Abstract
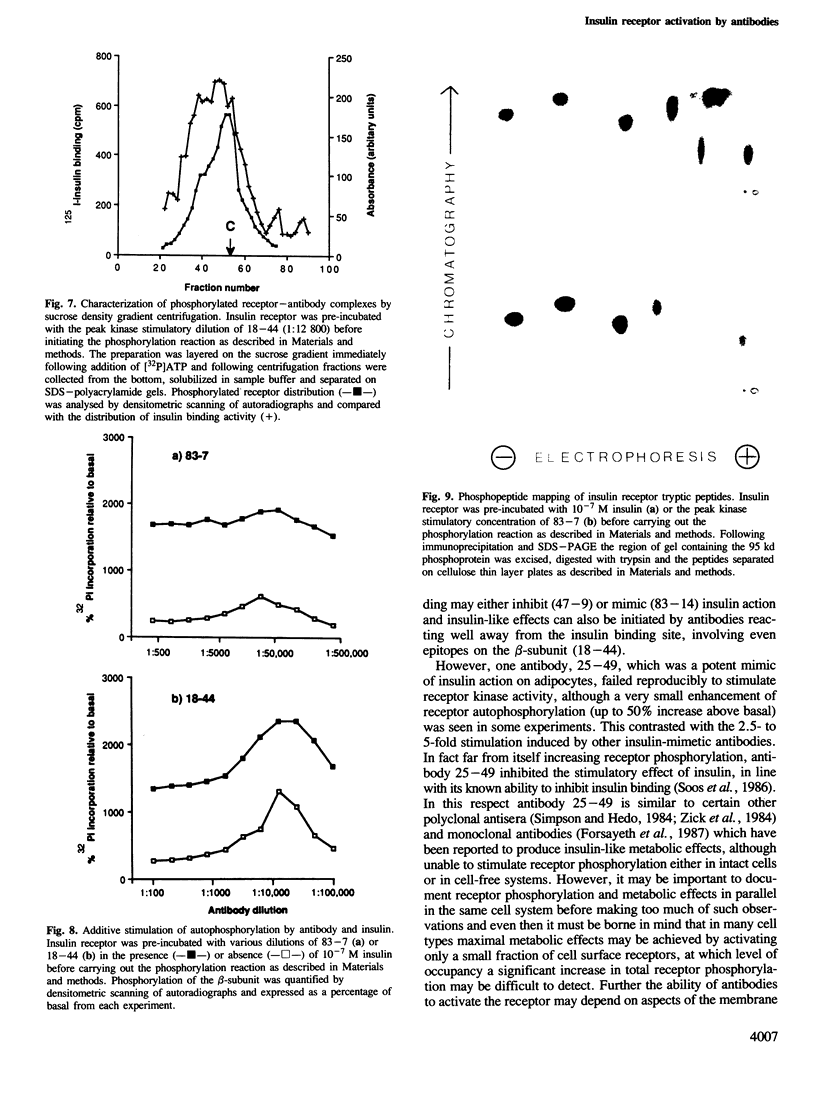
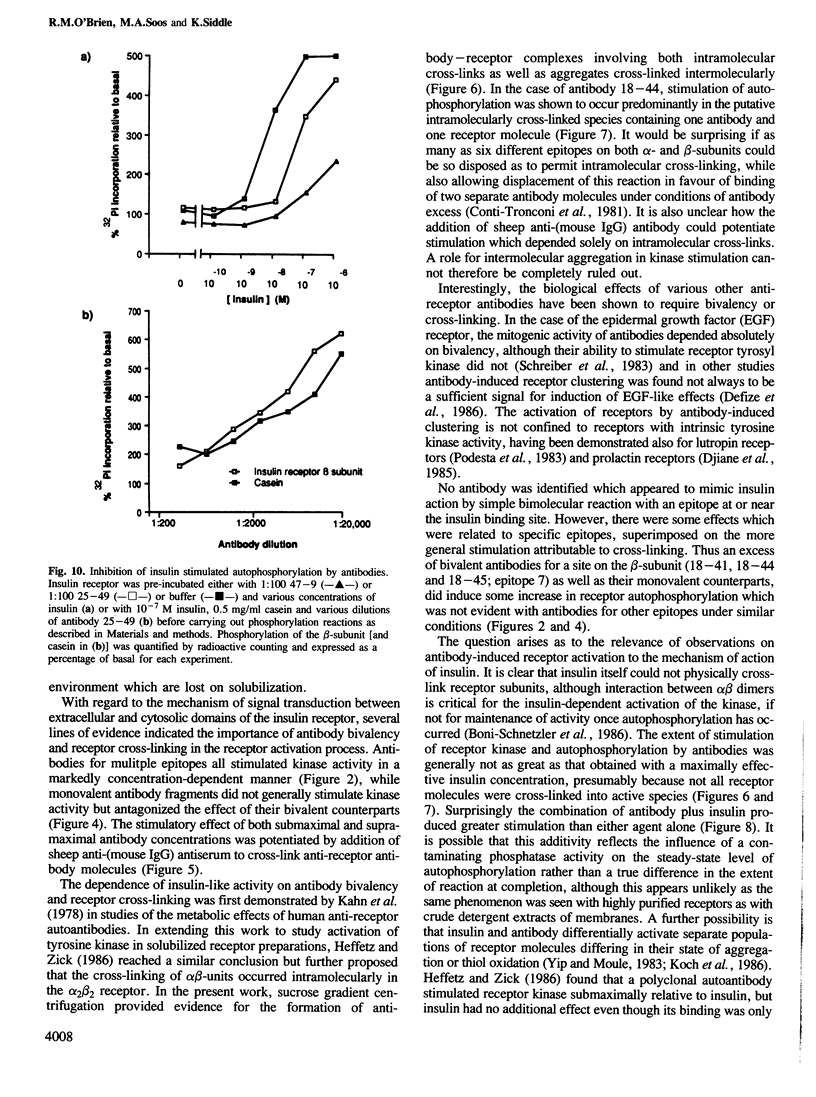
The effect of monoclonal anti-insulin receptor antibodies on the intrinsic kinase activity of solubilized receptor was investigated. Antibodies for six distinct epitopes stimulated receptor autophosphorylation and kinase activity towards exogenous substrates. This effect of antibodies was seen only within a narrow concentration range and monovalent antibody fragments were ineffective. Evidence was obtained by sucrose density-gradient centrifugation for the formation of antibody-receptor complexes which involved both inter- and intra-molecular cross-linking, although stimulation of autophosphorylation appeared to be preferentially associated with the latter. There was partial additivity between the effects of insulin and antibodies in stimulating autophosphorylation, although the sites of phosphorylation appeared identical on two-dimensional peptide maps. Antibodies for two further epitopes failed to activate receptor kinase, but inhibited its stimulation by insulin. The effects of antibodies on kinase activity paralleled their metabolic effects on adipocytes, except for one antibody which was potently insulin-like in its metabolic effects, but which antagonized insulin stimulation of kinase activity. It is concluded that antibodies activate the receptor by cross-linking subunits rather than by reacting at specific epitopes. The ability of some antibodies to activate receptor may depend on receptor environment as well as the disposition of epitopes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. D., Sönksen P. H. Characterization of two insulin-binding components of rat-liver plasma membranes. Biosci Rep. 1982 Oct;2(10):785–793. doi: 10.1007/BF01114938. [DOI] [PubMed] [Google Scholar]
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch W. J., Mullock B. M., Luzio J. P. Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin. Biochem J. 1987 Jun 1;244(2):311–315. doi: 10.1042/bj2440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Rubin J. B., Pilch P. F. Structural requirements for the transmembrane activation of the insulin receptor kinase. J Biol Chem. 1986 Nov 15;261(32):15281–15287. [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Cobb M. H., Rosen O. M. The insulin receptor and tyrosine protein kinase activity. Biochim Biophys Acta. 1984;738(1-2):1–8. doi: 10.1016/0304-419x(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 2. Binding to native receptor. Biochemistry. 1981 Apr 14;20(8):2181–2191. doi: 10.1021/bi00511a017. [DOI] [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Defize L. H., Moolenaar W. H., van der Saag P. T., de Laat S. W. Dissociation of cellular responses to epidermal growth factor using anti-receptor monoclonal antibodies. EMBO J. 1986 Jun;5(6):1187–1192. doi: 10.1002/j.1460-2075.1986.tb04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane J., Dusanter-Fourt I., Katoh M., Kelly P. A. Biological activities of binding site specific monoclonal antibodies to prolactin receptors of rabbit mammary gland. J Biol Chem. 1985 Sep 25;260(21):11430–11435. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorescu F., Herzberg V., King G., Meistas M., Elders J., Frazer T., Kahn C. R. Defects in insulin binding and autophosphorylation of erythrocyte insulin receptors in patients with syndromes of severe insulin resistance and their parents. J Clin Endocrinol Metab. 1987 Mar;64(3):549–556. doi: 10.1210/jcem-64-3-549. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Zick Y. Receptor aggregation is necessary for activation of the soluble insulin receptor kinase. J Biol Chem. 1986 Jan 15;261(2):889–894. [PubMed] [Google Scholar]
- Häring H. U., White M. F., Machicao F., Ermel B., Schleicher E., Obermaier B. Insulin rapidly stimulates phosphorylation of a 46-kDa membrane protein on tyrosine residues as well as phosphorylation of several soluble proteins in intact fat cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):113–117. doi: 10.1073/pnas.84.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptors. Annu Rev Pharmacol Toxicol. 1983;23:461–479. doi: 10.1146/annurev.pa.23.040183.002333. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R., Deger A., Jäck H. M., Klotz K. N., Schenzle D., Krämer H., Kelm S., Müller G., Rapp R., Weber U. Characterization of solubilized insulin receptors from rat liver microsomes. Existence of two receptor species with different binding properties. Eur J Biochem. 1986 Jan 15;154(2):281–287. doi: 10.1111/j.1432-1033.1986.tb09394.x. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Linde S., Hansen B., Sonne O., Holst J. J., Gliemann J. Tyrosine A14[125I]monoiodoinsulin: Preparation, Biologic Properties, and long-term stability. Diabetes. 1981 Jan;30(1):1–8. doi: 10.2337/diab.30.1.1. [DOI] [PubMed] [Google Scholar]
- Machicao F., Häring H., White M. F., Carrascosa J. M., Obermaier B., Wieland O. H. An Mr 180,000 protein is an endogenous substrate for the insulin-receptor-associated tyrosine kinase in human placenta. Biochem J. 1987 May 1;243(3):797–801. doi: 10.1042/bj2430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- Perrotti N., Accili D., Marcus-Samuels B., Rees-Jones R. W., Taylor S. I. Insulin stimulates phosphorylation of a 120-kDa glycoprotein substrate (pp120) for the receptor-associated protein kinase in intact H-35 hepatoma cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3137–3140. doi: 10.1073/pnas.84.10.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podestá E. J., Solano A. R., Attar R., Sánchez M. L., Molina y Vedia L. Receptor aggregation induced by antilutropin receptor antibody and biological response in rat testis Leydig cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3986–3990. doi: 10.1073/pnas.80.13.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio G., Dolais-Kitabgi J., Louvard D., Gautier N., Rossi B. Insulin and rabbit anti-insulin receptor antibodies stimulate additively the intrinsic receptor kinase activity. EMBO J. 1987 Feb;6(2):333–340. doi: 10.1002/j.1460-2075.1987.tb04759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Phosphorylation of synthetic insulin receptor peptides by the insulin receptor kinase and evidence that the preferred sequence containing Tyr-1150 is phosphorylated in vivo. J Biol Chem. 1986 Jul 25;261(21):10000–10005. [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Taylor R., Soos M. A., Wells A., Argyraki M., Siddle K. Insulin-like and insulin-inhibitory effects of monoclonal antibodies for different epitopes on the human insulin receptor. Biochem J. 1987 Feb 15;242(1):123–129. doi: 10.1042/bj2420123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Moule M. L. Structure of the insulin receptor of rat adipocytes. The three interconvertible redox forms. Diabetes. 1983 Aug;32(8):760–767. doi: 10.2337/diab.32.8.760. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Taylor S. I., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984 Apr 10;259(7):4396–4400. [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R., Pines M., Gierschik P., Spiegel A. M. Multisite phosphorylation of the alpha subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]