In this laboratory study, patients with peripheral artery disease performed plantar flexion exercise in the supine posture until symptoms of claudication occurred. Relative to age- and sex-matched healthy subjects we found that patients had a higher blood pressure response, a higher heart rate response, and a greater reduction in skeletal muscle oxygenation as determined by near-infrared spectroscopy. Our data suggest that muscle ischemia contributes to the augmented exercise pressor reflex in peripheral artery disease.
Keywords: exercise physiology, heart rate, sympathetic nervous system, muscle metabolism
Abstract
Peripheral artery disease (PAD) is an atherosclerotic vascular disease that affects 200 million people worldwide. Although PAD primarily affects large arteries, it is also associated with microvascular dysfunction, an exaggerated blood pressure (BP) response to exercise, and high cardiovascular mortality. We hypothesized that fatiguing plantar flexion exercise that evokes claudication elicits a greater reduction in skeletal muscle oxygenation (SmO2) and a higher rise in BP in PAD compared with age-matched healthy subjects, but low-intensity steady-state plantar flexion elicits similar responses between groups. In the first experiment, eight patients with PAD and eight healthy controls performed fatiguing plantar flexion exercise (from 0.5 to 7 kg for up to 14 min). In the second experiment, seven patients with PAD and seven healthy controls performed low-intensity plantar flexion exercise (2.0 kg for 14 min). BP, heart rate (HR), and SmO2 were measured continuously using near-infrared spectroscopy (NIRS). SmO2 is the ratio of oxygenated hemoglobin to total hemoglobin, expressed as a percent. At fatigue, patients with PAD had a greater increase in mean arterial BP (18 ± 2 vs. vs. 10 ± 2 mmHg, P = 0.029) and HR (14 ± 2 vs. 6 ± 2 beats/min, P = 0.033) and a greater reduction in SmO2 (−54 ± 10 vs. −12 ± 4%, P = 0.001). However, both groups had similar physiological responses to low-intensity, nonpainful plantar flexion exercise. These data suggest that patients with PAD have altered oxygen uptake and/or utilization during fatiguing exercise coincident with an augmented BP response.
NEW & NOTEWORTHY In this laboratory study, patients with peripheral artery disease performed plantar flexion exercise in the supine posture until symptoms of claudication occurred. Relative to age- and sex-matched healthy subjects we found that patients had a higher blood pressure response, a higher heart rate response, and a greater reduction in skeletal muscle oxygenation as determined by near-infrared spectroscopy. Our data suggest that muscle ischemia contributes to the augmented exercise pressor reflex in peripheral artery disease.
peripheral artery disease (PAD) is an atherosclerotic vascular disease that affects 200 million people worldwide (24, 52). Individuals with PAD have stenoses in one or more conduit arteries supplying their legs, which significantly impair the rise in blood flow and oxygen delivery to their leg muscles when they walk. However, leg blood flow impairments do not correlate well with exercise capacity or symptoms even after surgical revascularization procedures that restore flow or exercise training programs that improve walking tolerance (9, 22, 23, 35, 46, 47). Emerging evidence suggests that microvascular and/or mitochondrial abnormalities within the leg muscles partly explain the impaired walking ability of these patients (23, 24, 53, 57). Recent studies from our laboratory (48, 49, 59) and others (2, 37) indicate that the blood pressure (BP) response to exercise is augmented in PAD; heightened BP responses are predictive of mortality in PAD (12, 13) and cardiovascular morbidity in other at-risk populations (33, 34, 42). Understanding physiological mechanisms that contribute to impaired walking, morbidity, and mortality in PAD are imperative for improving treatment.
Assessment of muscle oxygen saturation (SmO2) with near-infrared spectroscopy (NIRS) has increasingly been used to evaluate exercise intolerance and potential interventions for PAD (6, 16, 18, 19, 40). Numerous reports show that NIRS-derived measures of gastrocnemius SmO2 decrease prematurely and to a significantly greater extent in patients with PAD during graded treadmill walking compared with healthy age-matched controls (3, 4, 14, 31, 41, 44). Larger reductions in gastrocnemius SmO2 during graded exercise in individuals with PAD have been attributed to local hemodynamic limitations in the ischemic muscle (32, 58), but systemic BP must also be considered when interpreting NIRS data. Surprisingly, no studies have examined the link between exercise-induced changes in SmO2 and BP in PAD. The influence of BP and exercise intensity on muscle oxygenation is important in PAD because higher BP responses to dynamic exercise are linked with increased risk of mortality (12, 13). Furthermore, many patients with PAD chronically take medications to lower their BP and it is possible that low BP during exercise could prevent adequate muscle perfusion. It is also important to note that the molecular mechanisms of pain and fatigue in ischemic muscle, which are targets of many experimental therapies in PAD (8, 51), are likely influenced by BP and muscle oxygenation (30).
The purpose of the present investigation was to characterize the intensity- and time-dependent relationships between exercise BP and muscle oxygen desaturation in PAD. We used supine dynamic plantar flexion exercise to standardize the mechanical work performed by each subject and to enhance our ability to track changes in medial gastrocnemius deoxygenation alongside changes in systemic BP. We hypothesized that fatiguing plantar flexion exercise that evokes claudication elicits a greater reduction in SmO2 (i.e., greater deoxygenation of the active muscle) and a higher rise in BP in PAD compared with age-matched healthy subjects. Second, we hypothesized that low-intensity steady-state plantar flexion elicits similar NIRS and BP responses between groups.
METHODS
Ethical approval.
The experiments were approved by the Pennsylvania State University College of Medicine Institutional Review Board and all procedures conformed to guidelines stated in the Declaration of Helsinki. All procedures were verbally explained and voluntary, written informed consent was obtained from all participants before the study. All participants were recruited from the Penn State Milton S. Hershey Medical Center vascular outpatient clinic list or from our list of previously enrolled research participants.
Subjects and design.
These acute laboratory studies used a repeated-measures design, and group (PAD, healthy) served as a between-subjects factor. Eight patients (5 men and 3 women) with PAD (Fontaine stages I and II; symptomatic intermittent claudication) and eight healthy adults matched for age, sex, and body mass index participated in experiment 1 (Table 1). The sample size for experiment 1 was determined after the first four subjects had completed testing. Specifically, we determined that if the true difference between groups in the mean SmO2 signal from baseline to the end of exercise was 45% with a standard deviation of 25%, then we would need to study eight total subjects in each group (i.e., four additional subjects per group) to reject the null hypothesis with 90% power and α = 0.05. Seven patients (5 men and 2 women) and seven healthy adults participated in experiment 2 (Table 2). Five patients and five healthy adults completed the thigh cuff occlusion pilot study. Healthy volunteers were normotensive, nonobese, and nonsmokers who were recreationally active yet not competitive athletes. Exclusion criteria for healthy control subjects were a diagnosis of any acute or chronic medical condition or taking any prescribed medication. All participants abstained from exercise, caffeine, and alcohol for 24 h and refrained from food for at least 2 h preceding the study. Patients with PAD did not alter their medication routine (Tables 1 and 2) on the day of the study.
Table 1.
Demographics and baseline parameters in experiment 1
| Measurement Unit | PAD | PAD Range | Healthy | Healthy Range | P |
|---|---|---|---|---|---|
| Men/Women | 5/3 | 5/3 | |||
| Age, yr | 63 ± 2 | 51–74 | 62 ± 3 | 58–74 | 0.749 |
| Height, m | 1.67 ± 0.04 | 1.52–1.85 | 1.73 ± 0.03 | 1.60–1.83 | 0.187 |
| Weight, kg | 79.3 ± 5.3 | 59.0–102.1 | 74.8 ± 4.3 | 58.2–97.5 | 0.522 |
| Body mass index, kg/m2 | 28.5 ± 2.0 | 22.2–34.1 | 25.4 ± 0.8 | 22.7–29.1 | 0.114 |
| ABI least symptomatic | 0.83 ± 0.04* | 0.70–0.96 | 1.02 ± 0.04 | 0.91–1.24 | 0.005 |
| ABI most symptomatic | 0.60 ± 0.04* | 0.43–0.71 | 1.07 ± 0.04 | 0.92–1.23 | <0.001 |
| Systolic blood pressure, mmHg | 137 ± 8 | 109–164 | 119 ± 3 | 104–131 | 0.055 |
| Diastolic blood pressure, mmHg | 73 ± 2 | 65–80 | 75 ± 2 | 65–83 | 0.477 |
| Mean arterial pressure, mmHg | 97 ± 5 | 80–126 | 89 ± 2 | 77–96 | 0.170 |
| Heart rate, beats/min | 64 ± 4 | 49–78 | 56 ± 3 | 43–74 | 0.101 |
| ATT, mm | 5.63 ± 0.81 | 3.07–8.50 | 5.28 ± 0.51 | 3.22–6.79 | 0.719 |
| Calf circumference, cm | 33.8 ± 0.7 | 30.5–36.5 | 34.4 ± 0.3 | 33.0–35.5 | 0.564 |
| Lesion location | |||||
| Aortoiliac | 1 | ||||
| Femoropopliteal | 6 | ||||
| Infrapopliteal | 2 | ||||
| Multiple | 6 | ||||
| Medications | |||||
| Anticoagulants | 6 | ||||
| Antiaggregants | 7 | ||||
| Statins | 5 | ||||
| Antihypertensives | 8 | ||||
| β-Blockers | 3 | ||||
| Drugs labeled for PAD | 1 |
Values are means ± SE and also as min-max values.
ABI, ankle brachial index; ATT, adipose tissue thickness over medial gastrocnemius; PAD, peripheral artery disease.
P < 0.05 compared with healthy subjects were obtained with independent samples via t-test.
Table 2.
Demographics and baseline parameters in experiment 2
| Measurement Unit | PAD | PAD Range | Healthy | Healthy Range | P |
|---|---|---|---|---|---|
| Men/Women | 5/2 | 5/2 | |||
| Age, yr | 64 ± 3 | 52–74 | 64 ± 3 | 51–75 | 0.940 |
| Height, m | 1.69 ± 0.04 | 1.52–1.85 | 1.74 ± 0.04 | 1.60–1.82 | 0.407 |
| Weight, kg | 80.7 ± 5.2 | 62.8–102.0 | 75.5 ± 4.5 | 57.7–87.6 | 0.476 |
| Body mass index, kg/m2 | 28.2 ± 1.6 | 21.9–34.2 | 25.6 ± 1.0 | 22.5–29.5 | 0.226 |
| ABI least symptomatic | 0.87 ± 0.02* | 0.76–0.96 | 1.00 ± 0.04 | 0.91–1.16 | 0.019 |
| ABI most symptomatic | 0.69 ± 0.03* | 0.60–0.83 | 1.04 ± 0.04 | 0.92–1.23 | <0.001 |
| Systolic blood pressure, mmHg | 129 ± 7 | 111–168 | 115 ± 4 | 101–128 | 0.128 |
| Diastolic blood pressure, mmHg | 75 ± 3 | 68–91 | 75 ± 3 | 64–83 | 0.688 |
| Mean arterial pressure, mmHg | 92 ± 3 | 84–106 | 87 ± 3 | 76–94 | 0.250 |
| Heart rate, beats/min | 65 ± 4 | 53–78 | 63 ± 5 | 50–87 | 0.722 |
| ATT, mm | 4.42 ± 0.50 | 3.07–6.98 | 5.87 ± 0.44 | 4.08–6.79 | 0.055 |
| Calf circumference, cm | 33.4 ± 0.7 | 30.5–35.4 | 34.6 ± 1.0 | 32.0–39.0 | 0.334 |
| Lesion location | |||||
| Aortoiliac | 1 | ||||
| Femoropopliteal | 4 | ||||
| Infrapopliteal | 2 | ||||
| Mutiple | 4 | ||||
| Medications | |||||
| Anticoagulants | 3 | ||||
| Antiaggregants | 5 | ||||
| Statins | 3 | ||||
| Antihypertensives | 4 | ||||
| β-Blockers | 2 | ||||
| Drugs labeled for PAD | 0 |
Values are means ± SE and also as min-max values.
ABI, ankle brachial index; ATT, adipose tissue thickness over medial gastrocnemius; PAD, peripheral artery disease.
P < 0.05 compared with healthy subjects were obtained with independent samples via t-test.
Measurements.
For all experiments, participants were outfitted with a 3-lead electrocardiogram to measure heart rate (HR) (Cardiocap/5; GE Healthcare), a finger BP cuff (Finometer; FMS, The Netherlands), and an automated arm BP cuff (Phillips Sure Signs VS3). Local SmO2 was monitored noninvasively on the medial gastrocnemius using continuous-wave NIRS (Moxy muscle oxygen monitor; Fortiori Design, Hutchinson, MN). The NIRS device uses a Monte Carlo model to trace the propagation of photons through a tissue with an adipose tissue thickness up to ~12.0 mm. The mathematical model treats a medium (tissue) as four layers (epidermis, dermis, adipose, and muscle) by which the adipose tissue thickness is unknown. The use of NIRS in exercise physiology research has been previously reviewed (21, 65). Adipose tissue thickness was measured at the NIRS probe site using ultrasound imaging (iE33; Philips) to ensure proper signal depth was achieved (39, 60). Leg pain (0 = no pain, 5 = strong pain, 10 = most severe pain ever experienced) and rating of perceived exertion (6 = very, very light, 11 = light, 15 = hard, and 20 = maximum exertion) were obtained during each minute of exercise using the Borg scales (7). Additionally, subjects completed the walking impairment questionnaire. This pen-and-paper questionnaire quantifies a person’s functional capacity in terms of walking distance, walking speed, and ability to climb stairs (61). Scores are weighted differently depending on the difficulty of the task; scores range from 0 to 100, with lower scores indicating lower walking performance.
Thigh cuff occlusion pilot study.
In an effort to validate the Moxy NIRS device, a pilot study using a 5-min thigh cuff occlusion (rapidly inflated to 190–200 mmHg) was conducted to provoke limb ischemia and subsequent reactive hyperemia for 3 min. Subjects were situated in the supine posture with their most symptomatic leg slightly elevated. We performed these trials to validate our NIRS device compared with the published literature (14, 39, 60). For these studies, skin blood flow (Laser Doppler Perfusion; Moor Instruments, UK) was measured proximal and distal to the thigh cuff, in addition to HR, BP, and NIRS. Patients with stents located in the femoropopliteal region were excluded from these thigh cuff occlusion experiments because the stents were not designed to withstand large external forces. Time constant resaturation kinetics were measured at half-time and time to peak resaturation immediately following deflation of the thigh cuff (68).
Experiment 1: fatiguing plantar flexion exercise.
All subjects were situated in the supine posture and were outfitted with HR, BP, and NIRS. A wooden sandal was secured to the participant’s foot, connected to a fixed pulley system and attached to a load bucket at the head of the bed (48). The sandal has no stop point so that tension is applied at all times during exercise. Following a 3-min baseline period, patients underwent a 14-min bout of plantar flexion exercise with their most symptomatic leg (30 contractions/min). Weight was added progressively (0.5 kg/min) beginning with 0.5 kg during the first minute. Maximum load was 7.0 kg during the final minute. This exercise protocol is comparable to published reports (20, 56) and was designed to be sufficiently challenging as to elicit claudication symptoms; because of this we expected the patients with PAD to terminate exercise early either because of pain greater than 5/10 or because they could not maintain the cadence. Healthy subjects exercised with the left or right leg corresponding to the most symptomatic leg of each patient with PAD.
Experiment 2: low-intensity, steady-state plantar flexion exercise.
Based on the data obtained in experiment 1, we conducted experiment 2 to test our hypothesis that low-intensity, nonpainful, steady-state exercise would elicit similar physiological responses between patients with PAD and healthy subjects. For these trials, we used a different plantar flexion device that allows for a 1-s unweighted pause in between contractions. This device has been previously described by our group (50). To summarize, participants performed 20 contractions per minute with a 1-s pause in between contractions for a total of 14 min with weight fixed at 2 kg. This modality exercises the gastrocnemius and is known to raise popliteal blood flow in healthy subjects (50). We have previously confirmed that this protocol does not significantly reduce SmO2 or raise BP and HR in healthy men (50). Because of the low intensity and the rest period between contractions we speculated that this workload could be performed in patients with PAD without experiencing claudication.
Data collection and statistical analysis.
All continuous NIRS data were recorded electronically at 2 Hz and transmitted wirelessly via ANT+ to a laptop computer for offline analysis (PeriPedal v2.4.8; Napoleon, IN). HR and BP data were collected continuously at 200 Hz and analyzed offline (model 16SP Powerlab; ADInstruments, New Castle, Australia). Mean arterial pressure (MAP) was determined offline from manually tracing the Finometer waveform in Powerlab. Whereas other exercise studies have used systolic BP as the primary BP variable (12, 13), we used MAP because it most closely reflects the variable regulated by the sympathetic nervous system. Statistical analyses were performed using IBM SPSS 23.0 software, and graphics were produced using Prism 6 software (GraphPad, San Diego, CA). All exercise data were averaged in 20-s increments and were checked for normality with the Kolmogorov-Smirnov test. For experiment 1, repeated-measures ANOVAs (baseline, 0.5 kg, 1.0 kg, 1.5 kg, and fatigue) were conducted for the physiological variables, and if a significant interaction was observed, the Holm adjustment to the Bonferroni correction was implemented. Due to symptomatic leg pain in experiment 1, patients with PAD terminated exercise at lower workloads. The fatigue time point represents the final 20 s of the last workload performed by each patient with PAD. There is no specified fatigue time point for healthy controls because all but one completed the protocol without experiencing true fatigue. Therefore, data from the corresponding control subjects are presented at the same time points as their matched PAD counterparts. For experiment 2, repeated-measures ANOVAs (baseline and all 14 min of exercise) were conducted. Pain and rating of perceived exertion data were analyzed using the Mann-Whitney test. Data are presented as means ± SE unless otherwise stated and values of P < 0.05 were considered statistically significant. Cohen’s d (mean 1 − mean 2/combined standard deviation) was calculated on primary outcomes to determine effect size. Cohen’s d values > than 0.8 were considered “strong” effect sizes (10).
RESULTS
Thigh cuff occlusion pilot study.
As expected, 5 min of thigh cuff occlusion reduced distal skin blood flow in both patients with PAD (from 20 ± 10 to 0 au) and healthy subjects (from 28 ± 5 to 0 au). The NIRS SmO2 value also decreased both in patients with PAD (from 65 ± 6 to 8 ± 3%) and healthy subjects (from 76 ± 3 to 11 ± 5%). The percent reduction in SmO2 at the very end of occlusion was comparable between patients with PAD (−86 ± 7%) and healthy controls (−91 ± 6%, P = 0.680). Immediately after deflating the thigh cuff, there was an increase in distal skin blood flow in both groups but the values at 1 min of recovery were attenuated in PAD (from 0 to 35 ± 10 au) compared with healthy subjects (from 0 to 62 ± 37 au, P < 0.001). In a similar way, SmO2 values at 1 min were attenuated in patients with PAD (46 ± 18%) compared with healthy subjects (84 ± 3%, P = 0.026). The peak resaturation time was 139 ± 87 s in patients with PAD and 44 ± 22 s in healthy subjects, consistent with prior studies that used other NIRS devices (11, 44). Skin temperature before (32.5 ± 0.3°C), during (32.4 ± 0.4°C), and after (32.4 ± 0.4°C) cuff inflation was stable.
Experiment 1: fatiguing plantar flexion exercise.
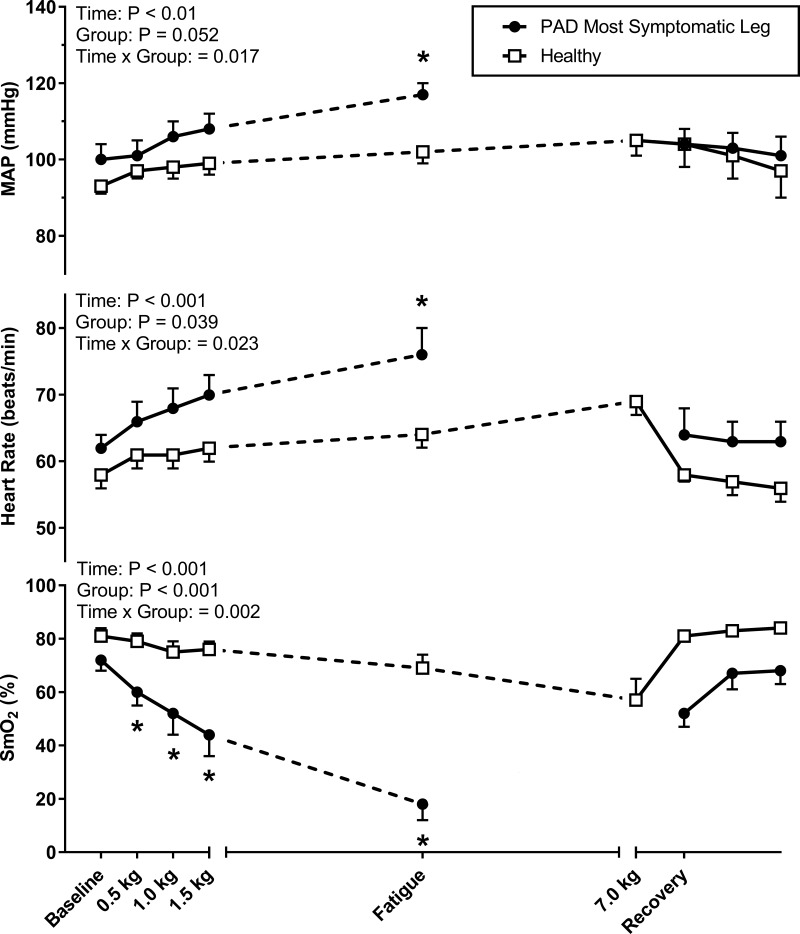
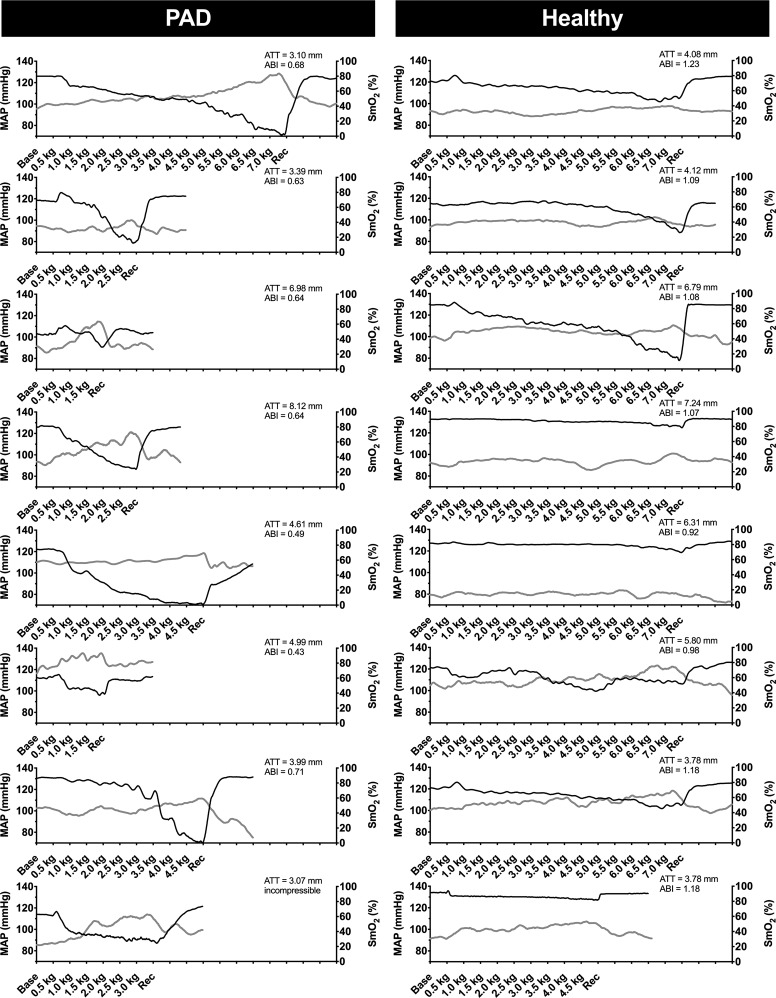
The patients with PAD in experiment 1 had a self-reported mean walking impairment score of 50 ± 9 (range, 16–87), whereas the healthy controls all scored 100 (which is the maximum). As shown in Fig. 1, exercise with the most symptomatic leg evoked an augmented increase in MAP in patients with PAD. At the end of exercise, MAP was significantly higher in patients with PAD (P = 0.02, Cohen’s d = 1.73). The increase in HR at end-exercise was also augmented in patients with PAD (P = 0.016, Cohen’s d = 1.11). Consistent with our hypothesis, SmO2 in the medial gastrocnemius was markedly reduced in patients with PAD, whereas little change in SmO2 was observed in healthy subjects. Indeed, when comparing the PAD SmO2 values at fatigue with the time-matched data from healthy subjects, there was an approximately threefold greater reduction in patients with PAD (P < 0.001, Cohen’s d = 3.07). In Fig. 2, the individual MAP and SmO2 time-course data are shown for the most affected leg. In all patients with PAD (Fig. 2, left) the highest MAP was associated with the lowest NIRS values, whereas no consistent relationship was observed in healthy subjects (Fig. 2, right). The median rating of perceived exertion at the end of exercise was 15 (range, 11–18) in patients with PAD and 15.5 (range, 9–19) in healthy subjects (P = 0.929). The median pain rating at the end of exercise was 5 (range, 1–6) in patients with PAD and 0 (range 0–0) in healthy subjects (P < 0.001).
Fig. 1.
Mean arterial pressure (MAP), heart rate (HR), and muscle oxygen saturation (SmO2) responses to single-leg plantar flexion exercise performed in the supine posture in experiment 1. Eight patients with peripheral artery disease (PAD, black circles) and eight healthy subjects matched for age, sex, and body mass index (white squares) exercised for a maximum of 14 min at 20 contractions per minute (0.5 kg to 7.0 kg) with the most symptomatic leg. The fatigue time point represents the last workload performed by each patient with PAD and the corresponding workload of each matched healthy subject. Seven healthy subjects and one patient with PAD completed the entire 14-min protocol (7.0 kg). Values are means ± SE with *P < 0.05 considered statistically significant.
Fig. 2.
Individual data from experiment 1. Mean arterial pressure (MAP, solid gray lines) and muscle oxygen saturation (SmO2, solid black lines) responses to fatiguing exercise in eight patients with peripheral artery disease (PAD) and eight healthy subjects. Patients with PAD underwent single-leg dynamic plantar flexion exercise in the supine posture until fatigue or a maximum of 14 min at 20 contractions per minute (0.5 kg to 7.0 kg) with the most symptomatic leg. ABI, ankle brachial index; ATT, adipose tissue thickness over the medial gastrocnemius.
Experiment 2: low-intensity, steady-state plantar flexion exercise.
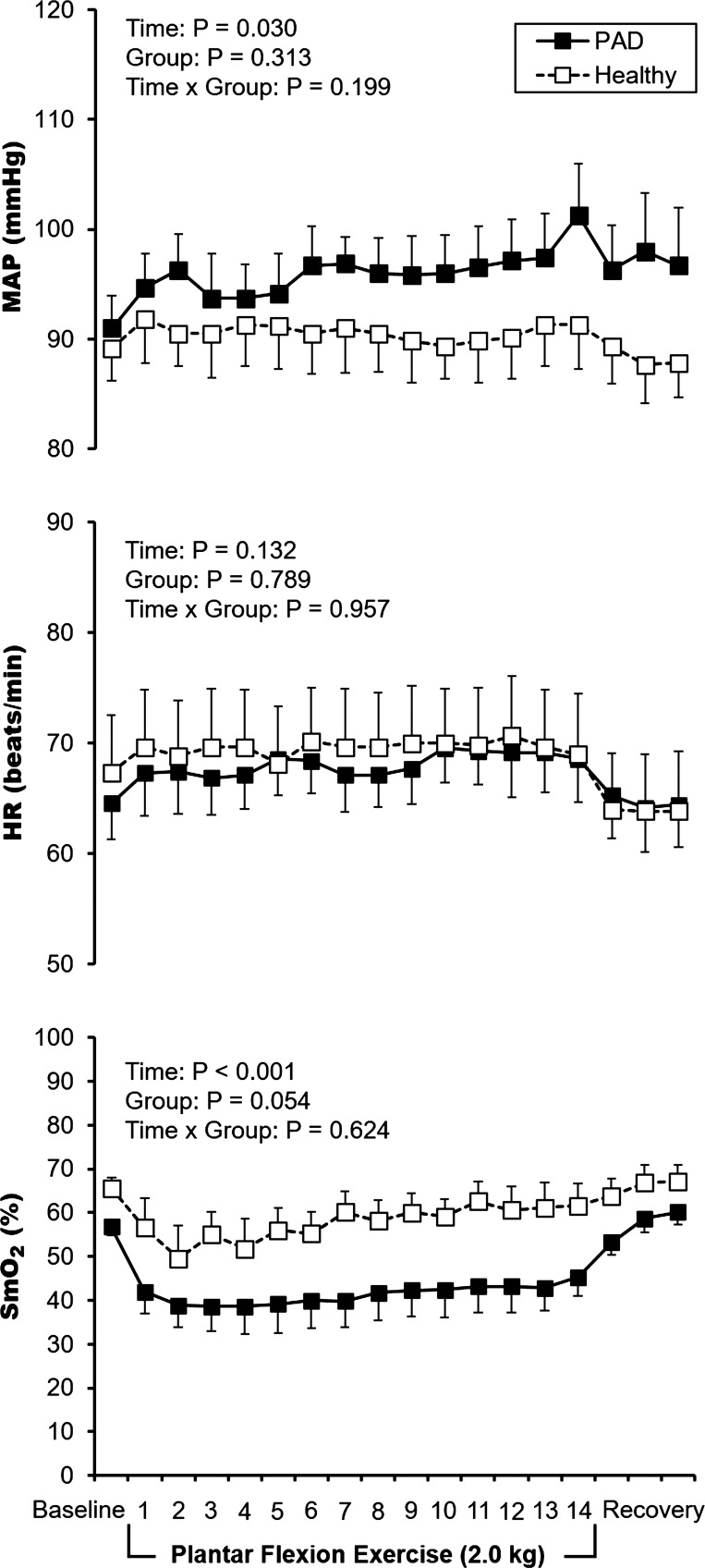
The patients with PAD in experiment 2 had a self-reported mean walking impairment score of 58 ± 12 (range, 16–87), whereas the healthy controls all scored 100 (which is the maximum). The mean responses to 14 min of low-intensity, steady-state plantar flexion exercise are displayed in Fig. 3. For MAP, there was a main effect for time (P = 0.030) but no main effect for group (P = 0.313) nor group-by-time interaction (P = 0.199). HR remained unchanged from baseline (main effect for time P = 0.132). Regarding SmO2 in the medial gastrocnemius, we observed a main effect for time (P < 0.001) but no group-by-time interaction (P = 0.624). However, because SmO2 was lower in patients with PAD at baseline (P = 0.020), we calculated percent changes at 1 and 14 min of exercise. At 1 min of exercise, the percent change in SmO2 was not different between patients with PAD (−26 ± 7%) and healthy subjects (−12 ± 7%, P = 0.190). At 14 min of exercise the percent change in SmO2 appeared to be different between patients with PAD (−21 ± 6%) and healthy subjects (−5 ± 5%) but this comparison did not reach statistical significance (P = 0.102). The median rating of perceived exertion at the end of exercise was 10 (range 6–17) in patients with PAD and 7 (range, 7–11) in healthy subjects (P = 0.694). The median pain rating at the end of exercise was 0 (range, 0–4) in patients with PAD and 0 (range, 0–0) in healthy subjects (P = 0.369). Taken together, the 2-kg workload in experiment 2 was perceived as low intensity and not painful, and had minimal effects on BP, HR, and NIRS SmO2 both in patients with PAD and healthy subjects.
Fig. 3.
Mean data from experiment 2. Mean arterial pressure (MAP), heart rate (HR), and muscle oxygen saturation (SmO2) responses to single-leg plantar flexion exercise in the supine posture in eight patients with peripheral artery disease (PAD, solid black circles) and eight healthy subjects (white squares). All participants exercised for 14 min at 20 contractions per min (2.0 kg) with the most symptomatic leg. Values are means ± SE.
DISCUSSION
The purpose of this study was to examine the relationship between muscle oxygen saturation (determined using NIRS) in the gastrocnemius muscle and the pressor response during plantar flexion in patients with PAD and healthy subjects. This study provides three novel findings. First, during fatiguing plantar flexion exercise that evokes claudication we observed a greater fall in SmO2 in patients with PAD compared with healthy controls. Second, the fall in gastrocnemius oxygen saturation in patients with PAD was observed before the rise in blood pressure. Third, low-intensity, steady-state plantar flexion exercise at 2 kg elicited similar effects on hemodynamics and SmO2 in both groups. These findings suggest that there is a threshold of oxygen desaturation necessary before the exercise pressor reflex is engaged. We speculate that the faster and greater drop in SmO2 in patients with PAD contributes to the rapid onset exercise pressor reflex observed in individuals with PAD but more definitive experiments are needed to isolate the cause-and-effect mechanism.
The cardiovascular adjustments to exercise are modulated by both feedforward and feedback control (17). The exercise pressor reflex is a neurally mediated reflex activated when group III and group IV afferents within skeletal muscle respond to mechanical and metabolic stimuli (29). There is increasing evidence in humans and animals that chronic low-flow states lead to upregulation of sensory receptors that mediate the exercise pressor reflex (36). In prior reports (48, 49) we have shown that the BP response to plantar flexion is accentuated in humans with PAD and this pressor response can be partly normalized by acute infusions of ascorbic acid and ketorolac. The current data expand upon those prior observations by suggesting that a necessary upstream prerequisite for engaging this reflex is tissue deoxygenation. Examination of individual subject data (Fig. 2) suggests that in many of the subjects with PAD the BP curve seemed to be inversely related to the fall in muscle oxygen saturation. To strengthen our findings from experiment 1, we performed additional studies using a less strenuous, less painful exercise paradigm. Consistent with our hypothesis, the physiological responses during the nonischemic exercise paradigm in experiment 2 were similar in the two groups (Fig. 3). Taken together, our data are consistent with studies in animal models of acute ischemia that demonstrate the crucial role muscle ischemia plays in evoking the exercise pressor reflex (36). These results support the hypothesis that a drop in calf muscle oxygen saturation corresponds to an increase in BP. More studies are needed to clarify the relationship between BP and SmO2 to understand this proposed desaturation threshold.
The clinical spectrum of PAD is large and ranges from asymptomatic to critical limb ischemia at its severest. Although the patients with PAD in our studies reported impaired walking ability compared with the healthy age-matched controls, it is not known whether the patients with PAD had also developed muscle myopathies as has been observed by others in studies of severe PAD (53). Patients with PAD also have changes in muscle capillarization (5, 57) and mitochondrial density (43, 54). These factors could contribute to a mismatch in oxygen delivery and/or utilization during walking exercise. The extent of histological changes in PAD and how leg muscle pathophysiology affects exercise BP and muscle oxygen delivery has yet to be determined.
Whereas early deoxygenation in the legs has potential clinical applications for indicating ischemia, NIRS is not used in diagnosing or monitoring patients during walking exercise. In this report, we used a continuous-wave NIRS device to measure microvascular responses in the medial gastrocnemius. Our thigh cuff occlusion studies provide evidence that our device is valid, reliable, and comparable to other NIRS devices used in prior studies (11, 27, 44). Specifically, complete occlusion of arterial blood flow with suprasystolic thigh cuff occlusion for 5 min reduced SmO2 to <11% in both groups, and subsequent hyperemia was evident in both groups but expectedly delayed and attenuated in patients with PAD. NIRS is one of several research tools that can be used to measure muscle oxygen transport and utilization. In this study, we used NIRS in conjunction with plantar flexion exercise to minimize the effects of hydrostatic pressure and venous congestion on the NIRS responses. These factors may confound data interpretation during upright treadmill walking. Other techniques such as magnetic resonance imaging (MRI), muscle biopsies, muscle microdialysis, and sampling blood from the femoral vein can provide additional information about muscle oxygenation, but these techniques have drawbacks. For instance, previous MRI experiments have quantified hemodynamic and metabolic limitations in exercising PAD muscle (1, 15, 26–28, 54, 55, 62, 63, 67, 69), but images can be acquired only during the postexercise rest periods (when BP and HR have returned toward baseline). Muscle biopsies have documented altered enzyme kinetics, myofibril architecture, and impaired oxidative phosphorylation in PAD (5, 38, 43, 57, 66), but serial biopsies are problematic in longitudinal designs, and this invasive technique requires specialized training and equipment to process tissue samples. Measurement of metabolites and growth factors from muscle dialysate or femoral or popliteal vein blood samples have also been used to clarify pathophysiological mechanisms in PAD (25, 64). However, in our experience, many patients with PAD (and their referring physicians) are less willing to participate in these longer-duration, invasive experiments.
Limitations.
To our knowledge, the present study is the first to use the Moxy NIRS device in patients affected by PAD. However, the device has been successfully used to track changes in SmO2 in competitive athletes (11). NIRS signal penetration depths are relatively shallow, thus measurements are taken from the superficial portion of the medial gastrocnemius. It is important to note that variations in adipose tissue thickness and extremely high SmO2 levels (i.e., >90%) dampen the NIRS signal (11, 45). We believe this is a result of reaching the edge of the NIRS optical window and thus produces a flat line in SmO2. It should also be noted that patients with PAD have diverse symptom presentations and lesion severity and are medically managed with a variety of medications that affect cardiovascular physiology. Perhaps the two key questions not addressed by this report are whether engagement of the exercise pressor reflex acts to restore flow to the ischemic limb and whether this reflex contributes to the exaggerated pressor response observed with ambulation in patients with PAD. We believe these questions can be addressed in future studies using NIRS during appropriately designed experiments.
Conclusions.
In summary, we found that patients with PAD have a greater reduction in oxygen saturation in the gastrocnemius during fatiguing plantar flexion exercise that evokes claudication compared with healthy subjects of similar age. This drop in SmO2 precedes the rise in BP during exercise and might help explain the exaggerated exercise pressor reflex in PAD.
GRANTS
Support for this study was provided by National Center for Advancing Translational Sciences Grants UL1 TR-000127 and KL2 TR-000126, and by a grant from the Pennsylvania Department of Health using Tobacco CURE funds (both to M. D. Muller). Support was also provided by National Heart, Lung, and Blood Institute Grants P01 HL-096570 to L. I. Sinoway, R25 HL-115473 to J. C. Luck, and by American Heart Association Grant 15PRE24470033 to A. J. Miller.
DISCLAIMERS
The Pennsylvania Department of Health and the National Institutes of Health specifically disclaim responsibility for any analyses, interpretations, or conclusions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.L., D.N.P., U.A.L., L.I.S., and M.D.M. conceived and designed research; J.C.L., A.J.M., D.N.P., and M.D.M. performed experiments; J.C.L., A.J.M., and M.D.M. analyzed data; J.C.L., F.A., J.F.R., D.N.P., U.A.L., L.I.S., and M.D.M. interpreted results of experiments; J.C.L. and M.D.M. prepared figures; J.C.L., D.N.P., L.I.S., and M.D.M. drafted manuscript; J.C.L., A.J.M., F.A., J.F.R., D.N.P., U.A.L., L.I.S., and M.D.M. edited and revised manuscript; J.C.L., A.J.M., F.A., J.F.R., D.N.P., U.A.L., L.I.S., and M.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Herr and Kris Brandt for technical support, Cheryl Blaha and Aimee Cauffman for nursing support, and Kris Gray and Jen Stoner for administrative guidance. The NIRS Moxy monitors were provided on loan from Colleen Millsaps of the Millsaps Training Facility (Cairo, GA).
REFERENCES
- 1.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol 54: 628–635, 2009. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 3.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol 100: 143–151, 2007. doi: 10.1007/s00421-007-0412-0. [DOI] [PubMed] [Google Scholar]
- 4.Bauer TA, Brass EP, Hiatt WR. Impaired muscle oxygen use at onset of exercise in peripheral arterial disease. J Vasc Surg 40: 488–493, 2004. doi: 10.1016/j.jvs.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Baum O, Torchetti E, Malik C, Hoier B, Walker M, Walker PJ, Odriozola A, Graber F, Tschanz SA, Bangsbo J, Hoppeler H, Askew CD, Hellsten Y. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 310: R943–R951, 2016. doi: 10.1152/ajpregu.00480.2015. [DOI] [PubMed] [Google Scholar]
- 6.Beckitt TA, Day J, Morgan M, Lamont PM. Calf muscle oxygen saturation and the effects of supervised exercise training for intermittent claudication. J Vasc Surg 56: 470–475, 2012. doi: 10.1016/j.jvs.2011.11.140. [DOI] [PubMed] [Google Scholar]
- 7.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Brass EP. Intermittent claudication: new targets for drug development. Drugs 73: 999–1014, 2013. doi: 10.1007/s40265-013-0078-3. [DOI] [PubMed] [Google Scholar]
- 9.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion. Vasc Med 9: 293–301, 2004. doi: 10.1191/1358863x04vm572ra. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 11.Cornachione K, McLaren J, Heil DP. Use of a wireless NIRS monitor to track changes in muscle oxygenation for laboratory-based Nordic skiing test protocol. Science and Skiing VI: 369–376, 2014. [Google Scholar]
- 12.de Liefde II, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, Poldermans D. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol 102: 921–926, 2008. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 13.de Liefde II, Verhagen HJ, Stolker RJ, van Domburg RT, Poldermans D. The value of treadmill exercise test parameters together in patients with known or suspected peripheral arterial disease. Eur J Prev Cardiol 19: 192–198, 2012. doi: 10.1177/1741826711399986. [DOI] [PubMed] [Google Scholar]
- 14.Egun A, Farooq V, Torella F, Cowley R, Thorniley MS, McCollum CN. The severity of muscle ischemia during intermittent claudication. J Vasc Surg 36: 89–93, 2002. doi: 10.1067/mva.2002.123678. [DOI] [PubMed] [Google Scholar]
- 15.Esterhammer R, Schocke M, Gorny O, Posch L, Messner H, Jaschke W, Fraedrich G, Greiner A. Phosphocreatine kinetics in the calf muscle of patients with bilateral symptomatic peripheral arterial disease during exhaustive incremental exercise. Mol Imaging Biol 10: 30–39, 2008. doi: 10.1007/s11307-007-0118-z. [DOI] [PubMed] [Google Scholar]
- 16.Figoni SF, Kunkel CF, Scremin AM, Asher A, Banks NL, Rivera A, Tin JK, Cohen B. Effects of exercise training on calf tissue oxygenation in men with intermittent claudication. PM R 1: 932–940, 2009. doi: 10.1016/j.pmrj.2009.08.453. [DOI] [PubMed] [Google Scholar]
- 17.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 18.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 3: e001107, 2014. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner AW, Parker DE, Montgomery PS, Blevins SM, Nael R, Afaq A. Sex differences in calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. J Vasc Surg 50: 77–82, 2009. doi: 10.1016/j.jvs.2008.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green S, Thorp R, Reeder EJ, Donnelly J, Fordy G. Venous occlusion plethysmography versus Doppler ultrasound in the assessment of leg blood flow during calf exercise. Eur J Appl Physiol 111: 1889–1900, 2011. doi: 10.1007/s00421-010-1819-6. [DOI] [PubMed] [Google Scholar]
- 21.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12: 062105, 2007. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 22.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123: 87–97, 2011. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood AE, Cayton T, Sarvanandan R, Lane R, Chetter I. A review of the potential local mechanisms by which exercise improves functional outcomes in intermittent claudication. Ann Vasc Surg 30: 312–320, 2016. doi: 10.1016/j.avsg.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 116: 1527–1539, 2015. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 25.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J, Askew CD, Hellsten Y. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985) 115: 1777–1787, 2013. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huegli RW, Schulte AC, Aschwanden M, Thalhammer C, Kos S, Jacob AL, Bilecen D. Effects of percutaneous transluminal angioplasty on muscle BOLD-MRI in patients with peripheral arterial occlusive disease: preliminary results. Eur Radiol 19: 509–515, 2009. doi: 10.1007/s00330-008-1168-6. [DOI] [PubMed] [Google Scholar]
- 27.Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol 47: 2289–2295, 2006. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 25: 1013–1020, 2007. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 30.Kellawan JM, Bentley RF, Bravo MF, Moynes JS, Tschakovsky ME. Does oxygen delivery explain interindividual variation in forearm critical impulse? Physiol Rep 2: 2, 2014. doi: 10.14814/phy2.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, Brennan J, Rankin A, Frostick SP. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg 34: 1103–1110, 2001. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- 32.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 110: 1582–1591, 2011. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laukkanen JA, Kurl S, Rauramaa R, Lakka TA, Venäläinen JM, Salonen JT. Systolic blood pressure response to exercise testing is related to the risk of acute myocardial infarction in middle-aged men. Eur J Cardiovasc Prev Rehabil 13: 421–428, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Le VV, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis 51: 135–160, 2008. doi: 10.1016/j.pcad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Leeper NJ, Myers J, Zhou M, Nead KT, Syed A, Kojima Y, Caceres RD, Cooke JP. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg 57: 728–733, 2013. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Xing J. Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front Physiol 3: 247, 2012. doi: 10.3389/fphys.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. doi: 10.1161/01.CIR.46.2.257. [DOI] [PubMed] [Google Scholar]
- 38.Mäkitie J, Teräväinen H. Histochemical changes in striated muscle in patients with intermittent claudication. Arch Pathol Lab Med 101: 658–663, 1977. [PubMed] [Google Scholar]
- 39.Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: a parameter to be considered in a clinical setting? Angiology 61: 530–536, 2010. doi: 10.1177/0003319710362975. [DOI] [PubMed] [Google Scholar]
- 40.Manfredini F, Lamberti N, Malagoni AM, Zambon C, Basaglia N, Mascoli F, Manfredini R, Zamboni P. Reliability of the vascular claudication reporting in diabetic patients with peripheral arterial disease: a study with near-infrared spectroscopy. Angiology 66: 365–374, 2015. doi: 10.1177/0003319714534762. [DOI] [PubMed] [Google Scholar]
- 41.Manfredini F, Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P. A dynamic objective evaluation of peripheral arterial disease by near-infrared spectroscopy. Eur J Vasc Endovasc Surg 38: 441–448, 2009. doi: 10.1016/j.ejvs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Manolio TA, Burke GL, Savage PJ, Sidney S, Gardin JM, Oberman A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: the CARDIA study. Am J Hypertens 7: 234–241, 1994. doi: 10.1093/ajh/7.3.234. [DOI] [PubMed] [Google Scholar]
- 43.Marbini A, Gemignani F, Scoditti U, Rustichelli P, Bragaglia MM, Govoni E. Abnormal muscle mitochondria in ischemic claudication. Acta Neurol Belg 86: 304–310, 1986. [PubMed] [Google Scholar]
- 44.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol 49: B128–B134, 1994. doi: 10.1093/geronj/49.3.B128. [DOI] [PubMed] [Google Scholar]
- 45.McCully KK, Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28: 123–127, 2000. [PubMed] [Google Scholar]
- 46.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 286: 1599–1606, 2001. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 47.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, Criqui MH. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol 57: 962–970, 2011. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, Sinoway LI. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol 309: H523–H528, 2015. doi: 10.1152/ajpheart.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller MD, Li Z, Sica CT, Luck JC, Gao Z, Blaha CA, Cauffman AE, Ross AJ, Winkler NJ, Herr MD, Brandt K, Wang J, Gallagher DC, Karunanayaka P, Vesek J, Leuenberger UA, Yang QX, Sinoway LI. Muscle oxygenation during dynamic plantar flexion exercise: combining BOLD MRI with traditional physiological measurements. Physiol Rep 4: 4, 2016. doi: 10.14814/phy2.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller MD, Reed AB, Leuenberger UA, Sinoway LI. Physiology in medicine: peripheral arterial disease. J Appl Physiol (1985) 115: 1219–1226, 2013. doi: 10.1152/japplphysiol.00885.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, Hiatt WR, Ho M, Ikeda K, Ikeno F, Jaff MR, Jones WS, Kawahara M, Lookstein RA, Mehran R, Misra S, Norgren L, Olin JW, Povsic TJ, Rosenfield K, Rundback J, Shamoun F, Tcheng J, Tsai TT, Suzuki Y, Vranckx P, Wiechmann BN, White CJ, Yokoi H, Krucoff MW. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol 65: 931–941, 2015. doi: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg 42: 101–112, 2008. doi: 10.1177/1538574408315995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg 31: 944–952, 2000. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 55.Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, Dimaria JM, Christopher JM, Kramer CM. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging 5: 1224–1230, 2012. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quaresima V, Homma S, Azuma K, Shimizu S, Chiarotti F, Ferrari M, Kagaya A. Calf and shin muscle oxygenation patterns and femoral artery blood flow during dynamic plantar flexion exercise in humans. Eur J Appl Physiol 84: 387–394, 2001. doi: 10.1007/s004210100390. [DOI] [PubMed] [Google Scholar]
- 57.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol (1985) 111: 81–86, 2011. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roseguini BT, Hirai DM, Alencar MC, Ramos RP, Silva BM, Wolosker N, Neder JA, Nery LE. Sildenafil improves skeletal muscle oxygenation during exercise in men with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 307: R396–R404, 2014. doi: 10.1152/ajpregu.00183.2014. [DOI] [PubMed] [Google Scholar]
- 59.Ross AJ, Gao Z, Luck JC, Blaha CA, Cauffman AE, Aziz F, Radtka JF III, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Coronary exercise hyperemia is impaired in patients with peripheral arterial disease. Ann Vasc Surg 38:260–267, 2017. doi: 10.1016/j.avsg.2016.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 115: 1757–1766, 2013. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagar SP, Brown PM, Zelt DT, Pickett WL, Tranmer JE. Further clinical validation of the walking impairment questionnaire for classification of walking performance in patients with peripheral artery disease. Int J Vasc Med 2012: 190641, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schocke MF, Esterhammer R, Ostermann S, Santner W, Gorny O, Fraedrich G, Jaschke WR, Greiner A. High-energy phosphate metabolism during calf ergometry in patients with isolated aorto-iliac artery stenoses. Invest Radiol 41: 874–882, 2006. doi: 10.1097/01.rli.0000246148.09129.42. [DOI] [PubMed] [Google Scholar]
- 63.Schunk K, Romaneehsen B, Rieker O, Düber C, Kersjes W, Schadmand-Fischer S, Schmiedt W, Thelen M. Dynamic phosphorus-31 magnetic resonance spectroscopy in arterial occlusive disease: effects of vascular therapy on spectroscopic results. Invest Radiol 33: 329–335, 1998. doi: 10.1097/00004424-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Sørlie D, Myhre K. Effects of physical training in intermittent claudication. Scand J Clin Lab Invest 38: 217–222, 1978. doi: 10.3109/00365517809108414. [DOI] [PubMed] [Google Scholar]
- 65.Vardi M, Nini A. Near-infrared spectroscopy for evaluation of peripheral vascular disease. A systematic review of literature. Eur J Vasc Endovasc Surg 35: 68–74, 2008. doi: 10.1016/j.ejvs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y, Askew CD. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246: 98–105, 2016. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 67.West AM, Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Berr SS, Harthun NL, Weltman AL, Annex BH, Kramer CM. Percutaneous intervention in peripheral artery disease improves calf muscle phosphocreatine recovery kinetics: a pilot study. Vasc Med 17: 3–9, 2012. doi: 10.1177/1358863X11431837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willingham TB, Southern WM, McCully KK. Measuring reactive hyperemia in the lower limb using near-infrared spectroscopy. J Biomed Opt 21: 091302, 2016. doi: 10.1117/1.JBO.21.9.091302. [DOI] [PubMed] [Google Scholar]
- 69.Zatina MA, Berkowitz HD, Gross GM, Maris JM, Chance B. 31P nuclear magnetic resonance spectroscopy: noninvasive biochemical analysis of the ischemic extremity. J Vasc Surg 3: 411–420, 1986. [DOI] [PubMed] [Google Scholar]