Abstract
NOD2 was the first susceptibility gene identified for Crohn’s disease (CD), one of the major forms of inflammatory bowel disease (IBD). The field of NOD2 research has opened up many questions critical to understanding the complexities of microbiota-host interactions. In addition to sensing its specific bacterial components as a cytosolic pattern recognition receptor, NOD2 also appears to shape the colonization of intestinal microbiota. Activated NOD2 triggers downstream signaling cascades exampled by the NF-κB pathway to induce antimicrobial activities, however, defective or loss of NOD2 functions incur a similarly activated inflammatory response. Additional studies have identified the involvement of NOD2 in protection against non-microbiota-related intestinal damages as well as extraintestinal infections. We survey recent molecular and genetic studies of NOD2-mediated bacterial sensing and immunological modulation, and integrate evidence to suggest a highly reciprocal but still poorly understood cross talk between enteric microbiota and host cells.
Keywords: IBD, Crohn’s disease, NOD2, MDP, microbiota, microbial sensor
the gastrointestinal (GI) tract contains a large population of commensal bacteria in symbiotic association with the human body. These bacteria aid in nutrient breakdown and are important for energy assimilation for the host. In healthy individuals, the intestinal epithelial cells (IECs) and the underlying immune cells tolerate commensal microbiota but respond to incursion by pathogenic microorganisms. Important mechanisms exist to maintain a delicate interaction between the host and microbiota. In addition to the innate barrier function presented by the mucosal layer, pattern recognition receptors (PRRs), in particular members of the membrane-bound Toll-like receptors (TLRs) [(58); reviewed in Takeda and Akira (48) and Yu and Gao (57)] and cytosolic nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs), play important roles in microbial recognition and immune modulation [reviewed in Claes et al. (9)].
Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is a member of the NLR family, consisting of two NH2-terminal caspase recruitment domains (CARDs), a central nucleotide-binding domain (NBD), and several COOH-terminal leucine-rich repeats (LRRs) (36). NOD2 was the first susceptibility gene identified for Crohn’s disease (CD) (20, 35), one of the major forms of inflammatory bowel disease (IBD). Because of its strong association with CD pathogenesis, NOD2-mediated bacterial sensing and immunological modulation have been extensively investigated over the past decade. By integrating insights from cell biology, immunology, and mouse genetics studies, we discuss the consensus view on how NOD2 may modulate the host-microbe cross talk with emphasis on the mechanism of NOD2 bacterial sensing, activation of downstream signaling effectors, and impact of NOD2 on intestinal homeostasis and pathogenesis.
Bacterial Sensing by NOD2
The bacterial peptidoglycan (PGN), common to both gram-positive and gram-negative bacteria, is made up of β(1→4)-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc), which are further cross-linked by short peptides to establish the major constituent of the bacterial cell wall (43). Cleavage of PGN by specific glycoside hydrolases, such as lysozyme, releases muramyl dipeptide (MDP), which consists of a MurNAc linked to d-Ala and d-Glu (14, 21). NOD2 specifically recognizes and senses MDP (14, 15, 21). This MDP-sensing capability by NOD2 has been suspected to be critical for modulating intestinal epithelia and microbiota interaction. In contrast to wild-type NOD2, CD-associated NOD2 variants show aberrant MDP sensing (14, 21), which could impair bacterial killing and cause microbial dysbiosis. Defective MDP sensing by NOD2 can ultimately compromise intestinal epithelial barrier and induce abnormal host immune response, resulting in exacerbated intestinal injury and CD pathogenesis, aspects that will be discussed through the course of this review.
The intracellular traffic and molecular delivery of MDP have been carefully investigated to understand NOD2-mediated MDP sensing and initiation of downstream NOD2 signaling. Surface plasmon resonance assay was used to demonstrate that MDP directly binds to NOD2 (15). The optimal NOD2-MDP binding took place within a pH range of 5.0–6.5 (15), a similar pH condition where dynamin-dependent MDP endocytosis occurs (26). Pull-down analyses carried out using biotinylated MDP and purified recombinant NOD2 (30) suggested that the NBD of NOD2, from amino acids 216 to 821, mediated the NOD2-MDP interaction (30). Whether NOD2-MDP binding is influenced by NOD2’s association with ATP/ADP has been controversial (15, 30).
The di/tripeptide transporter PepT1 is predominantly found at the brush border along the entire rat small intestine (34). Human peptide transporter hPepT1, which is mostly absent in normal colonic epithelia, showed an increased expression level in inflamed colonic mucosa of CD patients (29). This aberrantly expressed hPepT1 exhibited uptake of MDP in human colonic Caco2 cells, suggesting that MDP internalization may be enhanced during colonic inflammation (52). Likewise, an in vivo study in MDP perfused rats suggested that MDP transport through intestinal PepT1 induces Nod2-mediated intestinal inflammation (28). In addition to PepT1, two other endosomal oligopeptide transporters, SLC15A3 and SLC15A4, were shown to regulate MDP transport via bacterial ligands internalized endosomes (31). SLC15A4 levels were significantly elevated in inflamed colons of IBD patients (26), similarly to hPepT1. Human embryonic kidney (HEK)-293 cells stably expressing an IBD-associated NOD2, 3020insC, which was truncated in the LRR domain, showed greatly reduced NOD2-SLC15-phagosome interactions, accompanied by an attenuated pathogen sensing (31). Thus the LRR domain of NOD2 was likely responsible for its interaction with the above-mentioned SLC15 transporters and pathogen sensing.
Internalization of PGN, of which MDP is a structural unit, could also be accomplished by direct shedding of PGN from invasive species such as Shigella. This was shown to trigger NF-κB pathway activation through NOD1 rather than NOD2 and TLR in HEK-293 cells (32). Similar activation of NF-κB pathway by NOD1 in gastric epithelial cells could also be induced by PGN that was delivered through the type IV secretion system from the invasive gram-negative Helicobacter pylori (2). In addition, outer membrane vesicles (OMVs) from gram-negative bacteria, such as Vibrio cholerae, induced NOD1/2-mediated responses in HEK-293T cells (7). Recent in vivo studies demonstrated that polysaccharide A in OMVs from Bacteroides fragilis required mouse Nod2 and autophagy-related 16-like 1 (Atg16l1) to stimulate immune response and suppress inflammation in a 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis model (8). Moreover, PGN in the intestinal lumen was shown to be captured by calcium phosphate nanominerals and transported via M cells to antigen-presenting cells in the Peyer’s patch in both human and murine tissues (38). Peyer’s patches are aggregated lymphoid follicles present in the gut-associated lymphoid tissue and are surrounded by follicle-associated epithelium, which contains the M cells (23). In summary, the existence of multiple cellular types as well as internalization pathways appears to be critical for commensal bacterial sensing and pathogen surveillance during homeostasis and intestinal inflammation.
In addition to providing a direct signal to host cells via NOD2-mediated sensing, MDP also influences the antibacterial functions of host intestinal cells. Paneth cells store antimicrobial peptides (AMP) such as lysozyme in dense core vesicles (DCVs) before secreting them into intestinal lumen. MDPs induce the localization of Nod2 to these DCVs in mouse Paneth cells, followed by a recruitment of leucine-rich repeat kinase 2 (Lrrk2) and Rab2a. This process was critical for sorting lysozyme into DCVs in Paneth cells and for protecting the intestinal epithelium (60). Of note, LRRK2 is also a susceptibility gene for CD (6), suggesting that defective sorting of AMPs by MDP-NOD2 may be, in part, responsible for CD pathogenesis.
MDP was previously considered to be the minimal bioactive PGN motif; however, recent studies showed that GlcNAc itself triggered inflammasome activation through hexokinase dissociation in the cytoplasm (56). This finding will stimulate further research into the PGN moieties of intestinal microbiota and the mechanisms contributing to the maintenance and disruption of mucosal and microbial homeostasis.
NOD2’s Downstream Signaling Events
The cellular effectors and signaling pathways downstream of NOD2 have been studied to understand its contribution to the maintenance of mucosal immunity. Receptor-interacting serine/threonine protein kinase 2 (RIPK2) is one of the well-studied interaction partners of NOD2, and NOD2-RIPK2 downstream signaling cascade can be briefly summarized as follows: MDP stimulation initiates NOD2 binding to RIPK2 (10), and RIPK2 then gets ubiquitinated and recruits TAK1 and IKK complex (16), followed by phosphorylation of IκBα to activate the NF-κB pathway (16, 36).
Studies delineating the delivery routes of MDP also examined the NOD2 signaling mechanism initiated by MDP detection. Upon sensing of MDP from internalized invasive Salmonella typhimurium (S. Tm), NOD2 in HEK-293 cells recruited RIPK2 to endolysosomes to establish the signaling complex required for responding to pathogens (31). In an exacerbated intestinal injury model using MDP-perfused rats, RIPK2 was implicated as the NOD2 downstream signaling factor (28).
Identification of the positive and negative regulators of NOD2 pathway is imperative to understanding NOD2 cellular function. Interferon regulatory factor 4 (IRF4) is a negative regulator of NOD2-induced NF- κB signaling (55). In human dendritic cells, IRF4 expression stimulated by NOD2-mediated MDP sensing inhibits polyubiquitination of RIPK2, which subsequently downregulates NF-κB expression. This results in reduced colonic inflammation in experimental colitis models (55). As one of NOD2’s downstream effectors, NF-κB in physiological conditions induces transcription of inflammatory genes upon its nuclear translocation. The IRF-4 mediated check on NF-κB expression contributes to the importance of regulation of intestinal immune responses in pathological conditions.
A genome wide small interfering (si)RNA study in HEK-293 cells stably expressing NOD2 and NF-κB luciferase reporter identified a plethora of NOD2 effectors and regulators of NOD2-NF-κB signaling related to autophagy, ubiquitination, and endosomal sorting pathways (54). Fifteen of the identified genes, including IL-21, IL-19, and STAT3, were associated with CD risk, supporting the involvement of NOD2 and its regulators in CD pathogenesis (54). Likewise, RNA interference (RNAi) screens were done to identify additional positive and negative regulators of NOD2 pathway (27). The FERM and PDZ domain-containing protein 2 (FRMPD2) was shown to be critical for NOD2 localization at plasma membrane and found to positively regulate NOD2 signaling from the basolateral domain of IECs. FRMPD2, whose expressional level reduced significantly in CD patients, was placed upstream of RIPK2 (27).
A recent addition to the above NOD2 downstream signaling network is the tumor progression locus 2 (TPL2), a homolog of the human MAP3K8. An IBD-risk locus (rs1042058) is present in the TPL2 region (17). In monocyte-derived macrophages (MDM), IKK complex is required to induce NOD2-mediated phosphorylation of TPL2, which subsequently activates ERK and NF-κB, as well as the secretion of IL-1β and IL-18 cytokines (17). In both healthy controls and CD patients, rs1042058 GG carrier MDMs exhibited upregulation of TPL2 and downstream cytokine secretion compared with AA carriers (17). Therefore, the authors report that rs1042058 GG IBD-risk polymorphism in TPL2 may act as a gain-of-function leading to an increase in TPL2 levels and enhanced NOD2-mediated cytokine secretion. These studies on regulators of NOD2 signaling cascade contributed toward an understanding of NOD2-mediated maintenance of intestinal homeostasis.
Another important NOD2 interaction partner is CARD9, also a CD susceptibility gene (62). Upon stimulation, NOD2 binds and utilizes CARD9 to regulate innate immune responses (18). Card9−/− mice infected with Listeria monocytogenes exhibited an increased bacterial load with a delayed induction of strong proinflammatory responses (18). Thus several NOD2 downstream signaling molecules and pathways have been uncovered with complicated positive and negative feedbacks. If future functional studies can assess their individual contributions to epithelial homeostasis and IBD progression, and carefully delineate primary and secondary effectors, relevant targets may be selected for disease intervention.
Impact of NOD2 on Intestinal Epithelia and Immune Cells
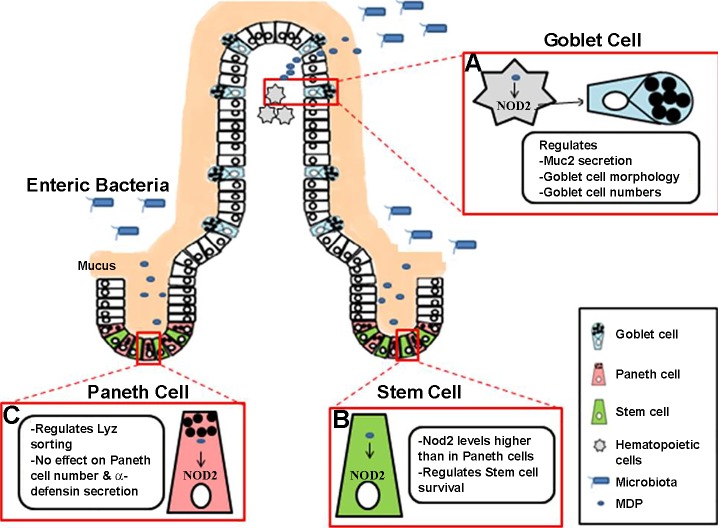
The crypt base columnar (CBC) intestinal stem cells (ISCs) give rise to the entire differentiated population of IECs. RT-PCR analysis carried out on sorted cells from crypts of Lgr5-EGFP knockin mice revealed that CBC cells had a fivefold higher level of Nod2 mRNA than the neighboring Paneth cells (Fig. 1) (33). To further decipher the role of NOD2 expression in CBC cells, organoids were used as a model of study. ISCs from isolated murine crypts can be grown in Matrigel supplemented with essential growth factors to form self-sustained organoids that resemble many features of intestine (42). ISCs sorted and isolated from wild-type mice generated more organoids than ISCs from Nod2-deficient mice (33). Although the addition of MDP provided a further increase in organoids yielded from wild-type stem cells, no difference was observed in the mutant condition. Moreover, crypts from doxorubicin-treated wild-type mice yielded more organoids on MDP stimulation, whereas organoids grown from Nod2-deficient mice injected with doxorubicin failed to recover even on addition of MDP. Doxorubicin is a DNA intercalating agent that is detrimental to ISCs. These results suggest that Nod2-mediated MDP sensing is critical to stem cell survival and renewal (33). In a similar study performed on colonic epithelial cells (CECs), Nod2 mRNA levels was augmented in proliferating crypt epithelial cells compared with the differentiating cells (12). From an in vivo context, wild-type mouse CECs showed increased proliferation and provided protection from invasion by Salmonella enterica serovar Typhimurium, compared with CECs of Nod2-deficient mice. Addition of MDP could not rescue the cell number of Nod2-deficient CECs, compared with the increase observed in MDP-treated wild-type CECs in vitro. Thus Nod2 can promote colonic epithelial cell growth in response to MDP sensing.
Fig. 1.
NOD2-mediated bacterial sensing and modulation of intestinal epithelial homeostasis. Binding of bacterial MDP to cytosolic NOD2 receptors appears to have comprehensive influences on intestinal mucosal homeostasis. A: incursion of pathogenic microorganisms or detrimental expansion of commensal bacteria may be sensed by hematopoietic NOD2-expressing cells that regulate goblet cell number and morphology, as well as the expression and secretion of antimicrobial gene products such as mucin 2. B: crypt base columnar (CBC) stem cells express higher levels of NOD2 compared with the Paneth cells. MDP sensing by NOD2 appears to influence stem cell renewal or/and survival. C: NOD2, as well as NOD2-mediated bacterial sensing, may regulate lysozyme sorting in Paneth cells, although the expression of several antimicrobial peptides, such as α-defensin, is not dependent on NOD2.
Nod2 global deletion has no effect on Paneth cell numbers (45, 50). This was corroborated by another study where Nod2 deficiency affected the expression levels of AMPs and mucin independently of Paneth and goblet cell number in the ileum (1). Specifically, Nod2 deficiency reduced Paneth cell-specific Reg3β and Reg3γ, but not cryptdin, whereas it increased goblet cell markers, Muc4, intestinal trefoil factor, and Fc-γ binding protein (1). However, during Paneth cell differentiation of Caco2 cells, NOD2 signaling through MDP decreased the expression of other Paneth cell-specific AMPs, including the enteric α-defensin (HD)5, HD6, lysozyme, and secretory phospholipase A2 (sPLA2) (49). In contrast, several studies reported that Nod2 deletion does not impact secretion of any AMP (41, 45) or the bactericidal activity of α-defensins (45). A study using resection specimens from CD patients showed that the degree of abnormality in Paneth cell morphology was proportional to the number of NOD2 risk variants (51). However, Ramanan et al. (40) observed that Paneth cell morphology did not alter in Nod2-deficient mice. In addition, NOD2 sensing of indigenous bacteria was shown to affect lysozyme sorting into the DCVs in Paneth cells (60) (Fig. 1). This sensing was proposed to facilitate localization of Nod2, Lrrk2, and Rab2a to the DCVs, thereby preventing degradation of lysozyme in lysosomes (60).
NOD2 has also been implicated in regulating goblet cell morphology and mucin secretion (Fig. 1). Mucin forms a major component of the mucosal layer that establishes a barrier between host epithelium and microbiota and provides the first line of defense in case this balance is compromised. Nod2-deficient mice were characterized by reduced Muc2 expression and decreased number of mucin granules per goblet cell (40). Wang et al. (53) challenged wild-type and Nod1/Nod2 double-knockout mice with the commensal parasite Trichuris muris and demonstrated that sensing of the microbiota by Nod1 and Nod2 was important for increasing goblet cell number and mucin secretion, which might help to counter the parasitic load in the intestine.
NOD2 is expressed by at least two distinct cell populations in the intestine: IECs and hematopoietic immune cells (3, 22, 25). By using reciprocal bone marrow transfer assays between Nod2 wild-type and Nod2-deficient mice, Alnabhani et al. (3) demarcated that IEC-specific Nod2 impacted microbial dysbiosis and antimicrobial secretion, whereas hematopoietic cell-specific Nod2 influenced the immune response and barrier function. They attributed microbial imbalance to IEC Nod2, whereas another study implicated hematopoietic Nod2 for microbial imbalance (40), a discrepancy that requires further investigation. Thus, whether NOD2 in different cell types plays distinct roles in intestinal-microbial homeostasis is still unclear but warrants further investigations.
CD has been characterized by an increase in T-cell population and proinflammatory responses (44). Nod2-deficient mice with acute T-cell activation using T cell-activating anti-CD3 monoclonal antibody presented disturbed intestinal crypt-villous architecture and increased levels of IL-17A, TNF-α, and IFN-γ compared with wild-type mice (59). On treatment with broad-spectrum antibiotics, T cell-activated Nod2-deficient mice could restore normal crypt architecture and cytokine levels 3 days after anti-CD3 injection. Further investigation revealed that acute T-cell activation in cell lineage-specific deletion of Nod2 by either Villin-Cre or Lyz2-Cre did not affect the intestinal architecture. However, the Lyz2-Cre mouse model exhibited an increase in cytokines such as TNF-α and IL-22, similarly to the Nod2-deficient mice. Collectively, this suggests that Nod2 sensing of gut microbiota modulates intestinal homeostasis following acute T-cell activation (59). Previously, Barreau et al. (5) demonstrated that antibodies against CD4+ T cells and IFN-γ levels in the Peyer’s patch of Nod2-deficient mice could reduce the elevated transcellular permeability and bacterial translocation across Peyer’s patch by regulating myosin light chain kinase (MLCK) expression. Moreover, inhibition of MLCK in these mice attenuated levels of CD4+ T cells and IFN-γ along with rescuing the barrier defect, thereby suggesting a reciprocal interaction between immune and epithelial cells regulated by Nod2. Collectively, NOD2 seems to modulate the intestinal epithelial differentiation and the mucosal defense mechanisms (Fig. 1).
Impact of NOD2 on Microbiota
Nod2-deficient mice showed a significant expansion of Bacteroides vulgatus, an anaerobic commensal bacterium, compared with wild-type mice (40). This expansion was associated with increased IFN-γ-expressing CD8+/TCRγδ+ intraepithelial lymphocytes (IELs), despite a decrease in the total number of IELs and defective goblet cell morphology. Treatment of the microbiota by metronidazole, an antibiotic against anaerobic bacteria, restored the epithelial morphology and cytokine expression (40). The phenotype of decrease in IEL population in Nod2-deficient mice was also reported in a previous study (22). The paper on expansion of B. vulgatus supports the notion that NOD2 mediates cross talk between intestinal microbiota and the immune system. Ramanan et al. (39) further investigated the hypothesis that CD pathophysiology characterized by expansion of Bacteroides species due to Nod2 mutation could be treated with a helminth infection. Colonization with helminthes ameliorated all abnormalities with a strong Th2 response and expansion of Clostridiales over Bacteroides (39). Taken together, the findings suggest that NOD2 mutation leads to exacerbated intestinal inflammation associated with microbial dysbiosis and specific expansion of the Bacteroides.
Pseudomonas fluorescens antigen was detected in the serum of almost 54% of CD patients (47). P. fluorescens infection enhanced IL-1β production and MLCK levels, thereby increasing the paracellular permeability in ileal mucosa (4). Nod2 secreted by macrophages is required for inducing IL-1β production in response to P. fluorescens; however, addition of MDP was able to attenuate the defect in epithelial barrier and IL-1β production (4).
The response of NLR sensing of intestinal microbiota is not restricted to local homeostasis. Recently, long-range effects of NLR signaling on host defense against pathogenic microorganisms have been reported. Specifically, NLR stimulation in the intestine stimulates bacterial killing in the lungs through reactive oxygen species released by alveolar macrophages. In the absence of NLR stimulation, mice treated with antibiotic exhibited decreased immune response in the lung tissue as observed through IL-6 and TNF-α levels (11).
The protective function of NOD2 extends to even non-microbiota-related conditions, such as ischemia-reperfusion-mediated intestinal damage. In response to this type of damage, Nod2-dependent sensing of commensal bacteria restores epithelial morphology in IECs through induction of autophagy (37). Nod2 signaling is also being considered for intestinal sepsis treatment. Septic rat intestines treated with saikosaponin A showed reduced Nod2 expression accompanied by an inhibition of proinflammatory cytokines IL-6 and TNF-α via NF-κB pathway (61). It is interesting to note that NOD2 signaling in the intestinal surface is being studied for its protective function in conditions apart from CD. NOD2 is vital for defense at the intestinal barrier as well as at distant sites. Future studies should identify mechanisms by which NOD2 establishes such an extensive regulation.
Collectively, studies of Nod2-deficient mice have documented a reduced α-defensin expression and a changed microbiota composition (24), as well as a significant expansion of B. vulgatus in these knockout animals (40). However, some studies have shown that by controlling for the genetic background of the mice (in this case C57BL/6), the housing conditions, and adequate number of mice, the above differences in defensin expression and microbiota composition were negated (41, 45, 46). To resolve the impact of Nod2 on intestinal microbiota composition, Al Nabhani et al. (1) used embryo transfer approach to minimize variability of environmental factors. Interestingly, Nod2 wild-type and Nod2-deficient embryos transferred to a Nod2 wild-type mother shared gut microbial composition similar to that of Nod2 knockout mice rather than their wild-type mother, Thus it proved that Nod2 deletion is linked with dominant and transmissible microbial dysbiosis. This microbial dysbiosis further affects the secretion of AMPs and mucin but does not impact immune response and gut epithelial barrier function. Similarly, mice lacking another NLR family member, Nlrp6, harbored an impaired microbiota (13). Wild-type mice cohoused with Nlrp6−/− mice exhibited dysbiotic microbiota and increased susceptibility to dextran sodium sulfate (DSS)-induced colitis and azoxymethane (AOM)-DSS induced colorectal cancer similarly to single-housed Nlrp6−/− mice rather than single-housed wild-type mice (13, 19). These results shed light on the importance of experimental design to dissect role of host gene expression on gut microbiota, intestinal epithelial, and host immunological responses.
Closing Remarks
NOD2 sensing of the intestinal microbiota allows nuclear translocation of NF-κB that initiates transcription of inflammatory cytokines. NOD2 mutations disrupt the balance between gut microbiota, immune response, and intestinal epithelium, which also results in exacerbated inflammation and eventually IBD. Ramanan et al. (40) showed that small intestinal abnormalities and inflammation in Nod2−/− mice were dependent on expansion of B. vulgatus, providing a possible explanation for the complex relationship between NOD2 and immune response to intestinal microbiota. Future studies are needed to acquire insights into such mechanisms and their role in disease IBD pathophysiology. It is also interesting to define the potential contribution of NOD2-mediated cellular autophagy to disease progression. The observation that intestinal NOD2 signaling may transcend intestinal barrier to distant sites indicates that monitoring intestinal bacteria by this pathway may be crucial for systemic microbial surveillance for human health. This makes it a plausible target for improving the overall well-being in clinic.
GRANTS
This work is supported by National Institutes of Health Grants DK102934, DK085194, DK093809, and CA178599, an Initiative for Multidisciplinary Research Teams award from Rutgers University, National Science Foundation BIO/IDBR Award 1353890, and American Cancer Society Research Scholar Grant RSG-15-060-01-TBE (to N. Gao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G. interpreted results of experiments; I.B. prepared figures; I.B. and N.G. drafted manuscript; I.B. and N.G. edited and revised manuscript; I.B. and N.G. approved final version of manuscript.
REFERENCES
- 1.Al Nabhani Z, Lepage P, Mauny P, Montcuquet N, Roy M, Le Roux K, Dussaillant M, Berrebi D, Hugot JP, Barreau F. Nod2 deficiency leads to a specific and transmissible mucosa-associated microbial dysbiosis which is independent of the mucosal barrier defect. J Crohn’s Colitis 10: 1428–1436, 2016. doi: 10.1093/ecco-jcc/jjw095. [DOI] [PubMed] [Google Scholar]
- 2.Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol 183: 8099–8109, 2009. doi: 10.4049/jimmunol.0900664. [DOI] [PubMed] [Google Scholar]
- 3.Alnabhani Z, Hugot JP, Montcuquet N, Le Roux K, Dussaillant M, Roy M, Leclerc M, Cerf-Bensussan N, Lepage P, Barreau F. Respective roles of hematopoietic and nonhematopoietic Nod2 on the gut microbiota and mucosal homeostasis. Inflamm Bowel Dis 22: 763–773, 2016. doi: 10.1097/MIB.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 4.Alnabhani Z, Montcuquet N, Biaggini K, Dussaillant M, Roy M, Ogier-Denis E, Madi A, Jallane A, Feuilloley M, Hugot JP, Connil N, Barreau F. Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1β expression through hematopoietic NOD2 signaling. Inflamm Bowel Dis 21: 543–555, 2015. doi: 10.1097/MIB.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 5.Barreau F, Madre C, Meinzer U, Berrebi D, Dussaillant M, Merlin F, Eckmann L, Karin M, Sterkers G, Bonacorsi S, Lesuffleur T, Hugot JP. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut 59: 207–217, 2010. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 6.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ, Parkes M, Georges M, Daly MJ; NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium . Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 40: 955–962, 2008. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielig H, Rompikuntal PK, Dongre M, Zurek B, Lindmark B, Ramstedt M, Wai SN, Kufer TA. NOD-like receptor activation by outer membrane vesicles from Vibrio cholerae non-O1 non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect Immun 79: 1418–1427, 2011. doi: 10.1128/IAI.00754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DPB, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 352: 1116–1120, 2016. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claes AK, Zhou JY, Philpott DJ. NOD-like receptors: guardians of intestinal mucosal barriers. Physiology (Bethesda) 30: 241–250, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Clark NM, Marinis JM, Cobb BA, Abbott DW. MEKK4 sequesters RIP2 to dictate NOD2 signal specificity. Curr Biol 18: 1402–1408, 2008. doi: 10.1016/j.cub.2008.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun 82: 4596–4606, 2014. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruickshank S-M, Wakenshaw L, Cardone J, Howdle PD, Murray PJ, Carding SR. Evidence for the involvement of NOD2 in regulating colonic epithelial cell growth and survival. World J Gastroenterol 14: 5834–5841, 2008. doi: 10.3748/wjg.14.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757, 2011. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872, 2003. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 15.Grimes CL, Ariyananda LZ, Melnyk JE, O’Shea EK. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 134: 13535–13537, 2012. doi: 10.1021/ja303883c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Núñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J 27: 373–383, 2008. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedl M, Abraham C. A TPL2 (MAP3K8) disease-risk polymorphism increases TPL2 expression thereby leading to increased pattern recognition receptor-initiated caspase-1 and caspase-8 activation, signalling and cytokine secretion. Gut 65: 1977–1811, 2016. doi: 10.1136/gutjnl-2014-308922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol 8: 198–205, 2007. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 19.Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, Flavell RA. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci USA 110: 9862–9867, 2013. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411: 599–603, 2001. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 21.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nuñez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 278: 5509–5512, 2003. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, Han J, MacDonald HR, Tschopp J, Tian Z, Zhou R. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med 210: 2465–2476, 2013. [Erratum in J Exp Med 210: 2791, 2013]. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung C, Hugot JP, Barreau F. Peyer’s patches: the immune sensors of the intestine. Int J Inflamm 2010: 823710, 2010. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734, 2005. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 25.Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nuñez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for Paneth cells. Gastroenterology 125: 47–57, 2003. doi: 10.1016/S0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem 284: 23818–23829, 2009. doi: 10.1074/jbc.M109.033670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipinski S, Grabe N, Jacobs G, Billmann-Born S, Till A, Häsler R, Aden K, Paulsen M, Arlt A, Kraemer L, Hagemann N, Erdmann KS, Schreiber S, Rosenstiel P. RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition. Proc Natl Acad Sci USA 109: 21426–21431, 2012. doi: 10.1073/pnas.1209673109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma G, Shi B, Liu J, Zhang H, YinTao Z, Lou X, Liang D, Hou Y, Wan S, Yang W. Nod2-Rip2 signaling contributes to intestinal injury induced by muramyl dipeptide via oligopeptide transporter in rats. Dig Dis Sci 60: 3264–3270, 2015. doi: 10.1007/s10620-015-3762-1. [DOI] [PubMed] [Google Scholar]
- 29.Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, Hediger MA, Madara JL. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120: 1666–1679, 2001. doi: 10.1053/gast.2001.24845. [DOI] [PubMed] [Google Scholar]
- 30.Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem 287: 23057–23067, 2012. doi: 10.1074/jbc.M112.344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Mazière A, Klumperman J, Schlatter M, Delamarre L, Mellman I. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509: 240–244, 2014. doi: 10.1038/nature13133. [DOI] [PubMed] [Google Scholar]
- 32.Nigro G, Fazio LL, Martino MC, Rossi G, Tattoli I, Liparoti V, De Castro C, Molinaro A, Philpott DJ, Bernardini ML. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell Microbiol 10: 682–695, 2008. doi: 10.1111/j.1462-5822.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 33.Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15: 792–798, 2014. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun 220: 848–852, 1996. doi: 10.1006/bbrc.1996.0493. [DOI] [PubMed] [Google Scholar]
- 35.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603–606, 2001. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 36.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J Biol Chem 276: 4812–4818, 2001. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Chanona E, Mühlbauer M, Jobin C. The microbiota protects against ischemia/reperfusion-induced intestinal injury through nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling. Am J Pathol 184: 2965–2975, 2014. doi: 10.1016/j.ajpath.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell JJ, Thomas-McKay E, Thoree V, Robertson J, Hewitt RE, Skepper JN, Brown A, Hernandez-Garrido JC, Midgley PA, Gomez-Morilla I, Grime GW, Kirkby KJ, Mabbott NA, Donaldson DS, Williams IR, Rios D, Girardin SE, Haas CT, Bruggraber SF, Laman JD, Tanriver Y, Lombardi G, Lechler R, Thompson RP, Pele LC. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat Nanotechnol 10: 361–369, 2015. doi: 10.1038/nnano.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YA, Loke P, Cadwell K. Helminth infection promotes colonization resistance via type 2 immunity. Science 352: 608–612, 2016. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity 41: 311–324, 2014. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson SJ, Zhou JY, Geddes K, Rubino SJ, Cho JH, Girardin SE, Philpott DJ. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes 4: 222–231, 2013. doi: 10.4161/gmic.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 43.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36: 407–477, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shale M, Schiering C, Powrie F. CD4+ T-cell subsets in intestinal inflammation. Immunol Rev 252: 164–182, 2013. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shanahan MT, Carroll IM, Grossniklaus E, White A, von Furstenberg RJ, Barner R, Fodor AA, Henning SJ, Sartor RB, Gulati AS. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut 63: 903–910, 2014. doi: 10.1136/gutjnl-2012-304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanahan MT, Carroll IM, Gulati AS. Critical design aspects involved in the study of Paneth cells and the intestinal microbiota. Gut Microbes 5: 208–214, 2014. doi: 10.4161/gmic.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton CL, Kim J, Yamane A, Dalwadi H, Wei B, Landers C, Targan SR, Braun J. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology 119: 23–31, 2000. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 48.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 17: 1–14, 2005. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 49.Tan G, Li RH, Li C, Wu F, Zhao XM, Ma JY, Lei S, Zhang WD, Zhi FC. Down-regulation of human enteric antimicrobial peptides by NOD2 during differentiation of the Paneth cell lineage. Sci Rep 5: 8383, 2015. doi: 10.1038/srep08383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Limbergen J, Geddes K, Henderson P, Russell RK, Drummond HE, Satsangi J, Griffiths AM, Philpott DJ, Wilson DC. Paneth cell marker CD24 in NOD2 knockout organoids and in inflammatory bowel disease (IBD). Gut 64: 353–354, 2015. doi: 10.1136/gutjnl-2013-305077. [DOI] [PubMed] [Google Scholar]
- 51.VanDussen KL, Liu TC, Li D, Towfic F, Modiano N, Winter R, Haritunians T, Taylor KD, Dhall D, Targan SR, Xavier RJ, McGovern DP, Stappenbeck TS. Genetic variants synthesize to produce Paneth cell phenotypes that define subtypes of Crohn’s disease. Gastroenterology 146: 200–209, 2014. doi: 10.1053/j.gastro.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-κB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127: 1401–1409, 2004. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Kim JJ, Denou E, Gallagher A, Thornton DJ, Shajib MS, Xia L, Schertzer JD, Grencis RK, Philpott DJ, Khan WI. New role of nod proteins in regulation of intestinal goblet cell response in the context of innate host defense in an enteric parasite infection. Infect Immun 84: 275–285, 2015. doi: 10.1128/IAI.01187-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner N, Burberry A, Franchi L, Kim YG, McDonald C, Sartor MA, Núñez G. A genome-wide siRNA screen reveals positive and negative regulators of the NOD2 and NF-κB signaling pathways. Sci Signal 6: rs3, 2013. doi: 10.1126/scisignal.2003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe T, Asano N, Meng G, Yamashita K, Arai Y, Sakurai T, Kudo M, Fuss IJ, Kitani A, Shimosegawa T, Chiba T, Strober W. NOD2 downregulates colonic inflammation by IRF4-mediated inhibition of K63-linked polyubiquitination of RICK and TRAF6. Mucosal Immunol 7: 1312–1325, 2014. doi: 10.1038/mi.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, Underhill DM. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166: 624–636, 2016. doi: 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu S, Gao N. Compartmentalizing intestinal epithelial cell Toll-like receptors for immune surveillance. Cell Mol Life Sci 72: 3343–3353, 2015. doi: 10.1007/s00018-015-1931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu S, Nie Y, Knowles B, Sakamori R, Stypulkowski E, Patel C, Das S, Douard V, Ferraris RP, Bonder EM, Goldenring JR, Ip YT, Gao N. TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. EMBO J 33: 1882–1895, 2014. doi: 10.15252/embj.201487888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanello G, Goethel A, Rouquier S, Prescott D, Robertson SJ, Maisonneuve C, Streutker C, Philpott DJ, Croitoru K. The cytosolic microbial receptor Nod2 regulates small intestinal crypt damage and epithelial regeneration following T cell-induced enteropathy. J Immunol 197: 345–355, 2016. doi: 10.4049/jimmunol.1600185. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, Li W, Wei H, Liu Z. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol 16: 918–926, 2015. doi: 10.1038/ni.3233. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H, Li S, Zhang H, Wang G, Xu G, Zhang H. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-κB activation. Exp Ther Med 10: 823–827, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhernakova A, Festen EM, Franke L, Trynka G, van Diemen CC, Monsuur AJ, Bevova M, Nijmeijer RM, van’t Slot R, Heijmans R, Boezen HM, van Heel DA, van Bodegraven AA, Stokkers PC, Wijmenga C, Crusius JB, Weersma RK. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet 82: 1202–1210, 2008. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]