Abstract
The basal, intermediate, and superficial cell layers of the urothelium undergo rapid and complete recovery following acute injury; however, the effects of chronic injury on urothelial regeneration have not been well defined. To address this discrepancy, we employed a mouse model to explore urothelial changes in response to spinal cord injury (SCI), a condition characterized by life-long bladder dysfunction. One day post SCI there was a focal loss of umbrella cells, which are large cells that populate the superficial cell layer and normally express uroplakins (UPKs) and KRT20, but not KRT5, KRT14, or TP63. In response to SCI, regions of urothelium devoid of umbrella cells were replaced with small superficial cells that lacked KRT20 expression and appeared to be derived in part from the underlying intermediate cell layer, including cells positive for KRT5 and TP63. We also observed KRT14-positive basal cells that extended thin cytoplasmic extensions, which terminated in the bladder lumen. Both KRT14-positive and KRT14-negative urothelial cells proliferated 1 day post SCI, and by 7 days, cells in the underlying lamina propria, detrusor, and adventitia were also dividing. At 28 days post SCI, the urothelium appeared morphologically patent, and the number of proliferative cells decreased to baseline levels; however, patches of small superficial cells were detected that coexpressed UPKs, KRT5, KRT14, and TP63, but failed to express KRT20. Thus, unlike the rapid and complete restoration of the urothelium that occurs in response to acute injuries, regions of incompletely differentiated urothelium were observed even 28 days post SCI.
Keywords: bladder, cytokeratin, BrdU, TP63 (p63)
the urothelium is a stratified epithelial tissue that lines the inner surface of the renal pelvis, the ureters, and the urinary bladder (26). It forms a high-resistance barrier to solutes, water, and pathogens, and thus protects the underlying muscular, nervous, and vascular tissues. Moreover, the urothelium conveys information about its milieu to the central nervous system via a local urothelial:afferent signaling pathway (5). In adult rodents, the urothelium is composed of different cell types arranged in three- to four-cell strata (Fig. 1). Facing the urinary space is a superficial layer composed of large “umbrella cells,” which form junctional complexes and express KRT20 and uroplakins (UPKs) (34, 59). The dimensions of these cells depend upon bladder fullness and can vary between 250-µm diameter in filled bladders to 40 µm in empty ones. Below the superficial cell layer lies 1–2 intermediate cell layers, which comprise relatively small cells (~10–20 µm in diameter) that express the tumor promoter p53-like family member TP63 and the Hedgehog signaling pathway ligand SHH (Fig. 1) (45, 50). A preponderance of these cells express KRT5; however, a small fraction of these cells, which are found just below the umbrella cell layer, express UPKs, but not KRT5 (19, 34, 45, 50). The final stratum, the basal cell layer, comprises cells that like those in the intermediate cell layers also express TP63, SHH, and KRT5. However, only basal cells express the hemidesmosome marker β4 integrin (30, 53), and a population of these cells also express KRT14 (Fig. 1) (30, 34, 42). This latter finding, coupled with recent data that the KRT14+ basal cells of adult mice are pluripotent (see below) (42), indicates that some (and perhaps all) of the cells in the basal cell layer are phenotypically and functionally distinct from those in the intermediate cell layers.
Fig. 1.
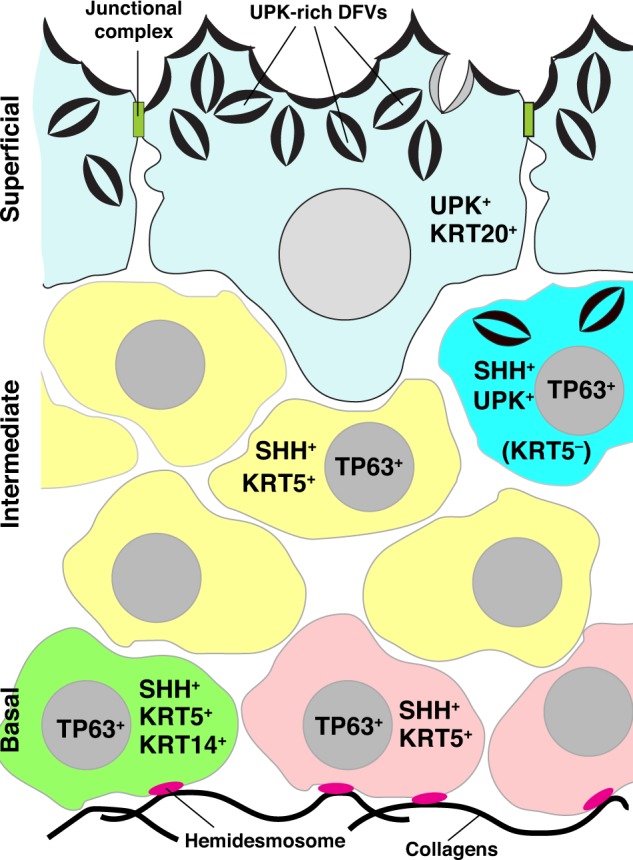
Phenotype of cells present in the urothelium of adult mouse bladders. The urothelium has 3 cell layers: basal, intermediate, and superficial. Associated with the basal cell layer are two cell populations: one is TP63+, SHH+, KRT5+, and KRT14+ (green-colored cell) and proliferates in response to urothelial injury, and the other cell type is TP63+, SHH+, KRT5+, and KRT14− (mauve-colored cells). Basal cells are the only cells that form hemidesmosomes and express the β4-integrin receptor. Intermediate cells also have two phenotypes. The majority of cells (yellow colored) are TP63+, SHH+, and KRT5+, whereas a smaller population express TP63 and SSH, but not KRT5 (cyan colored). This latter population of cells also expresses UPKs (which are present in discoidal- and/or fusiform-shaped vesicles; DFVs), and can give rise to superficial cells in response to acute injury. Umbrella cells are UPK+ and KRT20+ (pale blue colored). They are attached to one another by junctional complexes and their cytoplasm is filled with DFVs.
Under normal conditions, urothelial cell turnover is estimated to be ~200 days in rodents (23, 24). However, in response to acute injuries caused by exposure to chemical irritants (e.g., chitosan, cyclophosphamide, protamine sulfate, saccharin), uropathogenic bacteria, overdistension, subtotal cystectomy (removal of ~75% of the bladder), or changes associated with birth, the urothelium undergoes rapid repair and regeneration (4, 12, 16, 17, 19, 31, 33, 39–42, 44, 46, 47, 50, 58). In acute injuries, regeneration begins within minutes to hours, is most often associated with urothelial proliferation, and terminates with the restoration of a functional and normal-appearing urothelium within days. The regeneration response requires signals generated from both the stroma and from within the urothelium and includes BMP4, non-canonical and canonical Wnt signaling pathways, Delta-Notch, ELF3, various growth factors, retinoids, Sonic Hedgehog (SHH and GLI1), and TP63 pathways (4, 7, 15, 19, 33, 40, 41, 45, 50). However, in many cases the precise mechanisms are not well understood.
The identity of the progenitor cell(s) that promotes urothelial regeneration in response to injury remains controversial and may vary depending on the injury involved (4). In response to infections by piliated strains of uropathogenic Escherichia coli (E. coli), cells in the basal layer that express KRT5 and SHH proliferate and are reported to give rise to the other cells in the urothelium (12, 40, 50). However, a recent report indicates that it is specifically the KRT14+ subpopulation of basal cells (which are also KRT5+ and SHH+) that act as urothelial progenitor cells. Indeed, in response to repeated bouts of cyclophosphamide-induced injury, KRT14+ basal cells can give rise to cells in all three urothelial cell layers (42). However, cell types other than basal cells may also play a role in urothelial regeneration after injury (60). For example, KRT5− cells in the intermediate layer are reported to undergo proliferation 1 day post exposure to E. coli (12), and KRT5−, UPK+ cells can also serve as progenitors for superficial umbrella cells in response to cyclophosphamide-induced injury (19). Other studies show that treatment with protamine sulfate or chitosan causes the selective loss of the superficial umbrella cell layer. Within minutes, this loss spurs the differentiation of newly exposed cells in the underlying intermediate layer, stimulating them to express proteins normally associated with umbrella cells, including TPJ1 (alias ZO-1) and UPKs (17, 31, 40, 50, 58). Thus cells in the intermediate layer may also regenerate the superficial umbrella cell layer in response to injury.
A common feature of the majority of current models for urothelial injury is the acute nature of the insult. However, there are fewer studies that have explored urothelial responses and regeneration in response to chronic injuries, those where the injury is long-term and often sustained. One example is spinal cord injury (SCI), a disorder that affects >200,000 people per year, occurs in response to physical, genetic, or infectious traumas to the spinal cord, and results in a lifetime loss of voluntary control over urinary bladder function (6, 54). SCI is accompanied by functional deficits (e.g., bladder sphincter dyssynergia and spastic bladder), as well as morphological changes including hypertrophy of the bladder and an apparent increase in intermediate cell layers (36, 51, 57). It is not known which, if any, urothelial cell types proliferate in response to SCI, or whether proliferation occurs continuously. Using a rat model, we previously showed that within 1 day of SCI the superficial umbrella cell layer begins to slough off, causing an increase in solute and water flux (1). This loss of umbrella cells is accompanied by the rapid expression of UPKs and junctional complexes in newly exposed cells in the underlying intermediate cell layer. By 2 wk, the urothelium and its associated tight junction barrier is reformed, and by 4 wk the surface cells are small (10–20 µm in diameter), but are otherwise normal in appearance. It is unknown if this difference in cell size reflects changes in urothelial differentiation as a result of SCI.
In our current studies, we use a mouse model to demonstrate that experimentally induced SCI causes a focal loss of umbrella cells, which are replaced by small superficial cells that express UPKs along with differentiation markers normally associated with cells found in the basal and intermediate cell layers (KRT5, KRT14, and TP63); however, these cells lack expression of KRT20. SCI is also associated with proliferation of KRT14− and KRT14+ cells, but by 7 days proliferation subsides and by 28 days focal regions of small superficial cells that coexpress KRT5, KRT14, TP63, and UPKs, but not KRT20 persist. Thus, unlike the complete regeneration of the urothelium that occurs in response to acute injuries, SCI results in patches of poorly differentiated urothelium weeks after the initial insult, possibly in response to continued injury as a result of altered neural input to the bladder.
MATERIALS AND METHODS
Animals.
All procedures were conducted in accordance with, and protocols were approved by, the Institutional Animal Care and Use Committee at the University of Pittsburgh. Female C57Bl/6 female mice (15–20 g, 5–8 wk) were purchased from Envigo (Indianapolis, IN).
Chemicals.
Unless indicated otherwise, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Spinal cord transection.
SCI was performed using our previously described methods (1). Briefly, mice were anesthetized using isoflurane (1.5–2% vol/vol in O2), and a laminectomy performed between the T8 and T9 vertebrae. The spinal cord was completely transected, and gelfoam (Pharmacia and Upjohn, Kalamazoo, MI) was placed between the cut ends of the spinal cord. The muscle and skin overlying the vertebrae were closed and the animals treated with a prophylactic antibiotic (Polyflex, 100 mg/kg sc; Boehringer Ingelheim Vetmedica, St. Joseph, MO) and an analgesic (ketoprofen, 5 mg/kg sc; Zoetis, Kalamazoo, MI). The bladders of mice with SCI were manually expressed twice a day until the spinal cord-to-bladder micturition reflex developed (~10–14 days). Sham animals received laminectomy without spinal cord transection. In preliminary studies, we attempted to express the bladders of sham-treated animals, but they rarely had full bladders and because of the resulting stress associated with this manipulation, we did not routinely perform this manipulation in the sham group. Animals were euthanized by inhalation of 100% CO2 followed by exsanguination due to cutting of the vena cava at 1, 3, 7, and 28 days post SCI and bladder tissue collected for immunofluorescence analysis.
Scanning electron microscopy (SEM).
Tissue was gently pinned flat (with minimal stretching) on a soft plastic sheet and fixed by immersion (90 min, room temperature) in 2.0% vol/vol glutaraldehyde, 2.0% vol/vol paraformaldehyde in 100 mM sodium cacodylate buffer, pH 7.4, containing 1 mM CaCl2, and 0.5 mM MgCl2. The tissue was then washed with 100 mM sodium cacodylate buffer, postfixed in 1.0% wt/vol OsO4, 1.0% wt/vol K4Fe(CN) 6, in 100 mM sodium cacodylate, pH 7.4, then dehydrated for 15 min each in a graded series of ethanol: 30%, 50%, 70%, 95%, 100% vol/vol. The dehydrated samples were critical point dried, sputter coated with gold-palladium, and viewed in a JEOL model JSM T-300 scanning electron microscope at 20 kV. Samples were photographed using an attached Nikon D40 digital camera. Digital images were imported into Photoshop CC 2015 (Adobe Systems, San Jose, CA), the contrast and brightness were corrected, and composite images were assembled using Adobe Illustrator CC 2015.
Tissue processing and immunolabeling.
The size and elastic properties of SCI bladders varies over time, and the thickness of the urothelium and number of cell layers depends on the degree of bladder filling/stretch. Thus it is difficult to make comparisons between tissues fixed in a filled state. Instead, we used the following protocol to ensure that the bladder wall and associated urothelium was fully “relaxed.” Excised mouse bladders were cut open along the midline, rinsed with Krebs buffer (110 mM NaCl, 25 mM NaHCO3, 5.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11 mM glucose, 2 mM CaCl2, pH 7.4 when gassed with 5% vol/vol CO2), and then incubated in Krebs buffer gassed with 5% vol/vol CO2 for 30 min at 37°C. The tissue was subsequently fixed with 4% wt/vol paraformaldehyde in 100 mM Na-cacodylate buffer, pH 7.4 for 30 min, and then cryoprotected in 30% wt/vol sucrose (dissolved in phosphate-buffered saline, PBS) for 2 h. Three bladders from each treatment group were embedded in a single cryomold (15 × 15 × 5 mm; Fisher Scientific) filled with Optimal Cutting Temperature (OCT) solution (Tissue-Tek, Sakura Finetek, Torrance, CA), and frozen on dry ice. The blocks were stored at −70°C in tightly sealed plastic bags. Cryosections were cut using a Leica Microsystems CM1950 cryostat (Buffalo Grove, IL; 4 µm sections), and collected on Superfrost Plus glass slides (ThermoFisher Scientific, Pittsburgh, PA).
Sections were washed in PBS at room temperature and then unreacted fixative quenched by incubating the tissue slices for 10 min at room temperature with 75 mM NH4Cl and 20 mM glycine, pH 8.0 dissolved in Block Solution (PBS containing 0.6% vol/vol fish skin gelatin and 0.05% wt/vol saponin) supplemented with 0.1% vol/vol Triton X-100. Following a wash with PBS, the tissue was incubated in Block Solution containing 5% vol/vol donkey or goat serum for 2 h at room temperature. This solution was aspirated, and replaced with primary antibodies (see Table 1) diluted in Block Solution and incubated overnight at 4°C in a humid chamber. The second day, the slides were washed and incubated with minimal cross-reactivity, fluorophore-labeled secondary antibodies (see Table 1). When possible, nuclei were counterstained with TO-PRO-3 (1:1,000; ThermoFisher Scientific) and overall tissue architecture visualized using tetramethylrhodamine isothiocyanate (TRITC)–labeled phalloidin (ThermoFisher Scientific; 1:200), which labels cortical actin filaments.
Table 1.
Antibodies used in this study
| Target | Cell Type Labeled | Concentration Used | Species and Ab Type | Vendor/Source (Catalog No.) | 2° Ab or Blocking Ab | Vendor (Catalog No.) |
|---|---|---|---|---|---|---|
| BrdU | Proliferating cells | 1:200 | Mouse mAb | EMD Millipore (NA61) | Alexa 488-Goat anti-mouse | ThermoFisher Scientific (A-11001) |
| KRT5 | Intermediate and basal cells | 1:1,000 | Rabbit pAb | BioLegend (905501) | Dylight 549-Goat anti-rabbit | Jackson Immuno Research (111–505–144) |
| KRT14 | Sub population of basal cells | 1:300 | Chicken pAb | BioLegend (906001) | TRITC-Donkey anti-chicken | Jackson Immuno Research (703–025–155) |
| KRT20 | Umbrella cells | 1:1,000 | Rabbit pAb | Abcam (ab53120) | Alexa 488-Goat anti-rabbit | Jackson Immuno Research (111–545–144) |
| MKI67 (alias Ki-67) | Proliferating cells | 1:100 | Rabbit pAb | Abcam (15580) | Alexa 488-Goat anti-rabbit | Jackson Immuno Research (111–545–144) |
| TP63 | Intermediate and basal cells | 1:100 | Goat pAb | R&D Systems (AF1916) | Dylight 650-Donkey anti-goat | Abcam (ab96938) |
| UPK3a | Umbrella cells and KRT5− intermediate cells | 1:5 | Mouse mAb supernatant | Apodaca laboratory (see Ref. 55) | Alexa 488-Goat anti-mouse Goat-anti mouse Fab | Jackson Immuno Research (115–545–166) (115–007–003) |
| Pan-UPK (anti-AUM) | Umbrella cells and KRT5− intermediate cells | 1:1,000 | Rabbit pAb | T.T. Sun, NY University | Alexa 488-Goat anti-rabbit | Jackson Immuno Research (111–545–144) |
In some experiments, the primary antibodies were mouse in origin. To perform mouse-on-mouse labeling, the fixed tissue was quenched and blocked with goat serum for 2 h as described above. The tissue was then incubated with 20 µg/ml of unconjugated goat anti-mouse Fab fragments (Jackson ImmunoResearch) overnight at 4°C in a humid chamber. The tissue was rinsed with PBS, fixed with 1% wt/vol paraformaldehyde dissolved in 100 mM sodium cacodylate, pH 7.4 for 10 min at room temperature, and then quenched, blocked, and incubated with primary antibodies for 2 h and secondary antibodies as described above. Controls included omission of the primary mouse antibody, but subsequent incubation with goat-anti-mouse secondary antibodies (which were specific for the heavy and light chains of the IgGs).
Image capture, quantitation, and processing.
Fluorescent-labeled images were captured using a 63×, 1.2-NA glycerol objective and the appropriate laser lines of a Leica TCS SP5 CW-STED confocal microscope (in normal confocal mode). HyD hybrid detectors were set at 900–1,200 V, and Z sections (0.13-µm step size) were collected using an average of eight line scans and six frame scans. The images were imported into Volocity 4-D software (PerkinElmer; Waltham, MA) and, after image reconstruction and contrast correction, exported as TIFF files. Composite images were prepared in Adobe Illustrator CC 2015.
Because the changes in the urothelium of SCI-injured mice were focal, we quantified the fraction of urothelium affected by SCI. Tissue sections were immunolabeled with antibodies to KRT20 and counterstained with TRITC-phalloidin and TO-PRO-3. Wide-field images of random portions of the urothelium were captured using a DM6000 microscope (Leica) outfitted with a 20× HC plan APO objective (NA = 0.8), and coupled to a Retiga 4000R CCD camera (QImaging; Surrey BC, Canada) controlled by Volocity software. Using the length tool in the Volocity Quantitation module we measured the total length of sampled urothelium and then measured the length of regions of urothelium that lacked KRT20 staining (and had small superficial cells). By dividing the length of the KRT20− regions by the total length of urothelium we determined the fraction of affected urothelium.
BrdU labeling and quantitation.
BrdU (100 mg/kg; Calbiochem 203806), dissolved in a 1:10 mixture of ethanol and normal saline (0.9% NaCl) was injected intraperitoneally 24 h, and then again 6 h before animals were euthanized at the indicated time point. The mouse was then euthanized and the bladder recovered and fixed as described above. To detect BrdU, sectioned bladder tissue was placed in sodium citrate buffer (10 mM trisodium citrate dihydrate, 0.05% vol/vol Tween-20, pH 6.0) for 30 min in a 95–98°C water bath, followed by incubation in ethanol-glycine solution (a 7:3 mixture of 100% ethanol and 50 mM glycine, pH 2.0) for 20 min at −20°C, before quenching, blocking, and antibody incubation as described above. For quantification of BrdU+ cells, three to four sections per bladder were analyzed. For each section, five random pictures of the urothelium, lamina propria, and smooth muscle were taken using an Olympus BX62 microscope equipped with a 40× objective and the number of BrdU+ nuclei counted and expressed as BrdU+ cells per area.
Statistic.
Data were analyzed in GraphPad Prism 6 (GraphPad, La Jolla, CA) using one-way ANOVA followed by appropriate post hoc tests. P < 0.05 was considered significant. Results are expressed as means ± SE.
RESULTS
Early loss of superficial umbrella cells is observed in mice with SCI.
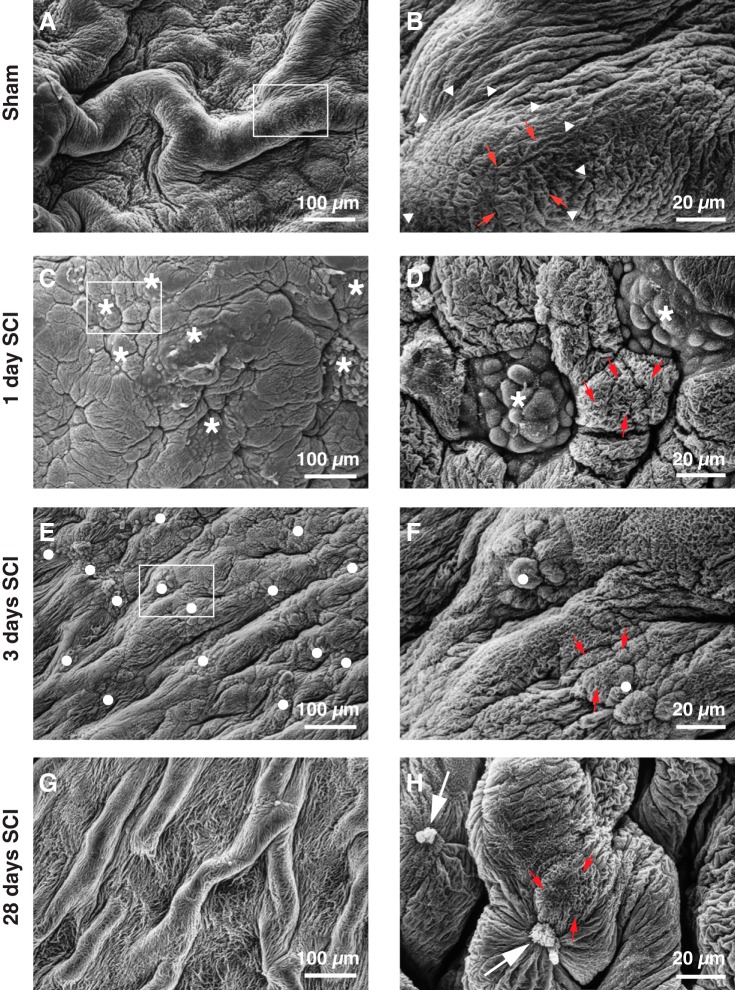
Using a rat model, we previously reported that umbrella cells are rapidly shed after SCI (1). We sought to confirm these findings and extend them to mice because there is a sizeable knowledge base about urothelial regeneration, potential stem cell populations, and cellular phenotypes in this species (19, 34, 42, 50, 60). We first examined the ultrastructure of the urothelium of sham-treated mice and those with SCI using scanning electron microscopy. At low magnification, large mucosal folds were observed in sham-treated animals (Fig. 2A), whereas at higher magnification, the mucosal surfaces of the polyhedral-shaped, superficial umbrella cells (~40 µm diameter in “relaxed” bladder samples; white arrowheads indicate the borders of single cells in Fig. 2B) were revealed. The apical surface of these cells exhibited characteristic microplicae (red-colored arrows, Fig. 2B), which abut the UPK-rich plaque regions that occupy the majority of the apical surface of umbrella cells (27). The zippered apical membrane, which lies directly above the tight junction (29), was apparent at the periphery of the cells (arrowheads, Fig. 2B), although sometimes obscured by the folds in the mucosa.
Fig. 2.
Effects of SCI on the mucosal surface of the bladder lumen. Tissues from sham-treated mice (A and B), or mice with SCI (C–H) were fixed at the indicated time and processed for scanning electron microscopy. The boxed regions in A, C, and E are magnified in B, D, and F. Arrowheads mark the position of the zippered apical membrane surrounding an individual umbrella cell. Small red-colored arrows indicate examples of microplicae, which border adjacent plaque regions. The asterisks in C and D indicate regions of the mucosal surface where umbrella cells are denuded, revealing the underlying intermediate cells. The latter lack microplicae and appear to be incompletely differentiated. In E and F, closed circles mark regions of urothelium populated by small superficial cells, which have undergone some differentiation and have microplicae at their apical surfaces. The large arrows in H mark clusters of small cells, possibly undergoing apoptosis or necrosis. The sham and SCI experiments included 3 animals per treatment group and were performed one time. Representative images are presented.
At 1 day postinjury, the mouse mucosa had a distinctly different morphology: large mucosal folds were less apparent, and the mucosa appeared somewhat smoother at lower magnification (Fig. 2C). Consistent with our previous findings in rats (1), we observed focal regions where superficial umbrella cells were lost, revealing cells from the underlying intermediate layer. The latter were contained within the polyhedral borders formed by adjacent normal-appearing umbrella cells (asterisks, Fig. 2D). These newly exposed cells were ~10 µm in diameter, and their surfaces were covered with short protrusions, but lacked microplicae, indicating that they had not yet undergone significant differentiation. In this manuscript, we refer to these as “small superficial cells,” and as noted below a defining feature of these cells is the lack of KRT20 expression. In rats, we observed hematuria at approximately 3 days post SCI, and a focal loss of the urothelium (1). However, this feature is not characteristic of the mouse model. Instead, at 3 days post SCI, the mucosal surface of the mouse began to regain its folds (Fig. 2E), and the focal patches of small superficial cells appeared to have undergone some differentiation as their surfaces now contained microplicae and some approached a diameter of ~15–20 µm (these areas are indicated by closed circles in Fig. 2F). A month after SCI, the urothelium appeared to be fully regenerated as mucosal folds were apparent (Fig. 2G), and the small superficial cells formed microplicae and zippered apical membranes at their cell surfaces (Fig. 2H). We also occasionally observed very small, shrunken-appearing cells, possibly undergoing necrosis or apoptosis (large arrows, Fig. 2H). These cells were not explored further.
In summary, SCI in mice, as in rats, results in a focal loss of umbrella cells, which by 1 day appear to be replaced by small superficial cells that are likely derived from newly exposed cells in the intermediate layer. With time, the mucosal surface takes on a more normal appearance, but regions of the superficial cell layer remain populated by smaller than normal cells.
Regions of urothelium damaged by SCI undergo rapid, but incomplete regeneration.
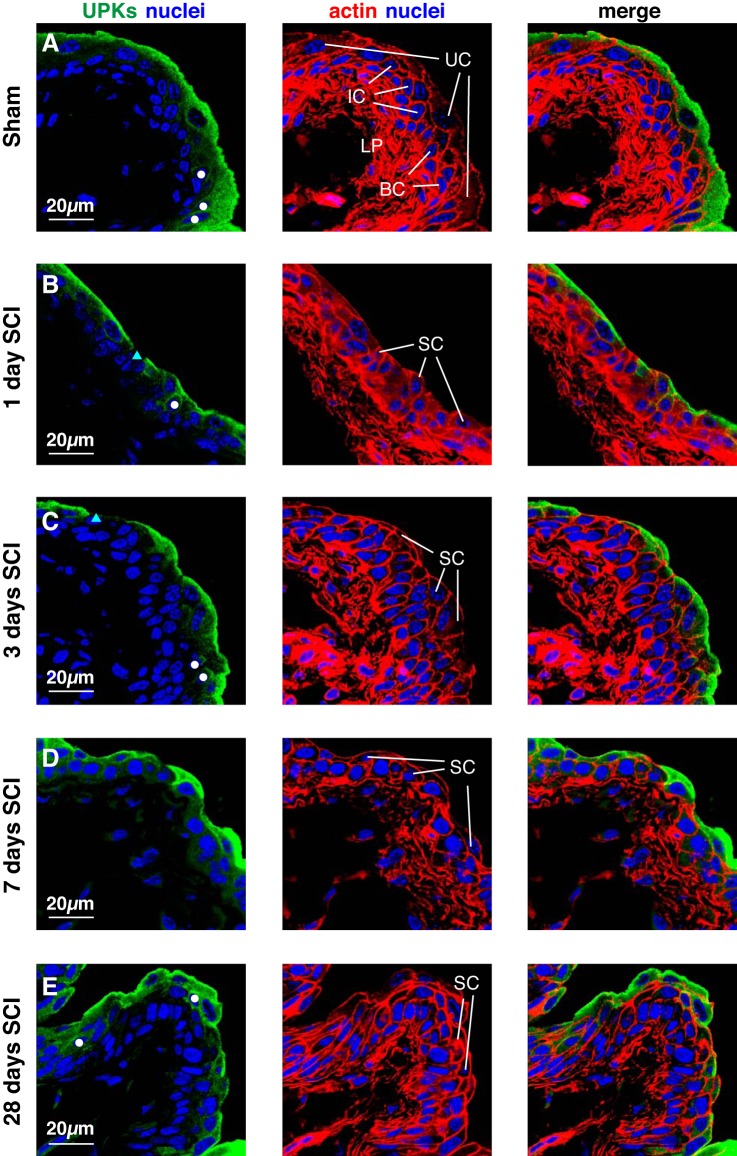
Although scanning electron microscopy provides a high-resolution view of the bladder mucosal surface, it does not give information about the underlying urothelial cell layers, particularly their state of differentiation. To address this issue, we examined the distribution of a number of well-established urothelial differentiation markers in the urothelium of sham mice and those with SCI including UPKs, TP63, KRT5, and KRT20 (19, 34). We provide summary figures that give a representative overview of the cellular phenotypes we observed for each treatment group over multiple experiments and using multiple mice (3–6) per experiment. We first examined the distribution of UPKs, which as expected were expressed in the large superficial umbrella cells (~40 µm diameter in relaxed tissue) of sham tissues (UC in Fig. 3A). In addition, a small number of cells in the subjacent intermediate cell layer (~10 µm diameter in relaxed tissue) were also UPK positive (white circles, Fig. 3A). However, UPK staining was not observed in the majority of cells that comprise the 1–2 layers of intermediate cells, nor was it found in basal cells (Fig. 3A), consistent with previous reports (19, 34). Essentially identical results were obtained for shams that were allowed to recover for 3, 7, or 28 days (n = three per time point), and are not shown.
Fig. 3.
UPK expression in bladder tissues from mice with SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were immunolabeled with a pan-UPK antibody, TRITC-phalloidin, to label the cortical actin cytoskeleton, and TO-PRO-3 to label nuclei. A representative region of urothelium (and subjacent lamina propria; LP) was imaged, and a projection of a Z-stack is shown for each condition. A: for reference, the middle panel shows the position of superficial umbrella cells (UC), intermediate cells (IC), and basal cells (BC). In B–E, regions where umbrella cells are replaced by small superficial cells (SC) are indicated. In A–E, white-colored circles mark intermediate cells that are UPK+, whereas cyan-colored triangles indicate superficial cells that are UPK−. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
In animals with SCI, we focused on those regions of the bladder where the larger, superficial umbrella cells were absent, and which as described above were replaced by small superficial cells that were most likely derived from the intermediate cell layer (or other cells in the subjacent cell layers) (small superficial cells are labeled “SC” in Fig. 3, B–E). However, we emphasize that “normal” appearing regions of urothelium were also present in SCI samples and bordered those regions containing small superficial cells. Whereas umbrella cells showed relatively weak phalloidin reactivity at their apical surfaces, the small superficial cells were often distinguished by relatively bright phalloidin-labeled apical surfaces (Fig. 3, B–E). Most of the small superficial cells expressed UPKs, and a few cells in the underlying intermediate layer were also UPK+ (white circles, Fig. 3, B–E). In some regions of the urothelium 3 days post SCI, there was an apparent increase in cell layers (now 3–5), possibly indicating a proliferation of the urothelium. At this time point, UPKs were expressed by the majority of small superficial cells and also by a limited number of cells in the intermediate cell layers (Fig. 3C), although some small superficial cells were negative for UPK expression (cyan-colored triangle, Fig. 3C). By 7–28 days, the proliferation of cell layers was less apparent, but regions of UPK+ small superficial cells persisted (Fig. 3, D and E).
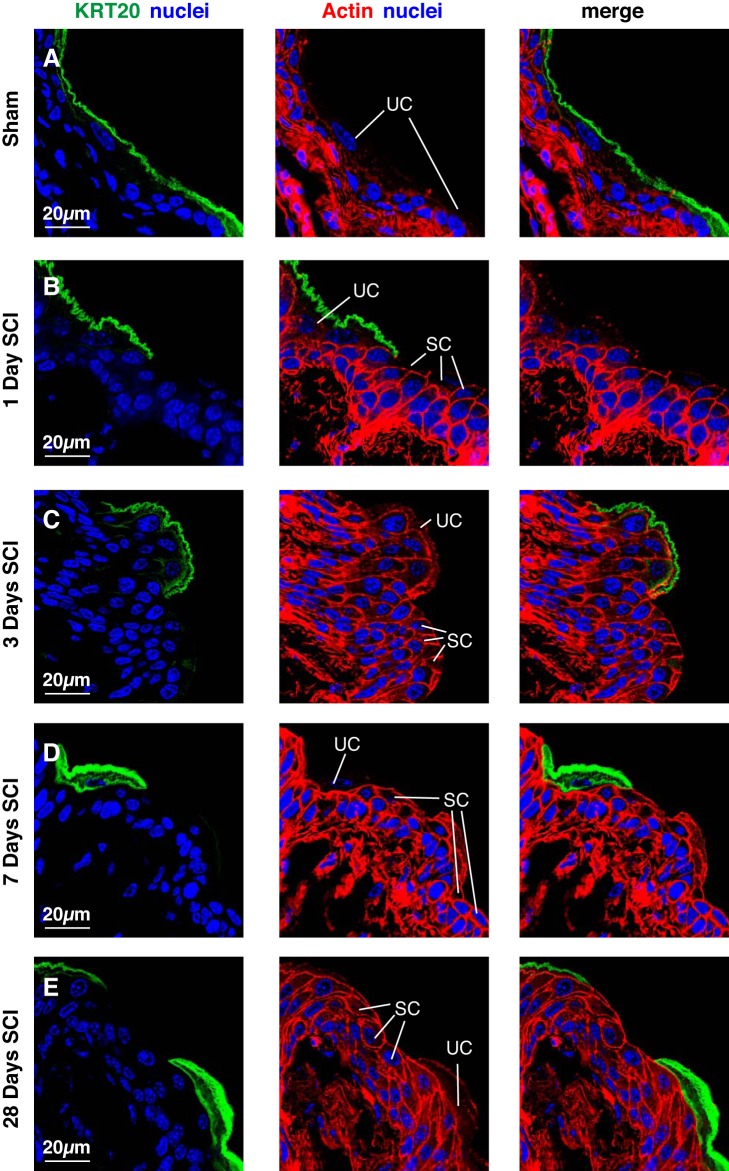
Besides the presence of small superficial cells, an additional indication that urothelial regeneration was incomplete following SCI occurred when we examined the distribution of two additional markers: KRT20, which is expressed solely by superficial umbrella cells, and TP63, which is expressed by all cells but superficial umbrella cells (Fig. 1) (19, 34). In sham tissues, KRT20 was exclusively expressed in the superficial umbrella cell layer (Fig. 4A). However, 1 day post SCI, we observed regions of urothelium populated by small superficial cells that lacked KRT20 staining (Fig. 4B). A similar phenotype was observed at 3 or 7 days post SCI (Fig. 4, C and D). Even after 28 days, small superficial cells that were KRT20− were still present (Fig. 4E). As KRT20 staining appeared to differentiate between those regions that contained superficial umbrella cells vs. those containing small superficial cells, we used it to determine the fraction of the urothelium that was altered in SCI tissues. We observed that 19.7 ± 6.9% of the urothelium was KRT20− 1 day post SCI, 20.5 ± 6.8% 3 days post SCI, 37.3 ± 12.2% 7 days post SCI, and 17.2 ± 4.0% 28 days post SCI (values are means ± SE; n = 3).
Fig. 4.
KRT20 expression in bladder tissues from mice with SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were immunolabeled with a KRT20-specific antibody, TRITC-phalloidin, and TO-PRO-3. A representative region of urothelium in which superficial umbrella cells (UC) bordered regions containing small superficial cells (SC) was imaged, and a projection of a Z-stack is shown for each condition. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
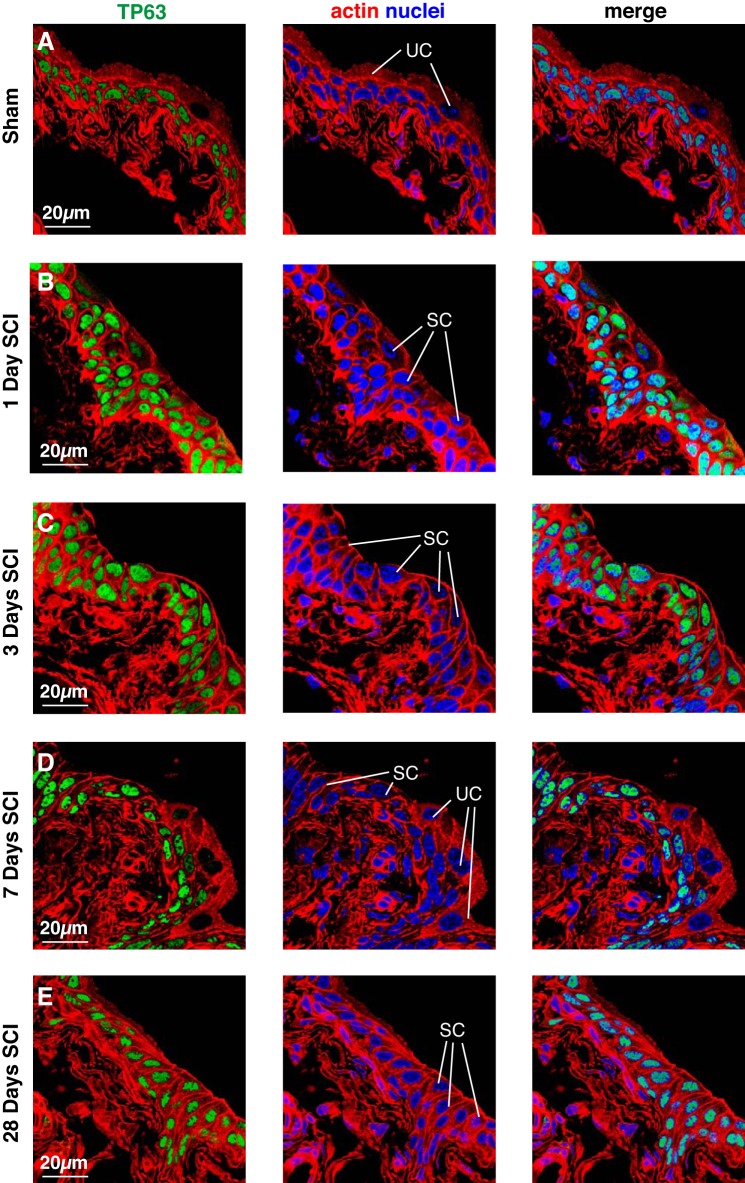
In sham tissues, TP63 was expressed in the cells that comprise the intermediate and basal cell layers, but was absent in superficial umbrella cells (Fig. 5A). In tissue obtained 1 day post SCI, TP63 had a similar distribution to sham tissues in those regions of the urothelium that contained superficial umbrella cells (data not shown). However, in regions populated by small superficial cells, TP63 expression was observed in all layers including the superficial cell layer (Fig. 5B). Similarly, TP63 was found in the small superficial cells of tissues 3, 7, and 28 days post SCI, as well as all of the underlying intermediate and basal cell layers (Fig. 5, C–E). However, it was absent in those regions of the superficial layer that contained umbrella cells (e.g., see Fig. 5D).
Fig. 5.
TP63 expression in bladder tissues from mice with SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were immunolabeled with a TP63-specific antibody, TRITC-phalloidin, and TO-PRO-3. A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. Examples of relatively large superficial umbrella cells (UC) and those regions where superficial umbrella cells are replaced by small superficial cells (SC) are indicated. Note that most small superficial cells are TP63+ in animals with SCI. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
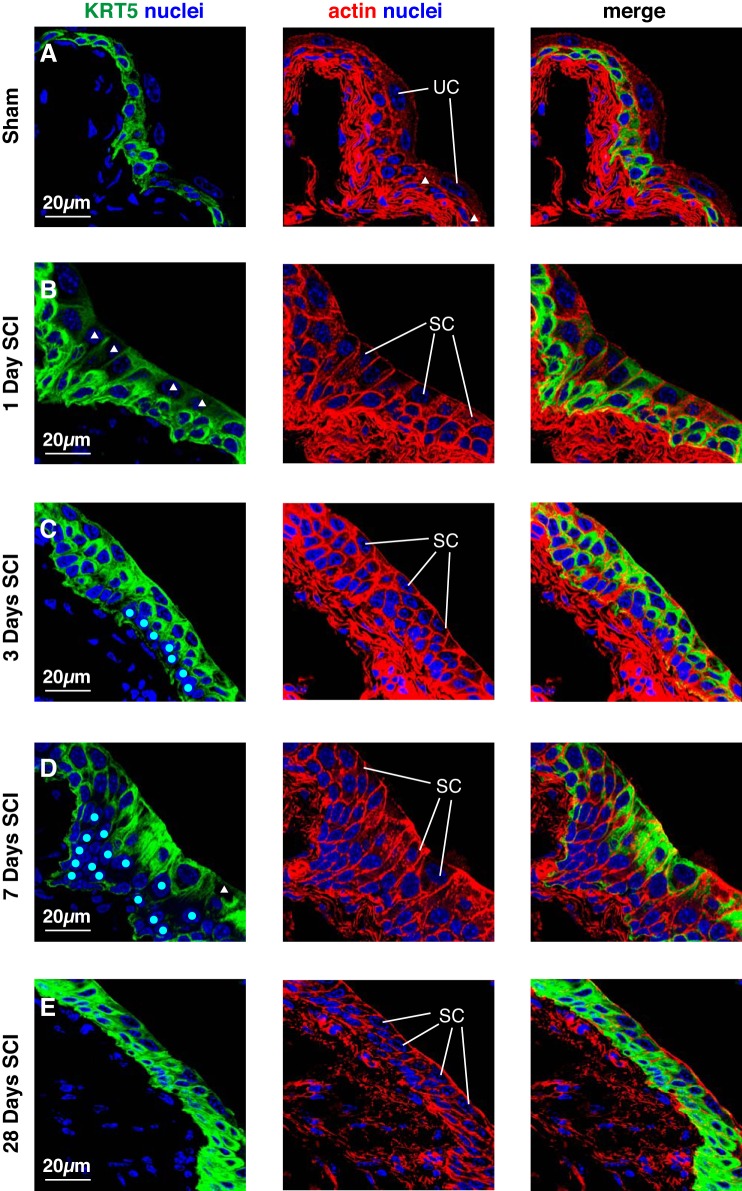
We also examined the distribution of KRT5. In sham tissues, superficial umbrella cells were devoid of KRT5 expression. Instead, KRT5 was localized to the cells in the basal and intermediate cell layers, although a small number of cells in the latter stratum were KRT5− as previously described (white triangles, Fig. 6A) (19). An identical staining pattern was observed post SCI in those regions of tissue that maintained a superficial umbrella cell layer. However, the distribution of KRT5 was different in those regions that were populated with small superficial cells. One day post SCI, the small superficial cells displayed a mixed KRT5+ or KRT5− phenotype (Fig. 6B), although in some regions KRT5+ cells predominated. At 3–7 days post SCI, the small superficial cells were still a mixture of KRT5+ or KRT5−, although the former were most prevalent (Fig. 6D). We occasionally observed regions of urothelium that contained both KRT5− and KRT5+ cells in the lower intermediate and basal cell layers (KRT5− cells are marked with cyan-colored circles in Fig. 6, C and D). However, these layers were most often KRT5+. At 28 days post SCI, the majority of cells in all three layers of the affected regions were KRT5+, although some small superficial cells were only weakly positive (Fig. 6E).
Fig. 6.
KRT5 expression in bladder tissues from mice with SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were immunolabeled with a KRT5-specific antibody, TRITC-phalloidin, and TO-PRO-3. A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. Examples of superficial umbrella cells (UC) and smaller superficial cells (SC) are indicated. White-colored triangles mark superficial cells that show limited staining for KRT5, whereas cyan-colored circles indicate intermediate and basal cells that are KRT5−. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
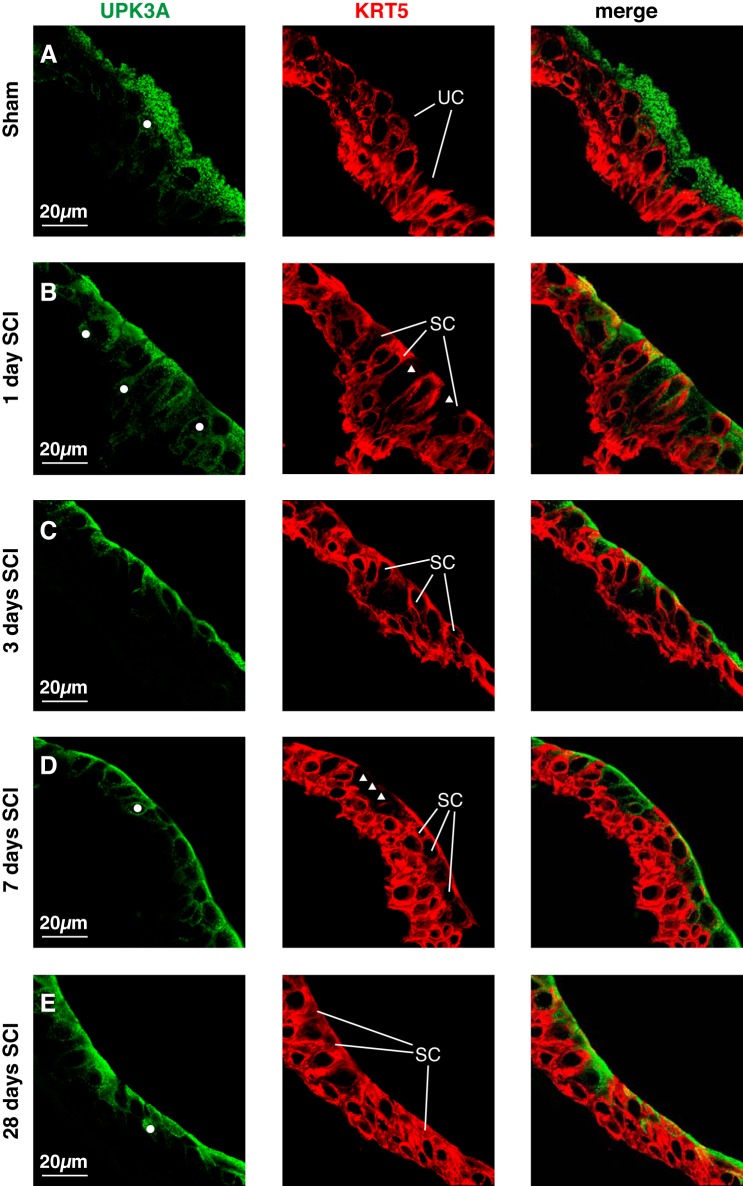
Superficial umbrella cells are normally UPK+, but KRT5− (19, 34). However, the results described above indicated that many of the small superficial cells were KRT5+ (Fig. 6, B–E). To more closely assess the phenotype of the urothelium post SCI, we colocalized UPK3a and KRT5. In sham tissues, umbrella cells were UPK3a+ and KRT5− as expected (Fig. 7A). In contrast, in samples taken 1 day post SCI, some small superficial cells were KRT5+ and UPK3a+ (Fig. 7B), whereas others were KRT5− and UPK3a+ (white triangles, Fig. 7B). After a longer duration of SCI, we similarly observed mixtures of small superficial cells, which varied somewhat in phenotype from region to region (Fig. 7, C–E). Despite the aberrant expression of KRT5 in small superficial cells, we observed that UPK3a staining remained primarily associated with the superficial layer and a small number of intermediate cells just below this layer (examples of the latter are labeled with white circles, Fig. 7).
Fig. 7.
UPK3A and KRT5 expression in urothelial cells post SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were labeled with antibodies to UPK3a and KRT5. A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. Examples of superficial umbrella cells (UC) and small superficial cells (SC) are indicated. UPK3A+ intermediate cells are indicated by white-colored circles, whereas UPK3A+, but KRT5− small superficial cells are indicated by white-colored triangles. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
In summary, in response to SCI, regions of superficial umbrella cells (up to ~40% after 7 days) are lost and replaced by small superficial cells that are UPK+, TP63+, KRT20−, and in many cases KRT5+, a phenotype that is not observed in the native urothelium. The presence of such cells even 28 days postinjury indicates that regeneration of the urothelium is altered by SCI.
KRT14+ cells are found throughout the urothelium of animals with SCI.
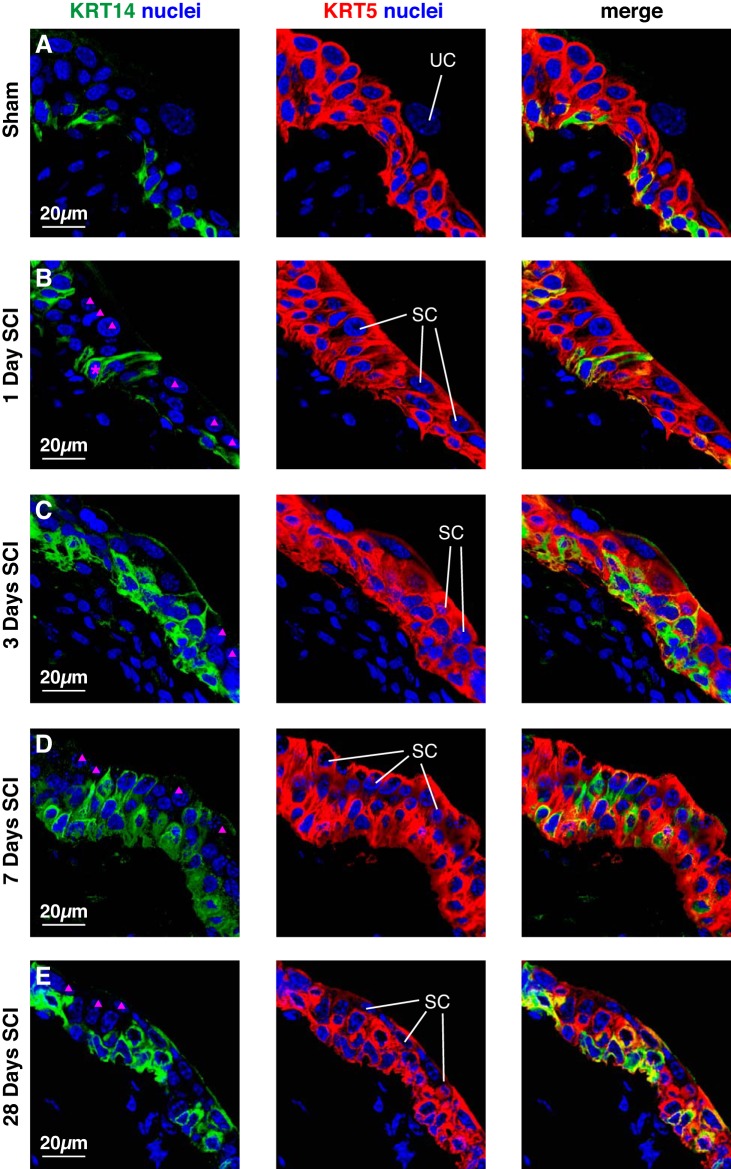
A recent report proposes that KRT14+ basal cells act as stem cells during urothelial regeneration and tumorigenesis (42). Thus we next sought to explore whether KRT14 expression was affected by SCI. In sham-treated tissues, KRT14 was expressed by a population of KRT5+ cells exclusively in the basal cell layer, but not in cells found in the intermediate layers or UPK+ superficial umbrella cells (Figs. 8A and 9A). At 1 day post SCI the distribution of KRT14 was aberrant in those regions of urothelium that contained small superficial cells (Figs. 8B and 9B). Whereas many of the small superficial cells were KRT14− (magenta triangles, Fig. 8B), a small number were KRT14+ (data not shown; and Fig. 10H). Strikingly, we observed that some KRT14+ cells in the basal and intermediate cell layers extended thin cytoplasmic projections that interdigitated between overlying cell layers and then terminated at the cell surface (cells labeled with magenta asterisk, Figs. 8B and 9B). This latter feature is more apparent in 3D reconstructions of image stacks, particularly when they are rotated (see Supplemental Movie S1, available with the online version of this article). These cells were mostly observed 1 day post SCI, but were occasionally observed at later stages. At 3–28 days post SCI we observed regions of urothelium where KRT14+ cells were distributed in the basal, intermediate, and superficial cell layers. However, not all cells in these layers were positive for KRT14 (magenta triangles, Fig. 8, C–E).
Fig. 8.
Distribution of KRT14 and KRT5 in the urothelium post SCI. Bladders from sham-treated mice (A) or those with SCI (B–E) were fixed and processed for confocal microscopy. Tissues were labeled with antibodies to KRT14 and KRT5, and with TO-PRO-3. A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. Examples of superficial umbrella cells (UC) and small superficial cells (SC) are indicated. Intermediate cells that are KRT14−, but KRT5+ are indicated by solid magenta triangles. The magenta-colored asterisk in B marks a KRT14+ basal cell that is extending a cell projection that terminates at the bladder lumen. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
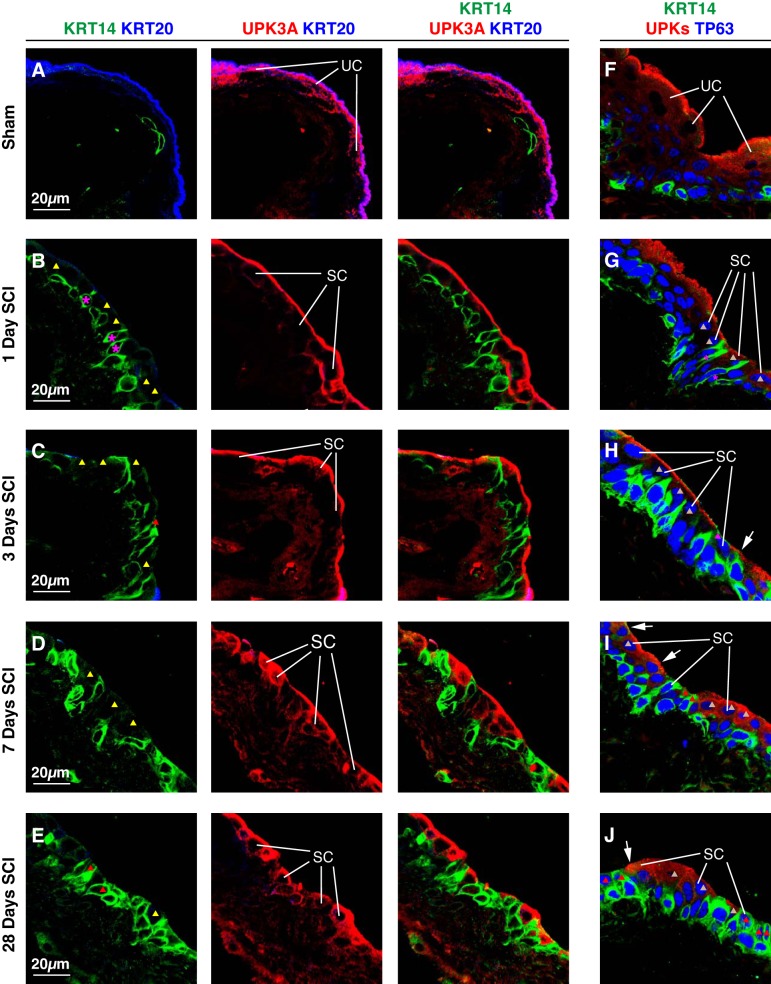
Fig. 9.
Relationship of KRT14, TP63, and UPK expression in small superficial cells post SCI. Bladders from sham-treated mice (A and F) or those with SCI (B–E, G–J) were fixed and processed for confocal microscopy. Tissues were labeled with antibodies to KRT14, KRT20, and UPK3A (A–E), or with antibodies to KRT14, TP63, and UPKs (F–J). A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. Examples of superficial umbrella cells (UC) and small superficial cells (SC) are indicated. Small superficial cells with limited or no KRT14 staining (KRT14−), but positive for UPK3A are marked with yellow-colored triangles, small superficial cells that are KRT14+, but UPK− are indicated by red-colored triangles, and small superficial cells that are TP63+ and UPK+, but KRT14− are marked with gray-colored triangles. The magenta-colored asterisks in B and G mark KRT14+ basal cell that are extending cell projections that terminate at the bladder lumen. The sham and SCI experiments included 3 animals per group and were performed on 5 separate occasions. Representative images are presented.
Fig. 10.
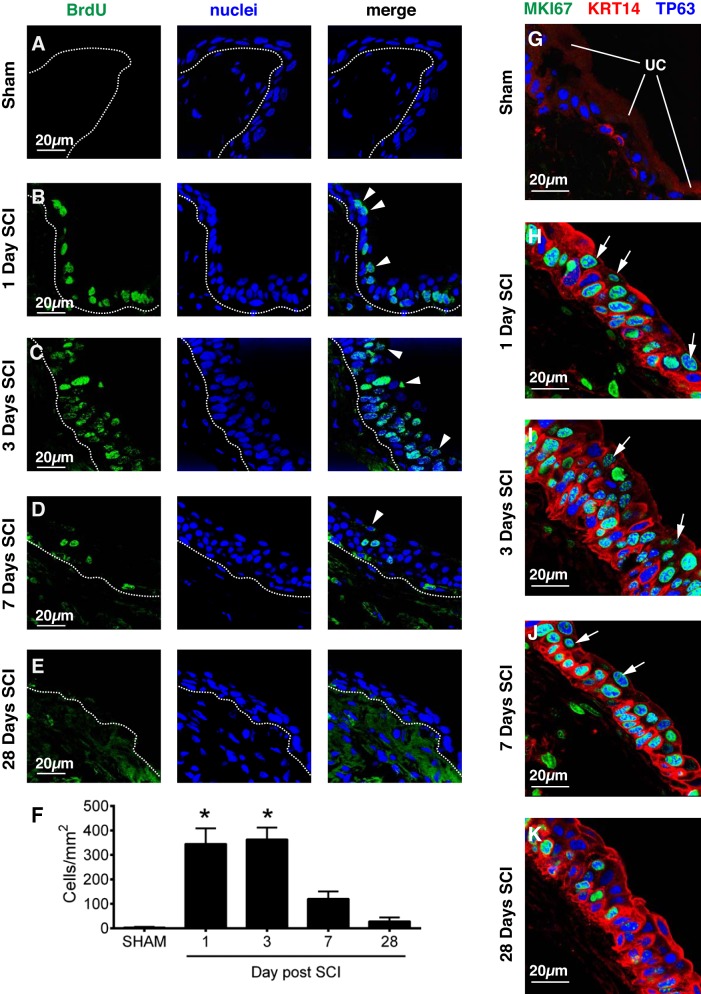
Urothelial proliferation accompanies SCI. Sham-treated mice (A) or those with SCI (B–E) were injected with BrdU 24 h and then 6 h before harvesting of their bladders. The bladder was fixed and then labeled with an anti-BrdU antibody and TO-PRO-3. A representative region of urothelium was imaged, and a projection of a Z-stack is shown for each condition. The position of the basement membrane is indicated by the dashed lines. Superficial cells that are BrdU+ are marked with arrowheads. F: the number of BrdU+ nuclei from randomly selected regions of urothelial tissue is reported as cells/mm2. Values significantly different from sham-treated samples, as assessed by ANOVA, are indicated with an asterisk (P < 0.05). Data are from three separate experiments; means ± SE; n = 5–6 mice per sham and SCI groups. G–K: bladders were recovered from sham-treated (G) or SCI (H–K) mice, fixed, and then labeled with antibodies to MKI67 (alias Ki-67), KRT14, and TP63. Proliferating cells (MKI67+) were found in all 3 cell layers following SCI. Although some of the proliferating cells were KRT14+, there are also ones that were KRT14−, but TP63+. Examples of the latter are marked with white-colored arrows. Data are from 2 separate experiments with 3 animals per group. Representative images are presented.
In adult mice, KRT14 and UPKs/KRT20 are associated with distinct cell populations (basal cells and superficial umbrella cells, respectively) (34, 40, 42), and we confirmed that this was true of urothelium taken from sham-treated animals (Fig. 9A). In contrast, 1 day post SCI we observed regions of urothelium where small superficial cells were KRT20−, but were mostly UPK3A+ and KRT14− (yellow triangles, Fig. 9B). However, cells that were UPK3A+ and KRT14+ or cells that were KRT14+, but UPK3A− were occasionally observed (data not shown). In tissues processed 3, 7, or 28 days post SCI we observed small superficial cells that were KRT14+ and UPK3A+, as well as ones that were negative for one of these markers, but positive for the other (magenta or yellow triangles, Fig. 9, C–E). We also performed a similar analysis examining the distributions of KRT14, TP63, and UPKs in small superficial cells. In general, these cells were KRT14+ and TP63+, but we also observed those that were TP63+ and UPK+, but CK14− (gray triangles in Fig. 9, G–J), or that were CK14+ and TP63+, but UPK− (pink triangles in Fig. 9, H–J). We also observed small superficial cells that expressed all three markers (white arrows in Fig. 9, H–J).
In summary, in response to SCI there is a change in the distribution of KRT14+ cells in those portions of urothelium populated by KRT20− small superficial cells. In these regions, KRT14+ cells are found throughout the urothelial cell layers, including in UPK+ or UPK− small superficial cells that also express TP63.
SCI is associated with proliferation of KRT14+ and KRT14− cells during urothelial regeneration.
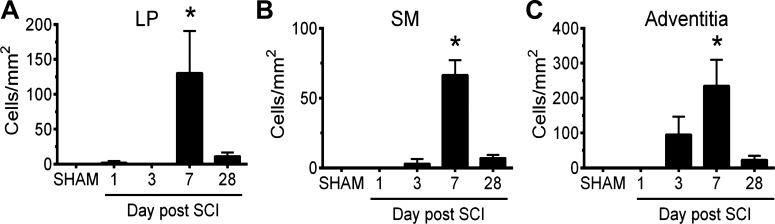
As noted above, urothelial injury is often accompanied by proliferation. To determine whether the urothelium proliferates after SCI, we examined tissue from mice injected with the thymidine analog BrdU. Consistent with previous reports using adult tissues (12, 40, 44), the number of BrdU+ cells in the sham-treated urothelium was very low, 0–5 cells/mm2 of urothelial area (Fig. 10, A and F). Likewise, few BrdU+ cells were found in the lamina propria (LP), smooth muscle (SM), or adventitia of the bladder wall in sham-treated tissues (Fig. 11). However, 1–3 days post SCI, there was a significant rise in BrdU+ cells within the urothelium (Fig. 10F). Positive cells were most abundant in the intermediate and basal layers, but were also observed in the superficial cell layer (arrowheads, Fig. 10, B and C). Regions of BrdU+ cells were occasionally observed 7 days post SCI. However, the numbers of these cells at 7 days post SCI were not significantly different than sham-treated tissues (Fig. 10F). Likewise, there was no significant increase in proliferation observed in tissues 28 days post SCI (Fig. 10, D–F). Interestingly, even regions of urothelium containing KRT20+ umbrella cells were also BrdU+, indicating that this was a urothelium-wide event (data not shown). In addition to the urothelium, BrdU+ cells were also seen in the LP, SM, and adventitia. Their numbers were smaller than those observed in the UT, and the peak proliferation time was at 7 days post SCI (Fig. 11).
Fig. 11.
Proliferation of cells in non-urothelial regions of the bladder following SCI. The number of BrdU+ nuclei from randomly selected regions of lamina propria (LP), smooth muscle (SM), or adventitia is reported as cells/mm2. Values significantly different from sham-treated samples, as assessed by ANOVA, are indicated with an asterisk (P < 0.05). Data are from 3 separate experiments; means ± SE; n ≥ 3 mice.
To confirm these results and to determine the phenotype of the proliferating cells, we labeled tissues with antibodies to MKI67 (alias Ki67), a nuclear protein that is routinely used to mark proliferating cells (20, 49), as well as TP63 and KRT14. Similar to the BrdU-labeling experiments, we observed that MKI67+ cells were largely absent from sham tissues, but were abundant 1–3 days post SCI (Fig. 10, G–I). Furthermore, by 28 days post-SCI there were fewer MKI67+ cells (Fig. 10K), consistent with the BrdU data described above. At 1 day post SCI we observed that many of the MKI67+ cells were KRT14+ (and TP63+) (Fig. 10H). However, we also observed cells that were MKI67+ and TP63+, but KRT14− (arrows, Fig. 10H), indicating that KRT14− cell populations were also proliferating. Such cells were also observed 3–7 days post SCI (arrows, Fig. 10, I and J). We were unable to colocalize both MKI67 and KRT5 because the only reliable antibodies we identified were of the same species; however, we did observe that BrdU-labeled cells were also positive for KRT5 and/or KRT14 (data not shown). Taken together, these results indicate that in response to SCI the urothelium proliferates after the initial injury, but with time this proliferation subsides. Furthermore, both KRT14+ and KRT14− cell populations undergo proliferation.
DISCUSSION
The bladder is one of few organs that can undergo functional and morphological regeneration, even when 75% of the bladder wall is removed by cystectomy (8, 44). Likewise, the normally quiescent urothelium has enormous regenerative capacity when exposed to acute chemical, bacterial, and mechanical injuries, restoring a functional and morphologically patent barrier within days of the initial insult (12, 16, 17, 19, 31, 33, 39–42, 44, 46, 47, 50, 58). The impact of chronic injuries is less well understood, although it is known that classical forms of interstitial cystitis, those with Hunner’s lesion, as well as cats with nonulcerating feline interstitial cystitis, exhibit a disrupted urothelium (18, 22, 32). Similarly, models for outlet obstruction are also associated with urothelial damage (9, 35, 48), and we previously showed that in rats SCI results in a rapid, focal loss of superficial umbrella cells (within 2 h), which are quickly replaced with smaller superficial cells (1). However, none of these previous studies carefully examined the long-term impact of these injuries on the differentiation status of the urothelium.
We observe that SCI causes a patchy, but selective loss of the umbrella cell layer of the mouse bladder, apparently stimulating the cells in the underlying intermediate cell layer to undergo a rapid differentiation process (Fig. 12). We believe this to be so because in rats the newly exposed superficial cells express TJP1 and UPK3s within 2 h of SCI (1). Moreover, in the current study we observe that small superficial cells are TP63+ (and variably positive for KRT5). However, it is formally possible that cells in deeper layers are proliferating and then migrating to the superficial cell layer within the intervening 24 h between SCI and tissue processing (see below). It is unclear why SCI causes the focal loss of superficial umbrella cells in ~20% of the urothelium after day 1, although in rats the loss of umbrella cells can be temporarily mitigated by treatment with the ganglionic blocker hexamethonium (1). Thus transmitter release from damaged nerves may be involved. However, we cannot rule out that some loss may arise from other phenomena such as overdistension and/or ischemia, particularly in early days, before the spinal cord-to-bladder micturition reflex develops. Previous studies have shown that ischemia is observed in the bladder after SCI (25), and short periods of ischemia can result in damage to the urothelium, although the lesions can be more pronounced than observed after SCI (28).
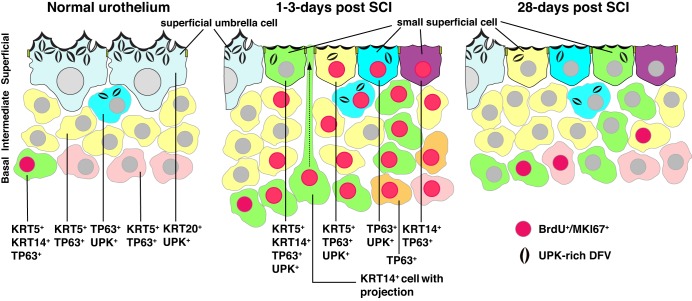
Fig. 12.
Changes in the urothelium in response to SCI. Cellular phenotypes observed in the indicated cell layer of normal (and sham-treated), 1–3 days post SCI, and 28 days post SCI urothelium. Note that the KRT20+ and UPK+ umbrella cells in normal urothelium are replaced in animals with SCI by smaller superficial cells with the indicated phenotypes. At 1 day post SCI in particular, KRT14+ basal cells are observed that send thin projections that terminate in the bladder lumen (one such cell is shown in the middle panel of the figure). This may represent a form of interkinetic nuclear migration. BrdU/MKI67+ nuclei are red-colored and indicate mitotically active cells.
Although acute injuries such as treatments with protamine sulfate and chitosan, or exposure to uropathogenic E. coli, also cause a rapid loss of superficial umbrella cells (11, 17, 41, 58), the newly exposed superficial cells undergo complete differentiation to umbrella cells. In contrast, in SCI, regions of superficial umbrella cells (up to ~40% after 7 days) are replaced by small superficial cells that express UPKs, but also coexpress TP63, KRT5, and/or KRT14, combinations not observed in the native urothelium (Fig. 12). Moreover, these small superficial cells lack expression of KRT20, further indicating they are not differentiated umbrella cells. Even 28 days post SCI, weeks after formation of a local spinal reflex, one still observes regions of small superficial cells that coexpress KRT5, KRT14, TP63, and UPKs, but not KRT20. The continued presence of focal regions of these altered cell types several weeks after the initial trauma to the spinal cord indicates the presence of an ongoing inflammatory process or derangement in signals that specify normal urothelial differentiation. The nature of these signals and their source is unknown, although one may surmise that they are occurring in response to altered nervous input and the resulting changes in bladder function that occur as bladder control is moved from the CNS to a local spinal reflex (14). Interestingly, proliferation begins to subside after 3 days post SCI, indicating that whatever the nature of the signal is, it does not involve a constant turnover of the urothelium.
Another hallmark of the post SCI urothelium is its early proliferation, which is also reported to occur following acute injuries. From 1 to 3 days post SCI, proliferating cells are detected in all three urothelial cell layers and are of two general types: KRT14− or KRT14+ (Fig. 12). However, without conducting lineage tracing studies it is possible that KRT14+ cells give rise to KRT14− cells or vice versa. The KRT14− population of cells includes TP63+ cells localized to the intermediate and superficial cell layers, which may be undergoing a local expansion to make up for those intermediate cells that replace the denuded superficial umbrella cells. The other proliferating cell population comprises KRT14+ (and TP63+) cells, which are reported to be bladder stem cells (42). However, this remains controversial because during mouse development progenitor cells (P cells), which give rise to cells in the intermediate and superficial cell layers (19), form at E11.5, several days in advance of KRT14+ basal cells (which are first detected at E16.5) (42). The expression of TP63 and UPKs, but not KRT20 in the KRT14+ small superficial cells, indicates that differentiation of KRT14+ cells is stopped some time before the expression of KRT20 in the bladders of animals with SCI.
Strikingly, 1 day post SCI we observe KRT14+ basal cells that send thin cytoplasmic extensions, some of which reach the bladder lumen (middle panel, Fig. 12). The functional significance of these cells and their protrusions is not known, but these cells may be undergoing interkinetic nuclear migration. In this process, the progenitor cell nuclei of stratified (and pseudostratified) epithelia migrate along the apico-basal axis via extensions that bridge the lumen and the basal lamina (37). In this way, the nuclei of KRT14+ cells can be “delivered” to areas of disrupted urothelium, facilitating the regeneration all three cell layers. Although this form of migration is observed in embryonic ureteric epithelium (38), we do not know the nature of the signals that promote these events, but it is possible they occur in response to the loss of an inhibitory factor normally released by superficial umbrella cells, or they could occur in response to positive signals emitted from adjacent cells in the urothelium or stroma. In the case of bacterial injury, stromal cells play an important role in promoting urothelial proliferation (40, 50), and we too observe stromal proliferation following SCI.
Importantly, changes in urothelial differentiation are likely to manifest in altered gene expression and urothelial function, as well as increased susceptibility to bacterial infections. Work to date indicates that the urothelium of patients with SCI exhibits modified expression of CDH1 (alias E-cadherin), EGFR, SPHK1 (alias sphingosine kinase 1), and TJP1 (3, 10, 13, 21, 56). We surmise that the expression of other proteins may similarly be affected, and these changes could further contribute to disrupted bladder function. For example, increased/decreased expression of proteins that affect communication between the urothelium and underlying nerves (e.g., urothelium-associated receptors, channels, and transmitters), could contribute to the increase in bladder spontaneous activity reported soon after SCI and the overactive bladder symptoms reported in patients with SCI (2, 43). Moreover, it is known that patients with SCI are particularly prone to episodes of bacterial cystitis (52). Although some of this sensitivity is associated with catheterization, it may also reflect the altered differentiation status of the urothelium, which may provide increased numbers of or additional cell surface “receptors” that could facilitate bacterial adhesion and colonization.
Summary
The urothelium forms an impermeable barrier and has important functions in sensory transduction. After acute chemical, bacterial, and physical injuries, the urothelium undergoes rapid and complete regeneration. However, much less is known about the renewal of the urothelium in response to chronic injuries. Our studies show that SCI is associated with a rapid, focal loss of the umbrella cell layer, which is replaced in part by underlying cells from the intermediate cell layer and likely other cell types in the urothelium including KRT14+ cells (Fig. 12). Interestingly, and unlike acute injuries, the urothelium of mice with SCI develops focal regions that exhibit an aberrant expression of keratins and other differentiation markers, indicating incomplete regeneration even 28 days postinjury. By learning more about the effects that injuries have on the urothelium, the cell types that promote urothelial regeneration, and the signaling pathways that are involved, the general hope is to devise treatments that will allow for partial or complete restoration of bladder function in those conditions where the bladder is compromised by obstruction, malformation, or diseases including SCI. In this regard, our research findings illuminate the changes in the urothelium that occur early on and chronically once the spinal cord-to-bladder reflex develops following SCI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-104287, R01-DK-099196, and P30-DK-079307 to G. Apodaca; by P01-DK-09324 to L. A. Birder; by P01-DK-09324 to A. Kanai; and by the Kidney Imaging Core of the Pittsburgh Center for Kidney Research (P30-DK-079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.A.K., D.R.C., W.G.R., A.W.-J., C.G., L.A.B., and G.A. conceived and designed research; F.A.K., D.R.C., W.G.R., A.W.-J., and C.G. performed experiments; F.A.K., D.R.C., W.G.R., A.W.-J., C.G., L.A.B., and G.A. analyzed data; F.A.K., D.R.C., W.G.R., L.A.B., and G.A. interpreted results of experiments; F.A.K., D.R.C., W.G.R., and G.A. prepared figures; F.A.K. and G.A. drafted manuscript; F.A.K., A.J.K., L.A.B., and G.A. edited and revised manuscript; F.A.K., D.R.C., W.G.R., A.W.-J., C.G., A.J.K., L.A.B., and G.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. T. T. Sun (NY University) for the kind gift of the pan-UPK antibody.
REFERENCES
- 1.Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol 284: F966–F976, 2003. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 2.Artim DE, Kullmann FA, Daugherty SL, Bupp E, Edwards CL, de Groat WC. Developmental and spinal cord injury-induced changes in nitric oxide-mediated inhibition in rat urinary bladder. Neurourol Urodyn 30: 1666–1674, 2011. doi: 10.1002/nau.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballouhey Q, Panicker JN, Mazerolles C, Roumiguie M, Zaidi F, Rischmann P, Malavaud B, Game X. Sphingosine Kinase 1 urothelial expression is increased in patients with neurogenic detrusor overactivity. Int Braz J Urol 41: 1141–1147, 2015. doi: 10.1590/S1677-5538.IBJU.2014.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsara ZR, Li X. Sleeping Beauty: awakening urothelium from its slumber. Am J Physiol Renal Physiol: 312: F732–F743, 2017. doi: 10.1152/ajprenal.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birder L, Andersson KE. Urothelial signaling. Physiol Rev 93: 653–680, 2013. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birder LA. Role of the urothelium in urinary bladder dysfunction following spinal cord injury. Prog Brain Res 152: 135–146, 2006. doi: 10.1016/S0079-6123(05)52009-0. [DOI] [PubMed] [Google Scholar]
- 7.Böck M, Hinley J, Schmitt C, Wahlicht T, Kramer S, Southgate J. Identification of ELF3 as an early transcriptional regulator of human urothelium. Dev Biol 386: 321–330, 2014. doi: 10.1016/j.ydbio.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A 16: 2541–2551, 2010. doi: 10.1089/ten.tea.2009.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celik-Ozenci C, Ustunel I, Erdogru T, Seval Y, Korgun ET, Baykara M, Demir R. Ultrastructural and immunohistochemical analysis of rat uroepithelial cell junctions after partial bladder outlet obstruction and selective COX-2 inhibitor treatment. Acta Histochem 107: 443–451, 2006. doi: 10.1016/j.acthis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen SF, Chang CH, Kuo HC. Effect of detrusor botulinum toxin a injection on urothelial dysfunction in patients with chronic spinal cord injury: a clinical and immunohistochemistry study before and after treatment. Spinal Cord 54: 889–894, 2016. doi: 10.1038/sc.2015.241. [DOI] [PubMed] [Google Scholar]
- 11.Tzan CJ, Berg J, Lewis SA. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J Membr Biol 133: 227–242, 1993. doi: 10.1007/BF00232022. [DOI] [PubMed] [Google Scholar]
- 12.Colopy SA, Bjorling DE, Mulligan WA, Bushman W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev Dyn 243: 988–998, 2014. doi: 10.1002/dvdy.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz CD, Coelho A, Antunes-Lopes T, Cruz F. Biomarkers of spinal cord injury and ensuing bladder dysfunction. Adv Drug Deliv Rev 82-83: 153–159, 2015. doi: 10.1016/j.addr.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. Sci World J 11: 214–234, 2011. doi: 10.1100/tsw.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer WI, Schuller AG, Vermey M, van der Kwast TH. Expression of growth factors and receptors during specific phases in regenerating urothelium after acute injury in vivo. Am J Pathol 145: 1199–1207, 1994. [PMC free article] [PubMed] [Google Scholar]
- 16.Erman A, Jezernik K, Stiblar-Martincic D, Romih R, Veranic P. Postnatal restoration of the mouse urinary bladder urothelium. Histochem Cell Biol 115: 309–316, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Erman A, Kerec Kos M, Žakelj S, Resnik N, Romih R, Veranič P. Correlative study of functional and structural regeneration of urothelium after chitosan-induced injury. Histochem Cell Biol 140: 521–531, 2013. doi: 10.1007/s00418-013-1088-7. [DOI] [PubMed] [Google Scholar]
- 18.Fall M, Logadottir Y, Peeker R. Interstitial cystitis is bladder pain syndrome with Hunner’s lesion. Int J Urol 21, Suppl 1: 79–82, 2014. doi: 10.1111/iju.12325. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, Sun TT, Aronow B, Rosen R, Mauney J, Adam R, Rosselot C, Van Batavia J, McMahon A, McMahon J, Guo JJ, Mendelsohn C. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26: 469–482, 2013. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodson WH III, Moore DH II, Ljung BM, Chew K, Florendo C, Mayall B, Smith HS, Waldman FM. The functional relationship between in vivo bromodeoxyuridine labeling index and Ki-67 proliferation index in human breast cancer. Breast Cancer Res Treat 49: 155–164, 1998. doi: 10.1023/A:1005926228093. [DOI] [PubMed] [Google Scholar]
- 21.Jiang YH, Liu HT, Kuo HC. Urothelial dysfunction and chronic inflammation in patients with spinal cord injuries at different levels and correlation with urodynamic findings. Neurourol Urodyn 34: 757–762, 2015. doi: 10.1002/nau.22650. [DOI] [PubMed] [Google Scholar]
- 22.Johansson SL, Fall M. Clinical features and spectrum of light microscopic changes in interstitial cystitis. J Urol 143: 1118–1124, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Jost SP. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch B Cell Pathol Incl Mol Pathol 57: 27–36, 1989. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- 24.Jost SP, Potten CS. Urothelial proliferation in growing mice. Cell Tissue Kinet 19: 155–160, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Kadekawa K, Majima T, Kawamorita N, Okada H, Yoshizawa T, Mori K, Tyagi P, Sugaya K, Yoshimura N. Effects of an alpha1A/D-adrenoceptor antagonist, naftopidil, and a phosphodiesterase type 5 inhibitor, tadalafil, on urinary bladder remodeling in rats with spinal cord injury. Neurourol Urodyn October 4, 2016. [Epub ahead of print]. doi: 10.1002/nau.23158. [DOI] [PubMed] [Google Scholar]
- 26.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol 167: 1195–1204, 2004. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korosec P, Jezernik K. Early cellular and ultrastructural response of the mouse urinary bladder urothelium to ischemia. Virchows Arch 436: 377–383, 2000. doi: 10.1007/s004280050462. [DOI] [PubMed] [Google Scholar]
- 29.Kreplak L, Wang H, Aebi U, Kong XP. Atomic force microscopy of Mammalian urothelial surface. J Mol Biol 374: 365–373, 2007. doi: 10.1016/j.jmb.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol 294: F1415–F1421, 2008. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 31.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 32.Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 278: F540–F553, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Ling S, Chang X, Schultz L, Lee TK, Chaux A, Marchionni L, Netto GJ, Sidransky D, Berman DM. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res 71: 3812–3821, 2011. doi: 10.1158/0008-5472.CAN-10-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, Clements D, Cullen-McEwen L, Fleming J, Gilbert T, Herzlinger D, Houghton D, Kaufman MH, Kleymenova E, Koopman PA, Lewis AG, McMahon AP, Mendelsohn CL, Mitchell EK, Rumballe BA, Sweeney DE, Valerius MT, Yamada G, Yang Y, Yu J. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns 7: 680–699, 2007. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu HT, Jiang YH, Kuo HC. Alteration of urothelial inflammation, apoptosis, and junction protein in patients with various bladder conditions and storage bladder symptoms suggest common pathway involved in underlying pathophysiology. Low Urin Tract Symptoms 7: 102–107, 2015. doi: 10.1111/luts.12062. [DOI] [PubMed] [Google Scholar]
- 36.Mimata H, Satoh F, Tanigawa T, Nomura Y, Ogata J. Changes of rat urinary bladder during acute phase of spinal cord injury. Urol Int 51: 89–93, 1993. doi: 10.1159/000282520. [DOI] [PubMed] [Google Scholar]
- 37.Miyata T, Okamoto M, Shinoda T, Kawaguchi A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insight into tissue mechanics. Front Cell Neurosci 8: 473, 2015. doi: 10.3389/fncel.2014.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motoya T, Ogawa N, Nitta T, Rafiq AM, Jahan E, Furuya M, Matsumoto A, Udagawa J, Otani H. Interkinetic nuclear migration in the mouse embryonic ureteric epithelium: Possible implication for congenital anomalies of the kidney and urinary tract. Congenit Anom (Kyoto) 56: 127–134, 2016. doi: 10.1111/cga.12150. [DOI] [PubMed] [Google Scholar]
- 39.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282: 1494–1497, 1998. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 40.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5: 463–475, 2009. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem 277: 7412–7419, 2002. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 42.Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7: 11914, 2016. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patra PB, Patra S. Research Findings on Overactive Bladder. Curr Urol 8: 1–21, 2015. doi: 10.1159/000365682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyton CC, Burmeister D, Petersen B, Andersson KE, Christ G. Characterization of the early proliferative response of the rodent bladder to subtotal cystectomy: a unique model of mammalian organ regeneration. PLoS One 7: e47414, 2012. doi: 10.1371/journal.pone.0047414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci USA 110: 8105–8110, 2013. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romih R, Jezernik K, Masera A. Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem Cell Biol 109: 263–269, 1998. doi: 10.1007/s004180050226. [DOI] [PubMed] [Google Scholar]
- 47.Romih R, Koprivec D, Martincic DS, Jezernik K. Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int 25: 531–537, 2001. doi: 10.1006/cbir.2000.0658. [DOI] [PubMed] [Google Scholar]
- 48.Romih R, Korosec P, Jezernik K, Sedmak B, Trsinar B, Deng FM, Liang FX, Sun TT. Inverse expression of uroplakins and inducible nitric oxide synthase in the urothelium of patients with bladder outlet obstruction. BJU Int 91: 507–512, 2003. doi: 10.1046/j.1464-410X.2003.03052.x. [DOI] [PubMed] [Google Scholar]
- 49.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 50.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114, 2011. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shunmugavel A, Khan M, Te Chou PC, Dhindsa RK, Martin MM, Copay AG, Subach BR, Schuler TC, Bilgen M, Orak JK, Singh I. Simvastatin protects bladder and renal functions following spinal cord injury in rats. J Inflamm (Lond) 7: 17, 2010. doi: 10.1186/1476-9255-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 113, Suppl 1A: 67S–79S, 2002. doi: 10.1016/S0002-9343(02)01061-6. [DOI] [PubMed] [Google Scholar]
- 53.Sugasi S, Lesbros Y, Bisson I, Zhang YY, Kucera P, Frey P. In vitro engineering of human stratified urothelium: analysis of its morphology and function. J Urol 164: 951–957, 2000. doi: 10.1016/S0022-5347(05)67224-2. [DOI] [PubMed] [Google Scholar]
- 54.Tai C, Roppolo JR, de Groat WC. Spinal reflex control of micturition after spinal cord injury. Restor Neurol Neurosci 24: 69–78, 2006. [PMC free article] [PubMed] [Google Scholar]
- 55.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Velzen D, Krishnan KR, Parsons KF, Soni BM, Fraser MH, Vaidyanathan S. Epidermal growth factor receptor in the vesical urothelium of paraplegic and tetraplegic patients: an immunohistochemical study. Spinal Cord 34: 578–586, 1996. doi: 10.1038/sc.1996.103. [DOI] [PubMed] [Google Scholar]
- 57.van Velzen D, Krishnan KR, Parsons KF, Soni BM, Howard CV, Fraser MH, Vaidyanathan S. Vesical urothelium proliferation in spinal cord injured persons: an immunohistochemical study of PCNA and MIB.1 labelling. Paraplegia 33: 523–529, 1995. doi: 10.1038/sc.1995.113. [DOI] [PubMed] [Google Scholar]
- 58.Veranic P, Erman A, Kerec-Kos M, Bogataj M, Mrhar A, Jezernik K. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem Cell Biol 131: 129–139, 2009. doi: 10.1007/s00418-008-0492-x. [DOI] [PubMed] [Google Scholar]
- 59.Veranic P, Jezernik K. Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskeleton 53: 317–325, 2002. doi: 10.1002/cm.10077. [DOI] [PubMed] [Google Scholar]
- 60.Yamany T, Van Batavia J, Mendelsohn C. Formation and regeneration of the urothelium. Curr Opin Organ Transplant 19: 323–330, 2014. doi: 10.1097/MOT.0000000000000084. [DOI] [PubMed] [Google Scholar]