We found that gut microbiota alters serotonin (5-HT)-evoked intestinal secretion in a 5-HT3 receptor-dependent mechanism and gut microbiota metabolite acetate alters 5-HT3 receptor expression in colonoids.
Keywords: irritable bowel syndrome, microbiome, motility, physiology, secretion, 5-hydroxytryptamine type 3
Abstract
Serotonin [5-hydroxytryptamine (5-HT)], an important neurotransmitter and a paracrine messenger in the gastrointestinal tract, regulates intestinal secretion by its action primarily on 5-HT3 and 5-HT4 receptors. Recent studies highlight the role of gut microbiota in 5-HT biosynthesis. In this study, we determine whether human-derived gut microbiota affects host secretory response to 5-HT and 5-HT receptor expression. We used proximal colonic mucosa-submucosa preparation from age-matched Swiss Webster germ-free (GF) and humanized (HM; ex-GF colonized with human gut microbiota) mice. 5-HT evoked a significantly greater increase in short-circuit current (ΔIsc) in GF compared with HM mice. Additionally, 5-HT3 receptor mRNA and protein expression was significantly higher in GF compared with HM mice. Ondansetron, a 5-HT3 receptor antagonist, inhibited 5-HT-evoked ΔIsc in GF mice but not in HM mice. Furthermore, a 5-HT3 receptor-selective agonist, 2-methyl-5-hydroxytryptamine hydrochloride, evoked a significantly higher ΔIsc in GF compared with HM mice. Immunohistochemistry in 5-HT3A-green fluorescent protein mice localized 5-HT3 receptor expression to enterochromaffin cells in addition to nerve fibers. The significant difference in 5-HT-evoked ΔIsc between GF and HM mice persisted in the presence of tetrodotoxin (TTX) but was lost after ondansetron application in the presence of TTX. Application of acetate (10 mM) significantly lowered 5-HT3 receptor mRNA in GF mouse colonoids. We conclude that host secretory response to 5-HT may be modulated by gut microbiota regulation of 5-HT3 receptor expression via acetate production. Epithelial 5-HT3 receptor may function as a mediator of gut microbiota-driven change in intestinal secretion.
NEW & NOTEWORTHY We found that gut microbiota alters serotonin (5-HT)-evoked intestinal secretion in a 5-HT3 receptor-dependent mechanism and gut microbiota metabolite acetate alters 5-HT3 receptor expression in colonoids.
View this article's corresponding video summary at https://www.youtube.com/watch?v=aOMYJMuLTcw&feature=youtu.be.
serotonin [5-hydroxytryptamine (5-HT)] is an important neurotransmitter and a potent secretagogue in the gastrointestinal (GI) tract (2, 29). The majority (90%) of the body’s 5-HT is derived from hydroxylation of the essential dietary amino acid l-tryptophan in the enterochromaffin (EC) cells (10). 5-HT released from the EC cells activates multiple 5-HT receptor subtypes to modulate GI functions including peristalsis (8), intestinal secretion (26, 29, 36), and sensation (9, 22). Seven different families of 5-HT receptors and at least 14 pharmacologically distinct 5-HT receptor subtypes have been described (1). Among these receptor subtypes, 5-HT3 and 5-HT4 receptors play an important role in regulating intestinal secretion (3, 13, 40). 5-HT3 receptors are the members of the Cys-loop ligand-gated ion channel family (4). In the GI tract, 5-HT3 receptors are located on multiple cell types including submucosal neurons, interstitial cells of Cajal, and few enteroendocrine cells including the EC cells (11). 5-HT4 receptors, on the other hand, are G protein-coupled receptors that stimulate cAMP production (30). 5-HT4 receptors are localized in the enteric neurons (35), smooth muscles (32), and epithelial cells including the EC cells (15). Effects of 5-HT mediated secretion in Ussing chamber involve activation of both neuronal and nonneuronal 5-HT3 and 5-HT4 receptors (6, 26, 31).
The gut lumen is home to trillions of microorganisms that include over 1,000 different bacterial species (16). However, the dynamic interaction between the gut microbiota and the host and how it modulates GI physiology are largely unknown. We have recently shown that human gut microbiota increases host 5-HT biosynthesis through an effect of short-chain fatty acid (SCFA; 34) and modulates GI transit in part via 5-HT3 and 5-HT4 receptors (20). Since 5-HT3 and 5-HT4 receptors are important mediators of intestinal secretion, in this study we investigated the physiological effects of microbial colonization on ex vivo host secretory response to exogenously applied 5-HT and on 5-HT3 and 5-HT4 receptor expression.
MATERIALS AND METHODS
Animals.
Swiss Webster germ-free (GF) breeders, used to produce the GF and the humanized (HM; ex-GF colonized with human gut microbiota) mice in this study, were obtained from Taconic Farms (Germantown, NY) and maintained in gnotobiotic isolators in the Mayo Clinic gnotobiotic facility. Humanization of GF mice was done by colonizing GF mice with 300 µl of prereduced PBS and fecal microbiota (a 1:1 suspension) from a healthy human donor (a 49-yr-old man) at 4–5 wk of age by gavage. All HM mice were colonized with human microbiota for 4–5 wk before experiments. GF and HM male mouse tissues were collected between 10 and 12 wk. Age-matched conventionally raised (CR) mice were purchased from Taconic Farms and maintained outside the isolator. All studies were conducted after approval from Mayo Clinic Institutional Animal Care and Use Committee. Tissue from 5-HT3A-green fluorescent protein (GFP) mice was obtained from University of Colorado, where conventionally housed FVB/N-Swiss Webster mice [Stock Tg(Htr3a-EGFP)DH30Gsat/Mmnc; RRID:IMSR_MMRRC:000273] were crossed with C57BL/6 line for two to four generations. Mice were intraperitoneally injected with 5-hydroxy-l-tryptophan (50 mg/kg; Sigma-Aldrich, St. Louis, MO) 1 h before euthanasia to increase 5-HT levels.
Tissue preparation.
Mice were asphyxiated with CO2, and euthanasia was confirmed by cervical dislocation. Proximal colon segments 2–3 cm below the cecum were removed and opened along the mesenteric border. The tissues were pinned flat on the Sylgard with mucosa side facing up. The luminal contents were then washed with chilled Krebs solution (composition in mM/l: 11.5 d-glucose/d-mannitol, 120.3 NaCl, 15.5 NaHCO3, 5.9 KCl, 1.2 NaH2PO4, 2.5 CaCl2·2H2O, and 1.2 MgCl2; pH 7.3–7.4). The colon segments were then flipped, and the underlying muscle layer was peeled away under a stereomicroscope with fine forceps. A 0.2-cm2 segment of mucosa-submucosa sheet was stored in RNAlater Stabilization Solution (Ambicon) for 1 h at 4°C and then moved to −20°C until ready for real-time PCR analysis. The remaining larger mucosa-submucosa epithelial sheets were used for Ussing chamber studies.
Colonoid preparation.
GF mice were asphyxiated with CO2, and euthanasia was confirmed by cervical dislocation. Entire colon segment below the cecum was removed in a sterile manner and collected in a cold, sterile Ca2+-Mg-free PBS (21-040-CV; Corning). The colon was cut along the mesenteric border, and the pellet was cleaned using a 10-ml sterile syringe filled with sterile PBS. With the use of sterile scissors, the colon segment was minced into ~1-mm2 pieces. The tissue chunks were then washed with cold Ca2+-Mg-free PBS until the supernatant was clear of any pellet debris. The clean tissue pieces were incubated in gentle cell dissociation media (7174; Stem Cell Technologies) for 15 min and rinsed vigorously using a 10-ml Pasteur pipette filled with freshly prepared filter-sterilized 0.1% PBS-BSA solution. The solution was then filtered through 70-µm nylon mesh (22363548; Fisher Scientific), and the filtrate-containing crypt cells were centrifuged in a 15-ml conical tube at 290 g at 4°C for 5 min. The centrifuged cell pellets were incubated in 6–7 ml of DMEM-F12 media (11330-032; Life Technologies) and recentrifuged at 290 g at 4°C for 5 min. The cell pellets were then broken down and mixed with 150 µl of IntestiCult Organoid Growth Medium (IOGM; 06000; Stem Cell Technologies) and 150 µl of reduced Matrigel (1:1 ratio, 356231; Corning). Seventy-five microliters of cell-Matrigel mixture were plated in a warm 24-well plate and supplemented with 750 µl (1:10 of Matrigel dome-IOGM mix) of warm IOGM. The colonoids were allowed to grow for 1 wk and passaged four times before they were incubated with sodium acetate (S2889; Sigma-Aldrich) and sodium butyrate (B5887; Sigma-Aldrich). Three technical replicates from five mice were plated uniformly in a warm 24-well plate. Quantitative real-time (qRT)-PCR was performed as described in materials and methods, Quantitative real-time PCR for 5-HT3 and 5-HT4 receptor mRNA expression.
Ussing chamber for measuring in vitro epithelial transport.
To measure 5-HT-induced in vitro epithelial transport, a proximal colon section 1–2 cm below the cecum was mounted in an Ussing chamber (Physiologic Instruments, San Diego, CA) with an aperture of 0.31 cm2. The chamber on the submucosal side was bathed with 4 ml of glucose-containing Krebs solution while the mucosal side was bathed with 4 ml of mannitol Krebs solution. The Krebs solution was bubbled with 97% O2 and 3% CO2 mixture. The tissues were short circuited by a voltage-clamp at zero potential using Ag/AgCl electrode that was inserted into a pipette tip filled with a salt-filled agar bridge (P2020-S; Physiologic Instruments) and connected to a voltage-clamp amplifier (VCC MC8; Physiologic Instruments). Transepithelial resistance (TER) was measured and averaged over a period of half an hour before application of drugs. The final TER (Ω·cm2) was calculated by multiplying the sample resistance with effective membrane area (0.31 cm2). Baseline short-circuit current (Isc) and change in short-circuit current (ΔIsc) were continuously recorded using Acquire & Analyze software (Physiologic Instruments). ΔIsc values were measured after application of drugs to the submucosal side. ΔIsc values were normalized to the tissue area for final calculations.
Quantitative real-time PCR for 5-HT3 and 5-HT4 receptor mRNA expression.
To detect whether there were changes in 5-HT3 and 5-HT4 receptor mRNA from mice proximal colon mucosa-submucosa preparation and in SCFA-treated GF colonoids, qRT-PCR was used. RNA was extracted and isolated using a tissue homogenizer and the RNeasy Mini Kit (Qiagen, Valencia, CA) with DNase I (Qiagen) treatment. RNA isolates were reverse transcribed with the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). The cDNA thus obtained was used for real-time PCR analysis. The qRT-PCR assays were performed in 25-µl reactions containing 1× LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) and 300-nM gene-specific primers (100 nM for housekeeping gene). Samples were analyzed in triplicate using a LightCycler 480 Instrument II (Roche). All cDNA synthesis reactions were set up with additional samples lacking reverse transcriptase as a control for genomic DNA contamination. Total mRNA expression was calculated after normalization to L32 mRNA (housekeeping) using the comparative cycle threshold (ΔΔCT) analysis method. Relative mRNA expression (fold difference) was calculated on the basis of the mRNA expression in humanized control after normalization. 5-HT3 and 5-HT4 primer sequences were synthesized at the Integrated DNA Technologies facility at Mayo Clinic (Rochester, MN). The primer sequences are listed in Table 1.
Table 1.
5-HT3 and 5-HT4 primer sequences
| Sequence No. | Receptor | Primer Sequence |
|---|---|---|
| 1 | 5-HT3B | Forward: 5′-CTGCATGGCCCTCTTGGTT-3′ |
| Reverse: 5′-AATCTAGACTCCTCGGCATC-3′ | ||
| 2 | 5-HT4 | Forward: 5′-AGTTCCAACGAGGGTTTCAGG-3′ |
| Reverse: 5′-CAGCAGGTTGCCCAAGATG-3′ | ||
| 3 | L32 | Forward: 5′-CCTCTGGTGAAGCCCAAGATC-3′ |
| Reverse: 5′-TCTGGGTTTCCGCCAGTTT-3′ |
Western blot analysis for 5-HT3 subunit protein expression.
Mucosa-submucosa from the proximal colon was quickly removed in cold Krebs solution to prevent protein degradation. Tissues were stored at −80°C until ready to be processed and homogenized. Homogenized tissues were lysed with 1× lysis buffer (9803s; Cell Signaling Technology) that contained protease inhibitor cocktail (539131; Calbiochem). Protein extracts (50 µg per lane) were loaded for SDS-PAGE along with the full-range rainbow molecular weight marker (MagicMark, LC5602; Invitrogen). Proteins were separated at 105 V on a 12% Mini-PROTEAN TGX gel (456-1044s; Bio-Rad) and electrotransferred for 45 min onto a polyvinylidene difluoride membrane (IPVH00010; Millipore) at 15 V. Tissues were later blocked for 1 h at room temperature with 5% milk in 1× Tris-buffered saline (170-6435; Bio-Rad) and 0.1% Tween 20 (TBS-T; 9005-64-5; Fisher Bioreagents) to prevent nonspecific binding. Membranes were then incubated overnight at 4°C with primary antibodies 5-HT3B (1:1,600, PA5-41033; Invitrogen) and GAPDH (1:10,000, G9545; Sigma-Aldrich). Membranes were then washed (3 × 10 min) with TBS-T and blotted with the horseradish peroxidase-conjugated secondary antibodies (anti-5-HT3B, 1:800; anti-GAPDH, 1:2,000) for 1 h at room temperature in blocking solution. Blots were detected with enhanced chemiluminescence (ECL) Western Blotting Substrate (33209; Thermo Fisher Scientific) and analyzed with ImageJ software (version 1.50i; National Institutes of Health). Relative protein abundance (fold difference) was determined relative to HM group after normalization to GAPDH.
Immunohistochemistry for 5-HT3 receptor expression.
Proximal colon tissues 2–4 cm below the cecum were harvested from conventionally housed 5-HT3A-GFP mice immediately following perfusion fixation and postfixed with 4% paraformaldehyde for 4 h. Tissues were cryoprotected in 20% sucrose and shipped, on ice, to Mayo Clinic for embedding in optimum cutting temperature compound. Cryostat sections were cut at 10 μm. The sections were washed (3 × 10-min intervals) with PBS and permeabilized with 0.1% Triton X-100. Tissues were blocked with 5% normal donkey serum and 0.5% BSA in PBS for 30 min at room temperature and immunoreacted with chicken anti-GFP antibody (1:500 dilution, A10262; Life Technologies) and goat anti-5-HT antibody (1:2,000 dilution, AB66047; Abcam) for 2 h at room temperature. Tissues were then washed (2 × 5-min intervals) with PBS. Antibody binding was visualized using Alexa Fluor 488-conjugated donkey anti-chicken antibody (1:500 dilution, 703-545-155; Jackson ImmunoResearch) and Alexa Fluor 594-conjugated donkey anti-goat antibody (1:500 dilution, 705-585-147; Jackson ImmunoResearch), respectively. Slides were mounted with a 1:1 mixture of Citifluor AF1 (17970-25; VWR) and SlowFade Gold Antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI; S36938; Thermo Fisher Scientific). Fluorescent images were obtained using an epifluorescence Olympus (BX51WI) microscope using ×20 and ×40 objectives.
Statistics.
Data are presented as means ± SE. Potential differences were tested either using ANOVA or Student’s t-test as appropriate. P < 0.05 was considered significant. Analysis was done in GraphPad Prism 6.0 software (San Diego, CA), and n stands for number of animals from which tissues were harvested.
Drugs.
The following drugs were used: serotonin (5-HT), forskolin (10 µM), ondansetron (1 µM), GR-113808 (100 µM), 2-methyl-5-hydroxytryptamine hydrochloride (10 µM), and tetrodotoxin (TTX; 500 nM). 5-HT hydrochloride was obtained from Sigma-Aldrich (CAS no. 153-98-0). Forskolin was obtained from Sigma-Aldrich (CAS no. 66575-29-9). Ondansetron hydrochloride (CAS no. 99614-01-04), GR-113808 (CAS no. 144625-51-4), and 2-methyl-5-hydroxytryptamine hydrochloride (CAS no. 845861-44-6) were all obtained from Tocris Bioscience (Bristol, United Kingdom). Drugs were dissolved and diluted in distilled water.
RESULTS
Microbial colonization does not affect intestinal permeability and baseline secretion.
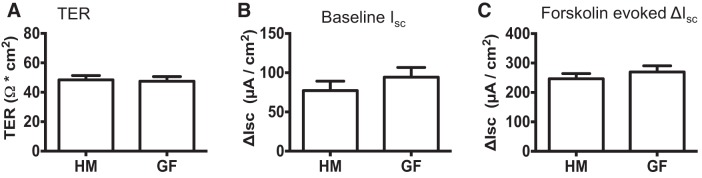
To test whether microbial colonization affects tissue integrity, baseline secretory response, and forskolin-evoked secretory response, we measured TER, baseline Isc, and forskolin-induced ΔIsc in GF and HM mice. We found that there was no significant difference in TER (47.5 ± 3.20 vs. 48.4 ± 2.95 Ω·cm2, n = 10, unpaired t-test; P > 0.05; Fig. 1A), baseline Isc (94.28 ± 12.47 vs. 77.16 ± 12 µA/cm2, n = 10, unpaired t-test; P > 0.05; Fig. 1B), or forskolin-evoked ΔIsc response (269 ± 20.9 vs. 247 ± 17.6 µA/cm2, n = 5, unpaired t-test; P > 0.05; Fig. 1C) between GF and HM mice.
Fig. 1.
Microbial colonization does not affect intestinal permeability and baseline secretion. TER (A), baseline Isc (B), and forskolin (10 µM)-evoked ΔIsc (C) in GF and HM mice.
Host secretory response to exogenously applied 5-HT is increased in the absence of gut microbiota.
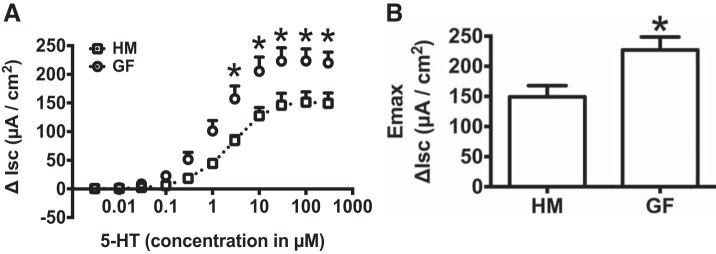
Since we did not observe changes in baseline secretion with microbial colonization, we next wanted to determine whether gut microbiota colonization modulates 5-HT-evoked secretory response. Hence we measured 5-HT-evoked ΔIsc in GF and HM mice. Application of cumulative concentrations of 5-HT increased ΔIsc in both GF and HM mice (Fig. 2). 5-HT-evoked ΔIsc was significantly higher in GF compared with HM mice (n = 6, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 2A). 5-HT-evoked maximum ΔIsc (Emax) was significantly higher in GF than HM mice (227.2 ± 21.4 vs. 149.3 ± 18.4 µA/cm2, n = 6, unpaired t-test; P < 0.05; Fig. 2B).
Fig. 2.
The maximal secretory response to 5-HT is greater in GF than HM mice. A: change in short-circuit current in response to cumulative concentration of 5-HT in HM and GF mice. B: 5-HT-induced Emax in GF and HM mice. *P < 0.05.
5-HT3 receptor mRNA and protein expression is significantly increased when gut microbiota is absent.
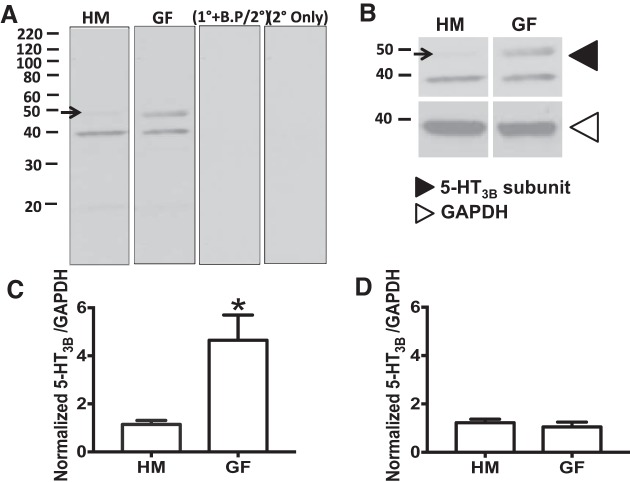
To determine whether increased secretory response to 5-HT seen in GF mice was due to an increase in 5-HT (5-HT3 and 5-HT4) receptor expression, we compared mRNA and protein expression in GF and HM mice (Fig. 3). We found a significant increase in 5-HT3B receptor mRNA expression in GF compared with HM mice (2.0 ± 0.38 vs. 1.0 ± 0.22, n = 8–9, unpaired t-test; P < 0.05; Fig. 3A). This difference was not observed in 5-HT4 receptor expression between GF and HM mice (0.78 ± 0.29 vs. 1.00 ± 0.17, n = 8–9, unpaired t-test; P > 0.05; Fig. 3B). To verify whether increased 5-HT3B receptor mRNA expression translated to higher protein expression in GF mice, we quantified 5-HT3 receptor protein using Western blot (Fig. 4A). The 5-HT3B antibody identified two bands at 50 kDa (expected size for 5-HT3) and 40 kDa (Fig. 4A; 25). Both bands were absent in the presence of a specific blocking peptide. GF mice had significantly higher expression of the 50-kDa band compared with HM mice (4.65 ± 1.05 vs. 1.15 ± 0.17, n = 12, unpaired t-test; P < 0.05; Fig. 4C); however, no difference in 40-kDa band intensity was observed (1.05 ± 0.20 vs. 1.22 ± 0.15, n = 13, unpaired t-test; P > 0.05; Fig. 4D).
Fig. 3.
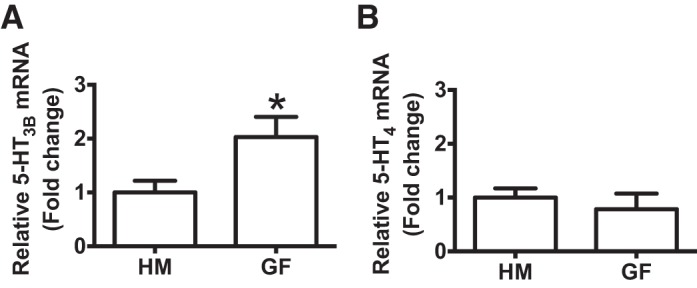
5-HT3B receptor mRNA expression is higher in GF mice than HM mice. A: relative expression of 5-HT3B mRNA in HM and GF mice. B: relative expression of 5-HT4 mRNA in HM and GF mice. *P < 0.05.
Fig. 4.
5-HT3B receptor protein expression is higher in GF mice than HM mice. A: 5-HT3B subunit antibody detected two bands at ~50 and 40 kDa. Controls include blockade of antibody binding by preincubating the primary antibody with its competing peptide (1°+B.P/2°) and secondary-only controls (2° Only). B: representative blot of 5-HT3B subunit and GAPDH expression in GF and HM mouse proximal colon. C and D: quantification of the 5-HT3B subunit band intensity present at 50 kDa (top band) and 40 kDa (bottom band), respectively, in HM and GF mice. *P < 0.05.
Colonization-dependent difference in secretory response to 5-HT in GF mice is mediated by altered 5-HT3 receptor expression.
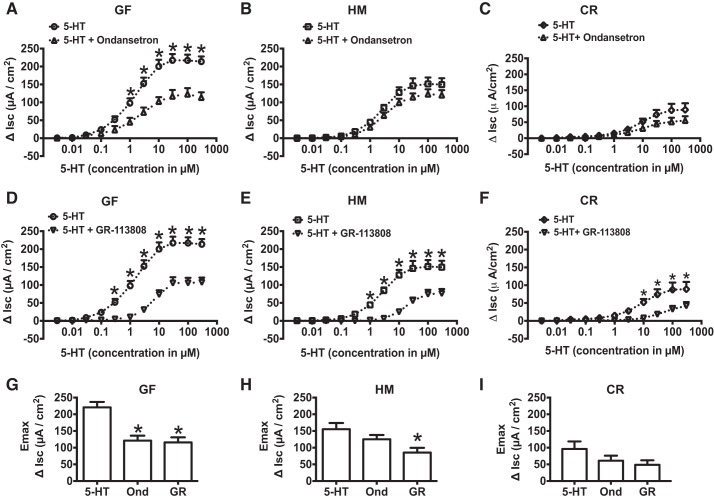
Since we observed an increase in 5-HT3 but not 5-HT4 receptor expression in GF mice, we next wanted to determine the role of 5-HT3 and 5-HT4 receptors in differential secretory response to 5-HT in GF and HM mice using specific 5-HT3 and 5-HT4 receptor antagonists (Fig. 5). Ondansetron (100 nM), a 5-HT3 receptor antagonist, significantly inhibited 5-HT-evoked ΔIsc in GF mice (n = 6–8, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 5A) but not in HM mice (P > 0.05; Fig. 5B). GR-113808 (30 nM), a 5-HT4 receptor antagonist, significantly inhibited 5-HT-evoked ΔIsc in both GF and HM mice (n = 6–8, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 5, D and E). 5-HT-evoked Emax in GF was significantly reduced by both ondansetron and GR-113808 (221 ± 16.3 vs. 121 ± 14.9 vs. 116 ± 15.3 µA/cm2, n = 6–8, 1-way ANOVA; P < 0.05; Fig. 5G), whereas 5-HT-evoked Emax in HM was significantly inhibited by GR-113808 (163 ± 18.5 vs. 69 ± 14.0 µA/cm2, n = 6, 1-way ANOVA; P < 0.05) but not by ondansetron (163 ± 18.5 vs. 120 ± 13.0 µA/cm2, n = 6, 1-way ANOVA; P > 0.05; Fig. 5H). We found that similar to HM mice, 5-HT-evoked ΔIsc in CR mice was significantly reduced following GR-113808 application (n = 6, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 5F) but not with ondansetron (n = 6, 2-way ANOVA with Bonferroni post hoc test; P > 0.05; Fig. 5C), suggesting that the effect is common to both mouse and human-derived gut microbiota. To further determine whether the increased secretory response in GF mice can be attributed to increased expression of 5-HT3 receptors, we used a 5-HT3 receptor-specific agonist, 2-methyl-5-hydroxytryptamine hydrochloride (100 μM), which evoked a significantly higher ΔIsc in GF compared with HM mice (86.6 ± 18.1 vs. 32.0 ± 5.6 µA/cm2, n = 5, unpaired t-test; P < 0.05; Fig. 6).
Fig. 5.
5-HT3 receptor antagonist inhibits 5-HT-evoked ΔIsc only in GF mice whereas 5-HT4 receptor antagonist significantly blocks ΔIsc in both HM and GF mice. A: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of ondansetron in GF mice. B: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of ondansetron in HM mice. C: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of ondansetron in CR mice. D: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of GR-113808 in GF mice. E: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of GR-113808 in HM mice. F: change in short-circuit current in response to cumulative concentration of 5-HT and 5-HT in the presence of GR-113808 in CR mice. 5-HT-induced Emax for GF (G), HM (H), and CR (I) mice in the presence of ondansetron (Ond) and GR-113808 (GR). *P < 0.05.
Fig. 6.
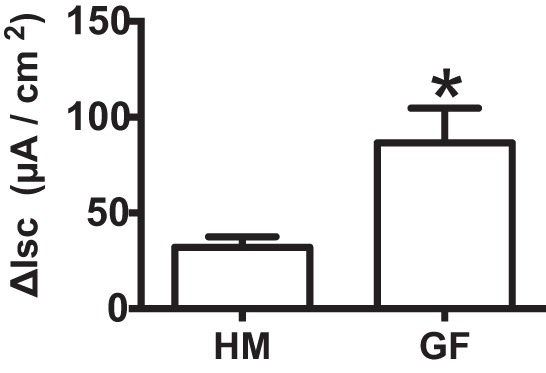
Increased secretory response to 5-HT in GF mice is mediated by 5-HT3 receptor. 5-HT3 receptor-selective agonist shows a significantly greater secretory response in GF mice compared with HM mice. *P < 0.05.
Epithelial 5-HT3 receptors likely contribute to increased host secretory response to 5-HT in GF mice.
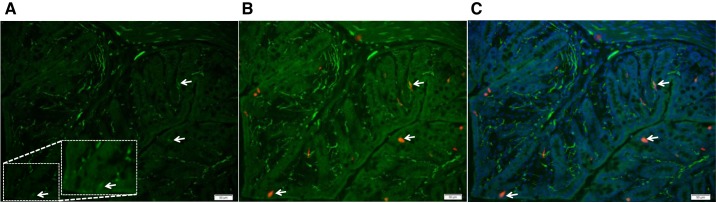
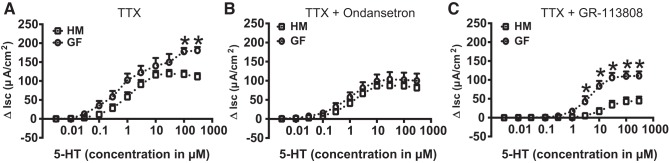
An earlier study identified 5-HT3 receptor expression in both the nerve fibers and the EC cells of rat small intestine (11). To determine the distribution of 5-HT3 receptors in mouse colon, we used 5-HT3A-GFP mouse tissues. Immunohistochemistry revealed 5-HT3A-GFP-positive cells in the mouse colonic epithelium and nerve fibers within the lamina propria (Fig. 7A). In the colonic epithelium, 5-HT3A-GFP colocalized with 5-HT and DAPI representing EC cells (Fig. 7, B and C) confirming mucosal expression of 5-HT3 receptors. Next, to ascertain whether the difference in ΔIsc response to 5-HT between GF and HM mice was in part due to the epithelial 5-HT3 receptors, we measured 5-HT-evoked ΔIsc in the presence of TTX (500 nM) to block the neuronal contribution. We found that the ΔIsc was significantly higher in GF compared with HM mice (n = 5, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 8A) in the presence of TTX suggesting a nonneuronal epithelial component contributing to the difference in 5-HT-evoked ΔIsc. To determine whether epithelial 5-HT3 receptors play a role in the differential 5-HT-evoked response, we measured ΔIsc in the presence of TTX and ondansetron. The above-noted difference in ΔIsc between GF and HM in the presence of TTX was lost in the presence of TTX and ondansetron (n = 5, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 8B) suggesting that epithelial 5-HT3 receptor likely contributes to the differential secretory response in GF and HM mice. Interestingly, the difference in 5-HT-evoked ΔIsc between GF and HM mice in the presence of TTX persisted in the presence of GR-113808 and TTX (n = 5, 2-way ANOVA with Bonferroni post hoc test; P < 0.05; Fig. 8C) suggesting that epithelial 5-HT4 receptors are likely involved in the differential secretory response to 5-HT.
Fig. 7.
5-HT3 receptors are expressed in the epithelial cells of colonic mucosa and in submucosal neuronal fibers. A: arrows highlight 5-HT3A-GFP-positive cells in the mouse colonic epithelium. A subset of 5-HT3-GFP-positive cells (B) colocalize with 5-HT and DAPI (C) in the proximal colon.
Fig. 8.
Epithelial 5-HT3 receptor is responsible in part for the differential secretory response to 5-HT. Change in short-circuit current in response to cumulative concentration of 5-HT in the presence of TTX (A), change in short-circuit current in response to cumulative concentration of 5-HT in the presence of TTX and ondansetron (B), and change in short-circuit current in response to cumulative concentration of 5-HT in the presence of TTX and GR-113808 (C) in GF and HM mice. *P < 0.05.
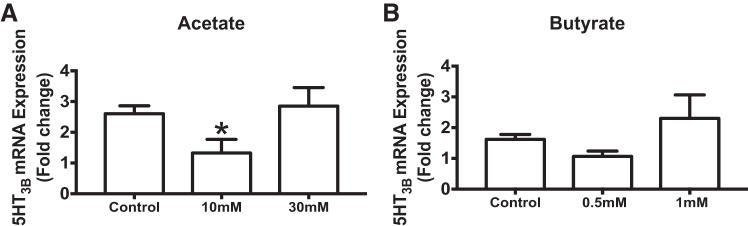
Incubation of colonoids with acetate significantly decreases 5-HT3 receptor expression in GF mice.
To determine the microbial mediators that affect 5-HT3 receptor expression, we treated GF mouse-derived colonoids with acetate and butyrate. We found that incubation of epithelial colonoids with 10 mM sodium acetate for 24 h significantly decreased 5-HT3 receptor expression compared with untreated controls (2.6 ± 0.26 vs. 1.33 ± 0.44, n = 5, repeated-measures 1-way ANOVA with Holm-Sidak’s multiple-comparisons test; P < 0.05; Fig. 9A). Although incubation with sodium butyrate did not significantly decrease 5-HT3 receptor expression, sodium butyrate (0.5 mM) showed a trend toward reduced 5-HT3 receptor expression (1.62 ± 0.16 vs. 1.07 ± 0.17, n = 5; P = 0.065; Fig. 9B).
Fig. 9.
Incubation of GF colonoids with acetate significantly decreases 5-HT3B receptor mRNA expression. A: effect of acetate on 5-HT3B receptor expression in GF colonoids. B: effect of butyrate on 5-HT3B receptor expression in GF colonoids. *P < 0.05.
DISCUSSION
The aim of this study was to investigate the effect of microbial colonization on 5-HT-evoked secretory response and the receptor subtype involved in this response. We chose to use human-derived gut microbiota for colonization given the significant differences in mouse and human gut microbiota and the greater translational applicability of our findings for human conditions. We found no difference in TER, baseline Isc, and ΔIsc in response to forskolin in GF and HM mice, suggesting that microbial colonization status does not affect tissue permeability or baseline secretion or cause defects in cAMP-dependent secretory response in mice. We next examined whether microbiota colonization status alters evoked secretory response to exogenously applied 5-HT, a potent secretagogue, which has been previously shown to mediate Cl− and HCO3− secretion (12, 19). We found that both GF and HM mice showed increased ΔIsc in response to exogenously applied 5-HT in a concentration-dependent manner, suggesting that 5-HT mediates the secretory response irrespective of microbial colonization. Interestingly, we found that GF mice had a significantly higher secretory response to exogenously applied 5-HT than HM mice, which implies that the presence of gut microbiota and associated changes result in a dampening of 5-HT-induced secretion. We hypothesized that the increase in secretory response to exogenously applied 5-HT in GF mice was due to increased 5-HT receptor expression in GF mice. Among various families of 5-HT receptors (5-HT1P, 5-HT2, 5-HT3, and 5-HT4) that potentially mediate secretion (26), 5-HT3 and 5-HT4 receptors have been best studied (29). We therefore investigated whether gut microbiota alters 5-HT3 or 5-HT4 receptor mRNA expression. The 5-HT3 receptor family comprises five different receptor subtypes—5-HT3A, 5-HT3B, 5-HT3C, 5-HT3D, and 5-HT3E—among which only 5-HT3A and 5-HT3B subtypes are well characterized (5). Studies suggest that although 5-HT3 receptors can exist as either a homopentameric 5-HT3A or a heteropentameric 5-HT3A/B receptor, only heteropentameric 5-HT3A/B receptors replicate functional native mammalian 5-HT3 channels (5, 7, 24, 27, 37, 38). Among 5-HT3A and 5-HT3B receptor subtypes, we chose to study 5-HT3B receptor expression for two reasons: first, 5-HT3B, and not 5-HT3A, receptor subunit mRNA expression undergoes adaptive changes in mice (21, 23), and second, 5-HT3B receptor expression modulates the biophysical and pharmacological properties (increased single-channel conductance, low permeability to calcium ions, and faster recovery from desensitization) of heteromeric 5-HT3A/B receptors to closely mimic native 5-HT3 receptors (5, 18). We found that 5-HT3B subunit mRNA expression was significantly higher in GF mice compared with HM mice whereas 5-HT4 receptor expression remained unchanged between the two groups of mice. We verified that increased 5-HT3B mRNA expression leads to increased 5-HT3B protein expression using Western blot. The protein band at ~50 kDa (expected molecular weight for 5-HT3B subunit in mice), was significantly higher in GF mice than HM mice. An additional protein band, which has been previously described, was also detected at ~40 kDa and was neutralized using control peptide. However, it remains unclear whether this additional band represents a posttranslational modification (25, 28, 33) or a nonspecific protein recognized by this particular antibody. The 5-HT3B subunit is a major determinant of 5-HT receptor function (5, 14), and our data show an increase in 5-HT3B receptor subunit in GF mice, which correlates with increased secretory response to 5-HT in GF mice. We further confirmed that increase in 5-HT3 receptor expression in GF mice contributes to increased colonic secretion as evidenced by a significantly greater response to specific 5-HT3 receptor agonist and significant inhibition by specific 5-HT3 receptor antagonist. We found that although 5-HT4 receptors also mediate secretion in mice colon, microbial colonization does not affect 5-HT4 receptor expression or 5-HT4-dependent secretory response to 5-HT. The effect on 5-HT3 receptor-dependent secretory response was seen in both HM and CR mice suggesting that a relatively conserved microbial function drives this process.
5-HT-evoked intestinal secretion could occur either via activation of 5-HT3 neural reflex arch or via direct autocrine mucosal activation of 5-HT3 receptors (13). We found that 5-HT3 receptors are expressed in EC cells in the colonic epithelium. An increased secretory response to 5-HT in GF mice was also seen following blockade of neural activity. Furthermore, the secretory response was inhibited by specific 5-HT3 receptor antagonist following blockade of neural activity, suggesting a role for epithelial 5-HT3 receptors in microbiota-mediated modulation of 5-HT secretory response. Our results support previously published studies that show that 5-HT3 receptor is not only present in the GI epithelium (11, 39) but also involved in mediating intestinal secretion via a nonneuronal pathway (17). Gut microbiota has been shown to increase 5-HT biosynthesis through an effect of SCFA (34), which may, in turn, affect 5-HT3 receptor expression. Our study suggests that bacterial fermentation product acetate can modulate 5-HT3B receptor expression in vitro in colonoids.
In conclusion, our study suggests that gut microbiota can alter host secretory response to 5-HT, in part by an effect on 5-HT3 receptor expression via acetate production. Future studies elucidating the mechanisms that lead to acetate-driven altered mucosal 5-HT3 receptor expression will allow a better understanding of the role of gut microbiota in pathogenesis of functional bowel disorders and the development of novel live biotherapeutic and microbial products to alter intestinal secretion.
GRANTS
This work was made possible by funding from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant 100638 (P. C. Kashyap), Global Probiotics Council (P. C. Kashyap), and Center for Individualized Medicine, Mayo Clinic (P. C. Kashyap). M. Grover is supported by NIDDK Grant 103911. A. Beyder is supported by NIDDK Grant 106456.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.B., B.A.S., and P.C.K. conceived and designed research; Y.B. and B.A.S. performed experiments; Y.B., B.A.S., and P.C.K. analyzed data; Y.B., B.A.S., D.R.L., M.G., A.B., G.F., and P.C.K. interpreted results of experiments; Y.B. and P.C.K. prepared figures; Y.B. drafted manuscript; Y.B., B.A.S., D.R.L., E.D.L., M.G., A.B., G.F., and P.C.K. edited and revised manuscript; Y.B., B.A.S., D.R.L., E.D.L., M.G., A.B., G.F., and P.C.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kristy Zodrow for administrative assistance. We also thank Lisa Till for help with gnotobiotic husbandry and experiments. E. D. Larson supplied 5-HT3A-GFP tagged mice tissues.
REFERENCES
- 1.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152, 1999. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Beubler E, Horina G. 5-HT2 and 5-HT3 receptor subtypes mediate cholera toxin-induced intestinal fluid secretion in the rat. Gastroenterology 99: 83–89, 1990. doi: 10.1016/0016-5085(90)91233-V. [DOI] [PubMed] [Google Scholar]
- 3.Budhoo MR, Harris RP, Kellum JM. The role of the 5-HT4 receptor in Cl- secretion in human jejunal mucosa. Eur J Pharmacol 314: 109–114, 1996. doi: 10.1016/S0014-2999(96)00474-8. [DOI] [PubMed] [Google Scholar]
- 4.Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem Soc Trans 32: 529–534, 2004. doi: 10.1042/bst0320529. [DOI] [PubMed] [Google Scholar]
- 5.Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397: 359–363, 1999. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- 6.Day J, King B, Haque SM, Kellum JM. A nonneuronal 5-hydroxytryptamine receptor 3 induces chloride secretion in the rat distal colonic mucosa. Am J Surg 190: 736–738, 2005. doi: 10.1016/j.amjsurg.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Dubin AE, Huvar R, D’Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, Jackson MR, Lovenberg TW, Erlander MG. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J Biol Chem 274: 30799–30810, 1999. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- 8.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology 111: 1281–1290, 1996. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 9.Galligan JJ, Patel BA, Schneider SP, Wang H, Zhao H, Novotny M, Bian X, Kabeer R, Fried D, Swain GM. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil 25: e373–e381, 2013. doi: 10.1111/nmo.12133. [DOI] [PubMed] [Google Scholar]
- 10.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 123: 217–226, 2002. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MB, Bindslev N. Serotonin receptors for chloride secretion in hen colon. Comp Biochem Physiol C 94: 189–197, 1989. doi: 10.1016/0742-8413(89)90166-7. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MB, Skadhauge E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp Biochem Physiol A Physiol 118: 283–290, 1997. doi: 10.1016/S0300-9629(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 14.Hapfelmeier G, Tredt C, Haseneder R, Zieglgänsberger W, Eisensamer B, Rupprecht R, Rammes G. Co-expression of the 5-HT3B serotonin receptor subunit alters the biophysics of the 5-HT3 receptor. Biophys J 84: 1720–1733, 2003. doi: 10.1016/S0006-3495(03)74980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854, 2012. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iglesias RC, Day J, Howell J, Kellum J. 5-HT3 receptor agonist 2-methyl-5-HT mediates nonneural chloride secretion in human jejunum in vitro (Abstract). J Am Coll Surg 205, Suppl: S18, 2007. doi: 10.1016/j.jamcollsurg.2007.06.037. [DOI] [Google Scholar]
- 18.Jensen AA, Davies PA, Bräuner-Osborne H, Krzywkowski K. 3B but which 3B and that’s just one of the questions: the heterogeneity of human 5-HT3 receptors. Trends Pharmacol Sci 29: 437–444, 2008. doi: 10.1016/j.tips.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172: 4655–4670, 2015. doi: 10.1111/bph.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G, Sonnenburg JL. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144: 967–977, 2013. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keating C, Nocchi L, Yu Y, Donovan J, Grundy D. Ageing and gastrointestinal sensory function: altered colonic mechanosensory and chemosensory function in the aged mouse. J Physiol 594: 4549–4564, 2016. doi: 10.1113/JP271403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keszthelyi D, Troost FJ, Jonkers DM, van Eijk HM, Dekker J, Buurman WA, Masclee AA. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism–a preliminary study. Neurogastroenterol Motil 27: 1127–1137, 2015. doi: 10.1111/nmo.12600. [DOI] [PubMed] [Google Scholar]
- 23.Liu MT, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol 283: G1398–G1411, 2002. doi: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- 24.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254: 432–437, 1991. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 25.Massoura AN, Dover TJ, Newman AS, Barnes NM. The identification of N-glycosylated residues of the human 5-HT3B receptor subunit: importance for cell membrane expression. J Neurochem 116: 975–983, 2011. doi: 10.1111/j.1471-4159.2010.07129.x. [DOI] [PubMed] [Google Scholar]
- 26.Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles TF, Dougherty DA, Lester HA. The 5-HT3AB receptor shows an A3B2 stoichiometry at the plasma membrane. Biophys J 105: 887–898, 2013. doi: 10.1016/j.bpj.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monk SA, Desai K, Brady CA, Williams JM, Lin L, Princivalle A, Hope AG, Barnes NM. Generation of a selective 5-HT3B subunit-recognising polyclonal antibody; identification of immunoreactive cells in rat hippocampus. Neuropharmacology 41: 1013–1016, 2001. doi: 10.1016/S0028-3908(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 29.Nagakura Y, Kontoh A, Tokita K, Tomoi M, Shimomura K, Kadowaki M. Combined blockade of 5-HT3- and 5-HT4-serotonin receptors inhibits colonic functions in conscious rats and mice. J Pharmacol Exp Ther 281: 284–290, 1997. [PubMed] [Google Scholar]
- 30.Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics 9: 501–504, 2008. doi: 10.2217/14622416.9.5.501. [DOI] [PubMed] [Google Scholar]
- 31.Ning Y, Zhu JX, Chan HC. Regulation of ion transport by 5-hydroxytryptamine in rat colon. Clin Exp Pharmacol Physiol 31: 424–428, 2004. doi: 10.1111/j.1440-1681.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- 32.Prause AS, Guionaud CT, Stoffel MH, Portier CJ, Mevissen M. Expression and function of 5-hydroxytryptamine 4 receptors in smooth muscle preparations from the duodenum, ileum, and pelvic flexure of horses without gastrointestinal tract disease. Am J Vet Res 71: 1432–1442, 2010. doi: 10.2460/ajvr.71.12.1432. [DOI] [PubMed] [Google Scholar]
- 33.Reeves DC, Lummis SC. Detection of human and rodent 5-HT3B receptor subunits by anti-peptide polyclonal antibodies. BMC Neurosci 7: 27, 2006. doi: 10.1186/1471-2202-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurai-Yamashita Y, Yamashita K, Kanematsu T, Taniyama K. Localization of the 5-HT(4) receptor in the human and the guinea pig colon. Eur J Pharmacol 383: 281–285, 1999. doi: 10.1016/S0014-2999(99)00642-1. [DOI] [PubMed] [Google Scholar]
- 36.Stoner MC, Kellum JM. Both serotonin and a nitric-oxide donor cause chloride secretion in rat colonocytes by stimulating cGMP. Surgery 130: 236–241, 2001. doi: 10.1067/msy.2001.115903. [DOI] [PubMed] [Google Scholar]
- 37.Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des 12: 3615–3630, 2006. doi: 10.2174/138161206778522029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AJ, Lummis SC. Discriminating between 5-HT3A and 5-HT3AB receptors. Br J Pharmacol 169: 736–747, 2013. doi: 10.1111/bph.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walstab J, Wohlfarth C, Hovius R, Schmitteckert S, Röth R, Lasitschka F, Wink M, Bönisch H, Niesler B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterol Motil 26: 810–820, 2014. doi: 10.1111/nmo.12334. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman TW, Binder HJ. Serotonin-induced alteration of colonic electrolyte transport in the rat. Gastroenterology 86: 310–317, 1984. [PubMed] [Google Scholar]