The current study provides new evidence that 25 or 50 mmHg of lower-body negative pressure reduces jugular venous pooling and intracranial pressure during simulated microgravity. Therefore, spaceflight countermeasures that sequester fluid to the lower body may mitigate cephalic venous congestion and vision impairment.
Keywords: spaceflight, tympanic membrane displacement, venous congestion, vision
Abstract
Long-term spaceflight induces a near visual acuity change in ~50% of astronauts. In some crew members, postflight cerebrospinal fluid (CSF) opening pressures by lumbar puncture are as high as 20.9 mmHg; these members demonstrated optic disc edema. CSF communicates through the cochlear aqueduct to affect perilymphatic pressure and tympanic membrane motion. We hypothesized that 50 mmHg of lower-body negative pressure (LBNP) during 15° head-down tilt (HDT) would mitigate elevations in internal jugular vein cross-sectional area (IJV CSA) and intracranial pressure (ICP). Fifteen healthy adult volunteers were positioned in sitting (5 min), supine (5 min), 15° HDT (5 min), and 15° HDT with LBNP (10 min) postures for data collection. Evoked tympanic membrane displacements (TMD) quantified ICP noninvasively. IJV CSA was measured using standard ultrasound techniques. ICP and IJV CSA increased significantly from the seated upright to the 15° HDT posture (P < 0.05), and LBNP mitigated these increases. LBNP at 25 mmHg reduced ICP during HDT (TMD of 322.13 ± 419.17 nl) to 232.38 ± 445.85 nl, and at 50 mmHg ICP was reduced further to TMD of 199.76 ± 429.69 nl. In addition, 50 mmHg LBNP significantly reduced IJV CSA (1.50 ± 0.33 cm2) during 15° HDT to 0.83 ± 0.42 cm2. LBNP counteracts the headward fluid shift elevation of ICP and IJV CSA experienced during microgravity as simulated by15° HDT. These data provide quantitative evidence that LBNP shifts cephalic fluid to the lower body, reducing IJV CSA and ICP.
NEW & NOTEWORTHY The current study provides new evidence that 25 or 50 mmHg of lower body negative pressure reduces jugular venous pooling and intracranial pressure during simulated microgravity. Therefore, spaceflight countermeasures that sequester fluid to the lower body may mitigate cephalic venous congestion and vision impairment.
long-duration spaceflight induces ophthalmic structural and/or functional changes in 35–50% of astronauts flying long-duration missions on the International Space Station (ISS). Vision impairment more frequently occurs on long-duration 6-mo missions than on short-duration 2-mo missions on the ISS, suggesting that mission duration is a contributing factor to the initiation and/or degree of impairment (12). Mader and coworkers, in a case series, document that seven astronauts had, some or combination of, decreased near vision, globe flattening, optic disc edema, choroidal folds, or retinal nerve fiber layer thickening after long-duration (~6 mo) ISS missions (20). In addition, lumbar punctures (in the lateral decubitus posture) performed on some of these ISS astronauts diagnosed with optic disc edema demonstrated moderately elevated opening pressure (15.4–20.9 mmHg, measured 12–60 days postflight) (20). Cranial compartment factors (24), such as venous volume (30), cerebrospinal fluid (CSF) volume (24), and brain parenchyma edema (6), can affect intracranial pressure (ICP). Currently the leading hypothesis holds that these structural and functional ocular adaptations that develop during or after spaceflight are caused by a chronic headward fluid shift during spaceflight (37). The change in hydrostatic pressure related to body position results in an increase of ICP by ~10 mmHg from the sitting to supine position. In addition, sitting-to-supine hydrostatic pressure changes affect intraocular pressure (IOP) by amounts on the order of 3–4 mmHg, and increase internal jugular vein (IJV) area by 66 mm2 (15). Typically we spend two-thirds of our daily life in the upright posture, and these venous and CSF compartments are adapted to maintain pressure and volumes within physiological limits in a gravitational environment. Cephalic venous pressure changes can affect ICP (9, 29). The few studies that report central venous pressure (CVP) during spaceflight suggest that CVP is near or below the preflight 1-g supine level (7, 13). However, IJV distention occurs during spaceflight, relative to dimensions on Earth in the supine posture (1). Therefore, it remains unclear whether cephalic venous pressures during spaceflight are elevated above supine levels in a 1-G environment.
Head-down tilt (HDT) results in elevated ICP. For example, the ICP of a monkey positioned at 6° HDT increases by 1.5 mmHg (17), and the ICP of a human positioned at 9° HDT increases by 5.3 mmHg (11), relative to supine (19). Another recent study provided further evidence of increased ICP in HDT with elevations of ICP by 4.9 and 10.6 mmHg in 10° and 20°, respectively, relative to supine (28). Skull pulsations, a noninvasive measure of ICP dynamics, increase by ~20% from the supine to 15° HDT posture (34).
Few established noninvasive or nonpharmacological strategies exist that reduce ICP. Lower-body negative pressure (LBNP) can cause fluid to shift away from central circulation (8). In addition, recent data suggest that 25 mmHg LBNP reduce cranial pulsations by 38% relative to the 15° HDT posture. However, it is unclear whether LBNP reduces measures of ICP and neck venous pressure during HDT, and how these relative changes compare with values in the sitting and supine posture (19). Moreover, it is unclear whether LBNP levels >25 mmHg during simulated HDT microgravity further reduce ICP and IJV cross-sectional area (CSA). Therefore, the purpose of this study was to examine whether LBNP at 50 mmHg would significantly decrease ICP at 15° HDT relative to 15° HDT with LBNP at 25 mmHg, and to 15° HDT, supine, and sitting postures. We hypothesize that 50 mmHg of LBNP during 15° HDT would maximally mitigate elevations in IJV CSA and ICP.
METHODS
Healthy adult volunteers, seven women and eight men (ages 19–60 yr), participated in this study. The Institutional Review Board at the University of California San Diego reviewed and approved all methods before the study was initiated. Subjects were notified of the study protocol and any associated risks, and subjects’ written informed consent was obtained before they participated in the study.
The subject’s body was positioned on a tilt table with waist and lower extremities inside the LBNP chamber, which has been described previously (19, 35). To maintain a negative pressure seal, a neoprene skirt was placed around the subject’s waist at the level of the iliac crest. Tilt angles in this study included supine (0°) and 15° HDT (HDT), and the LBNP conditions tested were HDT with −25 mmHg pressure [HDT + LBNP(25)] and HDT with −50 mmHg pressure [HDT + LBNP(50)]. To avoid confounding effects of order, the HDT and HDT + LBNP conditions were conducted in a balanced order. The 25- and 50-mmHg LBNP sessions occurred on two different days. Volunteers were positioned in the seated, supine, or HDT posture for a 5-min stabilization period, followed by data collection. LBNP was applied for 10 min followed by data collection.
Subjects’ ears were examined with an otoscope to verify the absence of ear canal blockage and/or any perforation or inflammation of the eardrum. Additionally, tympanometry (Amplivox Otowave 102) was performed to measure the auditory stimulus intensity required to elicit the lowest level of stimulus that produces a response. The same ear was used within a subject for all tympanometry and tympanic membrane displacement (TMD) measurement conditions. All subjects’ stapedius muscles contracted at 100 dB or less, indicating a normal healthy response (33). The TMD device produced tones that caused the middle ear’s stapedius muscle to contract, which resulted in tympanic membrane displacement and subsequently a measurable pneumatic volume change (Vm). The magnitude and direction of the TMD correlates with ICP magnitude (32). For example, low ICP levels will produce a laterally displaced tympanic membrane, and high ICP will produce a medial TMD (22, 23). Murthy and coworkers demonstrated a graded TMD response to posture change, from sitting to 15° HDT (26). The TMD device (Cerebral and Cochlear Fluid Pressure Analyzer) generated data used to quantify TMD volume displacements at each posture. Twenty TMD waveforms were collected at each posture and averaged for statistical analysis. Calculated ICP values were estimated using previously published direct measures of ICP in the sitting and supine posture (11). These seated and supine ICP values were used for a two-point calibration of TMD values, which yielded calculated ICP values for the seated and supine posture. ICP values were estimated based on the hydrostatic pressure column of the subject height at 15° HDT, therefore yielding estimated values for the HDT ± LBNP postures. IJV CSA was measured with an ultrasound device (Logiq 9; GE Medical Systems, Milwaukee, WI). The same operator measured the right IJV CSA at the level just caudal to the bifurcation of the common carotid artery on subjects’ necks. Doppler flow was used to distinguish the internal carotid artery from the IJV. Blood pressure and heart rate were measured with an automated blood pressure cuff over the brachial artery after the 5-min stabilization period (Microlife, Dunedin, FL).
Statistical analysis.
ICP, IJV CSA, blood pressure, and heart rate were analyzed using a repeated-measures ANOVA. Sitting ICP and IJV CSA data before and after HDT and HDT + LBNP were not significantly different, and thus were averaged for subsequent analysis. Significant differences were not detected between sessions 1 and 2 of seated, supine, or HDT conditions, and therefore these data were combined for statistical analysis. Similarly, supine ICP and IJV CSA values were not statistically different and were therefore averaged for analysis. Mean arterial blood pressure was calculated as one-third of the difference between the systolic and diastolic pressure plus diastolic pressure. Data are given as means ± SD. Statistical significance was set to P < 0.05.
RESULTS
Simulated microgravity significantly increased IJV CSA, relative to sitting and supine postures (P < 0.05). The IJV CSA significantly increased from sitting (0.13 ± 0.07 cm2) to supine (0.90 ± 0.39 cm2) posture (Fig. 1). IJV CSA was further increased during HDT to 1.50 ± 0.33 cm2. However, LBNP of 25 mmHg decreased IJV CSA to 1.18 ± 0.49 cm2, and it further decreased to 0.83 ± 0.42 cm2 (P = 0.003) with 50 mmHg LBNP during simulated HDT microgravity (Figs. 1 and 2). Relative to HDT posture, 10 min of 50-mmHg LBNP exposure during simulated microgravity decreased IJV CSA by 45%. IJV CSA observed during 25 and 50 mmHg LBNP was not significantly different from supine values.
Fig. 1.
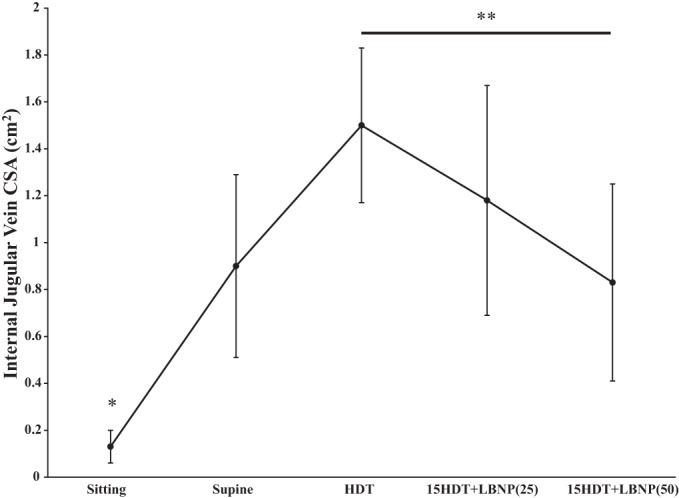
Internal jugular vein cross-sectional area (IJV CSA) during sitting, supine, head-down tilt (HDT), and HDT + lower-body negative pressure (LNBP) at −25 [LBNP(25)] and −50 [LBNP(50)] mmHg conditions. IJV CSA decreased significantly with LBNP (50 mmHg) relative to HDT with no LBNP. IJV CSA at sitting posture was significantly different from all other test conditions. 15HDT, 15° head-down tilt. **IJV CSA decreased significantly with LBNP (50 mmHg) relative to HDT with no LBNP. *IJV CSA at sitting posture was significantly different from all other test conditions.
Fig. 2.
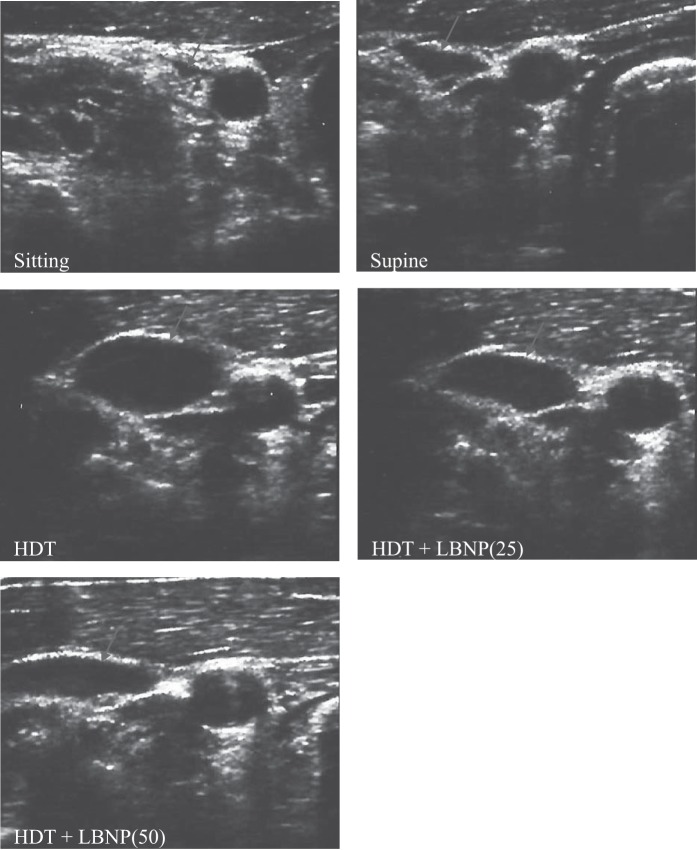
Representative IJV ultrasound images during sitting, supine, HDT, and HDT + LNBP at −25 and −50 mmHg conditions. These images demonstrate visually the progressive increase in IJV CSA (arrows) from sitting to supine to HDT posture. The application of LBNP reduced IJV CSA.
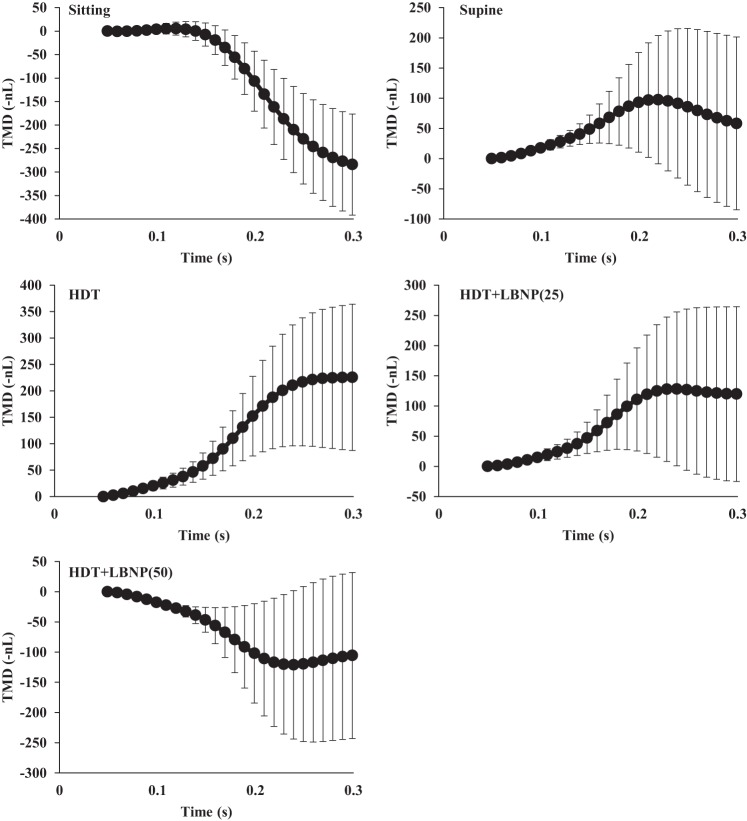
The direction of the TMD is inversely related to the ICP level. Therefore, for graphical clarity the noninvasive ICP TMD is expressed as –nl (−1 × nl). TMD, a noninvasive measure of ICP, indicated that ICP significantly increased, relative to the sitting condition, when the subject transitioned to the supine and HDT postures (P < 0.05). Both 25 and 50 mmHg LBNP significantly decreased TMD from HDT (322.13 ± 419.17 nl), to 232.38 ± 445.85 nl (P = 0.003) and 199.76 ± 429.69 nl (P = 0.005), respectively (Fig. 3). There was no significant difference in TMD between the supine, HDT + LBNP(25), and HDT + LBNP(50) conditions (Fig. 3). TMD decreased 40% after 10 min of 50 mmHg LBNP relative to HDT posture. Waveforms of the tympanic displacement were averaged across all subjects to graphically display the direction of change during sitting, supine, HDT, and LNBP at −25 and −50 mmHg conditions (Fig. 4). The sitting TMD moves in the outward lateral direction, after tone stimulation, indicating a low ICP. The supine and HDT postures caused the TMD to move in the inward medial direction, suggesting elevated ICP. Application of 25 and 50 mmHg LBNP caused the TMD to move outward, in the opposite direction of HDT values, suggesting LBNP reduced ICP. In addition, the change in TMD observed during simulated microgravity (HDT) was 38% of the average change from the seated to supine posture. Typical ICP values are 10 mmHg in the supine posture and 0 mmHg in the sitting posture (11), and the calculated ICP using TMD values during HDT is 24 ± 21 mmHg (Fig. 5). Furthermore, the calculated reductions in ICP by 25 and 50 mmHg LBNP are 12 and 14 mmHg, respectively. Heart rate was highest during the sitting posture, and blood pressure was elevated during HDT + LBNP(50); however, these variables were not significantly different across all conditions (Table 1).
Fig. 3.
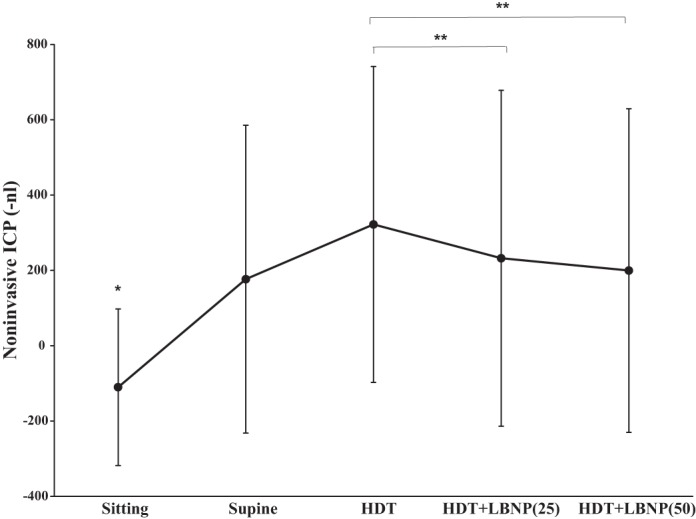
Tympanic membrane displacement (TMD) during sitting, supine, HDT, and HDT + LBNP at −25 and −50 mmHg conditions. The direction of the TMD is inversely related to the intracranial pressure (ICP) level. Therefore, for graphical clarity the noninvasive ICP TMD is expressed as –nl (−1 × nl). Noninvasive ICP significantly increased from sitting to supine to HDT posture. However, application of 25 and 50 mmHg LBNP significantly reduced noninvasive TMD measures of ICP. TMD in the sitting posture was significantly different from all other postures/tilt angles. **However, application of 25 and 50 mmHg LBNP significantly reduced noninvasive TMD measures of ICP. *TMD in the sitting posture was significantly different from all other postures/tilt angles.
Fig. 4.
TMD waveforms generated by the CCFP Analyzer were averaged across all subjects during sitting, supine, HDT, and HDT + LNBP at −25 and −50 mmHg conditions. The sitting TMD moves in the outward direction, after tone stimulation and indicating a low ICP. The supine and HDT conditions caused the TMD to move in the inward direction, and suggesting elevated ICP. Application of 25 and 50 mmHg LBNP during HDT caused the TMD to move outward, relative to HDT, suggesting that LBNP reduced ICP.
Fig. 5.
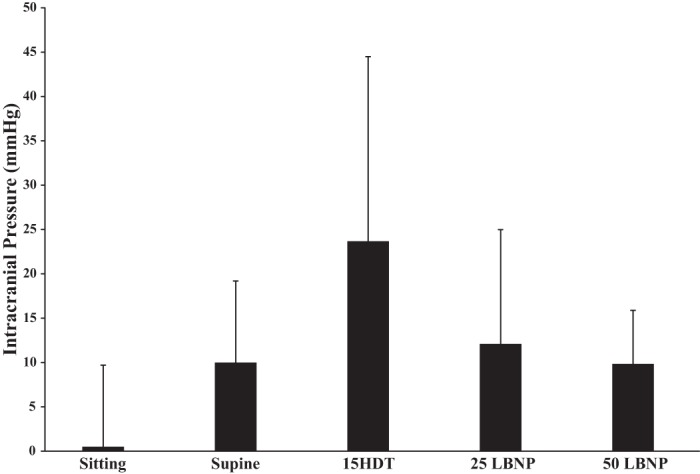
Calculated ICP values at sitting, supine, HDT, and HDT + LNBP at −25 and −50 mmHg conditions based on known values of directly measured ICP at supine posture.
Table 1.
Blood pressure and heart rate during sitting, supine, HDT, HDT with LBNP at −25 mmHg, and LBNP at −50 mmHg conditions
| Sitting | Supine | HDT | HDT + LBNP(25) | HDT + LBNP(50) | |
|---|---|---|---|---|---|
| Blood pressure, mmHg* | 87 ± 20 | 84 ± 20 | 82 ± 19 | 76 ± 28 | 85 ± 11 |
| Heart rate, beats/min | 68 ± 8 | 59 ± 8 | 59 ± 9 | 61 ± 6 | 74 ± 10 |
Values are means ± SD. HDT, head-down tilt; LBNP, lower-body negative pressure; HDT + LBNP(25), HDT with −25 mmHg pressure; HDT + LBNP(50), HDT with −50 mmHg pressure.
Reported as mean arterial pressure. Blood pressure and heart rate did not change significantly across all test conditions.
DISCUSSION
The results of this study support our hypothesis that exposure to LBNP(25 and 50 mmHg) mitigates the increase in IJV CSA and ICP caused by 15° HDT simulated microgravity. After 10 min of 50 mmHg LBNP during HDT, IJV CSA and ICP values were similar to those observed during supine posture. The current study provides new evidence that 25–50 mmHg LBNP reduces cephalic venous congestion and ICP during simulated microgravity.
The present data support our hypothesis that LBNP reduces IJV CSA during HDT. LBNP of 25 mmHg produced lower IJV CSA, but only 50 mmHg LBNP produced significant decreases in IJV CSA. IJV CSA has been shown to increase during 5° HDT (1.06 cm2) and 10° HDT (1.16 cm2) relative to supine posture (0.98 cm2) (3). Evidence exists that, during prolonged spaceflight, cephalic venous engorgement occurs (1). In addition, IJV engorgement occurs early during spaceflight and continues throughout a 6-mo mission (1, 2). Our study produced similar measurements, with a 44.5% decrease in IJV CSA during HDT + LBNP(50) exposure relative to 15° HDT, suggesting LBNP may lower ICP through decreasing venous engorgement of the head and neck.
Postflight CSF opening pressure in the lateral decubitus posture is moderately elevated (20) (15.4–20.9 mmHg vs. 12 mmHg for a normal healthy individual on Earth in supine posture) (11). Acute exposures to microgravity produce ICP measurements (determined by invasive measures) that are greater than those in upright posture, but not greater than those in supine posture (18). However, it remains unclear whether ICP is chronically elevated during long-duration spaceflight above levels typically observed on Earth in upright posture.
Tympanic membrane displacement is a unique technique to estimate relative changes in ICP. Quantification of TMD relies on computing the difference between the resting and contracted position of the tympanic membrane upon elicitation of the acoustic stapedial reflex. This technology requires normal function of middle- and inner-ear anatomy and absence of ear canal blockage. TMD measurements with Vm values less than −200 nl are associated with direct ICP measurements of patients with an ICP range of 23–40 mmHg (32). A Vm value of 530 nl correlates with a direct ICP measurement of 3.8 mmHg (32). In addition, TMD measurements were significantly different before and after shunting in patients within the age range of those in the present investigation (31). The present data follow a similar trend showing increasing ICP from the sitting to supine posture, as reported previously (26, 32). Moreover, 25 mmHg LBNP during HDT significantly reduced noninvasive measures of ICP. This noninvasive ICP measurement during HDT + LBNP(50), however, was not significantly different from measures during HDT + LBNP(25). This lack of further reduction in ICP with higher levels of applied LBNP suggests that a maximal fluid sequestration effect may occur. However, direct ICP and venous pressure measures at several LBNP levels will be necessary to characterize a dose-response curve. It is not possible to remove the hydrostatic pressure effects of gravity in this HDT model of microgravity. One limitation of this technology is that it does not allow production of a continuous real-time waveform; ICP waveform data often can provide important ICP compliance status. However, this technique does provide a noninvasive quantitative “snapshot” measure of ICP. Moreover, this technique provided reproducible data within 1 min.
Impairment of venous outflow may be a contributing factor to spaceflight-induced vision impairment. The documented spaceflight-induced vision changes may result from a fluid redistribution that alters transmural pressures of ocular and cerebral tissue. The Starling–Landis fluid pressure factors that regulate fluid shifts between blood and interstitial fluid are affected by the absence of gravity. In microgravity, the loss of tissue weight also reduces tissue fluid pressure and thus increases transcapillary filtration, tissue edema, and potentially CSF pressure at the optic nerve head. During simulated microgravity, transcapillary filtration increases in upper regions of the body because capillary blood pressure increases, causing facial edema (27). Furthermore, because elevated venous pressure inhibits the normal absorption of CSF in the cerebral venous sinuses, ICP may subsequently increase (16). It is well documented that LBNP causes venous pooling in the legs; for example, an acute exposure to 50 mmHg LBNP reduces the circulating blood volume by 500–1,000 ml and increases calf volume by 4% (14, 25, 26). The findings of the current study provide evidence for the efficacy of LBNP in reducing ICP, potentially by lowering postcapillary venous volume and pressure in the head and neck during simulated microgravity. Therefore, the translaminar pressure difference may be altered during spaceflight primarily by chronic moderately elevated ICP, unbalanced by an elevated IOP.
Long-term spaceflight and the resulting increase in ICP can cause structural adaptations of the eye, including optic disc edema (21). Additionally, translaminar pressure difference (IOP-ICP) is hypothesized to maintain normal tissue structure and function of the optic nerve entering the retina (4, 5). Increasing hydrostatic pressure at the eye by moving from sitting to HDT increases IOP (36). Macias and coworkers demonstrated decreased ICP waveforms (and IOP) after 10 min of 25 mmHg LBNP during HDT simulated microgravity (19). The mechanism resulting in the observed reduction of IOP by LBNP likely was due to a reduction in venous pooling at the choroid (19). The present IJV CSA data support LBNP as a modality to reduce cephalic venous volume at the level of the neck, thus providing additional evidence that LBNP may modulate choroid blood volume and affect IOP. Upon exposure to microgravity and elimination of hydrostatic gradients, an imbalance in the translaminar pressure difference may occur (37). Limited IOP data collected during spaceflight suggest that an acute increase in IOP occurs early during spaceflight and resolves over time (10). A fluid shift countermeasure such as LBNP may mitigate this cephalic venous congestion, and therefore reduce ICP during spaceflight. However, it remains to be determined whether cephalic venous congestion contributes to spaceflight-induced vision change.
In conclusion, the headward fluid shifts as evidenced by increased IJV CSA and elevated indicators of noninvasive ICP during HDT were normalized to supine levels after 10 min of 25 or 50 mmHg LBNP. Therefore, spaceflight countermeasures that sequester fluid to the lower body may mitigate cephalic venous congestion and vision impairment.
GRANTS
This study was supported by the National Space Biomedical Research Institute through NCC 9–58 to B. R. Macias, National Aeronautics and Space Administration Grant NNXAJ12G to A. R. Hargens, National Heart, Lung, and Blood Institute Grant 5 T35-HL-7491-35 to W. Watkins, an American Physiological Society Frontiers in Physiology Teacher Professional Development Fellowship to S. Seidl, and an American Physiological Society Undergraduate Summer Research Fellowship to E. M. Clary.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W., S.S., E.M.C., and B.R.M. performed experiments; W.W. and B.R.M. analyzed data; W.W., A.R.H., and B.R.M. interpreted results of experiments; W.W. and B.R.M. prepared figures; W.W. and B.R.M. drafted manuscript; W.W., A.R.H., and B.R.M. edited and revised manuscript; W.W., A.R.H., and B.R.M. approved final version of manuscript; A.R.H. and B.R.M. conceived and designed research.
REFERENCES
- 1.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol 86: 157–168, 2001. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- 2.Arbeille P, Provost R, Zuj K, Vincent N. Measurements of jugular, portal, femoral, and calf vein cross-sectional area for the assessment of venous blood redistribution with long duration spaceflight (vessel imaging experiment). Eur J Appl Physiol 115: 2099–2106, 2015. doi: 10.1007/s00421-015-3189-6. [DOI] [PubMed] [Google Scholar]
- 3.Beddy P, Geoghegan T, Ramesh N, Buckley O, O’Brien J, Colville J, Torreggiani WC. Valsalva and gravitational variability of the internal jugular vein and common femoral vein: ultrasound assessment. Eur J Radiol 58: 307–309, 2006. doi: 10.1016/j.ejrad.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115: 763–768, 2008. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci 49: 5412–5418, 2008. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betz AL, Iannotti F, Hoff JT. Brain edema: a classification based on blood-brain barrier integrity. Cerebrovasc Brain Metab Rev 1: 133–154, 1989. [PubMed] [Google Scholar]
- 7.Buckey JC Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW Jr, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol (1985) 81: 19–25, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Charles JB, Lathers CM. Summary of lower body negative pressure experiments during space flight. J Clin Pharmacol 34: 571–583, 1994. doi: 10.1002/j.1552-4604.1994.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 9.Davson H, Domer FR, Hollingsworth JR. The mechanism of drainage of the cerebrospinal fluid. Brain 96: 329–336, 1973. doi: 10.1093/brain/96.2.329. [DOI] [PubMed] [Google Scholar]
- 10.Draeger J, Schwartz R, Groenhoff S, Stern C. Self-tonometry under microgravity conditions. Clin Investig 71: 700–703, 1993. doi: 10.1007/BF00209723. [DOI] [PubMed] [Google Scholar]
- 11.Eklund A, Jóhannesson G, Johansson E, Holmlund P, Qvarlander S, Ambarki K, Wåhlin A, Koskinen L-OD, Malm J. The pressure difference between eye and brain changes with posture. Ann Neurol 80: 269–276, 2016. doi: 10.1002/ana.24713. [DOI] [PubMed] [Google Scholar]
- 12.Fogarty JA, Otto C, Kerstman E, Oubre C, Wu J. The Visual Impairment Intracranial Pressure Summit Report. Houston, TX: National Aeronautics and Space Administration, 2011. [Google Scholar]
- 13.Foldager N, Andersen TA, Jessen FB, Ellegaard P, Stadeager C, Videbaek R, Norsk P. Central venous pressure in humans during microgravity. J Appl Physiol (1985) 81: 408–412, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Foux A, Seliktar R, Valero A. Effects of lower body negative pressure (LBNP) on the distribution of body fluids. J Appl Physiol 41: 719–726, 1976. [DOI] [PubMed] [Google Scholar]
- 15.Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol 560: 317–327, 2004. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 46: 198–202, 1996. doi: 10.1212/WNL.46.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Keil LC, McKeever KH, Skidmore MG, Hines J, Severs WB. The effect of head-down tilt and water immersion on intracranial pressure in nonhuman primates. Aviat Space Environ Med 63: 181–185, 1992. [PubMed] [Google Scholar]
- 18.Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Levine BD. Effect of gravity and microgravity on intracranial pressure. J Physiol 595: 2115–2127, 2017. doi: 10.1113/JP273557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias BR, Liu JHK, Grande-Gutierrez N, Hargens AR. Intraocular and intracranial pressures during head-down tilt with lower body negative pressure. Aerosp Med Hum Perform 86: 3–7, 2015. doi: 10.3357/AMHP.4044.2015. [DOI] [PubMed] [Google Scholar]
- 20.Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118: 2058–2069, 2011. doi: 10.1016/j.ophtha.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Mader TH, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen H-C, Dervay JP, Barratt MR, Tarver WJ, Sargsyan AE, Kramer LA, Riascos R, Bedi DG, Pettit DR. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol 33: 249–255, 2013. doi: 10.1097/WNO.0b013e31829b41a6. [DOI] [PubMed] [Google Scholar]
- 22.Marchbanks RJ. A Study of Tympanic Membrane Displacement (PhD thesis). Uxbridge, UK: Brunel University, 1980. http://bura.brunel.ac.uk/handle/2438/6373. [Google Scholar]
- 23.Marchbanks RJ, Reid A, Martin AM, Brightwell AP, Bateman D. The effect of raised intracranial pressure on intracochlear fluid pressure: three case studies. Br J Audiol 21: 127–130, 1987. doi: 10.3109/03005368709077785. [DOI] [PubMed] [Google Scholar]
- 24.Marmarou A, Shulman K, LaMorgese J. A compartmental analysis of compliance and outflow resistance and the effects of elevated blood pressure. In: Intracranial Pressure II, edited by Lundberg N, Pontén U, Brock M. Berlin, Germany: Springer, p. 86–88, 1975. doi: 10.1007/978-3-642-66086-3_18. [DOI] [Google Scholar]
- 25.Murray RH, Thompson LJ, Bowers JA, Albright CD. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am Heart J 76: 799–811, 1968. doi: 10.1016/0002-8703(68)90266-4. [DOI] [PubMed] [Google Scholar]
- 26.Murthy G, Marchbanks RJ, Watenpaugh DE, Meyer JU, Eliashberg N, Hargens AR. Increased intracranial pressure in humans during simulated microgravity. Physiologist 35, Suppl: S184–S185, 1992. [PubMed] [Google Scholar]
- 27.Parazynski SE, Hargens AR, Tucker B, Aratow M, Styf J, Crenshaw A. Transcapillary fluid shifts in tissues of the head and neck during and after simulated microgravity. J Appl Physiol (1985) 71: 2469–2475, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Petersen LG, Petersen JCG, Andresen M, Secher NH, Juhler M. Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol 310: R100–R104, 2016. doi: 10.1152/ajpregu.00302.2015. [DOI] [PubMed] [Google Scholar]
- 29.Qvarlander S, Sundström N, Malm J, Eklund A. Postural effects on intracranial pressure: modeling and clinical evaluation. J Appl Physiol (1985) 115: 1474–1480, 2013. doi: 10.1152/japplphysiol.00711.2013. [DOI] [PubMed] [Google Scholar]
- 30.Raisis JE, Kindt GW, McGillicuddy JE, Giannotta SL. The effects of primary elevation of cerebral venous pressure on cerebral hemodynamics and intracranial pressure. J Surg Res 26: 101–107, 1979. doi: 10.1016/0022-4804(79)90085-4. [DOI] [PubMed] [Google Scholar]
- 31.Reid A, Marchbanks RJ, Bateman DE, Martin AM, Brightwell AP, Pickard JD. Mean intracranial pressure monitoring by a non-invasive audiological technique: a pilot study. J Neurol Neurosurg Psychiatry 52: 610–612, 1989. doi: 10.1136/jnnp.52.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel M, Burge DM, Marchbanks RJ. Tympanic membrane displacement testing in regular assessment of intracranial pressure in eight children with shunted hydrocephalus. J Neurosurg 88: 983–995, 1998. doi: 10.3171/jns.1998.88.6.0983. [DOI] [PubMed] [Google Scholar]
- 33.Samuel M, Burge DM, Marchbanks RJ. Quantitative assessment of intracranial pressure by the tympanic membrane displacement audiometric technique in children with shunted hydrocephalus. Eur J Pediatr Surg 8: 200–207, 1998. doi: 10.1055/s-2008-1071154. [DOI] [PubMed] [Google Scholar]
- 34.Ueno T, Ballard RE, Macias BR, Yost WT, Hargens AR. Cranial diameter pulsations measured by non-invasive ultrasound decrease with tilt. Aviat Space Environ Med 74: 882–885, 2003. [PubMed] [Google Scholar]
- 35.Watenpaugh DE, O’Leary DD, Schneider SM, Lee SMC, Macias BR, Tanaka K, Hughson RL, Hargens AR. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol (1985) 103: 1964–1972, 2007. doi: 10.1152/japplphysiol.00132.2007. [DOI] [PubMed] [Google Scholar]
- 36.Weinreb RN, Cook J, Friberg TR. Effect of inverted body position on intraocular pressure. Am J Ophthalmol 98: 784–787, 1984. doi: 10.1016/0002-9394(84)90698-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L-F, Hargens AR. Intraocular/intracranial pressure mismatch hypothesis for visual impairment syndrome in space. Aviat Space Environ Med 85: 78–80, 2014. doi: 10.3357/ASEM.3789.2014. [DOI] [PubMed] [Google Scholar]