 17.4 | 43 | 3 | 4 | 12 |
| KB30 | 158 | 9.9 | 31 | 2 | 3 | 6 |
Shown are the dried body weight of each male and the area of the nest's ceiling covered by eggs. Egg area is highly correlated with the number of eggs in a male's nest. The last four columns present the results of the genetic analysis. Shown are the numbers of embryos assayed, of females genetically inferred to have contributed eggs to the nest, of sneaker males that victimized the nest holder, and of assayed embryos that were fertilized by sneakers (i.e., were not the progeny of the nest-holding male). Genotypes of the progeny in KB16's nest were consistent with a single sire and three dams, but KB16 was excluded as the father for every embryo, suggesting a nest takeover. n/a, not applicable.
Successful sneaky fertilizations (i.e., as registered by eggs that were not fertilized by the nest holder) occurred in 52% of assayed nests (Table 2). The proportion of excluded embryos in sneaked nests varied from 3 to 71% (Table 2). In total, of 941 embryos assayed (excluding KB16's nest), 104 (11%) had resulted from fertilizations by males other than the guardian. The 23 assayed nests contained a total of 78 clutches, 21 of which contained sneaked eggs. This observation provides our estimate of C (= 0.269) for the model.
From the genetic data, we were able to determine the minimum number of sneakers that fertilized eggs in each male's nest. Most sneaked nests contained eggs from several different females fertilized partly by the nest holder and partly by multiple sneakers (Table 2). We found four instances in which a group of embryos with a single mother had at least three fathers, including the nest holder. In these cases, multiple sneakers must have entered the nest simultaneously. In addition, one nest contained embryos with two different mothers that apparently were the progeny of a single sneaker male. We also found evidence for multiple mating by the guardian male in every nest assayed (Table 2). Males mated with 2–6 females (mean = 3.4) per nest.
Cuckolded males were significantly smaller than noncuckolded males (t test, P = 0.03). We found no significant relationship between male body weight and detected number of mates (n = 22, r = 0.215, P = 0.336), but we did find a significant positive relationship between body weight and egg area (n = 22, r = 0.68, P < 0.001).
Cuckoldry and the Opportunity for Sexual Selection.
The results of the model show that the relationship between alternative mating strategies and the opportunity for sexual selection depends on the details of sneaker mating success. When all sneaking is done by successful nest holders (i.e., males with eggs in their own nests), the opportunity for sexual selection generally stays constant or increases slightly as a consequence of cuckoldry (Table 3). However, when all cuckoldry is done by nonnesting males (or males with empty nests), the opportunity for sexual selection usually is reduced dramatically.
Table 3.
Results of the model of sneaking and sexual selection in sand gobies
| % attempting to sneak of …
|
Mating success
|
Reproductive success
|
Opportunity for sexual selection | |||
|---|---|---|---|---|---|---|
| Non-nesting males, p0 | Successful nesters, pnh | Mean | Variance | Mean | Variance | |
| Proportion of males without a nest containing eggs (i.e., N0) = 0.425 | ||||||
| 0% | 0% | 1.95 | 3.61 | 13.58 | 229.22 | 0.94 |
| 0% | 100% | 2.49 | 5.86 | 13.61 | 225.02 | 0.95 |
| 100% | 0% | 2.47 | 2.46 | 13.65 | 170.03 | 0.40 |
| 100% | 100% | 2.49 | 4.13 | 13.61 | 199.52 | 0.67 |
| Proportion of males without a nest containing eggs (i.e., N0) = 0.689 | ||||||
| 0% | 0% | 1.04 | 2.87 | 7.34 | 170.33 | 2.63 |
| 0% | 100% | 1.34 | 4.68 | 7.33 | 168.01 | 2.60 |
| 100% | 0% | 1.34 | 2.62 | 7.38 | 135.56 | 1.47 |
| 100% | 100% | 1.34 | 3.18 | 7.32 | 144.30 | 1.78 |
The top half of the table shows results when N0 = 0.425; the bottom half shows results when N0 = 0.689 (N0 is the proportion of sexually mature males in the population that either have no nests or fail to attract females to their nests). The first two columns in the table show the parameters p0 and pnh, respectively. Other columns show the means and variances in mating success and reproductive success calculated from the simulations. The final column shows the opportunity for sexual selection, as given by the variance in mating success divided by the square of the mean (13). Reproductive success is measured here by egg area (cm2), which is highly correlated with the number of embryos in a nest.
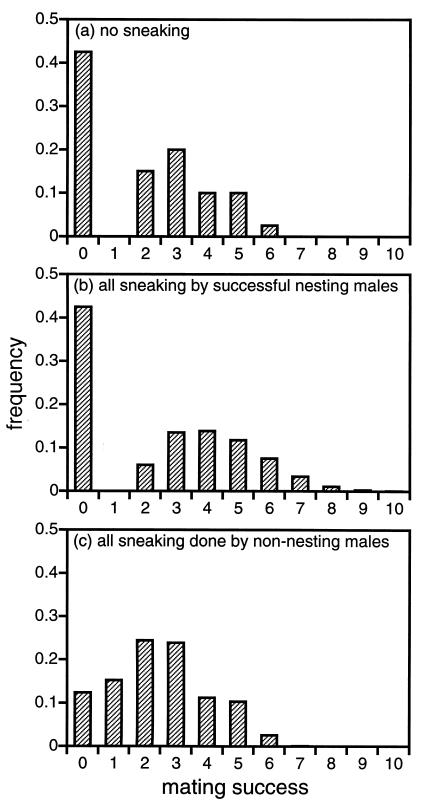
If all mature males in the population attempt to sneak (p0 = 1.0 and pnh = 1.0), then there is a modest reduction in the opportunity for sexual selection (Table 3). This result holds regardless of the number of nonnesting males that are assumed to be in the population. The main consequence of adding additional males without nests to the model is an overall increase in the opportunity for sexual selection, but retention of the same pattern with respect to the effects of sneaking (Table 3). When the variance in reproductive success is considered, we see essentially the same pattern: a small change in the variance when nest holders sneak and a large reduction when nonnesters sneak (Table 3). Fig. 1 shows mating success histograms for populations exhibiting three of the key sneaking scenarios described in Table 3.
Figure 1.
Distributions of mating success (i.e., the number of females with whom a male sired progeny) for sand goby males. Shown are (a) the observed distribution of mating success for males in the absence of sneaking (estimated directly from our parentage study), (b) the results of the model when all sneaking is done by males with eggs in their nests, and (c) the results of the model when all sneaking is done by nonnesting males (or unsuccessfully nesting males). This depiction assumes that N0 = 0.425.
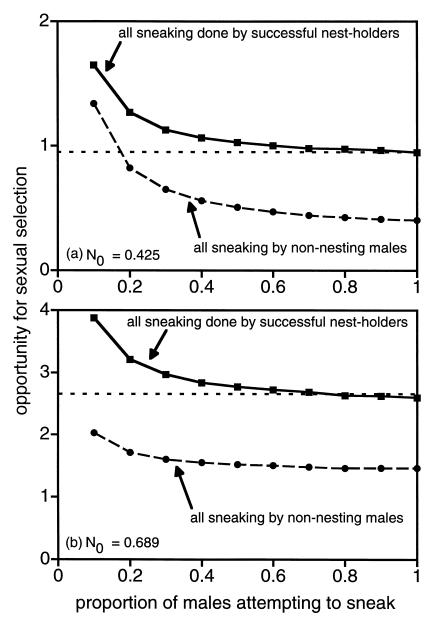
One parameter of particular importance is the expected mating success per sneaker, which is directly related to the proportion of males in a particular class of males that attempt to sneak. Fig. 2 presents the results of an analysis in which the proportion of males attempting to sneak varies. Under most circumstances, sneaking by nest-holding males results in either no change or in a modest increase in the opportunity for sexual selection (relative to the no-sneaking scenario). Similarly, in most situations, sneaking by nonnesters results in a marked decrease in the opportunity for sexual selection (relative to the case in which cuckoldry is absent). Exceptions to these patterns occur when the proportion of males in the population participating in sneaking is very small (<≈20%) and the mean mating success per sneaker is consequently quite high (>≈5 mates). Such high mating success per sneaker can result in a substantial increase in the opportunity for sexual selection (Fig. 2).
Figure 2.
The relationship between the proportion of males attempting to sneak and the opportunity for sexual selection based on variance in male mating success when (a) N0 = 0.425 or (b) N0 = 0.689. Squares (connected by a solid line) indicate the opportunity for sexual selection when all sneaking is done by a percentage of the nest holders (i.e., p0 = 0 and pnh ranges from 0.1 to 1.0). Circles (connected by a dashed line) indicate the results for the cases in which all sneaking is done by some percentage of nonnesting males (i.e., pnh = 0 and p0 ranges from 0.1 to 1.0). The horizontal dashed line in each graph corresponds to the opportunity for sexual selection in the absence of sneaking.
Discussion
This microsatellite assessment of parentage clearly demonstrates that successful sneaking is a common occurrence in the Klubban population of sand gobies, and that such alternative mating tactics can influence the opportunity for sexual selection, as defined by variance-based measures of the mating system (13, 15). We also show that male sand gobies are highly polygynous, even in the absence of sneaking behavior, a feature of the mating system that also influences the strength of sexual selection.
Our results indicate that sneaking is a common phenomenon in sand gobies. Forsgren (24) noticed sneaking attempts in about 12% of courtship interactions observed in the field and presented a much simpler scenario of sneaking than is suggested by our genetic results. For example, we found a much higher rate of successful sneaking. About half of the nests (and 27% of clutches) contained eggs fertilized by sneakers. Assuming that sneakers are sometimes unsuccessful, harassment by sneakers must be a nearly constant problem for nest-holding males. Previous observations also suggest that sneaking events are fairly simple, involving the nest holder, a female, and a sneaker (24). Our results indicate, however, that sneaking may be much more complicated, sometimes involving multiple simultaneous sneakers. In addition, one sneaker male fertilized eggs from two different females in a nest, suggesting either that two females spawned at the same time or that the sneaker remained in the vicinity of the nest between the two mating episodes.
Our most noteworthy finding is that cuckoldry does not always increase the opportunity for sexual selection, as is commonly supposed (7). Our observations agree well with theoretical results, which show that a negative covariance between within-pair success and cuckoldry (as occurs when nonnesters perform much of the sneaking) can reduce the variance in reproductive success (11). Thus, EPFs or other forms of alternative mating strategies should not always be equated with an increase in the intensity of sexual selection. Rather, a detailed understanding of the effects of mating tactics on the sexual selection process requires a more complete description of the genetic mating system, with particular emphasis on the reproductive success of individual males participating in alternative strategies.
In the present study (as in all other genetic studies of natural fish mating systems to date), we were unable to describe the complete genetic mating system of our population. Rather, we used a mathematical model to fill in some important parameters. Our model allowed for variation in the relative rates of sneaking by nesting and nonnesting males, but several lines of evidence suggest that nonnesting males perform most sneaking in sand gobies. First, if nest-guarding males leave their nests, even for short periods, predators quickly devour the developing progeny (C.K., personal observation). Second, some of our sampled nests were in close spatial proximity to one another, yet in no case did the inferred genotype of a sneaker match the genotype of a nest-guarding male. Third, laboratory observations have shown that small, sexually mature males, apparently uninterested in building nests of their own, sometimes bury themselves near the nests of parental males (20). Finally, in a closely related species, Pomatoschistus microps, cuckoldry seems to be a conditional strategy used by males that are unable to compete successfully to attract females to their nests (34, 35). Collectively, these observations imply that most sneakers are probably males that either fail to attract females to their nests or never build nests in the first place. Thus, in the Klubban population of sand gobies, the phenomenon of cuckoldry almost certainly decreases the opportunity for sexual selection. Our model shows that this conclusion is valid whether some successful nest-holding males sneak, as long as nonnesting males perform a substantial proportion of the cuckoldry.
The primary reason that alternative mating strategies decrease the opportunity for sexual selection in sand gobies (relative to a similar mating system with only a nest-holding strategy) is that these males are highly polygynous (mean mating success among successful males of 3.4). Thus, in the absence of alternative mating behavior, the opportunity for sexual selection is quite high. Cuckoldry simply reduces the ability of successful nest-guarding males to monopolize access to females, decreasing the variance in male mating success (12). A similar process could occur in species with less polygynous or even monogamous social mating systems. In such species, particularly if many males experience no mating success, there could be a large opportunity for sexual selection in the absence of cuckoldry.
Most studies of avian mating systems concerned with variation in mating success find that EPFs result in an increase in the opportunity for sexual selection (4–6, 8, 10), a pattern mainly caused by the fact that most bird species are socially monogamous and almost any deviation from such a mating system will increase the variance in male mating success. This feature of avian biology probably accounts for the positive correlation between EPFs and plumage brightness observed by Møller and Birkhead (7). However, even in some polygynous bird species, such as the red-winged blackbird (36), EPFs increase the opportunity for sexual selection. This pattern agrees well with our model, because many of the red-winged blackbird males who obtained EPFs were successful nest holders.
In summary, we have shown that the relationship between alternative mating strategies and variance in mating success is not a simple one, and that merely quantifying the frequency of cuckoldry will say little about the nature of sexual selection acting on a natural population. Studies aimed at a deeper understanding of sexual selection in nature need to face the difficult challenge of describing the complete mating and reproductive success of particular individuals who use various mating tactics.
Acknowledgments
We thank the Klubban Biological Station for providing excellent facilities for the fieldwork and W. S. Nelson for help in the laboratory. The field portion of this work was supported by funding from the Swedish Natural Sciences Research Council and the Knut and Alice Wallenberg Foundation (C.K.). The molecular work was supported by a National Institutes of Health training grant (A.G.J. and D.W.), the National Science Foundation (A.G.J.), the University of Georgia (J.C.A.), and the Pew Foundation (J.C.A.).
Abbreviation
- EPF
extra-pair fertilization
References
- 1.Hughes C. Ecology. 1998;79:383–399. [Google Scholar]
- 2.Birkhead T R, Møller A P. Sperm Competition in Birds. New York: Academic; 1992. [Google Scholar]
- 3.Avise J C. Molecular Markers, Natural History and Evolution. New York: Chapman & Hall; 1994. [Google Scholar]
- 4.Gibbs H L, Weatherhead P J, Boag P T, White B N, Tabak L M, Hoysak D J. Science. 1990;250:1394–1397. doi: 10.1126/science.250.4986.1394. [DOI] [PubMed] [Google Scholar]
- 5.Kempenaers B, Verheyen G R, van der Broeck M, Burke T, van Broeckhoven C, Dhondt A A. Nature (London) 1992;357:494–496. [Google Scholar]
- 6.Westneat D F. Behav Ecol. 1992;4:49–60. [Google Scholar]
- 7.Møller A P, Birkhead T R. Evolution (Lawrence, Kans) 1994;48:1089–1100. doi: 10.1111/j.1558-5646.1994.tb05296.x. [DOI] [PubMed] [Google Scholar]
- 8.Yezerinac S M, Weatherhead P J, Boag P T. Behav Ecol Sociobiol. 1995;37:179–188. [Google Scholar]
- 9.Møller A P, Ninni P. Behav Ecol Sociobiol. 1998;43:345–358. [Google Scholar]
- 10.Sheldon B C, Ellegren H. Anim Behav. 1999;57:285–298. doi: 10.1006/anbe.1998.0968. [DOI] [PubMed] [Google Scholar]
- 11.Webster M S, Pruett-Jones S, Westneat D F, Arnold S J. Evolution (Lawrence, Kans) 1995;49:1147–1157. doi: 10.1111/j.1558-5646.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen C W, Warner R R. In: Sperm Competition and Sexual Selection. Birkhead T R, Møller A P, editors. London: Academic; 1998. pp. 435–463. [Google Scholar]
- 13.Wade M J, Arnold S J. Anim Behav. 1980;28:446–461. [Google Scholar]
- 14.Whittingham L A, Lifjeld J T. J Avian Biol. 1995;26:283–288. [Google Scholar]
- 15.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 16.Arnold S J, Duvall D. Am Nat. 1994;143:317–348. [Google Scholar]
- 17.Kvarnemo C, Ahnesjö I. Trends Ecol Evol. 1996;11:404–408. doi: 10.1016/0169-5347(96)10056-2. [DOI] [PubMed] [Google Scholar]
- 18.Jones A G, Rosenqvist G, Berglund A, Arnold S J, Avise J C. Proc R Soc London Ser B. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesthagen I H. Sarsia. 1977;63:17–26. [Google Scholar]
- 20.Magnhagen C, Kvarnemo C. J Fish Biol. 1989;35:755–763. [Google Scholar]
- 21.Kvarnemo C. Proc R Soc London Ser B. 1994;256:151–156. [Google Scholar]
- 22.Kvarnemo C. Behav Ecol. 1996;7:208–212. [Google Scholar]
- 23.Lindström K, Seppä T. Proc R Soc London Ser B. 1996;263:1319–1323. [Google Scholar]
- 24.Forsgren E. Anim Behav. 1997;53:267–276. [Google Scholar]
- 25.Jones A G, Östlund-Nilsson S, Avise J C. Evolution (Lawrence, Kans) 1998;52:848–858. doi: 10.1111/j.1558-5646.1998.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 26.Gloor G, Engels W. Drosophila Inf Serv. 1992;71:148–149. [Google Scholar]
- 27.Jones A G, Avise J C. Mol Ecol. 1997;6:203–213. doi: 10.1046/j.1365-294x.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty R, Meagher T R, Smouse P E. Genetics. 1988;118:527–536. doi: 10.1093/genetics/118.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodds K G, Tate M L, McEwan J C, Crawford A M. Theor Appl Genet. 1996;92:966–975. doi: 10.1007/BF00224036. [DOI] [PubMed] [Google Scholar]
- 30.Jones A G, Avise J C. Evolution (Lawrence, Kans) 1997;51:1611–1622. doi: 10.1111/j.1558-5646.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 31.Forsgren E, Kvarnemo C, Lindström K. Evolution (Lawrence, Kans) 1996;50:646–654. doi: 10.1111/j.1558-5646.1996.tb03875.x. [DOI] [PubMed] [Google Scholar]
- 32.Raymond M, Rousset F. J Hered. 1995;86:248–249. [Google Scholar]
- 33.Jones A G. Mol Ecol Notes. 2001;1:33. [Google Scholar]
- 34.Magnhagen C. Anim Behav. 1994;47:1212–1215. [Google Scholar]
- 35.Magnhagen C. J Fish Biol. 1998;53:130–137. [Google Scholar]
- 36.Weatherhead P J, Boag P T. Ecology. 1997;78:884–896. [Google Scholar]