Targeted inactivation of copper-transporting ATPase 2 (Atp7b) in hepatocytes causes steatosis in the absence of inflammation.
Keywords: Wilson disease, copper-transporting ATPase 2, hepatocytes, obesity, copper
Abstract
Copper-transporting ATPase 2 (ATP7B) is essential for mammalian copper homeostasis. Mutations in ATP7B result in copper accumulation, especially in the liver, and cause Wilson disease (WD). The major role of hepatocytes in WD pathology is firmly established. It is less certain whether the excess Cu in hepatocytes is solely responsible for development of WD. To address this issue, we generated a mouse strain for Cre-mediated deletion of Atp7b and inactivated Atp7b selectively in hepatocytes. Atp7bΔHep mice accumulate copper in the liver, have elevated urinary copper, and lack holoceruloplasmin but show no liver disease for up to 30 wk. Liver inflammation is muted and markedly delayed compared with the age-matched Atp7b−/− null mice, which show a strong type1 inflammatory response. Expression of metallothioneins is higher in Atp7bΔHep livers than in Atp7b−/− mice, suggesting better sequestration of excess copper. Characterization of purified cell populations also revealed that nonparenchymal cells in Atp7bΔHep liver maintain Atp7b expression, have normal copper balance, and remain largely quiescent. The lack of inflammation unmasked metabolic consequences of copper misbalance in hepatocytes. Atp7bΔHep animals weigh more than controls and have higher levels of liver triglycerides and 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase. By 45 wk, all animals develop liver steatosis on a regular diet. Thus copper misbalance in hepatocytes dysregulates lipid metabolism, whereas development of inflammatory response in WD may depend on copper status of nonparenchymal cells. The implications of these findings for the cell-targeting WD therapies are discussed.
NEW & NOTEWORTHY Targeted inactivation of copper-transporting ATPase 2 (Atp7b) in hepatocytes causes steatosis in the absence of inflammation.
copper plays an essential role in human physiology as a cofactor of enzymes critically involved in the mitochondria function, production of neuroendocrine peptides, connective tissue formation, and many other processes. Liver is central for body copper homeostasis: it produces and secretes ceruloplasmin (CP; which accounts for ∼70% of the serum copper content) and eliminates excess copper via bile. The incorporation of copper into CP and export of excess copper out of the liver both require activity of copper-transporting ATPase 2 (ATP7B). Inactivation of ATP7B in humans or animals results in the loss of CP activity, marked accumulation of copper in the liver, and a broad spectrum of pathologies, known as Wilson disease (WD; 3, 12). Livers of WD patients show ballooned hepatocytes, inflammation, steatosis, and fibrosis; the extent of these manifestations varies between individuals, even among siblings. Animal models of WD recapitulate the key morphologic and functional changes in the liver. Although elevated copper is clearly a culprit, how exactly it triggers these various pathologies is not fully understood. Recent studies identified dysregulation of cholesterol biosynthesis (10, 22), activation of sphingomyelinases (15), inhibition of nuclear receptors (30), and a time-dependent loss of mitochondrial function in the liver (15, 31, 32) as important events associated with copper misbalance. Temporal relationships between these events remain to be established.
Hepatocytes constitute 90% of liver mass and are primary sites of ATP7B expression and copper accumulation. Metabolic changes and necrosis of hepatocytes are thought to trigger inflammation and fibrosis in the liver. Supporting this hypothesis, transplantation of fetal hepatocytes or a hepatocyte-directed expression of the recombinant ATP7B improves liver function in animal models of WD (13, 21, 26). These paradigm-setting studies have not examined the copper status or Atp7b expression in nonparenchymal liver cells (NPC), which comprise 10% of liver mass but represent 30–40% of total liver (17) cells. It remains unclear whether NPC merely respond to hepatocyte death or whether their copper metabolism is also altered and, therefore, they contribute to the onset of pathologic changes. We hypothesized that inactivating ATP7B selectively in hepatocytes would address this issue and help to identify the main metabolic events that initiate WD pathogenesis.
To test this hypothesis, we generated and characterized a new mouse strain with a targeted deletion of Atp7b in hepatocytes (Atp7bΔHep). We found that Atp7bΔHep mice display major biochemical features of WD (high copper accumulation, loss of copper incorporation into CP, increase in urinary copper export, and high expression of metallothioneins). At the same time, these animals show significantly less liver inflammation compared with animals with global inactivation of Atp7b, which accumulate copper to similar levels. Most significantly, Atp7bΔHep mice develop marked hepatic steatosis, a hallmark feature of WD. Our further studies revealed that upregulation of lipid metabolism occurred much earlier than changes in liver morphology and function and identified expression of metallothioneins, copper levels in nonparenchymal cells, and animal sex as factors that may influence liver response to copper overload.
MATERIALS AND METHODS
Animals.
The animals were housed at Johns Hopkins Animal Care Facilities, and the relevant experimental protocols have been approved by the Johns Hopkins Animal Care and Use Committee. The animals with a global Atp7b inactivation (Atp7b−/−, background strain C57BL/6x129S6/SvEv) have been previously described (2). To generate Atp7b−/− animals on a C57BL/6 background, Atp7b−/−;C57BL/6x129S6/SvEv mice were backcrossed for 10 generations with C57BL/6 mice. Atp7b−/− animals, independently of the strain background, develop liver pathology apparent with hematoxylin and eosin staining by 14–20 wk. Heterozygous Atp7b+/− (C57BL/6x129S6/SvEv background) mice do not accumulate copper and do not show pathologic changes in the liver at all tested ages.
Generation of liver-specific Atp7b knockout mice (Atp7bΔHep).
Atp7bLox/Lox mice were designed to have exon 2 of Atp7b flanked with a phosphoglycerate kinase (pGK)-gb2 loxP/flippase recognition target (FRT) neomycin resistance (neo) cassette and a loxP site for subsequent Cre-mediated removal of a 1.6-kb fragment and inactivation of Atp7b. The initial steps (construct design, blastocyte injection, generation of chimera mice, and removal of neo cassette) were done for a fee by Ingenious Targeting Laboratory (Ronkonkoma, NY). PCR amplification with two different sets of primers was used to determine deletion of the neo cassette and the presence/absence of the flippase (FLP) transgene. The neo cassette deletion was tested with the forward primer NDEL1, 5′-CGTCATAGCAAAGCTTGGTAACC-3′, and reverse primer NDEL2, 5′-CTTAAGTGCTGGATATGGGCATG-3′. The presence of the FLP transgene was tested using a forward primer FLP1, 5′ CACTGATATTGTAAGTAGTTTGC-3′, and reverse primer FLP2, 5′-CTAGTGCGAAGTAGTGATCAGG-3′. The retention of the distal loxP site was verified using a forward primer Lox1, 5′-ATAGTGACTGCACAACAGTGCC-3′, and reverse primer SDL2, 5′-GTCTGTCCCTGGGCTTAGAAATC-3′.
The Atp7bLox/Lox mice were mated with B6.Cg-Tg(Alb-Cre)21Mgn/J mice (Jackson Laboratory) that express Cre recombinase under albumin promoter. The first-generation (Lox+/−Cre+/−) animals were crossed to generate Lox/Lox:Cre+/− mice. These mice were identified by PCR using DNA from tail clip, NDEL1 and NDEL2 primers, and the Cre specific primers: forward 5′-AATGCTTCTGTCCGTTTGCCGGT-3′ and reverse 5′-CCAGGCTAAGTGCCTTCTCTACA-3′ (Fig. 1B). The Lox/Lox:Cre+/− mice had Atp7b deleted in hepatocytes and were named Atp7bΔHep. The animals were maintained on C57BL/6 background.
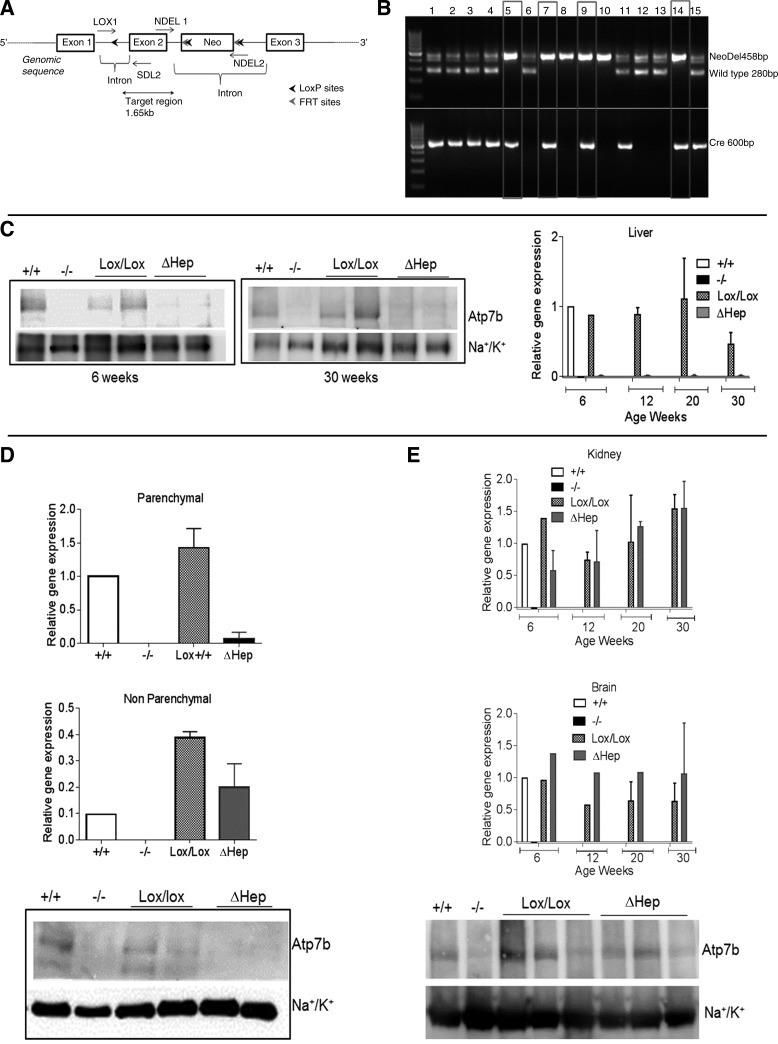
Fig. 1.
Cell-specific inactivation of Atp7b in hepatocytes of Atp7bΔHep mice. A: schematic showing the insertion of loxP sites, the neo cassette, and FRT sites with respect to exon 2 of Atp7b gene. B: verification of genotypes by PCR. The primer set Cre-5 and Cre-3 amplifies a 600-bp region in Alb-Cre-positive animals, and NDEL1 and NDEL2 primers generate a 458 band in animals with Atp7bLox/Lox genotype and 280 band for WT animals; lanes 5, 7, 9, and 14 are consistent with the Atp7bΔHep genotype. C: mRNA levels (right) and representative Western blot (left and middle) for Atp7b in livers of control (Lox/Lox) and Atp7bΔHep (ΔHep) littermates (n = 3–6 for each age and each genotype). Tissues from Atp7b+/+ and Atp7b−/− livers served as additional controls. D: detection of Atp7b mRNA and protein levels in parenchymal and nonparenchymal cells isolated from livers of Atp7bΔHep and Atp7bLox/Lox littermates (n = 2–3). Cells isolated from Atp7b+/+ and Atp7b−/− livers were used as controls. E: Atp7b mRNA levels in the kidneys and the brain at different ages (n = 1–3 for each age and each genotype) and Atp7b protein levels in kidneys.
Copper measurements.
All glassware was soaked in 10% trace metal-grade nitric acid, thoroughly rinsed with MiliQ water, and dried completely. For copper measurements in cells, frozen cell pellets were thawed, washed with HBSS, and then homogenized in HEPES buffer [20 mM HEPES pH 7.5, 1 mM EDTA, 1 mM EGTA, 8.5% sucrose, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 0.1% Igepal, 0.2% Triton X-100, and 1 tablet of EDTA-free protease inhibitor]. The homogenate was centrifuged at 10,000 g for 10 min. Protein concentration in the supernatant was measured using bicinchoninic acid (BCA) assay to be within the 3–20 μg/μl range for hepatocytes and 1–10 μg/μl for NPC. Thirty microliters of the lysate were added to 55 µl of concentrated HNO3 and digested at 55°C for 30 min. At the end of the digestion, the volume was adjusted to 200 µl with MiliQ water. For copper analysis in tissue, liver pieces (50–200 mg) were incubated at 95°C in 2-ml trace metal-grade nitric acid for 2 h with occasional agitation; the volume was adjusted to 10 ml with distilled water. The copper content was measured using Shimadzu 6650 graphite furnace atomic absorption spectrophotometer (AA) with autosampler Asc-6100. Copper concentration was quantified using standard curve and normalized to the tissue wet weight or protein concentration, for tissues and cells, respectively. To measure copper excretion in the urine, mice were placed in Tecniplast metabolic cages for 48 h with unlimited access to water and food during this period. The collected urine was centrifuged at 4,000 g for 5 min at 4°C to remove debris and food particles and then stored at −80°C until further analysis. To measure copper content by AA, 2 µl of urine were treated with 50 µl of 2% nitric acid followed by 50 µl of 50 mM NH4NO3 and final volume adjusted to make a dilution of 1:250.
Histology.
Animals were euthanized at different ages using CO2; blood was collected by cardiac puncture followed by perfusion with PBS through the left ventricle. Organs were excised, fixed in 10% buffered formalin, and then embedded, sectioned, and hematoxylin and eosin stained according to standard procedures at the Johns Hopkins Reference Histology Laboratory.
Western blot analysis.
Protein extracts were prepared on ice by homogenizing pieces of frozen tissues or cell pellets in 1 ml of homogenizing buffer (50 mM HEPES, 1:1,000 Igepal, 150 mM NaCl, 0.25 M sucrose, 0.4 mM AEBSF, and 1:100 protease inhibitor solution) using glass Dounce homogenizer. The samples received 30 strokes with each loose- and tight-fitting piston and then were centrifuged at 700 g for 15 min to remove debris. The supernatant (whole cell lysate) was collected. For isolation of membrane fractions, the supernatant was further centrifuged at 20,000 g for 40 min, and the pellet was resuspended (2 µl/mg tissue) in a sample buffer (2.7 M urea, 3.3% SDS, and 0.167 M Tris pH 6.7). Protein concentration was estimated by BCA assay. To detect Atp7b, 10 µg of membrane fraction were separated by 7.5% SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and immunostained using Abcam rabbit anti-Atp7b antibody in a 1:2,000 dilution.
Analysis of ceruloplasmin.
To measure total levels of CP, liver pieces were homogenized in 1-ml homogenizing buffer described above, 25 µg of lysates were mixed with a sample buffer (3.3% SDS, 2.7% urea, 5% β-mecaptoethanol, and 62.5 mmol/l Tris pH 6.8) and analyzed on 7.5% SDS-PAGE. For analysis of total CP in a serum, 1 µl of serum was mixed with the above sample buffer and analyzed on a 7.5% SDS-PAGE gel under denaturing conditions. To separate apo-CP and holo-CP, 1 µl of serum was treated with 1% SDS, 10% glycerol, and 62.5 mmol/l Tris pH 6.8 and analyzed under nondenaturing blue native gel conditions (27). The proteins were transferred onto a PVDF membrane and detected using goat anti-human CP antibody (Abcam) at a 1:5,000 dilution.
Quantitative PCR.
The RNA was isolated either from the frozen livers or frozen primary hepatocytes using Trizole, as suggested by the manufacturer. The RNA quality was determined by the ratio of absorbances at 260 and 280 nm, and concentration was determined by UV spectroscopy. cDNA was synthesized from 2 µg of RNA following the protocol supplied with the Roche Transcriptor First Strand cDNA synthesis kit [using oligo(dT)18 and random hexamer primers]. Quantitative PCR (qPCR) was performed using Power SYBR Green Master Mix from Applied Biosystems. The qPCR reaction mixture contained 12.5 µl of the SYBR Green Master Mix, 100 ng of cDNA, and 500 ng of each forward and reverse primer in a total volume of 25 µl. The mixture was incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The values of gene amplification were normalized to GAPDH mRNA; the relative mRNA abundance was quantified using the comparative cycle threshold method (23). The mRNA isolated from livers of 6-wk-old Atp7b+/+ mice served as a calibrator.
Isolation of primary hepatocytes.
Mice were first anesthetized using isoflurane and kept under anesthesia for the entire procedure. The liver was perfused through the portal vein first with HBSS containing 0.5 mM EGTA, followed by 200 ml of Worthington type 4 collagenase, dissolved in Williams media, at a rate of 10 ml/min. All solutions were prewarmed and maintained at 37°C during the perfusion. The collagenase-perfused liver was excised, transferred to a petri dish, and manually disrupted using forceps. The cells were transferred to a tube containing 40 ml of complete media (Williams, 10% FBS, 1% penicillin/streptomycin, and 1% Glutamax) and shaken vigorously. The cells were then passed through a 100-µm cell strainer followed by several washes with the complete media. The cells were then centrifuged at 95 g for 5 min to collect hepatocytes. To obtain NPC, the supernatant was then centrifuged at 2,500 g. Both hepatocytes and NPC were washed two times with 40-ml complete media and resuspended in 30 ml of the same media. For NPC, the resuspension volume was adjusted according to the pellet size. Cell viability was determined using Trypan Blue. Hepatocytes (typically 1.5 million cells) were plated on a 6-cm collagen-coated plate with 4-ml complete media containing 1× Gibco insulin (0.5 U/ml), transferrin (0.5 µg/ml), and selenium (5 ng/ml) (ITS) added. The rest of the hepatocytes and NPC were pelleted and stored at −80°C for RNA protein isolation or copper measurements. The purity of hepatocytes was tested first visually and then confirmed by separating cell lysates on SDS-PAGE gel and Western blotting using hepatocyte marker cytokeratin 18 (11, 18).
Flow cytometry analysis of liver leukocytes.
Livers were cut in small pieces and enzymatically digested (400 U/ml Liberase and 0.1% DNAse1; Roche Diagnostics, Indianapolis, IN). Leukocytes were then isolated using a Lymphoprep density gradient (Accurate Chemical & Scientific, Westbury, NY). Single-cell suspensions were subsequently stained for flow cytometry analysis. To characterize the myeloid population, an aliquot of the liver cell suspension was stained with live/dead (L/D) Aqua dye (Life Technologies, Grand Island, NY), cluster of differentiation 11b (CD11b)- peridinin-chlorophyll protein (PerCp)/Cy5.5 (M1/70; BioLegend, San Diego, CA), Ly6-C-Pacific Blue (HK1.4; BioLegend) and Ly6-G-AF647 (1A8; BioLegend), I-A/I-E-AF488 (M5/114.15.2; BioLegend), F4/80-phycoerythrin (PE)-Cy7 (BM8; BioLegend), CD11c- allophycocyanin (APC)-Cy7 (HL3; BD Biosciences, San Jose, CA), and CD86-AF700 (GL-1; BD Biosciences) antibodies. Polymorphonuclear cells were characterized as CD11bhiLy6C-Ly6GhiI-A/E−F4/80−, monocytes were characterized as CD11bhiLy6ChiLy6G−I-A/E+F4/80low, macrophages (Mϕ) were characterized as CD11b+ F4/80+ including CD11bintF4/80hi resident Kupffer cells and CD11bhiF4/80int recruited monocyte-derived macrophages (Ly6Chi profibrotic and Ly6Clo proresolution Mϕ), and CD11b dendritic cells were characterized as CD11chiI-A/Ehi. For the lymphocyte characterization we performed intracellular cytokine staining. Cells were stimulated for 3.5 h with 30 nM phorbol 12-myristate 13-acetate (PMA) and 1 μM ionomycin (eBioscience, Santa Clara, CA) in the presence of GolgiStop (BD Biosciences). Cells were washed and subsequently stained with L/D Aqua, CD4-PerCp/Cy5.5 (GK1.5; BioLegend), CD3-AF700 (17A2; BioLegend), CD8-PE/CF594 (53–6.7; BD Biosciences), γδ-T cell receptor (gd-TCR)-PE (GL3; BD Biosciences), CD19-APC-H7 (1D3; BD Biosciences), and natural cytotoxicity triggering receptor 1 (NKp46)-APC (29A1.4; BioLegend), followed by fixation and permeabilization (eBioscience) and intracellular cytokine staining using IL-17A-Pacific Blue (TC11-18H10.1; BioLegend), forkhead box p3 (Foxp3)-AF488 (8F5.1A9; eBioscience), and IFNγPE/Cy7 (XMG1.2; Affimetrix). Multiparameter flow cytometry was performed using a LSRII cytometer (BD Biosciences), and data were analyzed with FACSDiVa 6.1.3 software (BD Biosciences).
Data analysis.
Data were analyzed using GraphPad Prism version 6 and are expressed as means ± SD. The number of replicates, specific statistics methods, and P values are described in the figure legends. In all analyses, “ns” means “not statistically significant.”
RESULTS
Generation of Atp7bΔHep mice.
Atp7b has its highest expression in the liver compared with other tissues. We hypothesized that inactivation of Atp7b selectively in hepatocytes would result in Cu accumulation and may cause liver disease similar to WD. To test this model, we generated a mouse strain for cell-specific inactivation of Atp7b (Atp7bLox/Lox). The targeting construct was designed to flank exon 2 of the Atp7b gene with loxP sites for subsequent deletion of a 1.65-kb region by Cre recombinase (Fig. 1A). The targeted exon and the region to be removed by Cre were similar to those in mice with a globally inactivated Atp7b (Atp7b−/−; 2); this was done to simplify comparisons of the phenotypes. The Atp7bLox/Lox animals have normal reproductive ability, development, and life span.
To inactivate Atp7b in hepatocytes, the Atp7bLox/Lox mice were mated with the transgenic mice expressing Cre recombinase under an albumin promoter. The desired Atp7bLox/Lox/Albcre+/− genotype was verified by PCR (Fig. 1B). In Atp7bΔHep livers, Atp7b mRNA was reduced to 3–5% of the wild-type (WT) level (Fig. 1C, right). Similarly, Atp7b protein was markedly reduced in Atp7bΔHep livers compared with Atp7bLox/Lox or WT controls, but not fully absent (Fig. 1C, left and middle). This was in contrast to Atp7b−/− livers, which had no Atp7b. Traces of Atp7b in Atp7bΔHep liver could be due to incomplete deletion of Atp7b in hepatocytes or Atp7b expression in NPC (Kupffer cells, stellate cells, and endothelial cells), which has not so far been examined. To distinguish between these possibilities, we purified hepatocytes and NPC from livers of 6-wk-old animals of all four genotypes (Atp7b−/−, Atp7bΔHep, Atp7b+/+, and Atp7bLox/Lox, respectively) and quantified Atp7b mRNA and protein in these cell populations (Fig. 1D). As expected, Atp7b was absent in Atp7b−/− hepatocytes and Atp7b−/− NPC and was present in both cell populations from the Atp7b+/+ (WT) liver. In Atp7bΔHep hepatocytes, Atp7b mRNA was decreased by 98% compared with Atp7bLox/Lox control (Fig. 1D, top); a marked decrease in protein abundance was further confirmed by Western blotting (Fig. 1D, bottom). By contrast, the Atp7bΔHep NPC showed significant expression of Atp7b; the Atp7b mRNA levels were lower than in Atp7bLox/Lox control, but similar to those in the WT NPC (Fig. 1D, middle). Thus the WT and Atp7bΔHep NPC express Atp7b, and in Atp7bΔHep mice we achieved a preferential deletion of Atp7b in hepatocytes. Tissue specificity of Atp7b downregulation in Atp7bΔHep mice was illustrated by insignificant changes in the levels of Atp7b mRNA in kidneys and brains at different ages (Fig. 1E). The lack of compensatory upregulation of Atp7b was confirmed by Western blot analysis (shown for kidneys, where Atp7b signal is overall stronger).
Atp7bΔHep mice have disregulated copper homeostasis in the liver.
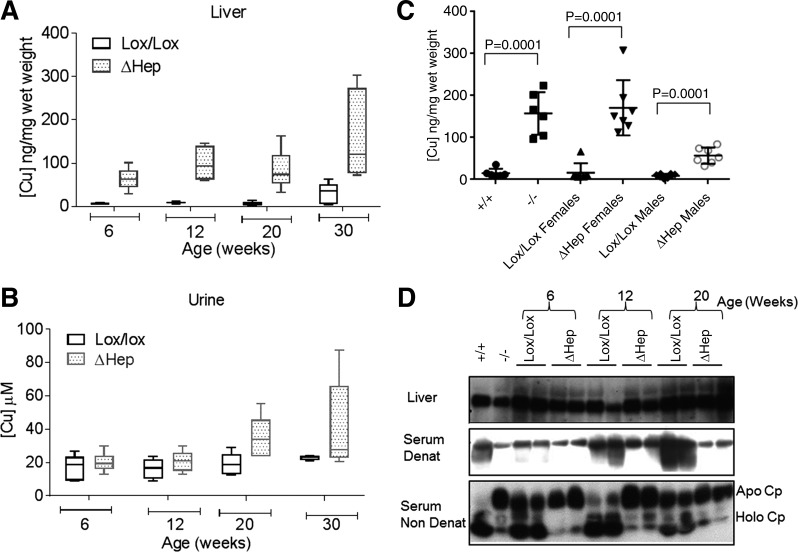
Inactivation of Atp7b in hepatocytes was expected to 1) decrease copper efflux from the liver resulting in copper accumulation; 2) prevent copper incorporation into CP; and, at the organism level, 3) increase copper in the urine. The Atp7bΔHep mice displayed all these features. The Atp7bΔHep livers had a 10–13-fold increase in copper content by 6 wk after birth (Fig. 2A), and overall, copper accumulation was similar to that in the Atp7b−/− livers. Urinary copper levels from Atp7bΔHep mice were normal at 6 and 12 wk after birth and elevated ~2-fold at 20 wk and later (Fig. 2B). The significant variability in copper levels in Atp7bΔHep mice prompted us to investigate influence of animals’ sex on copper accumulation. Female animals consistently showed much higher copper accumulation compared with males (Fig. 2C); within the sexes the copper values did not vary significantly.
Fig. 2.
Atp7bΔHep mice accumulate copper and lack holo-CP. A: copper levels in the Atp7bΔHep livers compared with Atp7bLox/Lox controls (n = 5–6). B: copper excretion in the urine of Atp7bΔHep mice and Atp7bLox/Lox littermates (n = 5–6). C: copper accumulation in Atp7bΔHep livers differs in males and females; sex dependence was not apparent in Atp7b−/− and Atp7b+/+ animals (n = 5–8 for each genotype and each sex; P value determined using 2-tailed unpaired t-test). D: ceruloplasmin in the liver and serum of Atp7bΔHep mice and control littermates. Top: total ceruloplasmin in the liver is unchanged in Atp7bΔHep mice. Middle: analysis of ceruloplasmin in a serum under denaturing conditions (Serum Denat) shows lower levels of CP in Atp7bΔHep mice compared with control Atp7bLox/Lox animals. Bottom: ratio of holo-CP and apo-CP in a serum under nondenaturing conditions (Serum Non Denat). Atp7b+/+ and Atp7b−/− were used as positive (holo-CP) and negative (apo-CP) controls.
Similar to WD patients and Atp7b−/− mice, the protein synthesis of CP was normal in Atp7bΔHep mice, but copper was not incorporated, yielding apo-CP. Secreted apo-CP is unstable in a serum and rapidly degrades (14). Consistent with these observations, in Atp7bΔHep mice, the protein production of CP in a liver was unaffected (Fig. 2D, top), whereas serum CP levels were significantly lower compared with Atp7bLox/Lox control, especially in older mice (Fig. 2D, middle). Analysis of serum proteins using nondenaturing electrophoresis (7) confirmed that CP was present mostly in the apo form (Fig. 2D, bottom) and was therefore less stable.
Atp7bΔHep mice do not show pathologic changes in liver morphology or function for up to 30 wk.
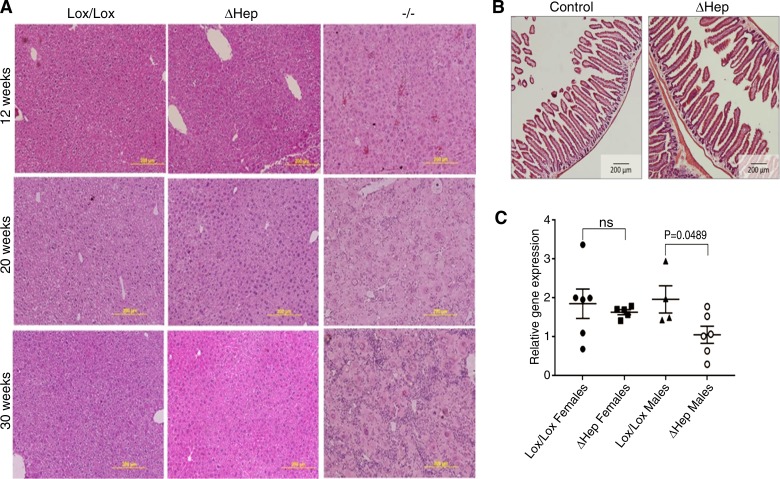
Copper misbalance in WD patients and animals with a globally inactivated Atp7b is associated with an inflammatory hepatitis, which in animals develops within 3 mo after birth (32). Accordingly, hematoxylin and eosin-stained liver sections from Atp7b−/− animals showed progressive inflammation, hepatocyte degeneration, and karyomegaly/cytomegaly at 12, 20, and 30 wk (Fig. 3A, right). In contrast, the histomorphology of Atp7bΔHep livers was very similar to control Atp7bLox/Lox for up to 30 wk (Fig. 3A, left and middle). Consistent with different liver histology, the activity of serum aminotransferases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) was high in serum from Atp7b−/− animals but normal in Atp7bΔHep serum indicating absence of significant liver damage in Atp7bΔHep animals (Table 1). Total protein, albumin, and bilirubin in Atp7bΔHep serum were also similar to controls (Table 1). We verified that absence of pathology in Atp7bΔHep liver was not due to significant upregulation of a homologous copper transporter, Atp7a: the levels of Atp7a mRNA were unchanged in the whole liver and in different cell populations at 6 wk and were only slightly elevated at 20 wk (data not shown). To examine a potential cross talk between the gut and liver tissue, we examined intestinal morphology (Fig. 3B), which was normal, and expression of Toll-like receptor 4 (TLR4) in the liver (involved in the gut-liver signaling). No changes in TLR4 expression were seen in female Atp7bΔHep mice compared with control Lox/Lox mice; decreased TLR4 levels were detected in Atp7bΔHep males (Fig. 3C).
Fig. 3.
Atp7bΔHep mice do not show pathology up to 30 wk, have normal intestinal morphology, and do not upregulate TLR4. A: representative histology (hematoxylin and eosin staining) of liver sections from Atp7bΔHep, Atp7b−/−, and Atp7bLox/Lox mice at different ages illustrates morphologic changes in Atp7b−/− liver, whereas histomorphology for Atp7bΔHep is similar to that of Atp7bLox/Lox mice. B: histology of intestinal sections from 52-wk-old Atp7bΔHep mice and controls demonstrates the lack of pathologic changes in intestine. C: TLR4 mRNA expression in 20-wk liver of Atp7bΔHep, and Atp7bLox/Lox mice (n = 4–7). Data were analyzed using a two-tailed unpaired t-test.
Table 1.
Biochemical characteristics of serum in control and mutant mice
| Parameter | Normal Range | Atp7b+/+(n = 2) | Atp7b−/− (n = 2) | Atp7bLox/Lox (n = 4) | Atp7bΔHep (n = 5) |
|---|---|---|---|---|---|
| ALP | 36–96 | 122 ± 26 | 219 ± 12* | 116 ± 11 | 104.6 ± 18 |
| Glucose | 62–175 | 215 ± 8 | 116 ± 12 | 203 ± 50 | 307 ± 74* |
| Total protein | 3.5–7.2 | 5.05 ± 0.15 | 4.35 ± 0.05 | 5.4 ± 0.3 | 5.3 ± 0.3 |
| Albumin | 2.5–3.0 | 2.95 ± 0.05 | 2.45 ± 0.05 | 3.3 ± 0.2 | 3.0 ± 0.2 |
| Bilirubin (total) | 0.1–0.9 | 0.25 ± 0.05 | 0.7 | 0.3 | 0.36 ± 0.1 |
| LDH | 500 ± 56 | 1,519 ± 14* | 730 ± 194 | 572 ± 104 | |
| ALT | 26–77 | 28.5 ± 15 | 425 ± 45* | 65 ± 16 | 30 ± 7 |
| AST | 54–269 | 128.5 ± 15 | 408 ± 4* | 211 ± 53 | 130 ± 18 |
| GGT | 3.5 ± 1.5 | 4.5 ± 0.5 | 4.6 ± 1.2 | 2.3 ± 0.9 |
Values are means ± SD. GGT, gamma-glutamyl-transferase.
Significant difference.
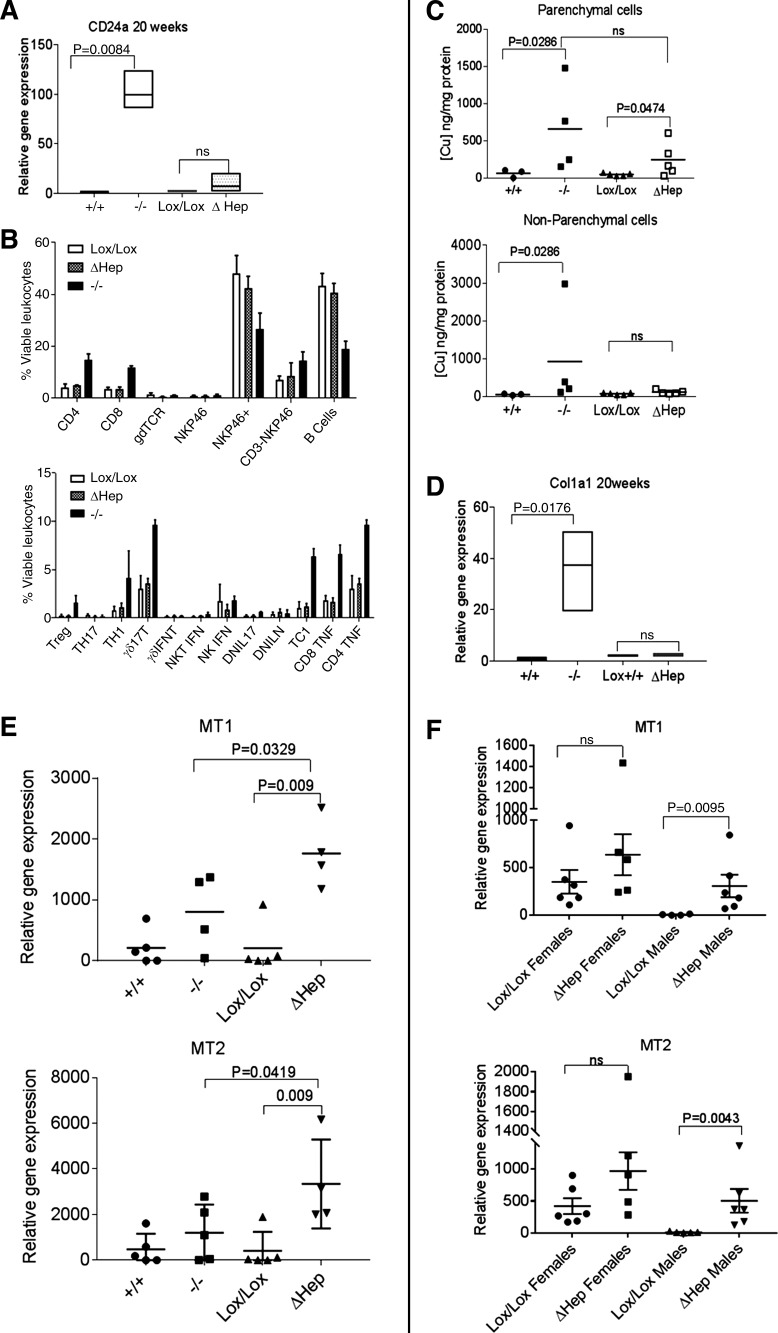
Inflammatory response in the liver of Atp7bΔHep mice is significantly milder than in Atp7b−/− mice. The marked difference in liver pathology between Atp7b−/− and Atp7bΔHep mice prompted us to characterize the liver inflammatory response in more detail. Using gene array data for 6-wk-old Atp7b−/− livers (4), we identified CD24a as the only molecule with known inflammatory function that was upregulated more than 1.5-fold at this age. Analysis of CD24a mRNA in Atp7b−/− liver at 20 wk revealed almost a 100-fold increase compared with WT control (Fig. 4A). By contrast, in Atp7bΔHep livers, CD24a was increased only threefold compared with Atp7bLox/Lox mice at 20 wk (Fig. 4A) and showed no change at 6 wk, indicating a significantly more muted immune response in Atp7bΔHep liver compared with Atp7b-null mice.
Fig. 4.
Atp7bΔHep and Atp7b−/− mice differ in copper accumulation in nonparenchymal cells, metallothionein, expression, and immune response. A: expression of CD24a is significantly increased in Atp7b−/−, but not in Atp7bΔHep livers at 20 wk after birth (n = 4–5 per genotype). The qPCR analysis was done using GAPDH as a control, and the amounts of transcripts were normalized to the transcript levels in Atp7b+/+ liver taken as 1. Data were analyzed using unpaired parametric two-tailed t-test. B: profiles of inflammatory cells in livers of 20-wk-old mice Atp7bLox/Lox, Atp7b−/−, and Atp7bΔHep characterized by flow cytometry (n = 3–4 per genotype; Treg, regulatory T cells; IFNT, interferon-τ; NKT, natural killer T cells; DNIL17, double-negative IL-17 and IL-N-producing T cells, respectively; TC1, type 1 CD8+ T cells). C: copper measurements in isolated hepatocytes and nonparenchymal cells. Parenchymal and nonparenchymal cells were isolated from 6-wk-old livers of Atp7b+/+, Atp7b−/−, Atp7bLox/Lox, and Atp7bΔHep mice (n = 3–5 per genotype; data were analyzed using unpaired 1-tailed t-test, because copper levels in Atp7b−/− samples were uniformly higher compared with controls). D: Col1a1 is significantly upregulated in Atp7b−/−, but not in Atp7bΔHep livers at 20 wk. E: differential expression of metallothionein 1 and 2 (MT1 and MT2) in 20-wk Atp7b−/− and Atp7bΔHep livers (n = 4–5). F: expression of MT1 and MT2 in the liver of 20-wk-old Atp7bΔHep males and females and age-matched controls. Data were analyzed using unpaired two-tailed t-test.
CD24a plays an important role in activation of T cells and leukocyte recruitment (8, 19); consequently, we evaluated the composition of immune cells in Atp7b−/− and Atp7bΔHep livers using cell sorting. The 20-wk Atp7b−/− livers showed dense infiltration by immune cells, with a higher proportion of CD4, CD8, γβ-T cells, natural killer (NK) cells, and macrophages compared with Atp7bΔHep and WT livers. Intracellular cytokine staining established a strong polarization toward a type 1 inflammatory response with accumulation of T helper 1 (Th1) cells (IFNγ-producing CD4+ T cells), cytotoxic T lymphocytes (IFNγ-producing CD8+ T cells), and a high number of TNF-α-producing CD4 and CD8 T cells (Fig. 4B). Consistent with their normal histology and function, the livers from Atp7bΔHep mice did not show inflammation, and their immune cell profile was similar to the WT.
NPC play an important role in development of inflammation and fibrosis (24, 28). We hypothesized that the expression of Atp7b in NPC of Atp7bΔHep liver precludes copper accumulation and activation of NPC in contrast to global knockouts, which accumulate copper throughout the liver tissue (20). To test this hypothesis, we measured copper levels in isolated cell populations (hepatocytes and NPC). As expected, hepatocytes from Atp7b−/− and Atp7bΔHep livers both had elevated copper content; the difference (8-fold and 6-fold increase, respectively) was not statistically significant (Fig. 4C, top). In contrast, copper was elevated in Atp7b−/− NPC and unchanged in Atp7bΔHep NPC (Fig. 4C, bottom), consistent with the loss of Atp7b expression in Atp7b−/− NPC and retention of expression in Atp7bΔHep NPC. Collagen type 1a (Col1a), secreted by activated NPC (stellate cells), is highly upregulated in Atp7b−/− liver (Fig. 4D) and is associated with fibrotic liver disease (6). Col1a expression in Atp7bΔHep liver was similar to that in the WT and Atp7bLox/Lox liver and significantly lower than expression in Atp7b−/− mice (Fig. 4D).
Atp7bΔHep livers express high levels of metallothioneins.
The lack of liver damage in Atp7bΔHep animals despite significant copper overload was surprising. Consequently, we investigated potential reasons for this unexpectedly low copper toxicity. Metallothioneins MT1 and MT2 bind copper with high affinity and limit copper reactivity. These proteins are usually upregulated in response to copper elevation; difference in metallothionein expression between the Atp7b−/− and Atp7bΔHep livers may contribute to dissimilar effects of high copper. Analysis of MT mRNA demonstrated that as expected, expression of MT1 and MT2 was upregulated in both Atp7b−/− and Atp7bΔHep livers. However, the levels of both MT1 and MT2 were significantly higher in Atp7bΔHep mice (Fig. 4E). This result supports the idea that copper in Atp7bΔHep mice might be less biologically available. Given sex-related differences in copper accumulation in Atp7bΔHep livers, we also compared MT levels between males and females. Atp7bΔHep mice of both sexes had high levels of MTs (14-fold and 2.2-fold increase for males and females, respectively). Upregulation was higher in males largely because of very low MT expression in control Atp7bLox/Lox strain, whereas control Atp7bLox/Lox females had high background MT levels (Fig. 4F).
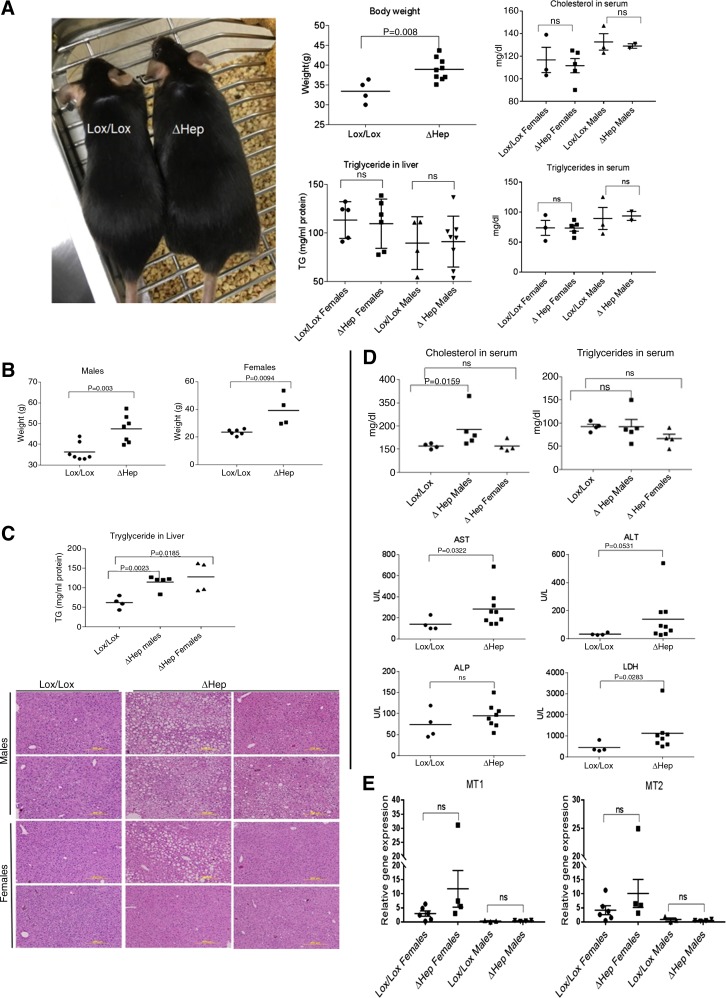
Atp7bΔHep mice have dysregulated lipid metabolism, increased body weight, and liver steatosis. We hypothesized that lack of copper accumulation in NPC of Atp7bΔHep livers would help to identify the main (initial) events associated with copper overload in hepatocytes. Consequently, we extended studies to include older animals and characterized the phenotype in more detail. Comparison of sex-matched littermates revealed that Atp7bΔHep males were significantly heavier compared with Atp7bLox/Lox control (Fig. 5A, left and top middle). At 20 wk after birth, despite obesity, there was no difference in hepatic triglycerides (Fig. 5A, bottom middle) and cholesterol (data not shown) among mice based on sex or genotype, and also there was no difference in levels of triglycerides and cholesterol in a serum (Fig. 5A, right). At 45 wk, both Atp7bΔHep males and females were significantly heavier than respective controls (Fig. 5B) and had elevated hepatic triglyceride levels (Fig. 5C, top). Circulating cholesterol levels were higher in males but unchanged in females. Serum triglycerides were comparable with controls for both males and females (Fig. 5D, top). Consistent with weight changes and metabolic measurements, liver histology from 45-wk-old Atp7bΔHep animals revealed a range of abnormalities including mild to severe steatosis, steatohepatitis, and necrosis (Fig. 5C, bottom). These abnormalities were more pronounced in male animals. Analysis of liver function showed that AST, ALT, and LDH levels were mildly elevated in Atp7bΔHep animals, indicating liver injury (Fig. 5D, bottom). Other parameters such as ALP were also elevated in sera, but differences were not statistically significant (Fig. 5D, bottom). Significant changes were observed in the expression of MT1 and MT2, which at 45 wk were significantly lower compared with 20 wk, for both sexes (Fig. 5E). The dam genotype and nursing patterns of the mice did not influence the steatotic phenotype.
Fig. 5.
Atp7bΔHep mice show dysregulated lipid metabolism and steatosis. A, left: representative image illustrating a size difference between the Atp7bΔHep male and a sex-matched Atp7bLox/Lox control. Middle top: weight difference between Atp7bΔHep and Atp7bLox/Lox male mice (n = 4–9). Middle bottom: triglyceride levels in Atp7bΔHep and Atp7bLox/Lox livers at 20 wk after birth (n = 4–8 per sex and per phenotype). Right: cholesterol and triglyceride levels in serum of 20-wk-old animals (n = 2–5). B: weight increase of Atp7bΔHep and Atp7bLox/Lox mice at 45 wk after birth (n = 4–7). C, top: triglyceride (TG) levels in liver at 45 wk (n = 4–5). Sex-related difference in triglyceride levels was not observed for control Lox/Lox animals, and the corresponding data were combined. Bottom: representative hematoxylin and eosin staining of liver sections. D, top: cholesterol and triglyceride levels in serum at 45 wk. Bottom: liver injury markers in a serum of 45-wk-old mice. E: MT1 and MT2 expression in the liver at 45 wk after birth (n = 4–9 per genotype; data were analyzed using unpaired 2-tailed t-test).
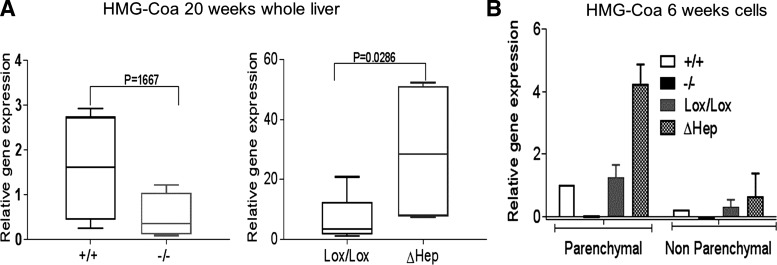
HMG-CoA reductase is upregulated in Atp7bΔHep livers before changes in liver histomorphology.
The above results established that Cu misbalance alters lipid metabolism before the development of significant liver disease. Measurements of expression of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis, confirmed this conclusion. It has been previously shown that lipid metabolism is inhibited in Atp7b−/− mice at the early stage of disease (10). Accordingly, expression of HMG-CoA was decreased in the Atp7b−/− liver (Fig. 6A, left). Analysis of isolated cell populations confirmed this conclusion. In both hepatocytes and NPC from Atp7b−/− livers, HMG-CoA was significantly downregulated. By contrast, the liver and hepatocytes purified from Atp7bΔHep mice showed significantly increased HMG-CoA levels, especially in Atp7bΔHep hepatocytes (Fig. 6, A, right, and B). This change occurs as early as 6 wk after birth preceding the onset of morphologic changes in the liver by many weeks.
Fig. 6.
Levels of HMG-CoA differ significantly between the Atp7bΔHep and Atp7b−/− strains. A: expression of HMG-CoA reductase in whole livers at 20 wk (n = 4 or 5 per each condition). Gene expression was normalized to Atp7b+/+ levels, and data were analyzed using Mann-Whitney two-tailed t-test. B: expression of HMG-CoA reductase in parenchymal and nonparenchymal cells isolated from livers of 6-wk-old mice (n = 3–5 each for Atp7bLox/Lox and Atp7bΔHep; cells from individual Atp7b+/+ and Atp7b−/− livers are used for reference). The values were normalized to GAPDH and compared with 6-wk-old Atp7b+/+ hepatocytes.
DISCUSSION
In this study, we examined whether copper accumulation in hepatocytes lacking the copper transporter Atp7b is sufficient to trigger a WD-like pathology in the liver. Our results do not support this simple model. Despite significant copper accumulation in hepatocytes, steatosis is the only typical WD pathology that we observed in Atp7bΔHep livers. Our data suggest that lipid misbalance is the major metabolic consequence of copper overload that occurs before other metabolic changes. This result reinforces a previously observed positive correlation between the liver copper content and steatosis scores in WD patients and provides direct evidence that hepatic steatosis is not a result of generic liver malfunction, but is caused by copper load in hepatocytes (16). The strikingly slow development of other pathologies (inflammation, fibrosis, and hepatocyte ballooning) is not due to species (human vs. mouse) differences, because mice (and rats) with global inactivation of Atp7b uniformly show pathologic changes in the liver within 2–4 mo after birth. Several factors may be required for the onset of inflammation and fibrosis. Our data suggest that expression levels of metallothioneins, copper misbalance in NPC, and sex may significantly augment liver response to copper overload. We demonstrate an important and previously unappreciated role of Atp7b in nonparenchymal cells, characterized the immune response in Atp7b−/− liver, and identified HMG-CoA reductase as an early marker of copper-induced lipid misbalance. Altogether, our results significantly update the mechanistic model of pathology development in WD.
Our studies were aided by generation of a new mouse strain with a targeted deletion of Atp7b in hepatocytes (Atp7bΔHep). These mice display the key biochemical hallmarks of WD, yet their inflammatory response is greatly muted compared with that of Atp7b−/− mice or human WD. The Atp7b−/− livers are rich in B cells, macrophages, and CD4 and CD8 T cells, i.e., cells producing cytokines such as TNF and interferon. In chronic hepatitis, these inflammatory mediators serve to activate hepatic stellate cells, which, in turn, results in liver fibrosis. It is notable that expression of Col1a, a marker of fibrosis, differs significantly between Atp7bΔHep mice, where it is similar to the WT mice, and Atp7b−/− mice, where it is highly elevated. Thus, in Atp7bΔHep mice, hepatic stellate cells remain quiescent and are unlikely to be triggering liver fibrosis. We observed similar differences for CD24a expression, which was robustly increased in the Atp7b−/− mice and only slightly changed in Atp7bΔHep livers. CD24a is a cell surface antigen for regulator B cells (5) and also a marker of reprogramming-responsive cells such as epiblast stem cells (25). Thus high expression of CD24a in Atp7b−/− liver may reflect activation of cells/programs necessary for liver repair. Further studies will test this hypothesis and determine relevance of CD24a upregulation to parenchymal regeneration observed in Atp7b−/− at the later stage of the disease (9). Altogether, our findings suggest that the development of WD phenotype in the liver is likely a result of copper overload in both hepatocytes and nonparenchymal cells. Thus WD therapies involving cell-specific targeting or cell replacement may not achieve optimal results if focused exclusively on hepatocytes. Differences in immunological response between two mutant strains also suggest that it may be worthwhile exploiting the immune system for treatment or delay of WD symptoms.
Characterization of Atp7bΔHep mice metabolism illustrates a role of liver copper homeostasis in the organism fat balance. Atp7bΔHep mice gain weight, have increased adipose tissue, and accumulate triglycerides long before pathologic changes are evident in the liver. The discrepancy between phenotype of Atp7b−/− liver (which shows downregulation of lipid metabolism) and Atp7bΔHep liver (which shows upregulation of fat and cholesterol production) remains puzzling. The differences could be due to distinct NPC signaling in their livers (see above) or a significant difference in expression levels of metallothioneins. A higher MT-to-Cu ratio in Atp7bΔHep liver may result in efficient sequestration of Cu and even a deficit of bioavailable copper, known to be associated with fat accumulation (1, 29). In Atp7b−/− liver, where the MT-to-Cu ratio is lower, copper could be more bioavailable and cause inhibition of lipid metabolism. We speculate that the MT-to-Cu ratio rather than total copper determines specific metabolic changes in the liver. The new strain represents a valuable tool for testing this hypothesis and elucidating the relationship between hepatic copper homeostasis, MT1 and MT2 expression, and lipid metabolism.
Finally, the Atp7bΔHep mice show differences between sexes in response to copper overload that have not been as apparent in other strains. Specifically, Atp7bΔHep females accumulate more copper and have higher triglyceride levels, but their liver histopathology (defined by the size of lipid droplets) is much less pronounced than that in males. This result indicates that histology reflects the extent of steatotic changes (which varies markedly in humans) less accurately than direct measurements of metabolites.
In summary, targeted deletion of Atp7b in hepatocytes yielded a wealth of new information. We found significant changes in liver lipid metabolism in response to copper overload, demonstrated that this change occurs before other pathologic changes in the liver, and identified several factors that may modulate liver response to copper overload. Our results may aid understanding of phenotypic diversity in WD and development of future cell-targeted therapies for WD.
GRANTS
This work was supported by the National Institute of General Medical Sciences Grants 67166 and 101502 to S. Lutsenko.
DISCLOSURES
L. Aronov is a former employee of Ingenious Targeting Laboratory; she and all other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
A.M., F.H., H.Y., L.A., and S.L. conceived and designed research; A.M., H.Y., L.K., H.F., H.P., A.B., R.M., and C.R.W.-K. performed experiments; A.M., H.Y., and S.L. analyzed data; A.M., H.Y., J.P.H., F.H., L.A., C.S., J.P., and S.L. interpreted results of experiments; A.M. and H.P. prepared figures; A.M. and J.P.H. drafted manuscript; S.L. edited and revised manuscript; S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge help of the late Dr. David Huso with the analysis of liver pathology of mice at the early stages of this project. We thank Dr. Martina Ralle for help with copper measurements and Christopher Mangles for help with maintenance of the mouse colony.
REFERENCES
- 1.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol 105: 1978–1985, 2010. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 2.Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8: 1665–1671, 1999. doi: 10.1093/hmg/8.9.1665. [DOI] [PubMed] [Google Scholar]
- 3.Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5: 327–337, 1993. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 4.Burkhead JL, Ralle M, Wilmarth P, David L, Lutsenko S. Elevated copper remodels hepatic RNA processing machinery in the mouse model of Wilson’s disease. J Mol Biol 406: 44–58, 2011. doi: 10.1016/j.jmb.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goode I, Xu H, Ildstad ST. Regulatory B cells: the new “it” cell. Transplant Proc 46: 3–8, 2014. doi: 10.1016/j.transproceed.2013.08.075. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton JP, Koganti L, Muchenditsi A, Pendyala VS, Huso D, Hankin J, Murphy RC, Huster D, Merle U, Mangels C, Yang N, Potter JJ, Mezey E, Lutsenko S. Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B(−/−) (Wilson disease) mice. Hepatology 63: 1828–1841, 2016. doi: 10.1002/hep.28406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD. Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem 277: 46632–46638, 2002. doi: 10.1074/jbc.M206246200. [DOI] [PubMed] [Google Scholar]
- 8.Hubbe M, Altevogt P. Heat-stable antigen/CD24 on mouse T lymphocytes: evidence for a costimulatory function. Eur J Immunol 24: 731–737, 1994. doi: 10.1002/eji.1830240336. [DOI] [PubMed] [Google Scholar]
- 9.Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol 168: 423–434, 2006. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 282: 8343–8355, 2007. doi: 10.1074/jbc.M607496200. [DOI] [PubMed] [Google Scholar]
- 11.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem 282: 22089–22101, 2007. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan JH, Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem 284: 25461–25465, 2009. doi: 10.1074/jbc.R109.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuda T, Teratani T, Ochiya T, Sakai Y. Transplantation of a fetal liver cell-loaded hyaluronic acid sponge onto the mesentery recovers a Wilson’s disease model rat. J Biochem 148: 281–288, 2010. doi: 10.1093/jb/mvq063. [DOI] [PubMed] [Google Scholar]
- 14.Kojimahara N, Nakabayashi H, Shikata T, Esumi M. Defective copper binding to apo-ceruloplasmin in a rat model and patients with Wilson’s disease. Liver 15: 135–142, 1995. doi: 10.1111/j.1600-0676.1995.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 15.Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rübben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Häussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 13: 164–170, 2007. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 16.Liggi M, Murgia D, Civolani A, Demelia E, Sorbello O, Demelia L. The relationship between copper and steatosis in Wilson’s disease. Clin Res Hepatol Gastroenterol 37: 36–40, 2013. doi: 10.1016/j.clinre.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Malarkey DE, Johnson K, Ryan L, Boorman G, Maronpot RR. New insights into functional aspects of liver morphology. Toxicol Pathol 33: 27–34, 2005. doi: 10.1080/01926230590881826. [DOI] [PubMed] [Google Scholar]
- 18.Mezey E, Rennie-Tankersley L, Potter JJ. Liver alcohol dehydrogenase is degraded by the ubiquitin-proteasome pathway. Biochem Biophys Res Commun 285: 644–648, 2001. doi: 10.1006/bbrc.2001.5226. [DOI] [PubMed] [Google Scholar]
- 19.Park K, He X, Lee HO, Hua X, Li Y, Wiest D, Kappes DJ. TCR-mediated ThPOK induction promotes development of mature (CD24-) gammadelta thymocytes. EMBO J 29: 2329–2341, 2010. doi: 10.1038/emboj.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralle M, Huster D, Vogt S, Schirrmeister W, Burkhead JL, Capps TR, Gray L, Lai B, Maryon E, Lutsenko S. Wilson disease at a single cell level: intracellular copper trafficking activates compartment-specific responses in hepatocytes. J Biol Chem 285: 30875–30883, 2010. doi: 10.1074/jbc.M110.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roybal JL, Endo M, Radu A, Gray L, Todorow CA, Zoltick PW, Lutsenko S, Flake AW. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson’s disease. Gene Ther 19: 1085–1094, 2012. doi: 10.1038/gt.2011.186. [DOI] [PubMed] [Google Scholar]
- 22.Sauer SW, Merle U, Opp S, Haas D, Hoffmann GF, Stremmel W, Okun JG. Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b(−/−) mice as a model for Wilson disease. Biochim Biophys Acta 1812: 1607–1615, 2011. doi: 10.1016/j.bbadis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61: 1066–1079, 2015. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakiba N, White CA, Lipsitz YY, Yachie-Kinoshita A, Tonge PD, Hussein SM, Puri MC, Elbaz J, Morrissey-Scoot J, Li M, Munoz J, Benevento M, Rogers IM, Hanna JH, Heck AJ, Wollscheid B, Nagy A, Zandstra PW. CD24 tracks divergent pluripotent states in mouse and human cells. Nat Commun 6: 7329, 2015. doi: 10.1038/ncomms8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, Wang H, Zhang J, Xie Y, Li L, Chen X, Edgerton BF, Bonami JR. Response of crayfish, Procambarus clarkii, haemocytes infected by white spot syndrome virus. J Fish Dis 28: 151–156, 2005. doi: 10.1111/j.1365-2761.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 27.Swamy M, Siegers GM, Minguet S, Wollscheid B, Schamel WW. Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci STKE 2006: pl4, 2006. doi: 10.1126/stke.3452006pl4. [DOI] [PubMed] [Google Scholar]
- 28.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12: 387–400, 2015. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 29.Tallino S, Duffy M, Ralle M, Cortés MP, Latorre M, Burkhead JL. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J Nutr Biochem 26: 996–1006, 2015. doi: 10.1016/j.jnutbio.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wooton-Kee CR, Jain AK, Wagner M, Grusak MA, Finegold MJ, Lutsenko S, Moore DD. Elevated copper impairs hepatic nuclear receptor function in Wilson’s disease. J Clin Invest 125: 3449–3460, 2015. doi: 10.1172/JCI78991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yurkova IL, Arnhold J, Fitzl G, Huster D. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b−/− mouse model of Wilson’s disease. Chem Phys Lipids 164: 393–400, 2011. doi: 10.1016/j.chemphyslip.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Zischka H, Lichtmannegger J, Schmitt S, Jägemann N, Schulz S, Wartini D, Jennen L, Rust C, Larochette N, Galluzzi L, Chajes V, Bandow N, Gilles VS, DiSpirito AA, Esposito I, Goettlicher M, Summer KH, Kroemer G. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest 121: 1508–1518, 2011. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]