These studies for the first time present comprehensive data on the relative characterization of vasoactive intestinal peptide (VIP) receptors in the intestinal mucosa. Vasoactive intestinal peptide receptor 1 (VPAC1) was identified as the predominant receptor with higher levels in the colon compared with the small intestine and was mainly localized to the apical membrane. In addition, the findings in the human tissues were consistent with VPAC1 expression in the mouse intestine and open possibilities to target colonic tissues with VIP for treating diseases such as inflammatory bowel disease.
Keywords: VIP, mouse intestine, human intestine, membrane localization
Abstract
Vasoactive intestinal peptide (VIP) is an endogenous neuropeptide with a broad array of physiological functions in many organs including the intestine. Its actions are mediated via G protein-coupled receptors, and vasoactive intestinal peptide receptor 1 (VPAC1) is the key receptor responsible for majority of VIP’s biological activity. The distribution of VPAC1 along the length of the gastrointestinal tract and its subcellular localization in intestinal epithelial cells have not been fully characterized. The current studies were undertaken to determine VPAC1 distribution and localization so that VIP-based therapies can be targeted to specific regions of the intestine. The results indicated that the mRNA levels of VPAC1 showed an abundance pattern of colon > ileum > jejunum in the mouse intestine. In parallel, the VPAC1 protein levels were higher in the mouse colon, followed by the ileum and jejunum. Immunofluorescence studies in mouse colon demonstrated that the receptor was specifically localized to the luminal surface, as was evident by colocalization with the apical marker villin but not with the basolateral marker Na+/K+-ATPase. In the human intestine, VPAC1 mRNA expression exhibited a distribution similar to that in mouse intestine and was highest in the sigmoid colon. Furthermore, in the human colon, VPAC1 also showed predominantly apical localization. The physiological relevance of the expression and apical localization of VPAC1 remains elusive. We speculate that apical VPAC1 in intestinal epithelial cells may have relevance in recognizing secreted peptides in the intestinal lumen and therefore supports the feasibility of potential therapeutic and targeting use of VIP formulations via oral route to treat gastrointestinal diseases.
NEW & NOTEWORTHY These studies for the first time present comprehensive data on the relative characterization of vasoactive intestinal peptide (VIP) receptors in the intestinal mucosa. Vasoactive intestinal peptide receptor 1 (VPAC1) was identified as the predominant receptor with higher levels in the colon compared with the small intestine and was mainly localized to the apical membrane. In addition, the findings in the human tissues were consistent with VPAC1 expression in the mouse intestine and open possibilities to target colonic tissues with VIP for treating diseases such as inflammatory bowel disease.
vasoactive intestinal peptide (VIP) is an endogenous, 28-amino acid neuropeptide, with a broad array of biological functions in both the central nervous system and peripheral organs including the intestine (8, 46). It belongs to a superfamily of structurally related brain-gut peptide hormones (28). These peptides include important neuroendocrine mediators such as PACAP (pituitary adenylate cyclase-activating polypeptide) and intestinal hormones such as glucagon (39). In the gastrointestinal tract (GIT), VIP is known to regulate many physiological processes in different regions. For example, in the upper intestine it mainly mediates smooth muscle relaxation for peristaltic movement and sphincter functions (6, 54). VIP is also important in secretion of luminal ions and fluid in the pancreas and jejunum (11, 30) and is imperative as an enteric neuropeptide for tonic inhibitory control of the small intestinal circular muscle in the ileum (13, 20). In the colon, VIP has been shown to be involved in smooth muscle relaxation (23), ion transport (4, 42), and mucus secretion (40), and in colon carcinoma cell models, VIP affects cellular biochemical processes such as glycogenolysis (45). Apart from these effects it also mediates epithelial regeneration (52) and tight junction barrier function (3, 38) in the intestinal epithelium. Furthermore, studies on VIP global knockout (KO) mice show severe abnormalities in the morphology and function of the GIT, validating the significance of VIP as a key enteric hormone (36).
The diverse physiological effects of VIP are mediated through binding of the peptide with the seven-transmembrane G protein-coupled receptors (GPCRs). VIP binds first, specifically to the NH2-terminus of its receptor and then to the other domains, which requires the entire 28-amino acid residues of the peptide (32, 49). The receptors for VIP are structurally related to the secretin/glucagon superfamily of receptors and are common to both VIP and PACAP (which shares almost 70% amino acid homology to VIP) (25). These receptors have been pharmacologically classified into two of which show higher affinity for VIP, termed VPAC1 and VPAC2, and one low-affinity receptor PAC1 (25, 32). Since VIP receptors are stimulatory or type 2 GPCRs (Gs), the molecular pathways of VPAC receptor activation and subsequent effects have been attributed mainly to increased intracellular cyclic AMP (cAMP) through adenylate cyclase. Among the three receptors VPAC1 has been identified as the predominant receptor in different organs such as liver, lung, thyroid, and reproductive organs (44).
With regard to intestinal epithelial cells, early studies conducted by Laburthe and colleagues with the aid of radiolabeled VIP binding showed high binding of VIP to the intestinal epithelial cells (12, 47). It should be noted that the discovery and characterization of the specific GPCRs for VIP and its classification were conducted only later and were not defined at the time these studies were undertaken (25, 31). In addition, the subcellular localization of these receptors in the intestinal epithelial cells has also not been characterized. Thus very limited knowledge is available regarding the expression of VIP receptors in the gastrointestinal mucosa.
Given the pleiotropic effects of VIP across different regions of the GIT, the current study investigated the expression of the known VIP receptors along the length of the human and mouse intestine. Our studies for the first time demonstrate the relative abundance of all VIP receptors from jejunum to the distal colon in mice and in the human intestine. We show that in humans and mice, the major receptor for VIP in the gut is VPAC1. In addition, our results demonstrate localization of VPAC1 predominantly to the luminal membranes in the human and mouse colon tissue. This study therefore provides valuable insights into the potential importance of the luminal enteric peptide VIP in health and its potential to be utilized in targeting strategies for VIP-based nanomedicines to various regions of the intestine for treating gut disorders, e.g., inflammatory bowel disease.
MATERIALS AND METHODS
Mice.
Male, 6–8 wk old C57BL/6 mice (n = 8) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were euthanized with carbon dioxide inhalation followed by cervical dislocation before harvesting of intestinal tissues. All animal studies performed were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center (Chicago, IL).
Human specimens.
Formalin-fixed, paraffin-embedded human colon sections from unaffected areas of the colon from IBD patients were kindly provided by the Department of Pathology, University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center.
Real-time PCR.
RNA was isolated from mice jejunum, ileum, and distal colon mucosal tissues with Qiagen RNeasy kits (Valencia, CA). Total human RNA from jejunum, ileum, and ascending and sigmoid colon was purchased from BioChain institute (Newark, CA). Equal amounts of RNA were reverse transcribed and amplified using Brilliant SYBR Green qPCR Master Mix kit (Stratagene, La Jolla, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control for each sample. Primers used are listed in Table 1.
Table 1.
Gene specific primer sequences
| Gene | Sequence (5′→3′) | Gene Accession No. |
|---|---|---|
| Human VPAC1 | F-TCATCCGAATCCTGCTTCAGA | NM_001251884 |
| R-AGGCGAACATGATGTAGTGTACT | ||
| Mouse VPAC1 | F-GATGTGGGACAACCTCACCTG | NM_011703 |
| R-TAGCCGTGAATGGGGGAAAAC | ||
| Human VPAC2 | F-CAGTGGCGTCTGGGACAAC | NM_003382 |
| R-CCGTCACTCGTACAGTTTTTGC | ||
| Mouse VPAC2 | F-GGTGAGCAGCATCCATCCAG | NM_009511 |
| R-TCGCTAGTGCAGTTTTTGCTTA | ||
| Human PAC1 | F-GTCGGAACCCTTCCCTCATTA | NM_001199635.1 |
| R-GGCCTTCACTGACAGGTAGTA | ||
| Mouse PAC1 | F-GGCTGTGCTGAGGCTCTACTTTG | NM_007407.4 |
| R-AGGATGATGATGATGCCGATGA | ||
| Human GAPDH | F-GAAATCCCATCACCATCTT | NM_002046.5 |
| R-AAATGAGCCCCAGCCTTCT | ||
| Mouse GAPDH | F-TGTGTCCGTCGTGGATCTGA | NM_001289726.1 |
| R-CCTGCTTCACCACCTTCTTGAT |
F, forward; R, reverse.
VPAC1 antibody.
Commercially available VPAC1 antibody was purchased from Thermofisher Scientific (catalog no. PA3-113; Waltham, MA) raised in rabbit against a synthetic peptide corresponding to human VPAC1 receptor residues, T (438) RVSPGARRSSSFQAEVSLV (457). The antibody was validated by using a peptide competition assay and confirmed for cross-reactivity in mice.
Western blot analysis.
Protein lysates from mouse intestinal mucosal scrapings from jejunum, ileum, and proximal and distal colons were extracted and 75 μg of protein from each region was used for Western analysis as described earlier (37). Briefly, protein samples were prepared with 4× Laemmli sample buffer (Bio-Rad, Hercules, CA) and boiled for 5 min. Samples were then loaded onto 10% Mini-Protean precast gel (Bio-Rad) and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in phosphate-buffered saline (PBS) for 1 h, followed by incubation with anti-rabbit VPAC1 primary antibody at a dilution of 1 to 250 or anti-rabbit GAPDH antibody (Sigma-Aldrich, St. Louis, MO) at a ratio of 1 to 10,000 in 1% milk in PBS overnight at 4°C. The bound antibodies were detected by horseradish peroxidase-conjugated anti-rabbit Ig secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by ECL detection system (Bio-Rad) per the manufacturer's instructions.
Peptide competition assay.
Western blot analysis was performed for 75 μg of protein from Caco2 and mouse distal colon tissue as above, and nitrocellulose membranes were processed for primary antibody incubation as described below. Peptide corresponding to human VPAC1 receptor residues, T (438) RVSPGARRSSSFQAEVSLV (457) was synthesized by pierce custom peptides (Thermofisher Scientific, Waltham, MA), and VPAC1 antibody (catalog no. PA3-113) was purchased from Thermofisher Scientific. In an Eppendorf tube, VPAC1 antibody was incubated at room temperature for 2 h with or without the peptide at a 5× excess. The resulting antibody mixtures were used in 1% milk and incubated overnight for Western analysis with and without competing peptide.
Immunofluorescence staining.
Formalin-fixed, paraffin-embedded, 5-μm sections from mouse proximal and distal colon regions were stained as described previously with some modifications (22). Briefly, slides were placed at 60°C for 30 min and then immersed in xylene twice for 20 min for deparafinization. The slides were then placed in a series of ethanol solutions (100, 95, 90,70, and 50%) in coupling jars for gradual rehydration. Afterward, the slides were immersed in distilled water for 5 min. Antigen retrieval was performed by submerging the slides in a steam bath of 0.1 M citrate buffer for 30 min at 100°C. The slides were then allowed to cool to room temperature and rinsed in wash buffer (Tris-buffered saline containing 0.05% Tween) for 5 min followed by 5 min in permeabilization solution of Tris-buffered saline containing 0.1% Triton X-100. The tissue sections were encircled with a water repellant pen, and the slides were then incubated for an hour in a moist, dark container with 10% normal goat serum (NGS) to block nonspecific antibody binding. This was followed by incubation of the slides with the primary antibody at a dilution of 1 to 100; antibodies used were VPAC1 (Thermofisher) and monoclonal anti-mouse villin (Abcam, Cambridge, MA) or monoclonal anti-mouse actin (Sigma-Aldrich) or monoclonal anti-mouse Na+/K+-ATPase (Thermofisher) 4°C overnight in wash buffer containing 1% NGS. Following several washes, the slides were incubated with anti-goat secondary antibodies conjugated to either anti-rabbit Alexa fluor 568 (red) or anti-mouse Alexa fluor 488 (green) (Thermofisher) for 1 h at a dilution of 1:100 in wash buffer containing 1% NGS. After a few washes, the slides were mounted with Prolong Gold Antifade-DAPI (Thermofisher) and sealed with clear nail polish. Slides were stored at −20°C until use. Images were acquired with the use of the Olympus BX51 fluorescent microscope or Zeiss Axiocam Acc1 (Oberkochen, Germany).
Statistical analysis.
All data were subjected to one-way ANOVA (Tukey) or Student’s t-test (paired) statistical analysis, and P < 0.05 or less was considered statistically significant.
RESULTS
mRNA expression of VIP receptors in the mouse intestine.
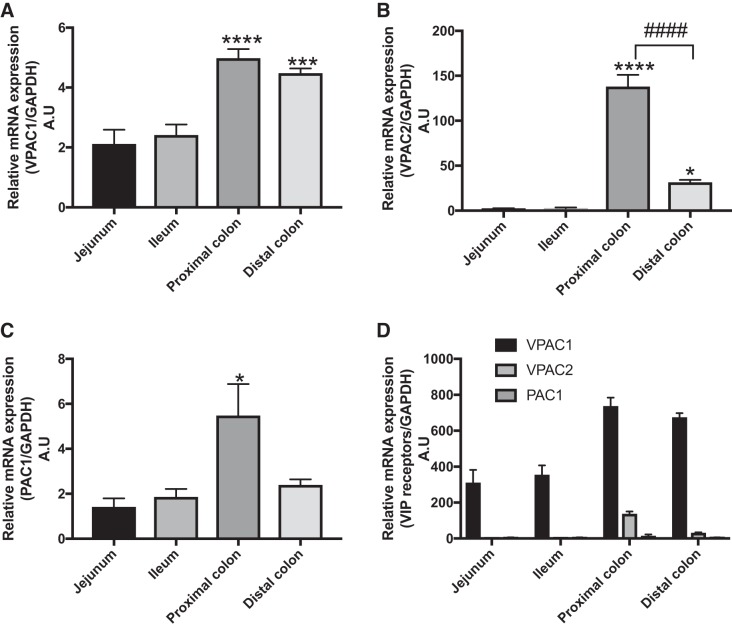
VIP is present abundantly in the intestine and mediates many important functions (28). Previous studies regarding the receptors for VIP in the intestine have been conducted only by radiolabeled iodine binding studies in rats, dogs, and humans. Additionally, there have been no studies conducted to determine the expression of VIP receptors in the widely used mouse model. Therefore, the present studies were undertaken to determine the specific receptor subtypes of VIP and their expression along the length of the intestine. As shown in Fig. 1, when the expression of individual receptors in regions from jejunum to distal colon were compared, VPAC1 mRNA was found to be significantly higher in the colon compared with the jejunum and ileum (Fig. 1A), with similar levels in the proximal and distal colons (Fig. 1A). VPAC2 mRNA distribution followed a pattern of proximal colon > distal colon > jejunum = ileum (Fig. 1B). PAC1 receptor expression was also highest in the proximal colon, whereas there was almost no difference in PAC1 levels among distal colon, ileum, and jejunum (Fig. 1C). PAC1 was also the least expressed receptor among the three receptors along the length of the intestine. When the expressions of all receptors (VPAC1, VPAC2, and PAC1) were compared along the intestine, the highest levels were observed in the colon followed by jejunum and ileum (Fig. 1D). In addition, VPAC1 mRNA was the most highly expressed receptor, being almost 300-fold higher in all regions than VPAC2 and PAC1 mRNA. (Fig. 1D). These results indicate that the predominant receptor of VIP, which may have the most functional relevance in the intestinal mucosa, appears to be VPAC1.
Fig. 1.
Vasoactive intestinal polypeptide (VIP) receptor mRNA expression along the length of the mouse intestine. mRNA isolated from mouse intestinal mucosa from jejunum, ileum, and proximal and distal colon were subjected to qPCR with specific primers for VIP receptors: VPAC1, VAPC2, and PAC1. Graphic representation of VPAC1 (A), VPAC2 (B), and PAC1 (C) receptor expressions normalized to internal control GAPDH. Expression of receptors was compared with their expression in jejunum (arbitrary units). D: compiled data of all receptor expressions compared with PAC1 expression in the jejunum (arbitrary units). Values represent means ± SE; n = 6. *P < 0.05 vs. jejunum and ileum, ***P < 0.0005 vs. jejunum and ileum,****P < 0.0001 vs. jejunum and ileum; ####P < 0.0001 vs. distal colon.
VPAC1 protein expression along the length of the mouse intestine.
The distribution of the mRNA levels clearly indicates that VPAC1 is the highly expressed receptor along the length of the mouse intestine. Therefore, to determine the protein levels of VPAC1 in regions of the intestine from jejunum to distal colon, Western analysis was performed. A specific antibody (PA3-113) raised against VPAC1 was utilized to determine protein levels across regions. When the antibody was incubated with 5× excess peptide, the band for VPAC1 at 55 kDa was significantly blocked (Fig. 2B), demonstrating the specificity of the antibody in identifying both human and mouse VPAC1 receptor. In agreement with mRNA levels, the 55-kDa glycoprotein VPAC1 was highly expressed in the proximal and distal colons (P < 0.05) compared with the jejunum and ileum (Fig. 2A). Since the levels were highest in the colon, we next aimed to identify the cellular and membrane localization of the receptor VPAC1 in the colon.
Fig. 2.
VPAC1 protein expression along the length of the mouse intestine. A: representative Western blot and graphic representation of densitometric analysis of VPAC1 protein level expressed in mucosal protein extracted from jejunum, ileum, and proximal and distal colons of the mouse. VPAC1 protein was detected at 55 kDa. Higher expression was observed in both proximal and distal colons. Values represent means ± SE; n = 8. *P < 0.05 vs. jejunum. B: antibody-peptide competition assay in Caco2 and mouse distal colon protein lysates. Data show specificity of VPAC1 antibody as band was competed out when antibody was incubated with 5× excess of peptide.
Localization of VPAC1 receptor in mouse colon.
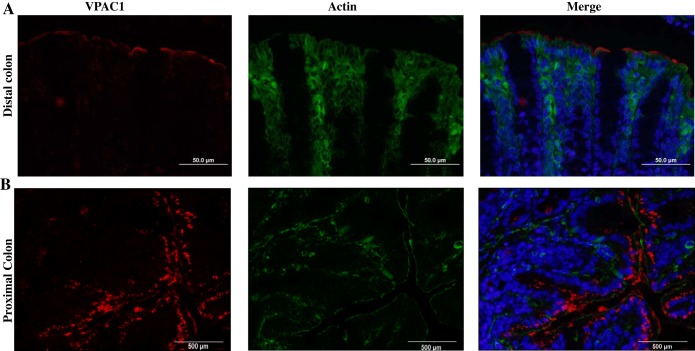
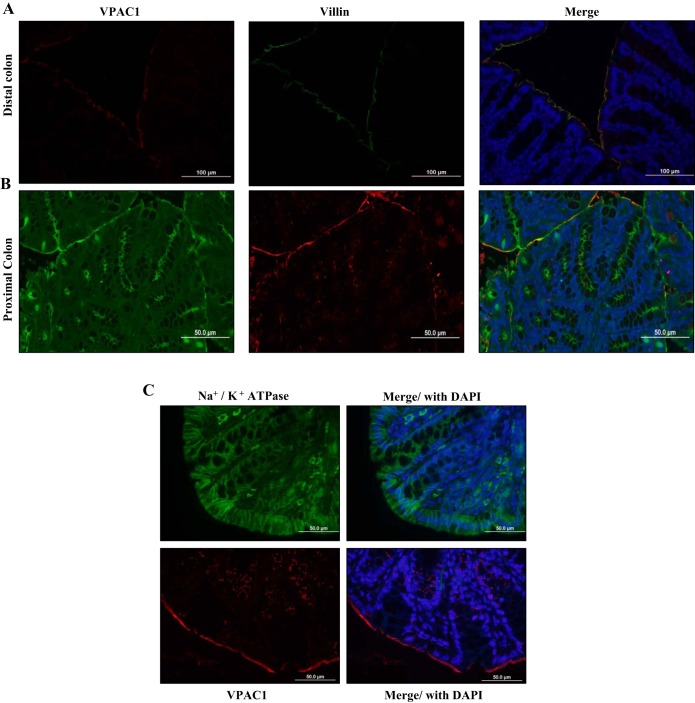
To determine the cellular localization of the receptor, immunofluorescence staining with specific VPAC1 antibody was performed in mouse colon, the region with highest expression of VPAC1. Interestingly, the VPAC1 receptor appeared to be localized to the apical membrane of the distal colon (Fig. 3A). In the proximal colon, some subapical staining was also observed in addition to the predominant staining on the apical membrane (Fig. 3B). To verify the apical localization, tissue sections were costained with the apical membrane marker villin (Fig. 4). The colocalization studies demonstrated that in the distal colon the receptor was almost exclusively located to the apical membrane of the mucosa, as confirmed by colocalization with villin (green) (Fig. 4A) and not to the basolateral marker Na+/K+-ATPase (green) (Fig. 4C). Similarly, in the proximal colon, apical localization was confirmed by colocalization of VPAC1 (green) with villin (red) (Fig. 4B). Villin was stained with two separate secondary antibodies in the proximal and distal colons to aid in differentiating between the two areas of the colon.
Fig. 3.
VAPC1 expression in mouse proximal and distal colon. Representative images of formalin-fixed, paraffin-embedded tissue sections from mouse distal (A) and proximal (B) colons of 5 μm stained with VPAC1 (Alexa fluor 568, red) and actin (Alexa fluor 488, green) and mounted with DAPI (blue) stained nuclei. Red staining was found predominantly in the luminal membrane in the distal colon, with some subapical staining present in the proximal colon.
Fig. 4.
Apical localization of VPAC1 in the mouse colon. Representative images of formalin-fixed, paraffin-embedded 5-μm tissue sections from mouse. A: distal colon stained with VPAC1 (Alexa fluor 568, red) and villin (Alexa fluor 488, green). B: proximal colon stainied with VPAC1 (Alexa fluor 488, green) and villin (Alexa fluor 568, red) mounted with DAPI (blue) stained nuclei. Yellow staining demonstrates colocalization of VPAC1 with apical marker villin. C: distal colon sections were stained separately with Na+/K+-ATPase (Alexa fluor 488, green) or VPAC1 (Alexa fluor 568, red) and DAPI, confirming apical localization. Separate staining of VPAC1 and Na+/K+-ATPase was performed due to noncompatibility of the primary antibodies when stained together.
VPAC1 mRNA expression along the length of the human intestine.
The results in mice demonstrated that the VPAC1 receptor is differentially expressed along the length of the mouse intestine. To determine if a similar pattern of expression is observed in the human intestine, we next performed qRT-PCR analysis in total human RNA (BioChain) obtained from the human jejunum, ileum, and ascending and sigmoid colons. In agreement with the mouse data, VPAC1 expression was highest in the colon, more specifically in the sigmoid colon (Fig. 5A). However, in contrast to the data in mouse colon, where there was no difference between proximal and distal colons, expression of VPAC1 in the human sigmoid colon appeared to be markedly (~4-fold) higher than in the ascending colon and small intestine. There was no difference in the expression levels of VPAC2 and PAC1 in various regions of the human intestine (Fig. 5, B and C). These data indicated that VPAC1 was the predominant VIP receptor in the human intestine as well and that its expression was relatively higher in the distal parts of the colon.
Fig. 5.
VIP receptor mRNA expression along the length of the human intestine. Total mRNAs isolated from human jejunum, ileum, and proximal and distal colons were subjected to qRT-PCR with specific primers for VIP receptors VPAC1, VAPC2, and PAC1. Graphic representation of VPAC1 (A), VPAC2 (B), and PAC1 (C) receptor expression normalized to internal control GAPDH. Expressions of receptors were compared with their expressions in jejunum where expression was set to 1. D: comparison of VIP receptor mRNA expression in all regions.
Localization of VPAC1 receptor in human colon sections.
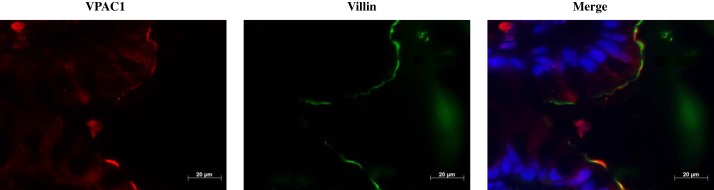
Since the mRNA expression showed the highest level of VPAC1 in the human sigmoid colon, it was of interest to determine the membrane localization of VPAC1 in the human colon. Formalin-fixed paraffin-embedded 5-μm sections of human colons were stained for immunofluorescence studies with VPAC1 (red) and villin (green) antibodies. Similar to the results observed in mice, the localization of VPAC1 in the human colon was also predominantly restricted to the apical membranes (Fig. 6).
Fig. 6.
Apical localization of VPAC1 in human colon tissue. Representative image of formalin-fixed, paraffin-embedded 5-μm section of human colon stained with VPAC1 (Alexa fluor 568, red) and villin (Alexa fluor 488, green) and mounted with DAPI (blue) stained nuclei. Yellow indicates colocalization of VPAC1 and villin, confirming apical localization. This representative image is from an unaffected colon of a 25-yr-old male patient.
DISCUSSION
Abundantly present in the intestine, VIP is an important enteric peptide with an array of physiological functions (3).
These biological effects are mediated via its binding to specific receptors, VPAC1, VPAC2, and, less avidly, to PAC1 (31). VPAC1 was first identified in the rat lung; thereafter, the human homolog was cloned and is considered to be the key receptor responsible for the majority of VIP’s biological functions (28). VPAC1 receptor is an approximately 55-kDa membrane-bound protein present in many tissues such as liver, lung, thyroid, central nervous system, immune cells, and tumors (17, 28, 44).
The studies conducted herein provide, for the first time, comprehensive data on the relative abundance of VIP receptors and their specific localization in the intestinal mucosa. Our results show comparable results to previous studies on the existence of VIP receptors in the intestine (29, 41, 47, 55). In addition, it addresses the gap in knowledge of the specific receptor subtypes of VIP in the intestinal mucosa and opens up investigation into the physiological relevance of these receptors. Earlier studies had attempted to determine the presence of VIP receptors in the gastrointestinal tract in the human, rat, and dog. The main approach of these studies involved performing radiolabeled VIP binding in either isolated epithelial cells or parafinized tissue sections. In addition, at the time when these studies were undertaken, the specific subtypes of VIP receptors had not been characterized and the reagents for immunodetection were also limited. One key reason for lack of studies could be that, after the recognition of anti-inflammatory characteristics of VIP, the major focus of VIP research shifted more to immune cells and non-gastrointestinal organs (17, 30). It should also be noted that in the mouse model the most widely used system in vivo, apart from one study conducted for VPAC2 receptor with radiolabeled ligand binding, there have been no studies conducted to determine the expression of VIP receptors in the intestine (26).
The key findings of our work are that the main receptor of VIP along the length of the mouse intestinal mucosa is VPAC1 and that the mRNA expression of VPAC1 is almost 300-times higher than the other receptors (Fig. 1). In addition, the expression was higher in the colon as compared with jejunum and ileum. However, in the human, VPAC1 was mostly expressed in the sigmoid colon (~4-fold) compared with other regions and it was similar in the jejunum, ileum and ascending colon (Fig. 5). The dissimilarity in expression pattern could be due to the RNA extracted from mucosal scrapings in mice and whole tissues in humans; this is potentially due to other VIP receptors being present in layers of the intestine from mucosa to muscle layers as compared with the mucosa alone. It could also be due to the difference in species. Protein data in mice also showed similar results to mRNA, with higher levels of VPAC1 in the colon (Fig. 2A). The most intriguing result from this study was the atypical apical localization of VPAC1 in the colonic epithelium (Figs. 3, 4, and 6). This is in contrast to previous studies, where radiolabeled VIP binding was indicated to be predominant in the basolateral membranes in the rat jejunum and rabbit ileum (18). In addition, several studies wherein effects of VIP were investigated in human colon carcinoma cell lines (Caco2 and HT29), VIP treatment was given basolaterally, implying the presence of the receptors in the basolateral membrane (2, 19, 51). However, none of these studies provide conclusive data about the specific localization of VPAC1 in intestinal epithelial cells.
This controversial finding of VPAC1 localization led us to undertake antibody authentication experiments, which would aid in validating our findings. This specific antibody raised in rabbit (PA3-113) has been used by other investigators in human and rodent models (5, 21, 48). However, to determine the specificity of the antibody in recognizing the protein of interest in human and mouse tissues, we performed a peptide competition assay (Fig. 2B). The blocking of the specific band with the addition of the specific peptide in both human and mouse tissue indicated to us that the detection of the protein was accurate. In addition, we used tissues from mice known to express less VPAC1 (kidney) as a negative control (53) and validated the results by performing qRT-PCR on RNA and then confirmed this finding by Western analysis showing comparable results (data not shown).
The mucosal lining of the colon plays a vital role in fluid reabsorption, and its impairment leads to pathological conditions that are predominant in the distal parts of the intestine, such as ulcerative colitis (35). Abundance of VPAC1 in the colon could, therefore, play a role under these conditions. Previous studies have provided valuable hints to the possible existence of VIP receptors in the epithelial cells and its potential presence on the apical membrane. Namely, the radioiodinated VIP binding studies in human colon sections demonstrated specific high binding of radiolabeled VIP to the colonic mucosa compared with other parts in tissue cross-sections, indicating the existence of VIP binding through receptors to the mucosa (55). In addition, multiple studies performed by Laburthe and colleagues, utilizing radiolabeled VIP, indicated the existence of VIP receptors on epithelial cells isolated from intestinal mucosa from rats and humans (7, 41, 47). However, these studies were conducted in cell suspensions isolated from human and rat intestines, and the term receptor was used to describe the specific binding of VIP to cells rather than identifying the target receptor protein itself (7, 41). With the advent of specific receptor subtype identification, another previous study utilized a radiolabeled analog of VIP with higher affinity for VPAC2 receptor (VPAC2 agonist) and showed minimal binding in the colonic epithelium (26). This is consistent with our current data demonstrating a significantly less expression of VPAC2 compared with VPAC1 in the colon.
The most important question arising from the presence of VPAC1 receptors in the luminal membrane of the intestine is its physiological relevance. Enteric neurons are known to secrete neuropeptides such as VIP into close proximity of the intestinal lumen due to the existence of nerve bodies in capillaries present in the mucosa (10). Another group has demonstrated that VIP is found in luminal contents after being stimulated (9). These studies provide a sound indication of potential physiological functions of luminal VIP in the intestine. Since VIP mediates functions only by binding to its receptors, luminal localization of VPAC1 may directly partake in mediation of these various functions.
The abundance and localization of VPAC1 in colon of mice and humans may indicate that some of the key functions of VIP, such as affecting epithelial regeneration (24), ion transport (42), fluid secretion (27), mucus secretion (40), and tight junction protein expression (38), can be directly mediated through the luminal receptors. The colonic epithelium undergoes constant regeneration due to being exposed to solid fecal contents that pass through the lumen. Furthermore, the very minimal presence of fluid in the colon requires mucus to be secreted to the lumen so that avid lubrication could allow rapid passage of the luminal contents. VIP affects epithelial regeneration and mucus secretion in mucosa including the intestine, which could indicate that VIP may affect these parameters in the colon. Apart from VIP, the receptor VPAC1 has lower affinity to other related peptides such as secretin and peptide histidine isoleucine and thus may also serve as a luminal signaling sensor of these secreted peptides.
The specific apical localization of VPAC1 also widens possibilities to target neuropeptide therapies to the colon, once administered luminally. Previously, we and others (14–16, 43) used VIP receptors as a targeting modality to various cancer cell populations. Being an immunomodulatory agent, VIP can also be successfully used to ameliorate colitis (1). If therapies with VIP can be administered via the oral route, the apical receptors would directly participate in the mediation of the anti-inflammatory and regenerative effects of VIP in reducing the disease pathology. The abundance of VPAC1 receptors in the lumen of the colon can be utilized to deliver VIP in drug delivery systems that resist the acidity of the stomach and are preferentially released in the colon. This can be achieved either by using enteric coated capsules (50) or by hydrogel systems (34) with VIP as an active agent so that release will take place specifically in the colonic lumen once administered orally.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-098170 (R. K. Gill); R01 DK-54016, R01 DK-81858, and R01 DK-92441 (P. K. Dudeja); and R01 DK-109709 (W. A. Alrefai); Department of Veterans Affairs Grants BX 002011 (P. K. Dudeja) and BX000152 (W. A. Alrefai); VA RCS (W. A. Alrefai) and VA SRCS Awards (P. K. Dudeja); and a TUBITAK Award (H. Onyüksel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J. conceived and designed research; D.J. performed experiments; D.J. and G.G. analyzed data; D.J., G.G., R.K.G., W.A.A., and P.K.D. interpreted results of experiments; D.J. prepared figures; D.J. drafted manuscript; D.J., G.G., R.K.G., W.A.A., H.O., and P.K.D. edited and revised manuscript; R.K.G., W.A.A., H.O., and P.K.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Special acknowledgment goes out to Dr. Marlene Gallegos, Staff Pathologist at Jesse Brown Veterans Affairs Medical Center and the staff at the core imaging facility at University of Illinois at Chicago Research Resource Center.
REFERENCES
- 1.Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology 124: 961–971, 2003. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM, Mendoza ME, Kennedy DJ, Raldua D, Thwaites DT. Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2). Br J Pharmacol 138: 564–573, 2003. doi: 10.1038/sj.bjp.0705049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker R, Dekker K, De Jonge HR, Groot JA. VIP, serotonin, and epinephrine modulate the ion selectivity of tight junctions of goldfish intestine. Am J Physiol 264: R362–R368, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne GH, Goldenring JR, Fleming FX, Rush S, Flint JS, Fielding LP, Binder HJ, Modlin IM. Inhibition of VIP-stimulated ion transport by a novel Y-receptor phenotype in rabbit distal colon. Am J Physiol 264: G848–G854, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Barbarin A, Séité P, Godet J, Bensalma S, Muller J-M, Chadéneau C. Atypical nuclear localization of VIP receptors in glioma cell lines and patients. Biochem Biophys Res Commun 454: 524–530, 2014. doi: 10.1016/j.bbrc.2014.10.113. [DOI] [PubMed] [Google Scholar]
- 6.Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J Clin Invest 73: 963–967, 1984. doi: 10.1172/JCI111320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broyart JP, Dupont C, Laburthe M, Rosselin G. Characterization of vasoactive intestinal peptide receptors in human colonic epithelial cells. J Clin Endocrinol Metab 52: 715–721, 1981. doi: 10.1210/jcem-52-4-715. [DOI] [PubMed] [Google Scholar]
- 8.Bryant MG, Polak MM, Modlin I, Bloom SR, Albuquerque RH, Pearse AG. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet 1: 991–993, 1976. doi: 10.1016/S0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- 9.Cassuto J, Fahrenkrug J, Jodal M, Tuttle R, Lundgren O. Release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut 22: 958–963, 1981. doi: 10.1136/gut.22.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa M, Furness JB, Buffa R, Said SI. Distribution of enteric nerve cell bodies and axons showing immunoreactivity for vasoactive intestinal polypeptide in the guinea-pig intestine. Neuroscience 5: 587–596, 1980. doi: 10.1016/0306-4522(80)90056-1. [DOI] [PubMed] [Google Scholar]
- 11.Coupar IM. Stimulation of sodium and water secretion without inhibition of glucose absorption in the rat jejunum by vasoactive intestinal peptide (VIP). Clin Exp Pharmacol Physiol 3: 615–618, 1976. doi: 10.1111/j.1440-1681.1976.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 12.Couvineau A, Rouyer-Fessard C, Darmoul D, Maoret J-J, Carrero I, Ogier-Denis E, Laburthe M. Human intestinal VIP receptor: cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem Biophys Res Commun 200: 769–776, 1994. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- 13.Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol 447: 119–131, 1992. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagar A, Kuzmis A, Rubinstein I, Sekosan M, Onyuksel H. VIP-targeted cytotoxic nanomedicine for breast cancer. Drug Deliv Transl Res 2: 454–462, 2012. doi: 10.1007/s13346-012-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagar S, Krishnadas A, Rubinstein I, Blend MJ, Onyüksel H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: in vivo studies. J Control Release 91: 123–133, 2003. doi: 10.1016/S0168-3659(03)00242-6. [DOI] [PubMed] [Google Scholar]
- 16.Dagar S, Sekosan M, Rubinstein I, Onyüksel H. Detection of VIP receptors in MNU-induced breast cancer in rats: implications for breast cancer targeting. Breast Cancer Res Treat 65: 49–54, 2001. doi: 10.1023/A:1006406617497. [DOI] [PubMed] [Google Scholar]
- 17.Delgado M, Abad C, Martinez C, Juarranz MG, Arranz A, Gomariz RP, Leceta J. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med (Berl) 80: 16–24, 2002. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 18.Dharmsathaphorn K, Harms V, Yamashiro DJ, Hughes RJ, Binder HJ, Wright EM. Preferential binding of vasoactive intestinal polypeptide to basolateral membrane of rat and rabbit enterocytes. J Clin Invest 71: 27–35, 1983. doi: 10.1172/JCI110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantini J, Martin J-M, Luis J, Rémy L, Tirard A, Marvaldi J, Pichon J. Restricted localization of functional vasoactive intestinal peptide (VIP) receptors in in vitro differentiated human colonic adenocarcinoma cells (HT29-D4). Eur J Cell Biol 46: 458–465, 1988. [PubMed] [Google Scholar]
- 20.Fox-Threlkeld JE, Manaka H, Manaka Y, Cipris S, Daniel EE. Stimulation of circular muscle motility of the isolated perfused canine ileum: relationship to VIP output. Peptides 12: 1039–1045, 1991. doi: 10.1016/0196-9781(91)90057-V. [DOI] [PubMed] [Google Scholar]
- 21.Fung C, Unterweger P, Parry LJ, Bornstein JC, Foong JP. VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum. Am J Physiol Gastrointest Liver Physiol 306: G748–G758, 2014. doi: 10.1152/ajpgi.00416.2013. [DOI] [PubMed] [Google Scholar]
- 22.Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol 294: G254–G262, 2008. doi: 10.1152/ajpgi.00354.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grider JR, Makhlouf GM. Colonic peristaltic reflex: identification of vasoactive intestinal peptide as mediator of descending relaxation. Am J Physiol 251: G40–G45, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Guan CX, Cui YR, Sun GY, Yu F, Tang CY, Li YC, Liu HJ, Fang X. Role of CREB in vasoactive intestinal peptide-mediated wound healing in human bronchial epithelial cells. Regul Pept 153: 64–69, 2009. doi: 10.1016/j.regpep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50: 265–270, 1998. [PMC free article] [PubMed] [Google Scholar]
- 26.Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology 145: 1203–1210, 2004. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- 27.Holst JJ, Fahrenkrug J, Knuhtsen S, Jensen SL, Poulsen SS, Nielsen OV. Vasoactive intestinal polypeptide (VIP) in the pig pancreas: role of VIPergic nerves in control of fluid and bicarbonate secretion. Regul Pept 8: 245–259, 1984. doi: 10.1016/0167-0115(84)90066-1. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron 8: 811–819, 1992. doi: 10.1016/0896-6273(92)90101-I. [DOI] [PubMed] [Google Scholar]
- 29.Korman LY, Sayadi H, Bass B, Moody TW, Harmon JW. Distribution of vasoactive intestinal polypeptide and substance P receptors in human colon and small intestine. Dig Dis Sci 34: 1100–1108, 1989. doi: 10.1007/BF01536382. [DOI] [PubMed] [Google Scholar]
- 30.Krejs GJ, Fordtran JS, Fahrenkrug J, Schaffalitzky de Muckadell OB, Fischer JE, Humphrey CS, O’Dorisio TM, Said SI, Walsh JH, Shulkes AA. Effect of VIP infusion in water and ion transport in the human jejunum. Gastroenterology 78: 722–727, 1980. [PubMed] [Google Scholar]
- 31.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept 108: 165–173, 2002. doi: 10.1016/S0167-0115(02)00099-X. [DOI] [PubMed] [Google Scholar]
- 32.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides 28: 1631–1639, 2007. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138: 843–853, 2010. doi: 10.1053/j.gastro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 14: 401–407, 2008. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, Waschek JA. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung’s disease. Peptides 28: 1688–1699, 2007. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra P, Soni V, Kumar A, Anbazhagan AN, Dudeja A, Saksena S, Gill RK, Dudeja PK, Alrefai WA. Epigenetic modulation of intestinal cholesterol transporter Niemann-Pick C1-like 1 (NPC1L1) gene expression by DNA methylation. J Biol Chem 289: 23132–23140, 2014. doi: 10.1074/jbc.M113.546283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche J-P, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 39.Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides 19: 1443–1467, 1998. doi: 10.1016/S0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 40.Plaisancié P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC. Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol 275: G1073–G1084, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Prieto JC, Laburthe M, Hoa DH, Rosselin G. Quantitative studies of vasoactive intestinal peptide (VIP) binding sites and VIP-induced adenosine 3′:5′-monophosphate production in epithelial cells from duodenum, jejunum, ileum, cecum, colon and rectum in the rat. Acta Endocrinol (Copenh) 96: 100–106, 1981. [DOI] [PubMed] [Google Scholar]
- 42.Racusen LC, Binder HJ. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology 73: 790–796, 1977. [PubMed] [Google Scholar]
- 43.Reubi C, Gugger M, Waser B. Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging 29: 855–862, 2002. doi: 10.1007/s00259-002-0794-5. [DOI] [PubMed] [Google Scholar]
- 44.Reubi JC, Läderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60: 3105–3112, 2000. [PubMed] [Google Scholar]
- 45.Rousset M, Laburthe M, Chevalier G, Boissard C, Rosselin G, Zweibaum A. Vasoactive intestinal peptide (VIP) control of glycogenolysis in the human colon carcinoma cell line HT-29 in culture. FEBS Lett 126: 38–40, 1981. doi: 10.1016/0014-5793(81)81027-7. [DOI] [PubMed] [Google Scholar]
- 46.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science 169: 1217–1218, 1970. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 47.Salomon R, Couvineau A, Rouyer-Fessard C, Voisin T, Lavallée D, Blais A, Darmoul D, Laburthe M. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. Am J Physiol 264: E294–E300, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Schulz S, Röcken C, Mawrin C, Weise W, Höllt V, Schulz S. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res 10: 8235–8242, 2004. doi: 10.1158/1078-0432.CCR-04-0939. [DOI] [PubMed] [Google Scholar]
- 49.Solano RM, Langer I, Perret J, Vertongen P, Juarranz MG, Robberecht P, Waelbroeck M. Two basic residues of the h-VPAC1 receptor second transmembrane helix are essential for ligand binding and signal transduction. J Biol Chem 276: 1084–1088, 2001. doi: 10.1074/jbc.M007686200. [DOI] [PubMed] [Google Scholar]
- 50.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials 31: 3384–3394, 2010. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 51.Thwaites DT, Kennedy DJ, Raldua D, Anderson CM, Mendoza ME, Bladen CL, Simmons NLH. H/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na/H exchanger. Gastroenterology 122: 1322–1333, 2002. doi: 10.1053/gast.2002.32992. [DOI] [PubMed] [Google Scholar]
- 52.Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol Motil 15: 239–242, 2003. doi: 10.1046/j.1365-2982.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 53.Waschek JA, Richards ML, Bravo DT. Differential expression of VIP/PACAP receptor genes in breast, intestinal, and pancreatic cell lines. Cancer Lett 92: 143–149, 1995. doi: 10.1016/0304-3835(95)03768-R. [DOI] [PubMed] [Google Scholar]
- 54.Wiley JW, O’Dorisio TM, Owyang C. Vasoactive intestinal polypeptide mediates cholecystokinin-induced relaxation of the sphincter of Oddi. J Clin Invest 81: 1920–1924, 1988. doi: 10.1172/JCI113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman RP, Gates TS, Mantyh CR, Vigna SR, Welton ML, Passaro EP Jr, Mantyh PW. Vasoactive intestinal polypeptide receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Neuroscience 31: 771–783, 1989. doi: 10.1016/0306-4522(89)90440-5. [DOI] [PubMed] [Google Scholar]