Abstract
Heme oxygenase 1 (HO-1) is an inducible stress-response enzyme that not only catalyzes the degradation of heme (e.g., released from erythrocytes) but also has an important function in various physiological and pathophysiological states associated with cellular stress, such as ischemic/reperfusion injury. HO-1 has a well-documented anti-inflammatory potential, and HO-1 has been reported to have a negative effect on adhesion and migration of neutrophils in acute inflammation in a model of peritonitis. This finding is supported by our recent observation that hematopoietic stem progenitor cells (HSPCs) from HO-1 KO mice are easy mobilizers, since they respond better to peripheral blood chemotactic gradients than wild type littermates. Based on these findings, we hypothesized that transient inhibition of HO-1 by nontoxic small-molecule inhibitors would enhance migration of HSPCs in response to bone marrow chemoattractants and thereby facilitate their homing. To directly address this issue, we generated several human hematopoietic cell lines in which HO-1 was upregulated or downregulated. We also exposed murine and human BM-derived cells to small-molecule activators and inhibitors of HO-1. Our results indicate that HO-1 is an inhibitor of hematopoietic cell migration in response to crucial BM homing chemoattractants such as stromal-derived factor 1 (SDF-1) and sphingosine-1-phosphate (S1P). Most importantly, our in vitro and in vivo animal experiments demonstrate for the first time that transiently inhibiting HO-1 activity in HSPCs by small-molecule inhibitors improves HSPC engraftment. We propose that this simple and inexpensive strategy could be employed in the clinical setting to improve engraftment of HSPCs, particularly in those situations in which the number of HSPCs available for transplant is limited (e.g., when transplanting umbilical cord blood).
Keywords: Stem cell homing, SDF-1, S1P, heme oxygenase 1, chemotaxis, adhesion, hematopoietic recovery
Introduction
Stromal-derived factor 1 (SDF-1), which binds to the seven-transmembrane-spanning Gαi protein-coupled receptor CXCR4, is unique among the family of chemokines as a chemoattractant of hematopoietic stem/progenitor cells (HSPCs)1,2,3. Since the CXCR4 receptor is expressed on HSPCs, SDF-1 plays an important role in regulating the trafficking of these cells and facilitates their homing and engraftment in bone marrow (BM) after transplantation. Furthermore, SDF-1 is involved in the retention of HSPCs in BM stem cell niches4,5,6,7. Nevertheless, the role of the SDF-1–CXCR4 axis in stem cell homing to BM has recently been challenged by several observations that support the involvement of SDF-1–CXCR4-independent homing mechanisms in which bioactive phospho-sphingolipids, such as sphingosine-1-phosphate (S1P)7,8,9,10 and ceramide-1-phosphate (C1P)11, may compensate for SDF-1–CXCR4 axis deficiencies in homing and retention of HSPCs in BM7,11,12,13.
Since SDF-1 is a major homing factor, several strategies have been proposed to enhance the chemotactic responsiveness of HSPCs to an SDF-1 gradient and thereby enhance homing to BM and accelerate engraftment of transplanted HSPCs. These strategies are based on ex vivo exposure of HSPCs to small cationic peptides (e.g., C3a, LL-37, or β-2 defensin)14,15,16, prostaglandin E2 (PGE-2)17, and hyaluronic acid (HA)20, which enhance the chemotactic responsiveness of HSPCs to an SDF-1 gradient14,15,16,18,32.
Recently we focused on heme oxygenase 1 (HO-1), which is an inducible stress-response enzyme that not only catalyzes the degradation of heme (e.g., released from erythrocytes), but also performs an important function in various physiological and pathophysiological states associated with cellular stress, such as ischemic/reperfusion injury17. As reported, HO-1 deficiency in humans and mice results in vulnerability to stress-mediated injury19,20. Moreover, HO-1 has been reported to have negative effects on adhesion and migration of neutrophils in acute inflammation21. We confirmed this observation in our recent paper and, significantly, demonstrated that HSPCs purified from HO-1 KO mice show enhanced migration in response to SDF-1 and S1P gradients22 and are easily mobilized into peripheral blood with G-CSF- and AMD3100-induced pharmacological mobilization22.
In support of a highly migratory state of HSPCs from HO-1 KO animals, mice lacking one HO-1 allele (HO-1+/−) showed accelerated hematopoietic recovery from myelotoxic injury, as HO-1+/− HSCs repopulated lethally irradiated recipients with more rapid kinetics. Unfortunately, mice transplanted with HO-1 KO HSPCs were ineffective in radioprotection and in serial repopulation of myeloablated recipients19.
Based on these observations, we hypothesized that transient inhibition of HO-1 by employing small-molecule inhibitors ex vivo could have a beneficial effect by increasing the chemotactic responsiveness of HSPCs to homing in response to SDF-1 and S1P gradients and that this strategy could enhance homing and engraftment of HSPCs after transplantation.
To address this issue directly, we first generated several human hematopoietic cell lines in which HO-1 was downregulated or overexpressed and performed in vivo transplant experiments in mice with bone marrow mononuclear cells (BM-MNCs) exposed to a small-molecule HO-1 inhibitor. We found that the chemotactic responsiveness of human and murine malignant and normal hematopoietic cells to SDF-1 and S1P gradients was enhanced in cells with downregulated HO-1 expression or activity. Most importantly, murine BMMNCs exposed to the HO-1 inhibitor SnPP engrafted faster in lethally irradiated recipients. We propose that this strategy to transiently inhibit HO-1 activity is safe and could be potentially employed in the clinic to accelerate engraftment of human HSPCs.
Material and Methods
Animals
We employed seventy two 6–8-week-old C57BL/6J wild type mice as well as six green fluorescent protein (GFP)-expressing mice as cell donors in our experiments. Animals were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and all experiments were approved by the Animal Care and Use Committee of the University of Louisville (Louisville, KY, USA).
Cells
Murine bone marrow-derived mononuclear cells (BM-MNCs) were collected by flushing murine tibias and femurs and then separated from erythrocytes and granulocytes by Ficoll-Paque centrifugation. Human peripheral blood (PB)- and umbilical cord blood (UCB)-derived CD34+ cells were isolated by employing MiniMacs anti-CD34+ magnetic beads according to the manufacturer’s protocol23. Raji, K562, Jurkat, and Nalm6 cell lines were purchased from ATCC (Manassas, VA, USA) and propagated in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Waltham, MA USA) supplemented with 10% fetal bovine serum (FBS) (Seradigm, Radnor, PA, USA), 1× GlutaMAX™ (Life Technologies), and 1× penicillin-streptomycin (Life Technologies). All cell lines were passaged every 2–3 days to maintain concentrations in an approximate range of 200,000–800,000 cells per ml.
Cell line electroporation
Twenty-four hours prior to electroporation, all cell lines were subdivided to ensure logarithmic growth at the time of electroporation. On the following day, cells were harvested via brief centrifugation (5 minutes at 400 × g) and washed twice with 1× phosphate buffered saline (PBS) (GE life sciences, Pittsburgh, PA, USA). An aliquot of cells (3 × 106 cells) was then resuspended in 300 μl of room-temperature (RT) RPMI 1640 medium without supplements, transferred to a 0.1-cm electrode gap Gene Pulser® Cuvette (Bio-Rad, Hercules, CA, USA), and 15 μg of plasmid DNA added with gentle agitation. Electroporations were carried out on each cell line using the Gene PulserXcell™ Electroporation System (Bio-Rad) with an applied voltage of 0.200 kV, a capacitance of 950 μF, and a resistance of 400 Ω. Following electroporation, the contents of each cuvette were transferred to individual wells of a 6-well tissue-culture-grade plate containing 2 ml of RPMI complete medium (without antibiotics). Cells were then incubated for 48 h at 37 °C and 5% CO2 prior to undergoing antibiotic-mediated selection.
HO-1 overexpression and shRNA-mediated knockdown
For the establishment of HO-1- overexpressing cell lines, cells were electroporated with 15 μg of a human HO-1 expression vector under the control of a cytomegalovirus (CMV) promoter (pCMV6-HO-1, vector SC320297; Origene, Rockville, MD, USA) or an empty control vector containing CMV-driven eGFP (pEGFP-C2; Clontech Laboratories, Mountain View, CA, USA). Forty-eight hours post electroporation, both pCMV-HO-1- and pEGFP-nucleofected cells underwent antibiotic-mediated selection using G418 (800–2000 μg/ml). In shRNA-mediated HO-1 knockdown experiments, cells were electroporated with either 15 μg of a Mission™ shRNA construct targeting human HO-1 (TRCN0000290436; Sigma-Aldrich, St. Louis, MO, USA) or a non-targeted shRNA control (SHC016; Sigma-Aldrich). As previously described, cells were placed under antibiotic-mediated selection using puromycin (2–5 μg/ml) 48 hours after electroporation. All cell lines were maintained under antibiotic-mediated selection pressure throughout the duration of their expansion prior to experimentation.
Detection of HO-1 by Western blotting
This experiment was carried out to determine the HO-1 protein expression levels in all of the transfected hematopoietic cell lines employed here in comparison with their respective control cell lines. The cells were harvested, centrifuged, and washed with PBS. Next, the total protein extracts were collected after cell lysis using Radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with protease and phosphatase inhibitors (Santa Cruz Biotechnology, Dallas, TX, USA) as described previously11. For each sample, the protein concentration was measured using the Pierce bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, IL, USA). The adjusted extracted proteins (40 μg/each sample) were then separated on a 4–12% SDS-PAGE gel, and fractionated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The membranes were blocked with 2.5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween (TBST) for 1 h at room temperature. After washing with TBST, the membranes were incubated with rabbit anti-HO-1 polyclonal antibody (Enzo Life Sciences, Farmingdale, NY, USA, diluted 1:1000) overnight at 4°C. To ensure equal protein loading in all the lanes, blots were stripped and then reprobed with rabbit anti-β-actin monoclonal antibody (Novus Biologicals, Littleton, CO, USA, diluted 1:1000). All membranes were treated with an enhanced chemiluminescence (ECL) reagent (Amersham Life Sciences) and subsequently exposed to film (Hyperfilm, Amersham Life Sciences, Pittsburgh, PA, USA). For band visualization, an automatic film processor supplied with fresh warm developer and fixer solutions was used9,14.
Real-time quantitative reverse-transcription PCR of cell lines
Total RNA was isolated from hematopoietic cells in which HO-1 was up- and down-regulated and their respective controls with the RNeasy Kit (Qiagen, Valencia, CA, USA). The RNA was reverse-transcribed with MultiScribe reverse transcriptase and oligo-dT primers (Applied Biosystems, Foster City, CA, USA). Quantitative assessment of mRNA levels was done by real-time RT-PCR on an ABI 7500 instrument with Power SYBR Green PCR Master Mix reagent. PCR conditions were as follows: 95°C (15 seconds), 40 cycles at 95°C (15 seconds), and 60°C (1 minute). According to melting point analysis, only one PCR product was amplified under these conditions. The relative quantity of a target, normalized to the endogenous β2-microglobulin gene as control and relative to a calibrator, is expressed as 2−ΔΔCt (fold difference), where Ct is the threshold cycle, ΔCt = (Ct of target genes) – (Ct of the endogenous control gene, β2-microglobulin), and ΔΔCt = (ΔCt of samples for the target gene) – (ΔCt of the calibrator for the target gene)23. The following primer pairs were used for analysis: 5′-GGG TGA TAG AAG AGG CCA AGA CT-3′ (forward) and 5′-AGC TCC TGC AAC TCC TCA AGA-3′ (reverse).
Transwell chemotaxis assay
Raji, K562, Jurkat, and Nalm6 cell lines were incubated overnight in RPMI 1640 medium containing low levels of bovine serum albumin (BSA, 0.5%) to render the cells quiescent. BM-MNCs from WT mice, human PB- and UCB-derived cells were made quiescent for 3 h with RPMI 1640 medium with 0.5% BSA and incubated for 1 h with different doses of the heme oxygenase 1 (HO-1) inhibitor - tin protoporphyrin IX dichloride (SnPP; Tocris Bioscience, Bristol, UK) or the activator - cobalt (III) protoporphyrin IX chloride (CoPP; Enzo Life Sciences Inc.). After incubation cells were washed and resuspended in assay medium (RPMI 1640 with 0.5% BSA). Subsequently, all cells were aliquoted at a density of 1 × 106 cells in 100 μl into 5-μm (for murine BM-MNCs and human CD34+ cells) and 1 × 105 cells in 100 μl into 8-μm (for human cell lines) polycarbonate membrane inserts in a Costar Transwell 24-well plate (Costar Corning, Cambridge, MA, USA) for 3 h of chemotaxis at 37°C. Medium with 0.5% BSA (650 μl/well) containing either no chemoattractant (negative control), SDF-1 (100 ng/ml), or S1P (0.1 μM) was added to the lower chambers of the plate. After 3 h of incubation, the cells from the lower chambers were collected. The number of human line cells and murine BM-derived cells were scored by FACS (Becton Dickinson, Franklin Lakes, NJ, USA). Briefly, the cells were gated according to their forward scatter (FSC) and side scatter (SSC) parameters and counted during a 30-s acquisition at a high flow rate. After chemotaxis murine and human PB- or UCB-derived cells from the lower chamber were resuspended in human methylcellulose base medium provided by the manufacturer (R&D Systems, Minneapolis, MN, USA), supplemented withmurine and human GM-CSF (25 ng/ml) and IL-3 (10 ng/ml), respectively, for determining the number of CFU-GM colonies. Cultures were incubated for 7 and 14 days (murine and human cells respectively) (37°C, 95% humidity, and 5% CO2), at which time they were scored under an inverted microscope for the number of colonies9.
Fibronectin adhesion assay
Murine BM-MNCs and human UCB or PB CD34+ cells (5×104/100 μl) were made quiescent for 3 hours with RPMI 1640 medium with 0.5% BSA and incubated with different doses of SnPP or CoPP for 1 h and then washed by centrifugation and suspended in RPMI 1640 medium. Raji, K562, Jurkat, and Nalm6 cell lines (1×104/100 μl) were made quiescent overnight in RPMI 1640 medium with 0.5% BSA. Subsequently, cell suspensions were added for 15 min at 37°C directly to 96-well plates coated before the experiment with fibronectin (10 μg/ml), incubated overnight at 4°C, and then blocked with medium with 0.5% BSA for 2 h. Non-adherent cells were then washed from the wells, and all adherent cells were counted using an inverted microscope23.
Toxicity assays
Murine BM-MNCs and human CD34+ cells (1 × 106/dish) were exposed to HO-1 inhibitor SnPP (10–75 μM) and murine cells also to HO-1 stimulator CoPP (30–90 μM) for 1 hour. Thereafter, cells were resuspended in human methylcellulose base medium provided by the manufacturer (R&D Systems, Inc.). To evaluate the number of clonogenic progenitor cells, BM-MNCs were supplemented with 25 ng/ml recombinant murine or human granulocyte/macrophage colony-stimulating factor (GM-CSF) and 10 ng/ml recombinant interleukin 3 (IL-3; Millipore, Billerica, MA, USA) for stimulating CFU-GM colonies and with erythropoietin (EPO, 5 unit/ml, Stem Cell Tech, Vancouver, BC, Canada) plus stem cell factor (SCF, 5 ng/ml) for stimulating burst-forming units (BFU-E). Cultures were incubated for 7 (murine) and 14 (human) days (37°C, 95% humidity, and 5% CO2), at which time they were scored under an inverted microscope for the number of colonies, as previously described9.
Short-term homing experiments
Mice were irradiated with a lethal dose of γ-irradiation (1000 cGy). After 24 h, the animals were transplanted (by tail vein injection) with 3 × 106 BM cells from GFP+ mice previously incubated with 50 μM of SnPP for 1 h or medium only (control group). At 24 h after transplant, BM cells from the femurs were isolated via Ficoll-Paque and divided. Some cells (5 × 105) were incubated with Vybrant® DyeCycle™ Ruby stain (Invitrogen, Carlsbad, CA, USA) and analyzed by FACS. The rest of the cells were plated in serum-free methylcellulose cultures and stimulated to grow CFU-GM colonies with mGM-CSF (25 ng/ml) and mIL-3 (10 ng/ml). After 7 days of incubation (37°C, 95% humidity, and 5% CO2), the number of colonies was scored under an inverted microscope22.
Evaluation of engraftment
For engraftment experiments, mice were irradiated with a lethal dose of γ-irradiation (1000 cGy). After 24 h, mice were transplanted by tail vein injection with 1.5 × 105 BM cells from WT mice previously incubated with 50 μM SnPP or medium alone. Femora of transplanted mice were flushed with phosphate-buffered saline (PBS) on day 12 post-transplant. Purified via Ficoll-Paque, BM cells were plated in serum-free methylcellulose cultures and stimulated to grow CFU-GM colonies with mGM-CSF (25 ng/ml) and IL-3 (10 ng/ml). After 7 days of incubation (37°C, 95% humidity, and 5% CO2) the number of colonies was scored under an inverted microscope. Spleens were also removed, fixed in Telesyniczky’s solution for CFU-S assays, and the colonies counted on the surface of the spleen22,24.
Recovery of leukocytes and platelets
For transplantation experiments, mice were irradiated with a lethal dose of γ-irradiation (1000 cGy). After 24 h, the mice were transplanted by tail vein injection with 2.5 × 105 BM cells incubated with 50 μM SnPP or medium alone. Transplanted mice were bled at various intervals from the retro-orbital plexus to obtain samples for white blood cell and platelet counts. Fifty microliters of PB were taken from the retro-orbital plexus of the mice into EDTA-coated Microvette tubes (Sarstedt Inc., Newton, NC, USA) and run within 2 h of collection on a HemaVet 950FS hematology analyzer (Drew Scientific Inc., Oxford, CT, USA) as described24.
Statistical analysis
All results are presented as mean ± SD. Statistical analysis of the data was done using Student’s t-test for unpaired samples (Excel, Microsoft, Redmond, WA, USA) with p ≤ 0.05 considered significant.
Results
Upregulation of HO-1 in established hematopoietic cell lines impairs their chemotactic response to SDF-1 and S1P gradients and enhances cell adhesion
To address the effect of HO-1 on migration and adhesion of hematopoietic cells, we established three human hematopoietic cell lines in which HO-1 had been overexpressed after transducing cells with an HO-1-encoding vector. Figure 1A shows that HO-1 was upregulated, as assessed by western blotting and real-time PCR, in Raji, K562, and Jurkat cell lines. This HO-1 overexpression was correlated with significant inhibition of the migration of these cells in response to SDF-1 and S1P gradients (Fig. 1C) as well as enhanced adhesion to fibronectin-coated plates (Fig. 1B).
Figure 1. Impact of HO-1 upregulation on chemotaxis and adhesion of human hematopoietic cell lines (K562, Raji and Jurkat).
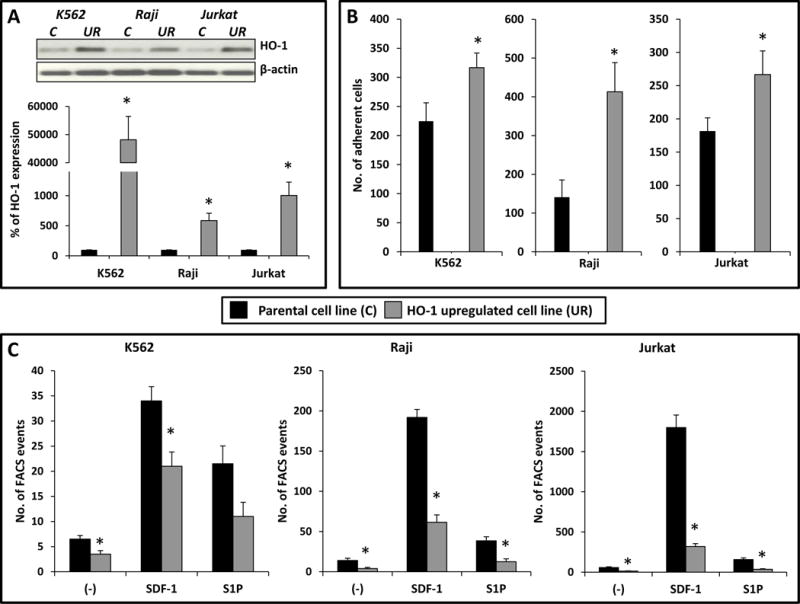
(A, top). Western blot detection of HO-1 expression levels in hematopoietic cell lines engineered for HO-1 overexpression. The same membranes were reprobed with β-actin to confirm equal loading of total protein. Legend: C- control, UR – upregulation. (A, bottom). HO-1 expression was evaluated at the mRNA level by real-time PCR. Results from three independent experiments are pooled together. *p ≤ 0.005. (B) Fibronectin adhesion assay for HO-1 overexpressing cell lines. The number of adherent cells is indicated, and data from three separate experiments are pooled together. *p ≤ 0.01. (C) The chemotactic responsiveness of HO-1-overexpressing cells to medium alone (−) and to stromal-derived factor 1 (SDF-1) or sphingosine-1-phosphate (S1P) gradients compared with migration of control parental cells. Results are combined from three independent experiments. *p ≤ 0.05.
Downregulation of HO-1 in established hematopoietic cell lines increases their chemotactic response to SDF-1 and S1P gradients and impairs cell adhesion
Next, we selected two cell lines with relatively high HO-1 activity and successfully downregulated HO-1 expression by employing a shRNA strategy (Fig. 2A). We found that downregulation of HO-1 in these cells was correlated with increased chemotactic responsiveness to SDF-1 and S1P gradients (Fig. 2C) and decreased adhesion to fibronectin- coated plates (Fig. 2B).
Figure 2. Impact of HO-1 downregulation on chemotaxis and adhesion of hematopoietic cell lines (Raji and Nalm6).
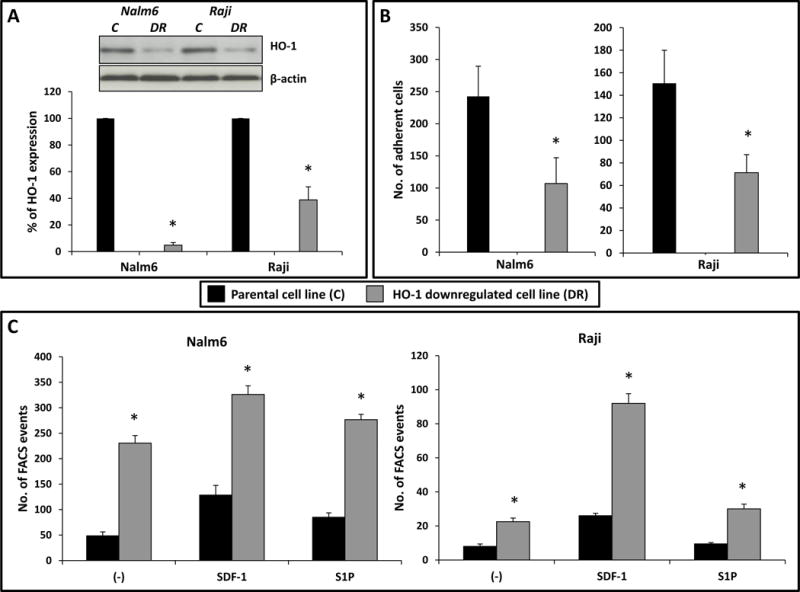
(A, top) Western blot detection of HO-1 expression levels in hematopoietic cell lines with downregulated HO-1. The membranes were reprobed with β-actin to confirm equal loading of total protein. Legend: C- control, DR – downregulation. (A, bottom) HO-1 expression at the mRNA level according to real-time PCR. Data from three independent experiments are pooled together. *p ≤ 0.005. (B) Adhesion to fibronectin of cell lines in which HO-1 had been downregulated. The number of adherent cells is indicated, and data from three separate experiments are pooled together. *p ≤ 0.01. (C) The chemotactic responsiveness of cells with downregulated HO-1 to medium alone (−) and to stromal-derived factor 1 (SDF-1) or sphingosine-1-phosphate (S1P) gradients in comparison with parental control cells. Results are combined from three independent experiments. *p ≤ 0.05
Downregulation of HO-1 in murine bone marrow mononuclear cells by small-molecule inhibitors of HO-1 increased their homing responses to SDF-1 and S1P gradients and accelerated their in vivo engraftment
Next, we employed a small-molecule inhibitor of HO-1 (SnPP) to downregulate HO-1 activity in BM-MNCs (Fig. 3). Our in vitro toxicity study (data not shown – available upon request) revealed that SnPP, in the doses employed in our study, is not toxic to BM hematopoietic clonogenic progenitors.
Figure 3. The influence of a small-molecule HO-1 inhibitor (SnPP) on chemotaxis and adhesion of murine BM-MNCs and on in vivo homing and engraftment of HSPCs.
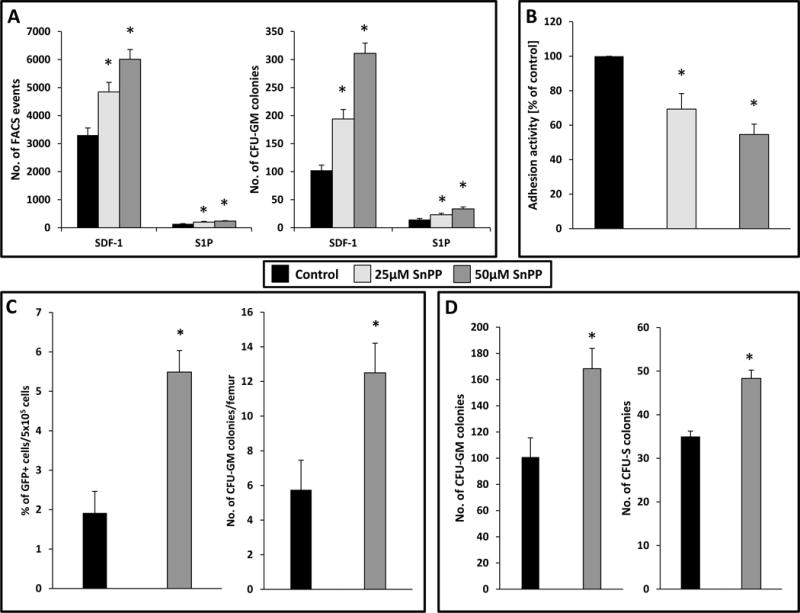
(A) The chemotactic responsiveness to SDF-1 and S1P gradients of murine BM-MNCs (evaluated by FACS) and clonogenic CFU-GM progenitors exposed to two different dosages of HO-1 inhibitor. Results are combined from three independent experiments.*p ≥ 0.05. (B) The effect of HO-1 inhibition by SnPP on adhesion of murine BM-MNCs to fibronectin. The results are shown as the percentage relative to control cells unexposed to SnPP (control). Data from four separate experiments are pooled together. *p ≤ 0.01. (C) Increase in homing of HO-1 inhibited HSPCs from GFP+ to the BM of normal wild type (WT) animals. Lethally irradiated WT mice (six animals per group) were transplanted with 3 × 106 GFP+ BM-MNCs pre-incubated with 50 μM SnPP for 1 h. Twenty-four hours after transplantation, femoral BMMNCs were harvested, the number of GFP+ cells in murine BM was evaluated by FACS (left), and the number of clonogenic GFP+ CFU-GM progenitors was enumerated in an in vitro colony assay (right). Both results were compared with the numbers obtained for WT mice transplanted with GFP+ BMMNCs untreated with SnPP. No colonies were formed in lethally irradiated and non-transplanted mice (irradiation control). The data in both panels represent the combined results from two independent experiments (n = 12). *p ≤ 0.05. (D) Defect in short-term engraftment of HSPCs exposed to SnPP in BM. Lethally irradiated mice (six mice per group) were transplanted with 1.5 × 105 BM-MNCs from WT mice incubated with medium alone (control) or SnPP (50 μM) for 1 h. Twelve days after transplantation, femoral BMMNCs were harvested and plated in in vitro cultures to enumerate the number of CFU-GM progenitors (left), and spleens were removed to count the number of CFU-S colonies (right). The data in both panels represent the combined results from two independent experiments (n = 12). *p ≤ 0.05.
We found that downregulation of HO-1 activity by SnPP in murine BM-MNCs enhanced chemotaxis of these cells in response to SDF-1 and S1P gradients (Fig. 3A, left panel). More importantly, it also increased the chemotactic responsiveness of in vitro clonogenic CFU-GM progenitors (Fig. 3A, right panel). At the same time, as we observed for established hematopoietic cells lines (Fig. 2B), we found that inhibition of HO-1 decreased adhesion of these cells to fibronectin-coated plates (Fig. 3B). In control experiments, we enhanced HO-1 activity in murine BM-MNCs by employing non-toxic doses of the HO-1 activator CoPP (data not shown – available upon request) and found the opposite effect on migration of murine BM-MNCs and clonogenic CFU-GM progenitors in response to SDF-1 and S1P gradients (data not shown – available upon request) and on adhesion of these cells to fibronectin-coated plates (data not shown – available upon request). Importantly, in kinetic inhibition studies we found that the effect of SnPP on enhanced migration of HSPCs is apparent for 6–9 hours, which exceeds the time of decreased adhesion of these cells, since HSPCs regain normal adhesiveness to fibronectin after 3–6 hours (data not shown – available upon request).
Next, we performed homing studies employing GFP+ cells that were exposed to SnPP and these cells were subsequently transplanted into lethally irradiated WT animals. Twenty-four hours after transplantation, we observed an increased number of GFP+ transplanted cells in which HO-1 was inhibited by SnPP as well as an increased number of CFU-GM progenitors that were able to grow GFP+ colonies after isolation from BM (Fig. 3C).
To evaluate the engraftment of BM-MNCs exposed to an HO-1 inhibitor, we first transplanted BM-MNCs into lethally irradiated mice and at 12 days after transplantation evaluated the number of CFU-GM progenitors that, after isolation form BM, were able to form in vitro colonies as well as the number of day-12 CFU-S colonies (Fig. 3D). Again, we observed a statistically significant beneficial effect of HO-1 inhibition on short-term engraftment.
Finally, to assess the effect of HO-1 inhibition by SnPP, we transplanted lethally irradiated mice with BM-MNCs exposed or unexposed to SnPP and evaluated the kinetics of leukocyte (Fig. 4A) and platelet (Fig. 4B) recovery in these animals. We found that mice transplanted with BM-MNCs in which HO-1 was inhibited by SnPP had significantly accelerated recovery of leukocyte and platelet counts in peripheral blood.
Figure 4. Accelerated recovery of peripheral blood counts in mice transplanted with BM-MNCs exposed to SnPP.
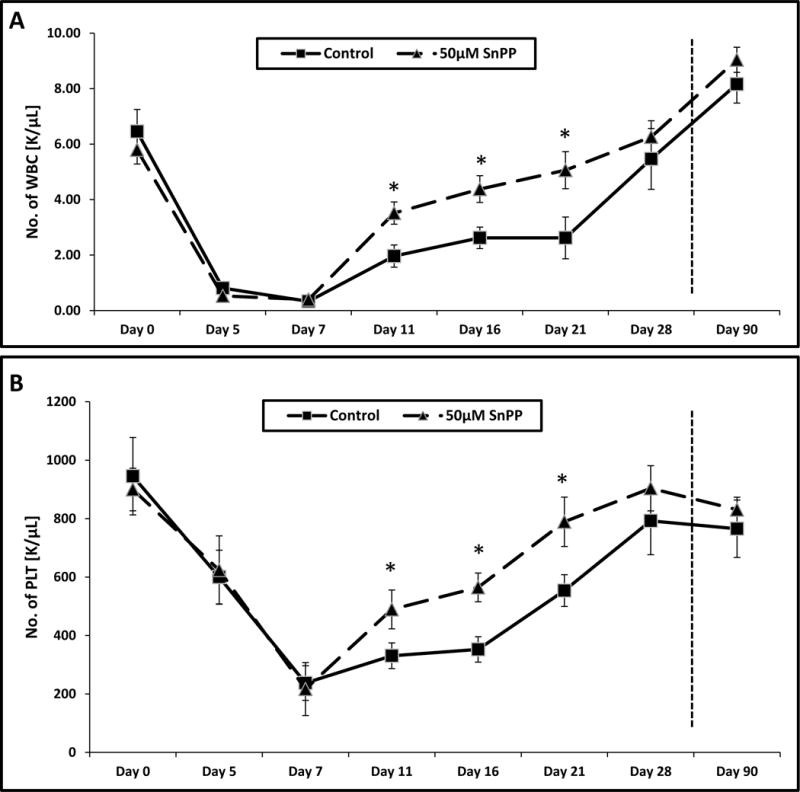
Lethally irradiated mice were transplanted with 2.5 × 105 BM-MNCs incubated with 50 μM SnPP. White blood cells (A) and platelets (B) were counted at intervals (at 0, 5, 7, 11, 16, 21, 28 and 90 days after transplantation). Results are combined from two independent experiments (six mice per group, n = 12). *p ≤ 0.05.
Downregulation of HO-1 in human peripheral blood umbilical cord blood (UCB)- and (PB)-derived cells by small-molecule inhibitors of HO-1 increases their migration in response to SDF-1 and S1P gradients
Based on encouraging data with murine BM-MNCs, we exposed human UCB- and PB-derived MNCs to the small-molecule HO-1 inhibitor SnPP and evaluated the responsiveness of these cells to SDF-1 and S1P homing gradients. As in murine cells, inhibition of HO-1 in human UCB and PB MNCs resulted in enhanced migration (Fig. 5A and B left panels) and decreased adhesion to fibronectin-coated plates (Fig. 5A and B right panels).
Figure 5. SnPP, a small-molecule inhibitor of HO-1, enhances chemotaxis and decreases adhesion of human peripheral blood (PB)- and umbilical cord blood (UCB)-derived cells.
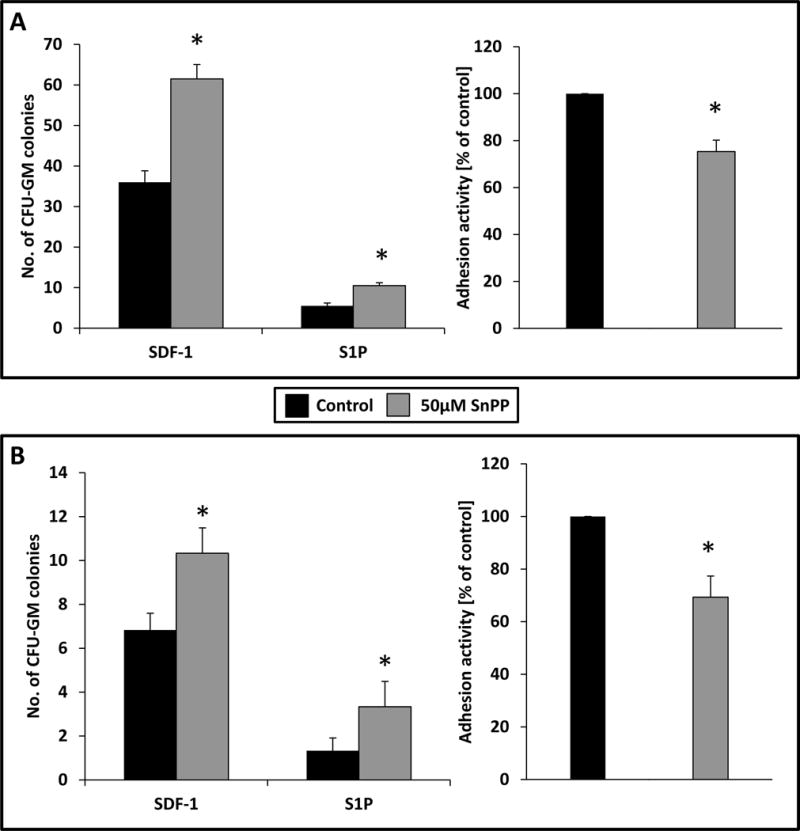
(A and B, left) - effect of HO-1 inhibition by SnPP on the chemotactic responsiveness of UCB- (A) and PB-derived (B) CFU-GM progenitors to SDF-1 and S1P gradients compared with cells exposed to medium alone (control). Results are evaluated by FACS and are combined from two independent experiments.*p ≥ 0.05. (A and B, right) Adhesion of UCB- (A) and PB-derived (B) cells to fibronectin. The results are shown as the percentage relative to control cells unexposed to SnPP. Results from two separate experiments are pooled together. *p ≤ 0.01.
Discussion
The most salient observation of this work is that HO-1 is a negative regulator of cell migration and adhesion for human and murine, normal and malignant hematopoietic cells. This observation has important implications for increasing the responsiveness of HSPCs to BM homing gradients and thus for developing better homing strategies for HSPCs after transplantation by employing small-molecule inhibitors of HO-1.
Homing of HSPCs is a process initiated by their chemotactic responsiveness. After their infusion into PB, HSPCs respond to BM-secreted chemoattractants and lodge in the hematopoietic microenvironment of BM2,5,6,13. Thus, by definition, the homing process precedes the subsequent expansion and engraftment of transplanted HSPCs. It is known that not all HSPCs infused into recipients home to BM, and in fact only ~10–20% find their way to BM niches, with the remainder of the cells “trapped” in lung, liver, and other organs25. Hence, it is obvious that by increasing the homing or seeding efficiency of HSPCs in the BM microenvironment, one could improve clinical outcomes for hematopoietic transplants, as better homing efficiency would accelerate engraftment and recovery of PB blood cell counts. Optimal seeding efficiency and homing of HSPCs is particularly important in UCB transplants and in poor mobilizers, in both of which cases the number of HSPCs may be below the threshold required for successful transplantation.
There are only a few chemoattractants for HSPCs involved in the BM homing process that have been identified so far. The most important is SDF-11,2,3,4,5,6,7; however, recent evidence indicates that an SDF-1 chemotactic gradient is supported by gradients of bioactive phosphosphingolipids (S1P, C1P)7 and certain extracellular nucleotides, such as UTP and ATP26,27.
Significant effort is currently being directed at increasing the responsiveness of HSPCs to SDF-1 gradients. Specifically, since the SDF-1 interaction with CXCR4+ HSPCs is attenuated by the dipeptidyl peptidase CD26, inhibition of the CD26 exposed on HSPCs has been proposed to enhance the chemotactic responsiveness to SDF-1 gradients28,29, and this strategy is currently being clinically tested in UCB transplant recipients. Another interesting strategy being tested in animal models is based on the modification of certain adhesion molecules on HSPCs by ex vivo treatment with fucosyltransferase, which increases the level of fucosylation or glycoengineering of these receptors and may improve HSPC homing to BM30,31. In parallel, evidence has accumulated that the chemotactic responsiveness of HSPCs to an SDF-1 homing gradient can be modulated by ex vivo exposure of the cells to certain small-molecule priming factors that enhance SDF-1 signaling through CXCR4 receptors on the cell surface14,15,16. These priming factors are part of the innate immune response and include C3a and desArgC3a cleavage fragments of the C3 component of the complement cascade and the antimicrobial cationic peptides cathelicidin (LL-37 fragment) and β2-defensin. Homing can also be enhanced by exposure of HSPCs ex vivo to PGE218 or hyaluronic acid32. Finally, it was recently reported that homing and engraftment can be accelerated by ex vivo inhibition of glycogen synthase kinase-3β (GSK-3β) in HSPCs by employing a small-molecule inhibitor33,34.
Based on evidence from the literature that HO-1 deficiency accelerates engraftment of HSPCs, we considered a novel strategy to transiently downregulate ex vivo HO-1 activity in HSPCs before transplantation by employing the small-molecule HO-1 inhibitor SnPP. Such a strategy would avoid the consequences of long-term downregulation of HO-1 seen in mice transplanted with HO-1 KO cells, which resulted in a disrupted response to acute stress after transplantation19. This transient inhibition of HO-1 activity is important, since, as in mice, it has been reported that inborn HO-1 deficiency in a human patient resulted in several complications, such as growth retardation, anemia, leukocytosis, thrombocytosis, coagulation defects, hyperlipidemia, development of amyloidosis, and premature death20. This is at least partially explained by the lack of an anti-inflammatory effect of HO-1, particularly because it is a negative regulator of activation of the complement cascade17,22,35.
We demonstrate here that, by taking advantage of the possibility of short-term inhibition of HO-1 activity in murine HSPCs, we can accelerate migration of these cells in response to SDF-1 and S1P homing gradients, which results in accelerated hematopoietic recovery from transplant. Since, in parallel experiments, human UCB-derived MNCs after inhibition of HO-1 responded more robustly to SDF-1 and S1P gradients, we propose that a similar strategy could be employed to increase homing efficiency of these cells in patients, in particular, when the number of UCBs to be transplanted is low, and an increase in seeding efficiency of these cells to BM would improve clinical outcomes.
These investigations with normal murine and human HSPCs are strongly supported by experiments that we performed using human cell lines in which HO-1 had been permanently downregulated or overexpressed. In all these cell lines, a decrease in HO-1 expression resulted in improved migration in response to SDF-1 and S1P, and, vice versa; overexpression resulted in defective responsiveness to gradients of both chemoattractants.
One important observation from our studies is that HO-1 deficiency not only affects migration, but also adhesion of HSPCs. This is an important question to address, since cells after homing/lodging to the BM microenvironment need to subsequently adhere to their specific niches and engraft. This, however, should not be a concern, as our experiments clearly show that, despite initially decreased adhesion to fibronectin-coated plates, transplanted BMNNCs exposed ex vivo to SnPP efficiently engraft and accelerate recovery of transplanted animals. This could be explained by the fact that HO-1 was only transiently inhibited and HSPCs recover from such treatment and regain their normal adhesive properties after homing to BM. In fact, we found that the chemotactic responsiveness of HSPCs after exposure to SnPP was enhanced for a longer period than the period of decreased adhesive properties of these cells. What is also very important, we provide evidence that SnPP is nontoxic against hematopoietic clonogenic progenitors, which supports the concept that it could be employed in a clinical setting to treat cells in the hematopoietic graft ex vivo.
In conclusion, we provide evidence that HO-1 is a negative regulator of cell migration and adhesion, and transient inhibition of HO-1 activity in HSPCs enhances their homing to BM and accelerates hematopoietic recovery after transplantation. Thus, we propose that this relatively simple strategy of ex vivo exposure of the graft to an HO-1 inhibitor may find practical application in clinical settings, particularly in UCB transplantation in which the number of HSPCs in the UCB unit is limited36,37.
Acknowledgments
This work was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and Maestro grant 2011/02/A/NZ4/00035 to MZR.
Footnotes
Conflicts of interest.
None.
References
- 1.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–67. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 2000;72:408–11. [PubMed] [Google Scholar]
- 4.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27:24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 6.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–92. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29:776–82. doi: 10.1038/leu.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Physiological recirculation of hematopoietic stem and progenitor cells through blood, lymph and extramedullary tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-Plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–85. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seitz G, Boehmler AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann NY Acad Sci. 2005;1044:84–9. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–16. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arana L, Gangoiti P, Ouro A, Trueba M, Gámez-Mũoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ, Ratajczak J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–62. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–90. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, Ratajczak J, Laughlin MJ, Ratajczak MZ. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26:736–45. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao YA, Wagers AJ, Karsunky H, Zhao H, Reeves R, Wong RJ, Stevenson DK, Weissman IL, Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood. 2008;112:4494–502. doi: 10.1182/blood-2007-12-127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–30. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 21.Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, Cunha FQ. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharmacol. 2006;149:345–54. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wysoczynski M, Ratajczak J, Pedziwiatr D, Rokosh G, Bolli R, Ratajczak MZ. Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev. 2015;11:110–8. doi: 10.1007/s12015-014-9547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Borkowska S, Mierzejewska K, Kucia M, Mendek-Czajkowska E, Suszynska M, Sharma VA, Deptala A, Song W, Platzbecker U, Larratt L, Janowska-Wieczorek A, Maciejewski J, Ratajczak J. Further evidence that paroxysmal nocturnal haemoglobinuria is a disorder of defective cell membrane lipid rafts. J Cell Mol Med. 2015;19:2193–201. doi: 10.1111/jcmm.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells reveals a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–61. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castello S, Podestà M, Menditto VG, Ibatici A, Pitto A, Figari O, Scarpati D, Magrassi L, Bacigalupo A, Piaggio G, Frassoni F. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp Hematol. 2004;32:782–7. doi: 10.1016/j.exphem.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Di Virgilio F. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–70. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 27.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Di Virgilio F, Baccarani M, Lemoli RM. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–42. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 28.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 29.Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–74. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers J, Huang Y, Wei L, Yan Q, Huang A, Zhou L. Fucose-deficient hematopoietic stem cells have decreased self-renewal and aberrant marrow niche occupancy. Transfusion. 2010;50:2660–9. doi: 10.1111/j.1537-2995.2010.02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sackstein R. Glycoengineering of HCELL, the human bone marrow homing receptor: sweetly programming cell migration. Ann Biomed Eng. 2012;40:766–76. doi: 10.1007/s10439-011-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 33.Dolnikov A, Xu N, Shen S, Song E, Holmes T, Klamer G, O’Brien TA. GSK-3β inhibition promotes early engraftment of ex vivo-expanded haematopoietic stem cells. Cell Prolif. 2014;47:113–23. doi: 10.1111/cpr.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goichberg P, Kalinkovich A, Borodovsky N, Tesio M, Petit I, Nagler A, Hardan I, Lapidot T. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–9. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 35.Kinderlerer AR, Pombo Gregoire I, Hamdulay SS, Ali F, Steinberg R, Silva G, Ali N, Wang B, Haskard DO, Soares MP, Mason JC. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood. 2009;113:1598–607. doi: 10.1182/blood-2008-04-152934. [DOI] [PubMed] [Google Scholar]
- 36.Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation—from niche to bedside. Nat Rev Clin Oncol. 2015;12:163–74. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43:498–513. doi: 10.1016/j.exphem.2015.04.011. [DOI] [PubMed] [Google Scholar]


