Abstract
Glucose homeostasis is highly controlled, and the function of the kidney plays an integral role in this process. The exquisite control of blood glucose relies, in part, on renal glucose filtration, renal glucose reabsorption, and renal gluconeogenesis. Particularly critical to maintaining glucose homeostasis is the renal reabsorption of glucose; with ~162 g of glucose filtered by the kidney per day, it is imperative that the kidney have the ability to efficiently reabsorb nearly 100% of this glucose back in the bloodstream. In this review, we focus on this central process, highlighting the renal transporters and regulators involved in both the physiology and pathophysiology of glucose reabsorption.
Keywords: glucose reabsorption, sodium-glucose cotransporter 1, sodium-glucose cotransporter 2, glucose transporter 2, proximal tubule
glucose homeostasis is crucial for maintaining a highly functioning individual; in particular, the brain relies on glucose as its main energy source and requires a constant supply from the blood for proper function (40). As such, glucose regulation is critical for the maintenance of life. The kidney plays an integral role in maintaining glucose homeostasis by participating in the following three central processes: glucose filtration by the glomerulus, gluconeogenesis in proximal tubule, and glucose reabsorption from the proximal tubule lumen (38, 39). Glucose is neither protein bound nor complexed to macromolecules, thus, it is freely filtered in the kidney by the glomerulus. The kidney is then tasked with the function of reabsorbing this filtered glucose load to avoid an unnecessary loss. The focus of this review is on this central process, highlighting the functions of the known glucose transporters in both health and disease.
Renal Glucose Handling
The glomerulus filters ~162 g of glucose/day (11), and under normal physiological conditions (normal plasma glucose levels and glomerular filtration rate), <1% of the filtered glucose appears in the final urine. The ability to recover this glucose load is due to the impressive reabsorptive capacity of the renal proximal tubule (38, 39). The proximal tubule expresses the following two different classes of glucose transporters: the SLC5 solute carriers otherwise known as the Na+-glucose cotransporters (Sglts) and the SLC2A transporters (Gluts). Localized on the apical membrane, the Sglts (Sglt1 and Sglt2) function to reabsorb the filtered glucose through secondary active transport, whereas the Glut transporters, localized on the basolateral membrane, efflux the glucose back in the interstitial space through facilitated transport (38, 39). The function and regulation of these transporters are discussed in more detail below.
The SLC5 carriers (Sglt1 and Sglt2).
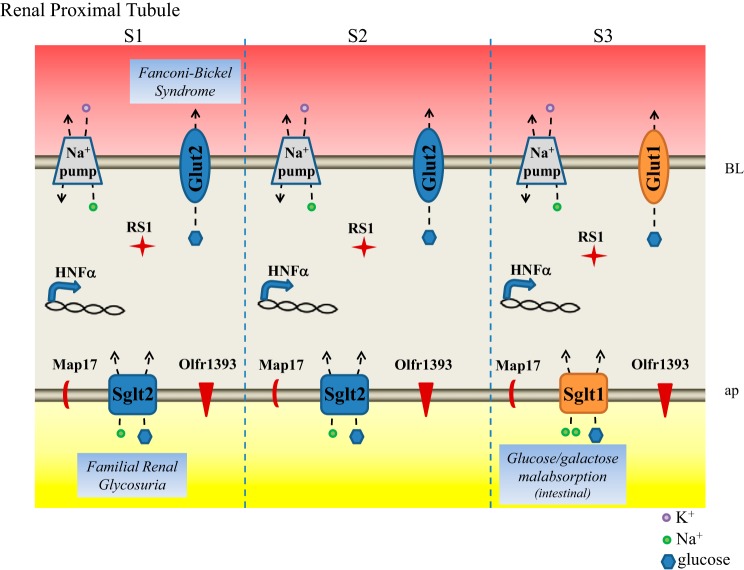
To date, 12 SLC5 carriers have been identified, all of which have a varied tissue distribution and transport diverse substrates ranging from glucose to short-chain fatty acids (19, 63). Apart from their protein homology, the unifying feature of all of these transporters is the coupling of substrate transport to an ion gradient (primarily Na+). The predominant SLC5 transporters linked to glucose transport in the kidney are 1) the high-affinity (Km 0.35 mM) low-capacity SLC5a1 (Sglt1) and 2) the low-affinity (Km 1.1 mM) high-capacity transporter SLC5a2 (Sglt2) (Fig. 1). Both Sglt1 and Sglt2 are 14 transmembrane domain transporters that rely on a favorable Na+ gradient to actively transport glucose across the lumen and into the polarized proximal tubule cells. The Na+ gradient is maintained by the Na+-K+ pump localized on the basolateral plasma membrane (Fig. 1).
Fig. 1.
Glucose transporters and their regulators in the renal proximal tubule. Na+-glucose cotransporters Sglt1 and Sglt2 localize to the apical plasma (ap) membrane of the proximal tubule where they face the newly forming urine and use the existing Na+ gradient to reabsorb all filtered glucose. The Na+ gradient is maintained by the Na+-K+-ATPase (labeled Na+ pump) on the basolateral (BL) membrane. Glut1 and Glut2 localize to the BL plasma membrane where they function to release the reabsorbed and newly generated glucose back in circulation. Known regulators of the glucose transporters that are discussed in text are labeled in the proximal tubule segments in which they are known to be found. Diseases associated with the glucose transporters are also noted.
Experimental evidence clearly indicates that Sglt2 handles the bulk of glucose reabsorption in the early proximal tubule (S1 and S2) and that Sglt1 transports the remaining glucose in the late proximal tubule (S3). However, the exact contribution (% of total glucose transport) of Sglt1 vs. Sglt2 remains somewhat unclear, since estimates differ depending on the method used. With the use of free-flow micropuncture of the proximal tubule from wild-type and Sglt2 knockout mice (KO), it was determined that Sglt2 is responsible for ~78–93% of glucose reabsorption (56). However, studies examining the fractional glucose excretion in Sglt1 KO animals revealed a total loss of 3% in the final urine, suggesting that Sglt2 can handle as much as 97% of the filtered glucose load (21). Confounding these studies is the fact that they were performed using KO animals where it is possible that there are changes in the expression or function of the remaining transporter. Indeed, in Sglt2 KO animals, Sglt1 mRNA and protein expression are decreased by ~40% (56), whereas there is no change in Sglt2 expression in Sglt1 KO mice (21). In lieu of genetic KO animals, studies have also taken advantage of specific Sglt2 inhibitors, including empagliflozin. Under conditions of maximal Sglt2 inhibition, wild-type mice had a fractional glucose reabsorption of ~44% (47), suggesting that Sglt2 is responsible for ~66% of glucose reabsorption, although one cannot rule out compensation by Sglt1 under these conditions. Thus, while the exact values are debatable, it is commonly stated that Sglt2 handles ~90% of all filtered glucose with the remaining ~10% reabsorbed by Sglt1 in the straight proximal tubule (S3 segment).
As discussed above, much of our understanding of Sglt1 and Sglt2 comes from studying KO mouse models (21, 56). As expected, the loss of either transporter lowers the handling capacity of the proximal tubule, resulting in glycosuria. Despite this, both Sglt1 and Sglt2 KO animals are euglycemic, with indistinguishable blood glucose values from wild-type animals at baseline. In addition, both KO models exhibit improved glucose tolerance, likely from their ability to more rapidly clear blood glucose through urinary wasting (29, 56). As expected, loss of Sglt2 leads to severe urinary glucose wasting, although Sglt2 KO animals can still maintain ~50% fractional glucose reabsorption. The residual glucose reabsorption in the Sglt2 KO is due to an increase in glucose handling by Sglt1 (47). Despite decreased protein expression of Sglt1, the increased delivery of glucose to the S3 segment in the Sglt2 KO along with the fact that Sglt1 typically operates at only 10–15% of its maximal transport capacity (2) can explain the compensation that is observed by Sglt1. Of note, the glycosuria and improved glucose tolerance seen in the KO animals is more pronounced in Sglt2 KO (compared with Sglt1 KO), consistent with Sglt2 playing a larger role in glucose reabsorption. In addition, because Sglt1 is responsible for the active absorption of glucose and galactose in the intestine, global loss of this transporter leads to severe glucose/galactose malabsorption (described in more detail below), and the mice must be maintained on a glucose-free diet to survive (21).
Despite the importance of these transporters from a therapeutic perspective (see below), there is very little understood about the regulation of Sglt1 and Sglt2, and what little is known has been primarily pieced together using in vitro model systems, including cell lines and oocytes (45). Recently, progress has been made with the identification of several regulatory proteins for Sglt2 and Sglt1 (Fig. 1). MAP17 is a two-transmembrane-domain protein that contains a PDZ-binding motif and is highly expressed in the renal proximal tubule (32). When overexpressed, it significantly increases Sglt2 activity in both oocytes and opossum kidney cells without changing the total expression of Sglt2 or plasma membrane localization (9). Interestingly, a mutation in MAP17 is also associated with familial renal glycosuria (see below), suggesting that MAP17 may be a physiological regulator of Sglt2 function (9). In a separate study, it was reported that RSC1A1 (RS1), a 67/68-kDa protein found in both the small intestine and all three segments of the kidney proximal tubule (24), serves as a regulator of Sglt1 (8, 33, 43, 57–59). RS1 localizes to the trans-Golgi network (TGN), plasma membrane, and nucleus in renal proximal tubule cells in culture (8, 33, 43, 57–59). RS1 and Sglt1 colocalize at the TGN where RS1 controls post-Golgi trafficking of Sglt1, and other transporters, in a dynamin-dependent manner. Recent studies have identified a key regulatory domain of RS1 (RS1-Reg) that contains multiple putative phosphorylation sites (8, 59). Upon phosphorylation, RS1 promotes Sglt1 retention in the TGN. When glucose levels are high, RS1 retention of Sglt1 is relieved, allowing Sglt1 to traffic to the plasma membrane and transport extracellular glucose. To date, the upstream signaling events that control the Sglt1-RS1 complex have yet to be elucidated.
More recently, an additional regulator of Sglt1 has emerged within the proximal tubule. Olfactory receptor 1393 (Olfr1393) is expressed in all three segments of the renal proximal tubule and localizes to the apical membrane (Fig. 1) where it contributes to glucose handling via regulation of Sglt1 (51). Olfr1393 KO mice present with similar renal phenotypes to that of the Sglt1/Sglt2 KO animals: euglycemic glycosuria and improved glucose tolerance. However, the glycosuria in Olfr1393 KO is relatively mild, consistent with the idea that Olfr1393 is not a transporter itself but rather functions to fine tune Sglt1 transport. In fact, luminal expression of Sglt1 was reduced by 22% in Olfr1393 KO animals, despite no changes in total Sglt1 protein expression and no alteration in Sglt2 localization. Together, the mild glucose wasting, coupled with an altered Sglt1 localization in the proximal tubule, suggests that Olfr1393 is a regulator of Sglt1 in the kidney. It should be noted that, unlike whole animal Sglt1 KO animals, Olfr1393 KO animals do not present with glucose/galactose malabsorption, suggesting that its regulation is renal specific or, alternatively, that Olfr1393 is not required for basal Sglt1 activity in the intestine. Although the previously mentioned study revealed eight novel ligands for Olfr1393, it remains to be determined how the activation of Olfr1393 leads to Sglt1 regulation. However, earlier studies in oocyte expression systems have provided a few hints as to the potential mechanism(s) of regulation. Previous studies have found that both Sglt1 and Sglt2 can be regulated by cAMP (20, 26, 28, 45, 64). Upon elevation of cAMP within the cell, these studies found that Sglt1 and Sglt2 plasma membrane expression and/or activity was increased. Whereas the downstream signaling components for Olfr1393 have not been identified, it is well known that olfactory receptors signal through increases in cAMP (62). Thus, it is quite possible that activation of Olfr1393 within the proximal tubule leads to increases in brush-border membrane expression of Sglt1. It is also tempting to speculate that perhaps Olfr1393 represents one of the missing signaling components for the RS1-Sglt1 regulatory complex. In the future, it would be intriguing to determine whether activation of Olf1393 can lead to an alteration in RS1 phosphorylation, which would promote post-Golgi trafficking of Sglt1.
Sglt1 and Sglt2 have also been shown to be regulated through protein modifications, including phosphorylation and glycosylation. Human Sglt1 contains multiple consensus sequence sites for both protein kinase A (PKA) and protein kinase C (PKC), and activation of both PKA and PKC has been shown to alter Sglt1 activity likely through regulating plasma membrane insertion (26, 45). Other kinases, including tau-tubulin-kinase 2 (TTBK2), AMP-activated protein kinase (AMPK), STE30/SPS1-related protein/alanine-rich kinase (SPAK), and serum- and glucocorticoid-inducible kinase 1 and 3 (SGK1/SGK3), have also been shown to contribute to Sglt1 activity and plasma membrane expression (4, 14, 16, 52). To date, it is not known whether any of these kinases can directly phosphorylate Sglt1 or Sglt2 or whether their activity is through the phosphorylation of other regulatory proteins. Although it remains to be seen whether Sglt1 or Sglt2 are directly phosphorylated, there is stronger evidence for glycosylation. Sglt1 is glycosylated within the extracellular loop between the fifth and sixth transmembrane domain, and this glycosylation is required for proper function (6, 53).
The Slc2A transporters (Gluts).
To date, a total of 14 Glut transporters have been identified (5, 42). These transporters are ubiquitously expressed and serve as the main glucose transporters in most tissues, including in adipocytes, liver, skeletal muscle, and pancreas. A handful of these transporters have been localized to the kidney, including Glut 1, Glut 2, Glut 4, Glut 5, Glut 9, and Glut 10 (5, 42). Although not involved in glucose transport, both Glut 5 and Glut 9 are highly expressed in the kidney where they serve to transport fructose and urate, respectively. The task of renal basolateral glucose reabsorption falls to two Gluts (Glut 1 and Glut 2) both of which localize to the basolateral membrane in the renal proximal tubule and function to return both filtered glucose and glucose generated de novo by the kidney (via gluconeogenesis) back into the circulation (Fig. 1). Glut 2 (Slc2A2) is the major glucose transporter in the kidney and is found in the proximal convoluted tubule (S1 and S2) where it pairs with Sglt2 to efflux the majority of glucose from the epithelial cells (34). Although most Gluts have a relatively high affinity for their substrate, Glut 2 is similar to Sglt2 in that it has an apparent low affinity (Km 17 mM) for glucose. Whereas Glut 2 KO mice have been generated, their phenotypes are varied because of the widespread distribution of Glut 2. Mice that are null for Glut 2 fail to survive to weaning without Glut 1 or Glut 3 overexpression because of the inability of the pancreatic β-cells to take up glucose (23). With rescued β-cell function, these KO mice present with severe glycosuria, indicating that this transporter is required for proper glucose reabsorption.
Whereas Glut 2 is the main glucose transporter in the kidney, Glut 1 (Slc2a1) is also present in the proximal straight tubule (S3) where it is coupled with Sglt1 to reabsorb the remaining filtered glucose (38, 39). Like most of the Gluts, Glut 1 has a high affinity for glucose (Km ~3 mM) (42). Although Glut 1 was the first glucose transporter to be characterized (it is highly expressed in erythrocytes), little is understood about its function in the kidney. Glut 1 KO mice are embryonic lethal, and heterozygous mice present with impaired motor activity and coordination explained by decreased glucose uptake in the brain (61). Renal-specific phenotypes were not examined.
Most of what is known about Glut regulation has come from examination in other systems, including the intestine and pancreas. In the kidney, it is known that Glut2 expression is increased during conditions of hyperglycemia in diabetic mouse models, and this process is transcriptionally mediated by hepatocyte nuclear factor (HNF)-1α and -4α in the proximal tubule (10) (Fig. 1). In addition, it has also been shown that, under conditions of hyperglycemia, Glut2 is transcytosed to the apical brush-border membrane, presumably to function as an additional glucose transporter under conditions that require increased reabsorption (37). As is the case for the entire family of Glut transporters, Glut1 and Glut2 are glycosylated on an extracellular loop that confers functionality to the proteins (66). Glut2 also contains PKA phosphorylation sites, and it has been shown that PKA can directly phosphorylate Glut2 in pancreatic β-cells to modulate the rate of glucose transport (55).
Pathophysiologies of Renal Glucose Handling
Now that we have reviewed the individual transporters that play physiological roles in renal glucose handling, we will discuss the roles of these transporters in related pathologies. First, we will discuss what is known about these transporters in the context of diabetes, and, subsequently, we will discuss mutations in individual transporters that have been tied to disease. Finally, we will briefly consider the important and emerging role of Sglt2 inhibitors as therapeutics.
Changes in transporter expression or function in diabetes.
When plasma glucose levels are abnormally high, as in diabetes mellitus, it is reasonable to expect that renal glucose transporter activity may be altered. On one hand, one may hypothesize that the expression/function of renal glucose transporters may be upregulated in diabetes to facilitate the efficient recovery of excess glucose from the filtrate. In support of this, it has been reported that type 1 diabetic patients have an increased transport maximum for glucose in the proximal tubule; however, this increase is elevated in parallel with an increase in glomerular filtration rate, indicating that glomerulotubular balance for glucose is maintained (41). On the other hand, however, one could argue that a decrease in transporter function in diabetes could also be logical, since plasma glucose levels are already too high and retaining additional glucose is counterintuitive from the standpoint of whole body homeostasis. To investigate these questions, several studies have examined the expression and/or function of renal glucose transporters in disease. As we will discuss below, the results have been mixed.
There is evidence that glucose transporters are upregulated in type 2 diabetic kidneys: human renal proximal tubule cells isolated from the urine of patients with type 2 diabetes have enhanced expression of both Sglt2 and GLUT2, although GLUT1 expression was reported to be downregulated (46). Similarly, it was reported that, in diabetic Zucker rats (a model of type 2 diabetes), mRNA expression of both Sglt1 and Sglt2 is increased (54), and a separate study reported that GLUT2 expression is also increased (with minor decreases in GLUT1 and no apparent change in Sglts) (30).
Conversely, however, studies examining glucose transporter expression in type 1 diabetic models have been more likely to report downregulation of renal glucose transporters. One study found that glucose uptake (studied in brush-border membrane preparations taken from mice with streptozotocin-induced diabetes) was consistently lower in diabetic animals (3). At some time points, this also corresponded with a decrease in Sglt2 expression. A separate study found that Na+-dependent glucose uptake was decreased in vesicles prepared from the renal cortex of an uncontrolled type 1 diabetic model (25). This transport was restored to control levels in vesicles from diabetic rats given daily insulin treatment. However, another study had an opposing finding: they reported that Sglt2 mRNA levels are increased in a type 1 diabetic model and that this change is also restored by insulin treatment (18). Yet another study reported that Sglt1 protein levels were upregulated in a type 1 diabetic rat model (60).
Although this is a logical area to investigate, we have found no clear consensus in the literature. On balance, although it seems that glucose transporters are upregulated in type 2 diabetes but downregulated in type 1, there are too many conflicting findings to state this as a general rule. Potential reasons for these conflicts include the precise model of type 1 or type 2 diabetes used and the time point when measurements were taken (both the age of the animal and the amount of time after disease onset). Indeed, ages are not always precisely reported (studies often describe age-matched animals without giving precise ages), so this is difficult to evaluate. The majority of the studies cited above only used male animals, and thus there may also be sex differences. In addition, some of the studies cited above investigated mRNA, whereas others investigated protein levels, and still others measured activity. Finally, the antibodies for Sglts in particular are not straightforward to work with (with commercially available antibodies generally considered less reliable than laboratory-generated antibodies), introducing an additional level of complexity. In summary, it is clear that renal glucose handling is dysregulated in diabetes. Although the literature gives us important clues, in the future it will be important to clearly establish functional changes in glucose transport, as a function of time, in both type 1 and type 2 diabetes.
Single nucleotide polymorphisms (SNPs), another important source of variability to consider, have been identified for both the Sglts and GLUTs. Although “disease-causing” mutations of Sglts and GLUTs will be discussed in the sections below, we should note here that some (non-disease-causing) SNPs do appear to track with an increased risk of diabetes. In particular, three different SNPs have been identified in Glut1, including one in the enhancer region of intron 2 (7, 36). This SNP occurs at the predicted binding site of upstream stimulatory transcription factors that help to regulate gene expression under conditions of hyperglycemia. Those with this SNP have a 2.4-fold increased risk of developing diabetic nephropathy. SNPs have also been mapped for Sglt2 in nondiabetic German populations (17). According to these studies, four different SNPs have been identified in Sglt2, and some of these marginally tracked with impaired glucose tolerance.
Fanconi/Fanconi-Bickel Syndrome: GLUT2.
Two conditions that are known to involve renal glucose transport are Renal Fanconi syndrome and the related Fanconi-Bickel syndrome. Renal Fanconi syndrome is characterized by general dysfunction of the renal proximal tubule, including, but not limited to, changes in glucose transport. As the first segment to come in contact with the forming urine, in a healthy individual, the proximal tubule is a workhorse: polarized cells, packed with mitochondria, and with the aid of the additional surface area afforded by dense apical microvilli and elaborate basolateral infoldings, work constantly to reabsorb the great majority of Na+, Cl−, K+, glucose, amino acids, and other substances. In Fanconi syndrome, however, the transport capacity of this critical segment is severely comprised. As a result, an excess of glucose, bicarbonate, K+, protein, and other substances is lost in the urine of these patients. Thus, as stated above, Fanconi syndrome certainly results in glycosuria and defects in renal glucose handling, but it is not a disease of glucose transport but rather of renal proximal tubule dysfunction. Fanconi syndrome has a number of causes, including exposure to certain drugs or heavy metals, as well as inherited mutations (31). One subset of Renal Fanconi syndrome, Fanconi-Bickel syndrome, is caused by mutations in GLUT2 (1, 22, 31) (Fig. 1). Because GLUT2 plays important roles not only in the kidney but in other tissues as well, GLUT2 mutations result in hepatorenal glycogen accumulation, as well as proximal tubule dysfunction and impaired metabolism of glucose and galactose (in fact, Fanconi-Bickel is classified as a glycogen storage disease).
Glucose-galactose malabsorption: Sglt1.
Glucose-galactose malabsorption is a disease caused by mutations in Sglt1 (Fig. 1). Although Sglt1 plays an important role in glucose reabsorption in both the intestine and the kidney, the phenotype in patients with glucose-galactose malabsorption is driven by the critical role of Sglt1 in intestinal glucose absorption. Glucose-galactose malabsorption presents in infancy as a life-threatening diarrhea, which is dependent on oral intake of lactose, glucose, or galactose. In patients with this disease, these substances are retained in the intestinal lumen, causing severe osmotic diarrhea that leads to dehydration. Disease-causing Sglt1 mutations occur along the length of the gene and in the promoter region, with the majority of mutations disrupting Sglt1 membrane targeting (65). Although patients with glucose-galactose malabsorption may also have relatively mild changes in renal glucose handling (15, 48), the phenotype in these patients is clearly driven by deficits in intestinal Sglt1 function.
Familial renal glycosuria: Sglt2.
Familial renal glycosuria, as the name implies, is an inherited disorder that leads to glucose wasting in the urine and was first recognized in 1955 (27). Familial renal glycosuria is caused by inactivating mutations in Sglt2 (Fig. 1). In contrast to Fanconi syndrome, in which glucose is one of many substances whose proper transport is disrupted, familial renal glycosuria is characterized by an isolated defect in renal glucose handling (although in severe forms it has been reported to secondarily affect the transport of amino acids) (35). Because Sglt2 is responsible for reabsorbing the majority of the filtered glucose, defects in Sglt2 function lead to glycosuria despite normal plasma glucose levels (euglycemic glycosuria). Sglt2 disease-causing mutations (nonsense, missense, deletions, and splicing mutations) have been found along the length of Sglt2 and do not appear to concentrate in a particular domain or segment (49, 50). The inheritance of Sglt2 mutations is thought to be primarily codominant although not all individuals with a mutation have similar phenotypes, and some but not all heterozygotes are affected. Glycosuria is typically most severe in cases of homozygosity (or compound heterozygosity) (49). Mutations in MAP17, discussed above as a regulator of Sglt2, have also been found to cause familial renal glycosuria (9). Of note, in most cases renal glycosuria is noted on a routine urinalysis, since most patients are asymptomatic.
Sglt2 inhibitors as therapeutics.
The phenotype of patients with renal familial glycosuria suggests that inhibiting Sglt2 function is well tolerated and that Sglt2 inhibitors may be potential therapeutics to lower blood glucose in type 2 diabetes. Although not the focus of this review, we would be remiss not to mention the important and emerging role of Sglt2 inhibitors as therapeutics. Inhibition of Sglt2 in the renal proximal tubule lowers the transport maximum for glucose (in healthy individuals and in those with type 2 diabetes), causing glucose to be “lost” in the urine. This glycosuria results in a reduction in fasting and postprandial glucose levels, and improved insulin sensitivity (12). In addition, it is associated with lowering of A1C levels, blood pressure, and body weight (13). Despite the recent success of these Sglt2 inhibitors as therapeutics for diabetics, it should be mentioned that, perhaps counterintuitively, familial renal glycosuria does not appear to be associated with better glycemic control over time (44).
Notably, although Sglt2 mediates reabsorption of ~90% of filtered glucose, Sglt2 inhibitors only cause 35–40% of filtered glucose to be lost in the urine (12). This discrepancy is explained by the fact that Sglt1, which is expressed distally to Sglt2, typically operates at only 10–15% of its maximum transport capacity (2). Inhibiting Sglt2 forces Sglt1 to operate at its maximal capacity, resulting in the recovery of ~60–65% of filtered glucose. Thus, the glycosuria of Sglt2 inhibitors is tempered by Sglt1; this is exemplified by the fact that Sglt1 KO mice treated with a Sglt2 inhibitor lose 100% of filtered glucose in the urine (47).
Conclusions
Each day, a healthy human kidney reabsorbs ~162 g of glucose from the forming urine back in the circulating blood (11). This impressive feat of transport is accomplished by the coordinated action of Sglt2 (apical) and Glut2 (basolateral) in the S1 and S2 segments, and by Sglt1 (apical) and Glut1 (basolateral) in the S3 segment. Although the majority (~90%) of glucose is reabsorbed by the low-affinity/high-capacity transporters in S1/S2, the S3 segment has high-affinity transporters that play a key role in ensuring that no glucose escapes past this segment to become lost in the urine. Normally, Sglt1 in the S3 segment works at only 10–15% of its maximum transport efficiency, but recently studies have highlighted the ability of this segment to “step up” and transport much higher amounts of glucose. Although there have been relatively few studies on the regulation of these important transporters, it is becoming clear that other proteins can regulate the function of Sglts. Better understanding of this regulation has the potential to lend great insight into the physiology, pathophysiology, and therapeutic potential of these transporters.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants F32-DK-096780 and K01-DK-106400.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.D.S. prepared figures; B.D.S. and J.L.P. drafted manuscript; B.D.S. and J.L.P. edited and revised manuscript; B.D.S. and J.L.P. approved final version of manuscript.
REFERENCES
- 1.Abbasi F, Azizi F, Javaheri M, Mosallanejad A, Ebrahim-Habibi A, Ghafouri-Fard S. Segregation of a novel homozygous 6 nucleotide deletion in GLUT2 gene in a Fanconi-Bickel syndrome family. Gene 557: 103–105, 2015. doi: 10.1016/j.gene.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 62: 3324–3328, 2013. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertoni Borghese MF, Majowicz MP, Ortiz MC, Passalacqua MR, Sterin Speziale NB, Vidal NA. Expression and activity of SGLT2 in diabetes induced by streptozotocin: relationship with the lipid environment. Nephron, Physiol 112: 45–52, 2009. doi: 10.1159/000214214. [DOI] [PubMed] [Google Scholar]
- 4.Alesutan I, Sopjani M, Dërmaku-Sopjani M, Munoz C, Voelkl J, Lang F. Upregulation of Na-coupled glucose transporter SGLT1 by Tau tubulin kinase 2. Cell Physiol Biochem 30: 458–465, 2012. doi: 10.1159/000339039. [DOI] [PubMed] [Google Scholar]
- 5.Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life 62: 315–333, 2010. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 6.Birnir B, Lee HS, Hediger MA, Wright EM. Expression and characterization of the intestinal Na+/glucose cotransporter in COS-7 cells. Biochim Biophys Acta 1048: 100–104, 1990. doi: 10.1016/0167-4781(90)90028-Z. [DOI] [PubMed] [Google Scholar]
- 7.Brosius FC III, Heilig CW. Glucose transporters in diabetic nephropathy. Pediatr Nephrol 20: 447–451, 2005. doi: 10.1007/s00467-004-1748-x. [DOI] [PubMed] [Google Scholar]
- 8.Chintalapati C, Keller T, Mueller TD, Gorboulev V, Schäfer N, Zilkowski I, Veyhl-Wichmann M, Geiger D, Groll J, Koepsell H. Protein RS1 (RSC1A1) downregulates the exocytotic pathway of glucose transporter SGLT1 at low intracellular glucose via inhibition of ornithine decarboxylase. Mol Pharmacol 90: 508–521, 2016. doi: 10.1124/mol.116.104521. [DOI] [PubMed] [Google Scholar]
- 9.Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe JY. MAP17 is a necessary activator of renal Na+/glucose cotransporter SGLT2. J Am Soc Nephrol 28: 85–93, 2017. doi: 10.1681/ASN.2015111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David-Silva A, Freitas HS, Okamoto MM, Sabino-Silva R, Schaan BD, Machado UF. Hepatocyte nuclear factors 1α/4α and forkhead box A2 regulate the solute carrier 2A2 (Slc2a2) gene expression in the liver and kidney of diabetic rats. Life Sci 93: 805–813, 2013. doi: 10.1016/j.lfs.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, LaCreta FP, Griffen SC. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36: 3169–3176, 2013. doi: 10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13: 11–26, 2017. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 13.Del Prato S, Nauck M, Durán-Garcia S, Maffei L, Rohwedder K, Theuerkauf A, Parikh S. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes Metab 17: 581–590, 2015. doi: 10.1111/dom.12459. [DOI] [PubMed] [Google Scholar]
- 14.Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 12: 862–870, 2004. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- 15.Elsas LJ, Hillman RE, Patterson JH, Rosenberg LE. Renal and intestinal hexose transport in familial glucose-galactose malabsorption. J Clin Invest 49: 576–585, 1970. doi: 10.1172/JCI106268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elvira B, Blecua M, Luo D, Yang W, Shumilina E, Munoz C, Lang F. SPAK-sensitive regulation of glucose transporter SGLT1. J Membr Biol 247: 1191–1197, 2014. doi: 10.1007/s00232-014-9719-z. [DOI] [PubMed] [Google Scholar]
- 17.Enigk U, Breitfeld J, Schleinitz D, Dietrich K, Halbritter J, Fischer-Rosinsky A, Enigk B, Müller I, Spranger J, Pfeiffer A, Stumvoll M, Kovacs P, Tönjes A. Role of genetic variation in the human sodium-glucose cotransporter 2 gene (SGLT2) in glucose homeostasis. Pharmacogenomics 12: 1119–1126, 2011. doi: 10.2217/pgs.11.69. [DOI] [PubMed] [Google Scholar]
- 18.Freitas HS, Anhê GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, Machado UF. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 149: 717–724, 2008. doi: 10.1210/en.2007-1088. [DOI] [PubMed] [Google Scholar]
- 19.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 12: 78–89, 2015. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 303: C348–C354, 2012. doi: 10.1152/ajpcell.00115.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grünert SC, Schwab KO, Pohl M, Sass JO, Santer R. Fanconi-Bickel syndrome: GLUT2 mutations associated with a mild phenotype. Mol Genet Metab 105: 433–437, 2012. doi: 10.1016/j.ymgme.2011.11.200. [DOI] [PubMed] [Google Scholar]
- 23.Guillam MT, Hümmler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Dériaz N, Thorens B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 17: 327–330, 1997. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 24.Haase W, Koepsell H. Electron microscopic immunohistochemical localization of components of Na+-cotransporters along the rat nephron. Eur J Cell Biol 48: 360–374, 1989. [PubMed] [Google Scholar]
- 25.Harris RC, Brenner BM, Seifter JL. Sodium-hydrogen exchange and glucose transport in renal microvillus membrane vesicles from rats with diabetes mellitus. J Clin Invest 77: 724–733, 1986. doi: 10.1172/JCI112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem 271: 14740–14746, 1996. doi: 10.1074/jbc.271.25.14740. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz L, Schwarzer S. Renal glycosuria; occurrence in two siblings and a review of the literature. J Pediatr 47: 634–639, 1955. doi: 10.1016/S0022-3476(55)80085-6. [DOI] [PubMed] [Google Scholar]
- 28.Ikari A, Harada H, Takagi K. Role of actin in the cAMP-dependent activation of sodium/glucose cotransporter in renal epithelial cells. Biochim Biophys Acta 1711: 20–24: 2005. doi: 10.1016/j.bbamem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, Shulman GI, Kibbey RG. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60: 890–898, 2011. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol 8: 943–948, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Klootwijk ED, Reichold M, Unwin RJ, Kleta R, Warth R, Bockenhauer D. Renal Fanconi syndrome: taking a proximal look at the nephron. Nephrol Dial Transplant 30: 1456–1460, 2015. doi: 10.1093/ndt/gfu377. [DOI] [PubMed] [Google Scholar]
- 32.Kocher O, Cheresh P, Lee SW. Identification and partial characterization of a novel membrane-associated protein (MAP17) up-regulated in human carcinomas and modulating cell replication and tumor growth. Am J Pathol 149: 493–500, 1996. [PMC free article] [PubMed] [Google Scholar]
- 33.Kroiss M, Leyerer M, Gorboulev V, Kühlkamp T, Kipp H, Koepsell H. Transporter regulator RS1 (RSC1A1) coats the trans-Golgi network and migrates into the nucleus. Am J Physiol Renal Physiol 291: F1201–F1212, 2006. doi: 10.1152/ajprenal.00067.2006. [DOI] [PubMed] [Google Scholar]
- 34.Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. The role of GLUT2 in dietary sugar handling. J Physiol Biochem 61: 529–537, 2005. doi: 10.1007/BF03168378. [DOI] [PubMed] [Google Scholar]
- 35.Magen D, Sprecher E, Zelikovic I, Skorecki K. A novel missense mutation in SLC5A2 encoding SGLT2 underlies autosomal-recessive renal glucosuria and aminoaciduria. Kidney Int 67: 34–41, 2005. doi: 10.1111/j.1523-1755.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- 36.Makni K, Mnif F, Boudawara M, Hamza N, Rekik N, Abid M, Rebaï A, Jarraya F, Granier C, Ayadi H. Association of glucose transporter 1 polymorphisms with type 2 diabetes in the Tunisian population. Diabetes Metab Res Rev 24: 544–548, 2008. doi: 10.1002/dmrr.866. [DOI] [PubMed] [Google Scholar]
- 37.Marks J, Carvou NJ, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol 553: 137–145, 2003. doi: 10.1113/jphysiol.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 53: 875–883, 2009. doi: 10.1053/j.ajkd.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl 79, Suppl: S1–S6, 2011. doi: 10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 40.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597, 2013. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 28: 101–109. 1971. doi: 10.3109/00365517109090668. [DOI] [PubMed] [Google Scholar]
- 42.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34: 121–138, 2013. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osswald C, Baumgarten K, Stümpel F, Gorboulev V, Akimjanova M, Knobeloch KP, Horak I, Kluge R, Joost HG, Koepsell H. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol Cell Biol 25: 78–87, 2005. doi: 10.1128/MCB.25.1.78-87.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottosson-Laakso E, Tuomi T, Forsén B, Gullström M, Groop PH, Groop L, Vikman P. Influence of Familial Renal Glycosuria Due to Mutations in the SLC5A2 Gene on Changes in Glucose Tolerance over Time. PLoS One 11: e0146114, 2016. doi: 10.1371/journal.pone.0146114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24: 463–469, 2015. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54: 3427–3434, 2005. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 47.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabino-Silva R, Mori RC, David-Silva A, Okamoto MM, Freitas HS, Machado UF. The Na(+)/glucose cotransporters: from genes to therapy. Braz J Med Biol Res 43: 1019–1026, 2010. doi: 10.1590/S0100-879X2010007500115. [DOI] [PubMed] [Google Scholar]
- 49.Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 5: 133–141, 2010. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- 50.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003. doi: 10.1097/01.ASN.0000092790.89332.D2. [DOI] [PubMed] [Google Scholar]
- 51.Shepard BD, Cheval L, Peterlin Z, Firestein S, Koepsell H, Doucet A, Pluznick JL. A renal olfactory receptor aids in kidney glucose handling. Sci Rep 6: 35215, 2016. doi: 10.1038/srep35215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sopjani M, Bhavsar SK, Fraser S, Kemp BE, Föller M, Lang F. Regulation of Na+-coupled glucose carrier SGLT1 by AMP-activated protein kinase. Mol Membr Biol 27: 137–144, 2010. doi: 10.3109/09687681003616870. [DOI] [PubMed] [Google Scholar]
- 53.Stringer DM, Zahradka P, Taylor CG. Glucose transporters: cellular links to hyperglycemia in insulin resistance and diabetes. Nutr Rev 73: 140–154, 2015. doi: 10.1093/nutrit/nuu012. [DOI] [PubMed] [Google Scholar]
- 54.Tabatabai NM, Sharma M, Blumenthal SS, Petering DH. Enhanced expressions of sodium-glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res Clin Pract 83: e27–e30, 2009. doi: 10.1016/j.diabres.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorens B, Dériaz N, Bosco D, DeVos A, Pipeleers D, Schuit F, Meda P, Porret A. Protein kinase A-dependent phosphorylation of GLUT2 in pancreatic beta cells. J Biol Chem 271: 8075–8081, 1996. doi: 10.1074/jbc.271.14.8075. [DOI] [PubMed] [Google Scholar]
- 56.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veyhl M, Keller T, Gorboulev V, Vernaleken A, Koepsell H. RS1 (RSC1A1) regulates the exocytotic pathway of Na+-D-glucose cotransporter SGLT1. Am J Physiol Renal Physiol 291: F1213–F1223, 2006. doi: 10.1152/ajprenal.00068.2006. [DOI] [PubMed] [Google Scholar]
- 58.Veyhl M, Wagner CA, Gorboulev V, Schmitt BM, Lang F, Koepsell H. Downregulation of the Na(+)- D-glucose cotransporter SGLT1 by protein RS1 (RSC1A1) is dependent on dynamin and protein kinase C. J Membr Biol 196: 71–81, 2003. doi: 10.1007/s00232-003-0626-y. [DOI] [PubMed] [Google Scholar]
- 59.Veyhl-Wichmann M, Friedrich A, Vernaleken A, Singh S, Kipp H, Gorboulev V, Keller T, Chintalapati C, Pipkorn R, Pastor-Anglada M, Groll J, Koepsell H. Phosphorylation of RS1 (RSC1A1) steers inhibition of different exocytotic pathways for glucose transporter SGLT1 and nucleoside transporter CNT1 and a RS1 derived peptide inhibits glucose absorption. Mol Pharmacol 89: 118–132, 2015. doi: 10.1124/mol.115.101162. [DOI] [PubMed] [Google Scholar]
- 60.Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol 28: 107–114, 2008. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet 15: 1169–1179, 2006. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 62.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27: 487–497, 2000. doi: 10.1016/S0896-6273(00)00060-X. [DOI] [PubMed] [Google Scholar]
- 63.Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med 34: 183–196, 2013. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol 200: 287–293, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Wright EM, Turk E, Martin MG. Molecular basis for glucose-galactose malabsorption. Cell Biochem Biophys 36: 115–121, 2002. doi: 10.1385/CBB:36:2-3:115. [DOI] [PubMed] [Google Scholar]
- 66.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 8: 113–128, 2007. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]