We found that the impairment in popliteal artery flow-mediated dilation caused by physical inactivity can be prevented by increased shear stress. These findings indicate that reduced leg blood flow-induced shear stress during physical inactivity may be a key underlying mechanism mediating the detrimental leg vascular effects of physical inactivity. Heating the foot area may be used as a nonpharmacological therapy to combat inactivity-induced leg vascular dysfunction, especially in people who are unable or unwilling to be active.
Keywords: physical inactivity, exercise, sedentary, flow-mediated dilation, shear stress
Abstract
We recently showed that 5 days of reduced daily physical activity impair popliteal artery, but not brachial artery, flow-mediated dilation (FMD). However, the mechanisms by which physical inactivity causes leg vascular dysfunction are unclear. We reason that a reduction in leg blood flow-induced shear stress is a primary underlying mechanism by which reduced daily physical activity impairs popliteal artery FMD. Thus the purpose of this study was to determine whether increased leg blood flow and shear stress during inactivity prevent the reduction in popliteal artery FMD. Bilateral popliteal artery FMD measures were performed at baseline and after 5 days of a transition from high (>10,000 steps/day) to low levels (<5,000 steps/day) of physical activity in 13 healthy and physically active men [20 ± 2 (SD) yr]. During the inactive period, one foot was submerged in ~42°C water (i.e., heated leg) three times a day for 30 min each period, to increase blood flow and thus shear stress, whereas the contralateral leg remained dry and served as internal control (i.e., nonheated leg). During heating, popliteal artery mean shear rate was increased in the heated leg (change of 119.3 ± 26.4%, P < 0.01) but slightly decreased in the nonheated leg (change of −21.8 ± 7.5%, P = 0.03). Popliteal artery FMD was impaired after 5 days of reduced daily physical activity in the control nonheated leg (P < 0.01) but was unchanged in the heated leg (P = 0.34). These results support the hypothesis that reduced leg blood flow-induced shear stress during physical inactivity is a key underlying mechanism mediating leg vascular dysfunction.
NEW & NOTEWORTHY We found that the impairment in popliteal artery flow-mediated dilation caused by physical inactivity can be prevented by increased shear stress. These findings indicate that reduced leg blood flow-induced shear stress during physical inactivity may be a key underlying mechanism mediating the detrimental leg vascular effects of physical inactivity. Heating the foot area may be used as a nonpharmacological therapy to combat inactivity-induced leg vascular dysfunction, especially in people who are unable or unwilling to be active.
it is estimated that a sedentary lifestyle contributes to 6–10% of all deaths from chronic diseases (5), which is equivalent to the number of deaths attributed to smoking (10). The impact of inactivity is especially profound in the cardiovascular system. Indeed, epidemiological data demonstrate that individuals who are most inactive have the highest risk for cardiovascular disease morbidity and mortality (4, 19). Despite the well-established relationship between inactivity and cardiovascular disease risk, there is a relative paucity of experimental data on the vascular effects of reduced daily physical activity. Furthermore, the mechanisms by which inactivity increases vascular burden remain largely unknown.
In an effort to evaluate the vascular effects of physical inactivity, we recently performed a study designed to emulate the degree of inactivity encountered in real life (6). Specifically, regularly active individuals meeting current guidelines of >10,000 steps/day were transitioned into a period of reduced activity (<5,000 steps/day) for 5 days. We found that reduced activity impaired popliteal artery flow-mediated dilation (FMD). Interestingly, brachial artery FMD was not affected by 5 days of reduced physical activity (6). The finding of decreased popliteal, but not brachial, artery FMD may be related to the fact that popliteal arteries, upon reduction of locomotion, were subjected to a greater decrease in blood flow and thus shear stress, relative to the brachial arteries. Because shear stress is an important stimulus for maintaining endothelial health (7, 11, 15, 20, 25, 35), it is plausible that with reduced activity, “knockdown” of shear stress in the legs is an underlying factor mediating the impairment in popliteal artery function. In this sense, the development of nonpharmacological therapies to combat inactivity-induced detrimental leg vascular effects is needed. In addition, the assessment of leg vascular function is important in light of studies demonstrating that the leg vasculature is highly susceptible to atherosclerosis, relative to other disease-resistant vasculatures such as the brachial artery (1, 13, 14, 32, 33).
It has been previously shown that local heating at 42°C is an effective stimulus for dilating the skin circulation, increasing limb blood flow and thus shear stress without producing major systemic cardiovascular effects (24, 26–28). Here, we reasoned that if reduced shear stress is indeed a primary mechanism by which reduced physical activity impairs popliteal artery FMD, an increase in leg blood flow and shear stress during inactivity would produce a vascular protective effect. Specifically, we examined whether preventing the sustained reduction in shear stress by intermittent foot heating during 5 days of reduced daily physical activity would abolish the detrimental effects of inactivity on popliteal artery FMD.
METHODS
Participants.
Thirteen apparently healthy (determined by a detailed heath history questionnaire), recreationally active men (age 20 ± 2 yr, height 177.4 ± 7.5 cm, weight 76.6 ± 10.5 kg, means ± SD) were enrolled in this study. Physical activity was self-reported, and “recreationally active” was defined as completing habitual physical activity for at least 6 consecutive months with a minimum frequency of 3 days per week in ≥30-min sessions and taking >10,000 steps/day. Subjects had to be asymptomatic, nonsmokers, normotensive, and nondiabetic. All subjects were confirmed to have no heart disease and were using no prescribed or over-the-counter medications.
All study procedures were approved by the University of Brasília research committee (CAAE 49631815.6.0000.0030) in accordance with the Declaration of Helsinki. All subjects participated in the present study voluntarily, receiving no financial incentive. Participants were informed that they could withdraw at any time, and each subject read and signed a specific informed consent form.
Experimental protocol.
Bilateral popliteal artery FMD was measured before and after 5 days of reduced daily physical activity. Before baseline measurements, participants wore an accelerometer (Vívofit; Garmin, Olathe, KS) during 5 days and were asked to follow their normal physical activity patterns. Subjects were then instructed to reduce daily physical activity during 5 consecutive days by transitioning from high (>10,000 steps/day) to low daily physical activity (<5,000 steps/day; 6). Subjects were also instructed to avoid any planned exercise sessions. Steps per day were measured using the accelerometer. To achieve the target goal of reduced steps, under certain circumstances, some subjects went home and/or commuted by taxi arranged by study personnel.
To test our initial hypothesis that shear stress is a primary underlying mechanism by which reduced daily physical activity impairs popliteal artery FMD, during the 5 days of inactivity, one foot was submerged in 40–42°C water (i.e., heated leg), whereas the contralateral foot remained in open air and served as internal control (i.e., nonheated leg). Leg assignment was randomized. The heating protocol was performed three times a day for 30-min each period and comprised one foot being submerged up to the ankle in a commercially available foot spa (Aqua Foot; Britânia, Curitiba, Brazil) with temperature of the water maintained at ~42°C using an automated thermometer (i.e., heated leg). A total of 15 sessions of heating were performed by each participant. All participants were trained in utilizing the heating device for weekend sessions. Compliance over the weekend was ensured by a phone call from one of the researchers. A total of 195 sessions of heating were performed by the participants, of which 62% (n = 121) were performed at the laboratory and 38% (n = 74) were conducted at home. The last heating session was performed 12–16 h before the post leg FMD.
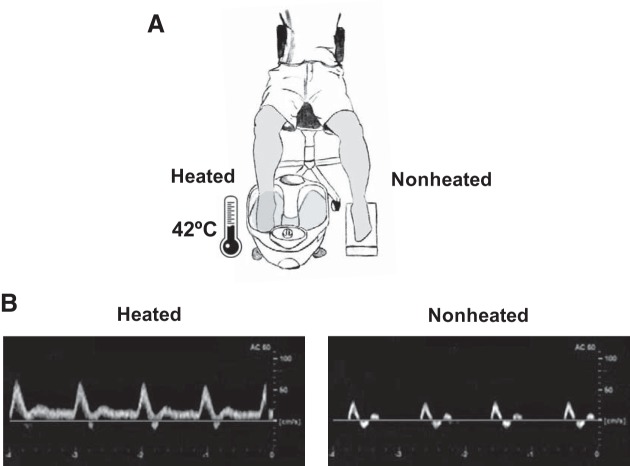
In addition, bilateral popliteal artery Doppler ultrasound, beat-to-beat heart rate (three-lead electrocardiography), and oscillometric arterial blood pressure were measured before and during one session of heating using automated equipment (DX-2022; Dixtal, Santo Amaro, Brazil). The popliteal artery and hemodynamics data were obtained during the final 10-min period of the protocol. Figure 1 provides a schematic illustration of the experimental setup (Fig. 1A) and original Doppler ultrasound recordings from one subject showing the remarkable increase in popliteal artery blood flow in the heated leg compared with the nonheated leg during one session of heating (Fig. 1B).
Fig. 1.
Schematic diagram of experimental protocol (A) and original Doppler ultrasound recordings from one subject illustrating the remarkable increase in popliteal artery blood velocity in the heated leg compared with the nonheated leg during one session of local heating (B).
The subjects were asked to refrain from consuming caffeine/alcohol and from engaging in physical exercise for 6 and 24 h, respectively, before the tests. Subjects were 2 h postprandial upon arrival at the laboratory. To avoid potential diurnal variations, subjects were always tested at the same time of day and in the same quiet, temperature-controlled room (~24°C). Upon arrival at the laboratory, height and weight were obtained via standard methods.
Popliteal artery FMD.
Bilateral popliteal artery FMD was measured at baseline and after 5 days of reduced daily physical activity. Initially, subjects rested in a supine position during a 15-min period to ensure the attainment and stabilization of cardiovascular variables. Popliteal artery diameter and blood velocity were measured using high-resolution duplex-Doppler ultrasound (Logiq P5; GE Medical Systems, Milwaukee, WI) following present guidelines (34). A 9-MHz linear array transducer was placed over the popliteal artery just distal to the popliteal fossa. Simultaneous diameter and velocity signals were obtained in duplex mode at a pulsed frequency of 5 MHz and corrected with an insonation angle of 60°. Sample volume was adjusted to encompass the entire lumen of the vessel without extending beyond the walls, and the cursor was set at midvessel. Popliteal artery FMD was assessed in both legs in the supine position as previously described (6, 18, 29, 30). Briefly, a rapid inflating cuff was placed on the lower leg. Two minutes of baseline hemodynamics were recorded, and then the cuff was inflated to a pressure of 220 mmHg for 5 min. Continuous diameter and blood velocity measures were recorded for 3 min following rapid cuff deflation. Recordings of all vascular variables were analyzed off-line using specialized edge detection software (Cardiovascular Suite; Quipu, Pisa, Italy). FMD percentage change was normalized to shear rate incremental area under the curve up to peak diameter (22, 23).
Statistical analysis.
The Shapiro-Wilk normality test and Levene’s test of homogeneity of variance were used to assess the normality of the distribution of data. All variables presented a normal distribution and equal variance. A 2 × 2 ANOVA for repeated measures was applied: time (pre × post) and condition (heated × nonheated leg). We then tested the sphericity of the data and used least significant difference post hoc test to detect differences when necessary. As an additional FMD analysis, peak (Dpeak) and baseline (Dbase) popliteal artery diameters were logarithmically transformed, and differences between them were determined. Logged scale differences between diameters were entered into an analysis of covariance model in which time and condition constituted a fixed factor and the logarithmically transformed Dbase constituted a covariate. Covariate-adjusted estimate of the means was then back-transformed and calculated as percentage changes according to previous reports (2, 3). A paired t test was used to compare hemodynamic data within group at rest (preheating) and during the heating protocol. SPSS (version 19; IBM, Armonk, NY) was used to perform all analyses. Data are reported as means ± SE, and the significance level adopted was P < 0.05.
RESULTS
The number of steps significantly decreased in the reduced daily physical activity phase compared with baseline (reduced daily physical activity phase: 3,842 ± 123 steps/day vs. active phase: 13,103 ± 455 steps/day, P < 0.01; Fig. 2, A and B).
Fig. 2.
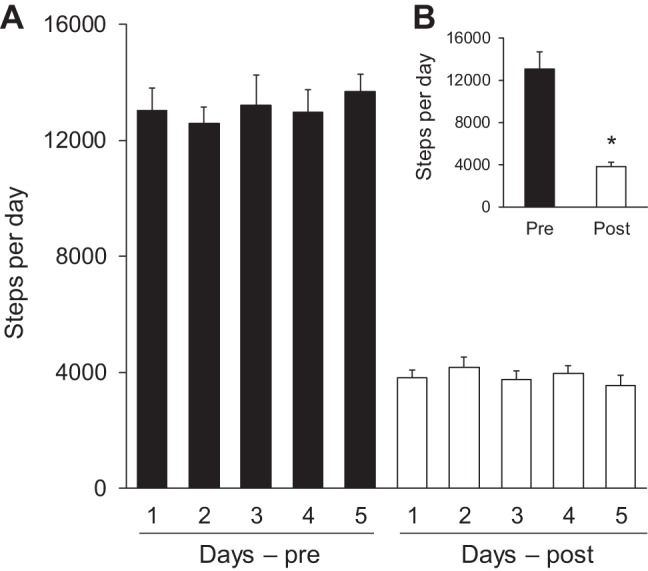
Number of daily steps during the 5 days of baseline (pre) and 5 days of reduced physical activity (post; A). B: 5-day averaged data. *P < 0.05 vs. pre.
Table 1 shows popliteal artery Doppler measurements and hemodynamics during one experimental session of heating. Baseline diameter was not impacted by heating in the heated or nonheated leg (P = 0.93). Both blood flow and velocity increased in the heated leg [change (Δ): 110.7 ± 27.6%, P < 0.01, and Δ: 104.7 ± 24.6%, P < 0.01, respectively] and slightly decreased in the nonheated leg (Δ: −25.7 ± 8.1%, P < 0.01, and Δ: −25.7 ± 6.7%, P = 0.01, respectively; Table 1). The heating protocol increased popliteal artery mean shear rate in the heated leg (Δ: 119.3 ± 26.4%, P < 0.01) and slightly decreased in the nonheated leg (Δ: −21.8 ± 7.5%, P = 0.03; Table 1 and Fig. 3). Heart rate and systolic, diastolic, and mean blood pressure were not affected by heating (P > 0.05 vs. preheating; Table 1).
Table 1.
Physiological variables before and during heating protocol at ~42°C
| Preheating | 42°C Water | |
|---|---|---|
| Popliteal Artery | ||
| Diameter, mm | ||
| Heated leg | 5.76 ± 0.22 | 5.78 ± 0.27 |
| Nonheated leg | 5.79 ± 0.16 | 5.78 ± 0.20 |
| Velocity, cm/s | ||
| Heated leg | 7.45 ± 0.99 | 14.35 ± 1.82*† |
| Nonheated leg | 7.76 ± 1.02 | 5.60 ± 0.89* |
| Blood flow, ml/min | ||
| Heated leg | 123.3 ± 19.3 | 224.2 ± 33.8*† |
| Nonheated leg | 128.3 ± 20.2 | 86.2 ± 11.5* |
| Shear rate, s−1 | ||
| Heated leg | 47.64 ± 5.40 | 104.32 ± 15.53*† |
| Nonheated leg | 49.68 ± 5.54 | 40.11 ± 7.46* |
| Hemodynamics | ||
| Heart rate, beats/min | 70 ± 2 | 71 ± 3 |
| Systolic BP, mmHg | 120 ± 3 | 118 ± 3 |
| Diastolic BP, mmHg | 66 ± 2 | 68 ± 2 |
| Mean BP, mmHg | 84 ± 2 | 84 ± 2 |
Values are means ± SE. Preheating, before heating protocol. BP, blood pressure.
P < 0.05 vs. preheating.
P < 0.05 vs. nonheated leg.
Fig. 3.
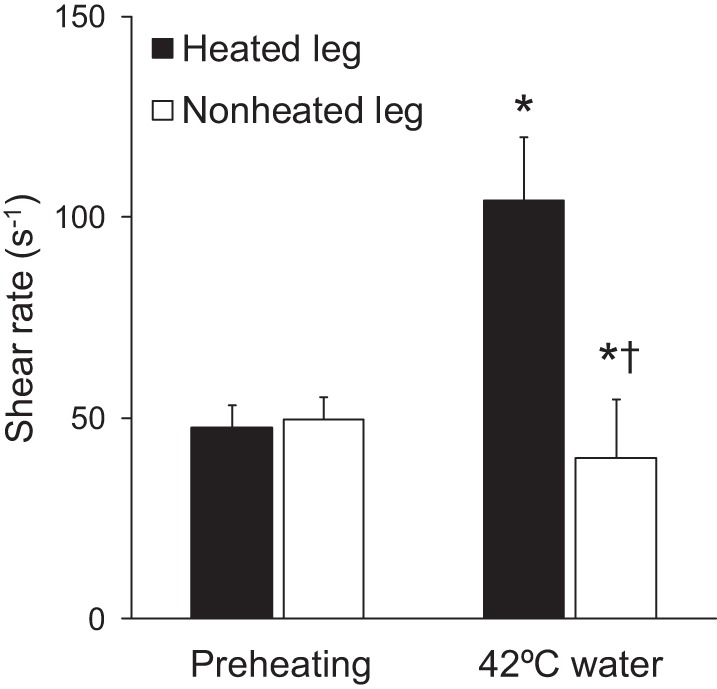
Mean shear rate measured before (preheating) and during one session of the experimental protocol, in which one leg was submerged in ~42°C water (i.e., heated leg) and the contralateral leg remained dry (i.e., nonheated leg). *P < 0.05 vs. rest. †P < 0.05 vs. heated leg.
Consistent with our hypothesis, 5 days of reduced physical activity decreased popliteal artery FMD in the control nonheated leg (pre: 6.69 ± 0.45% vs. post: 2.93 ± 0.36%, P < 0.01), and this impairment was prevented by intermittent local heating in the heated leg (pre: 6.82 ± 0.30% vs. post: 6.47 ± 0.36%, P = 0.34; Fig. 4). Likewise, popliteal artery FMD normalized to shear rate area under the curve was unchanged in the heated leg (P = 0.64) but significantly decreased after 5 days of inactivity in the nonheated leg (P < 0.01; Table 2). The hyperemic stimulus for FMD, shear rate area under the curve, was not significantly affected by inactivity or heating (Table 2). In addition, after 5 days of reduced daily physical activity, baseline popliteal artery diameter was not significantly different between legs (P = 0.25; Table 2). However, baseline diameter numerically trended toward a decrease in both the heated (Δ: −2.16 ± 0.87%) and nonheated legs (Δ: −0.7 ± 0.91%), which could inflate the postintervention FMD values. To address this, we performed an analysis of covariance model in which the difference in diameter is the outcome and Dbase is a covariate in a logarithmic-linked generalized linear model. The Dbase-adjusted FMD% remained unchanged in the heated leg (pre: 13.5 ± 0.6% vs. post: 12.7 ± 0.9%, P = 0.34) but was decreased in the nonheated leg (pre: 13.3 ± 0.8% vs. post: 6.1 ± 0.7%, P < 0.01).
Fig. 4.
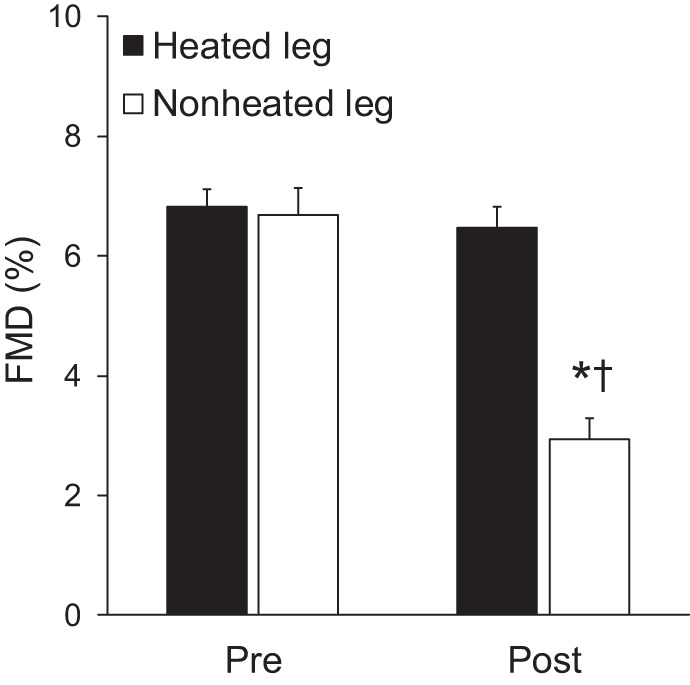
Popliteal artery flow-mediated dilation in the heated and nonheated legs during baseline (pre) and after 5 days of reduced daily physical activity (post). *P < 0.05 vs. pre. †P < 0.05 vs. heated leg.
Table 2.
Popliteal artery vascular measures at baseline and during FMD before and after 5 days of reduced daily physical activity in the heated and nonheated legs
| Baseline Diameter, mm | Peak Diameter, mm | SRAUC | Peak FMD-to-SRAUC Ratio, AU | Time to Peak, s | |
|---|---|---|---|---|---|
| Heated leg | |||||
| Pre | 5.78 ± 0.21 | 6.17 ± 0.22 | 22,262.91 ± 2,616.98 | 0.37 ± 0.06 | 98.69 ± 7.67 |
| Post | 5.66 ± 0.22 | 6.02 ± 0.24 | 22,254.92 ± 3,126.64 | 0.35 ± 0.05 | 78.77 ± 6.43 |
| Nonheated leg | |||||
| Pre | 5.81 ± 0.23 | 6.20 ± 0.24 | 20,628.46 ± 2,963.32 | 0.38 ± 0.05 | 85.62 ± 9.87 |
| Post | 5.77 ± 0.22 | 5.94 ± 0.23 | 19,825.03 ± 2,296.18 | 0.18 ± 0.03*† | 84.15 ± 6.03 |
SRAUC, shear rate area under the curve; AU, arbitrary units; pre and post, before and after 5 days of reduced daily physical activity, respectively.
P < 0.05 vs. pre.
P < 0.05 vs. heated leg.
DISCUSSION
The most salient finding of the present investigation is that the impairment in popliteal artery FMD caused by short-term physical inactivity can be prevented by increasing leg blood flow and shear stress. Indeed, we found that increasing leg blood flow-induced shear stress with intermittent foot heating throughout a 5-day period of reduced activity abolished the impairment in popliteal artery FMD. This finding supports the hypothesis that reduced leg vascular shear stress during inactivity may be an underlying physiological mechanism by which physical inactivity causes vascular dysfunction in the lower extremities.
Consistent with our previous work (6), we found that when regularly active individuals engaging in >10,000 steps/day reduce their number of steps to ~4,000 steps/day (i.e., the American average) for 5 days, popliteal artery FMD becomes impaired. The finding that impaired FMD manifested in the popliteal artery but not in the brachial artery (6) suggests that the mechanisms altering vascular function with changes in activity levels are largely regulated by local factors. A well-known local factor responsible for the modulation of vascular function is shear stress. With reduced locomotion, the vasculature of the lower extremities becomes exposed to a marked reduction in blood flow and, thus, shear stress, relative to the vasculature of the arms, which largely retain similar levels of activity. Thus it is likely that inactivity-induced reduction in popliteal artery FMD is attributable to the loss of shear stress. The concept that vascular adaptations can be mediated by shear stress-dependent mechanisms is indeed supported by cell and organ culture, as well as by in vivo animal and human studies experimentally altering shear stress (7, 9, 11, 15, 20, 25, 35).
In support of the hypothesis that reduced shear stress with inactivity is a prime underlying mechanism of impaired popliteal artery FMD, here we found that subjecting one leg to increased blood flow with foot heating throughout the inactivity period prevented the decline in popliteal artery FMD in that leg. That is, the present data indicate that leg vascular dysfunction caused by inactivity can be eliminated by “replenishing” the shear stress stimulus lost during inactivity. However, on the basis of the current data, we cannot exclude the possibility that inactivity impairs popliteal artery FMD through other mechanisms that are independent of shear stress and that increased shear stress with heating restores popliteal artery FMD back to normal.
As shown recently, acute (30, 31, 36) and chronic (8, 21) limb-specific heating improves vascular dilator function in both upper (8, 21, 36) and lower (30, 31) extremities of healthy humans through a shear stress-dependent mechanism. However, heating per se, independent of changes in physical activity or muscular contractions (8, 21), may also produce beneficial vascular effects. In this regard, previous studies indicate that elevated body core temperature increases expression of heat shock proteins, which are known to play a role in the modulation of vascular health (12, 16). Importantly, in the present study, heating was applied to only the foot, minimizing the direct effects of heat on the upstream popliteal artery.
Some methodological aspects of the present study should be considered. First, we studied only healthy, young, and physically active men, and thus the generalizability of the findings remains limited to this population. Second, part of our results should be interpreted with caution given the potential for a type II error. For example, a nonsignificant (P = 0.25) reduction in baseline popliteal artery diameter was observed between legs after the inactivity period. However, the additional allometric scaling analysis proposed by Atkinson and Batterham (2), in which logarithmically transformed resting popliteal artery diameter represents a covariate, reinforced our main findings that leg vascular function was impaired after 5 days of reduced daily physical activity in the nonheated leg but remained unchanged in the heated leg. Third, we cannot guarantee that 2 h postprandial is sufficient to eliminate the confounding effects of hyperglycemia and hyperinsulinemia on the FMD response. However, we believe that this is more indicative of normal daily life than the fasted state. Importantly, our approach is in line with recent studies of physical inactivity and FMD (6, 17, 18, 29, 30, 37). Fourth, although the prognostic value of popliteal artery FMD remains unknown, the finding that reduced daily physical activity lowered FMD by 3.8% (absolute units) in the nonheated leg should be considered in light of studies demonstrating that the leg vasculature is highly susceptible to atherosclerosis, relative to other disease-resistant vasculatures such as the brachial artery (1, 13, 14, 32, 33). Given this, the use of foot heating as a nonpharmacological therapy to combat inactivity-induced popliteal artery dysfunction may be particularly relevant in patients with increased risk factors for peripheral artery disease who are unable or unwilling to be active. Future studies, however, should be conducted in these populations to confirm this hypothesis.
In conclusion, we found that 5 days of reduced physical activity impair popliteal artery FMD and that this impairment can be abrogated by increasing leg vascular shear stress with recurrent foot heating. These findings support the hypothesis that reduced leg blood flow-induced shear stress during physical inactivity may be a key underlying mechanism mediating detrimental leg vascular effects associated with inactivity. Thus our study presents initial evidence that foot heating may be used as a nonpharmacological therapy to combat inactivity-induced leg vascular dysfunction.
GRANTS
This study was supported by grants and scholarships from the Brazilian National Council of Scientific and Technological Development (CNPq), the Foundation for Research Support of Federal District (FAPDF), and Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES). J. Padilla was supported by National Institutes of Health Grants K01-HL-125503 and R21-DK-105368.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.T., J.P., and L.C.V. conceived and designed research; A.L.T. and L.C.V. performed experiments; A.L.T. and L.C.V. analyzed data; A.L.T., J.P., and L.C.V. interpreted results of experiments; A.L.T. prepared figures; A.L.T., J.P., and L.C.V. drafted manuscript; J.P. and L.C.V. edited and revised manuscript; A.L.T., J.P., and L.C.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all volunteer subjects are greatly appreciated. We thank Marilia F. Silva and Bruno Freitas for their technical assistance and Milena Samora, Jeann L. Sabino-Carvalho, Diego Antonino, Paulo M. Maia-Lopes, and Mayara C. Souza for their excellent support with data collection and analysis.
REFERENCES
- 1.Aboyans V, McClelland RL, Allison MA, McDermott MM, Blumenthal RS, Macura K, Criqui MH; The Multi-Ethnic Study of Atherosclerosis . Lower extremity peripheral artery disease in the absence of traditional risk factors. Atherosclerosis 214: 169–173, 2011. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 226: 425–427, 2013. doi: 10.1016/j.atherosclerosis.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 31: 287–291, 2013. doi: 10.1097/HJH.0b013e32835b8164. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kampert JB, Kohl HW III, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276: 205–210, 1996. doi: 10.1001/jama.1996.03540030039029. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 115: 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 8.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med 177: 87–95, 2017. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 61: 615–621, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang Q, Chen Y, Zhang X, Yu G, Wan X, Wang J, Bo L, Zhu K. Heat shock protein A12B protects against sepsis-induced impairment in vascular endothelial permeability. J Surg Res 202: 87–94, 2016. doi: 10.1016/j.jss.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Kröger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 14.Li MF, Ren Y, Zhao CC, Zhang R, Li LX, Liu F, Lu JX, Tu YF, Zhao WJ, Bao YQ, Jia WP. Prevalence and clinical characteristics of lower limb atherosclerotic lesions in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Diabetol Metab Syndr 6: 71, 2014. doi: 10.1186/1758-5996-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 16.McCarty MF, Barroso-Aranda J, Contreras F. Regular thermal therapy may promote insulin sensitivity while boosting expression of endothelial nitric oxide synthase: effects comparable to those of exercise training. Med Hypotheses 73: 103–105, 2009. doi: 10.1016/j.mehy.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 17.McManus AM, Ainslie PN, Green DJ, Simair RG, Smith K, Lewis N. Impact of prolonged sitting on vascular function in young girls. Exp Physiol 100: 1379–1387, 2015. doi: 10.1113/EP085355. [DOI] [PubMed] [Google Scholar]
- 18.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 265: 1053–1057, 1953. doi: 10.1016/S0140-6736(53)90665-5. [DOI] [PubMed] [Google Scholar]
- 20.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535–H1543, 2009. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 22.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009. doi: 10.1159/000226227. [DOI] [PubMed] [Google Scholar]
- 23.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 24.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedrigi RM, Mehta VV, Bovens SM, Mohri Z, Poulsen CB, Gsell W, Tremoleda JL, Towhidi L, de Silva R, Petretto E, Krams R. Influence of shear stress magnitude and direction on atherosclerotic plaque composition. R Soc Open Sci 3: 160588, 2016. doi: 10.1098/rsos.160588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol (1985) 97: 499–508, 2004. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 27.Pyke KE, Hartnett JA, Tschakovsky ME. Are the dynamic response characteristics of brachial artery flow-mediated dilation sensitive to the magnitude of increase in shear stimulus? J Appl Physiol (1985) 105: 282–292, 2008. doi: 10.1152/japplphysiol.01190.2007. [DOI] [PubMed] [Google Scholar]
- 28.Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008. doi: 10.1152/ajpheart.01372.2007. [DOI] [PubMed] [Google Scholar]
- 29.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro- and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol 312: H89–H97, 2017. doi: 10.1152/ajpheart.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 33.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374, 1995. doi: 10.1161/01.CIR.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 34.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 36.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]