Abstract
Podocytes are highly differentiated epithelial cells wrapping glomerular capillaries to form the filtration barrier in kidneys. As such, podocyte injury or dysfunction is a critical pathogenic event in glomerular disease. Autophagy plays an important role in the maintenance of the homeostasis and function of podocytes. However, it is less clear whether and how autophagy contributes to podocyte injury in glomerular disease. Here, we have examined the role of autophagy in adriamycin-induced nephropathy, a classic model of glomerular disease. We show that autophagy was induced by adriamycin in cultured podocytes in vitro and in podocytes in mice. In cultured podocytes, activation of autophagy with rapamycin led to the suppression of adriamycin-induced apoptosis, whereas inhibition of autophagy with chloroquine enhanced podocyte apoptosis during adriamycin treatment. To determine the role of autophagy in vivo, we established an inducible podocyte-specific autophagy-related gene 7 knockout mouse model (Podo-Atg7-KO). Compared with wild-type littermates, Podo-Atg7-KO mice showed higher levels of podocyte injury, glomerulopathy, and proteinuria during adriamycin treatment. Together, these observations support an important role of autophagy in protecting podocytes under the pathological conditions of glomerular disease, suggesting the therapeutic potential of autophagy induction.
Keywords: adriamycin, apoptosis, autophagy, kidney, mTOR, podocytes
podocytes are highly specialized epithelial cells in Bowman’s capsule of kidneys that wrap around the capillaries of glomeruli. Functionally, podocytes are critical to the formation of the filtration barrier in glomeruli, the maintenance of the structural integrity of glomerular capillaries, and the production of pleiotropic factors for neighboring cells. Terminally differentiated, podocytes are incapable of proliferation, and the mechanism of podocyte replacement is limited (18). As a result, damage or dysfunction of podocytes may lead to proteinuria and glomerulosclerosis, contributing to the pathogenesis of primary glomerular diseases (e.g., focal segmental glomerulosclerosis; FSGS) as well as secondary glomerular diseases (e.g., diabetic nephropathy) (24).
As long-lived cells, podocytes rely on cellular quality control mechanisms to maintain their homeostasis, viability, and function. One such mechanism is autophagy, which delivers damaged or dysfunctional organelles and misfolded proteins or protein aggregates to lysosomes for degradation (13, 17). Indeed, podocytes exhibit a relatively high level of autophagy that may be pivotal to the maintenance of cellular homeostasis (13, 17, 29). In this regard, Hartleben et al. (6) demonstrated that autophagy deficiency in podocytes resulted in endoplasmic reticulum stress and accumulation of oxidized and ubiquitinated protein, followed by podocyte injury and late onset of glomerulopathy in aging mice. In humans, Liu and colleagues (28) demonstrated a negative correlation between podocyte autophagic activity and the progression of glomerular diseases. Specifically, podocytes from minimal change disease (MCD) patients showed higher autophagic activity than those from FSGS patients. Moreover, repeat renal biopsies of MCD patients revealed a decrease in podocyte autophagic activity during the progression to FSGS (28). More recently, Tagawa et al. (19) revealed the impairment of autophagy in podocytes of kidney biopsy from diabetic patients with massive proteinuria. These and other studies have suggested the possible involvement of autophagy dysregulation in podocytes in the pathogenesis of glomerular diseases; however, the conclusion remains to be established by inhibitory studies, especially those using autophagy gene knockout models.
In the present study, we have examined podocyte autophagy in adriamycin-induced nephropathy, a classic model of chronic kidney disease characterized by glomerular pathologies including FSGS (10). We have demonstrated the induction of autophagy by adriamycin in cultured podocytes and the podocytes in mouse kidneys. In cultured podocytes, we show that adriamycin -induced apoptosis is suppressed by the autophagy inducer rapamycin, whereas it is increased by the autophagy inhibitor chloroquine. We have further established an inducible, podocyte-specific, autophagy-deficient mouse model. In this model, adriamycin induces more severe podocyte injury, which is accompanied by glomerulosclerosis and proteinuria. These results suggest that autophagy is an intrinsic cytoprotective mechanism in podocytes in renal diseases.
MATERIALS AND METHODS
Cell Culture
Conditionally immortalized mouse podocytes were originally generated by the laboratory of Dr. Peter Mundel and were cultured as previously described (15). Briefly, the cells were maintained at the permissive condition of 33°C in RPMI 1640 medium with 10% fetal bovine serum (BenchMark), 1% Antibiotic-Antimycotic (GIBCO), and recombinant mouse interferon-γ. To induce differentiation, the cells were transferred to the nonpermissive condition of 37°C in collagen I-coated dishes without interferon-γ. After 7–14 days of differentiation, cells were subjected to experimental treatment.
Apoptosis Assay
Apoptosis was quantified by flow cytometry after cell staining with Annexin V-FITC Apoptosis Detection Kit I (BD PharMingen). Briefly, cells were washed twice with cold PBS and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Then, 5 μl of FITC Annexin V and propidium iodide (PI) were added to 100 μl cell suspension for 15 min in the dark. Following the incubation, the mixture was diluted with 400 μl 1× binding buffer and analyzed by the BD Accuri C6 flow cytometer (BD Biosciences).
Transfection and Detection of GFP-LC3
Cells were seeded at a density of 4 × 105 on glass coverslips in 35-mm dishes and cultured to 70% confluence. Then the green fluorescent protein (GFP)-LC3 plasmid was transfected with FuGENE 6 Transfection Reagent (Promega, Madison, WI) for 36 h according to the manufacturer’s instruction. Then, the GFP-LC3-transfected cells were treated with adriamycin (Sigma-Aldrich, D1515) as indicated. Microphotographs of GFP-LC3 labeled cells were obtained by Zeiss 780 laser confocal fluorescence microscopy. ImageJ software (National Institutes of Health) was used for GFP-LC3 puncta counting.
Generation of Podo-Atg7-KO Animals
All mice in the study were housed and used in the animal facility of Augusta University following a protocol approved by the Institutional Animal Care and Use Committee.
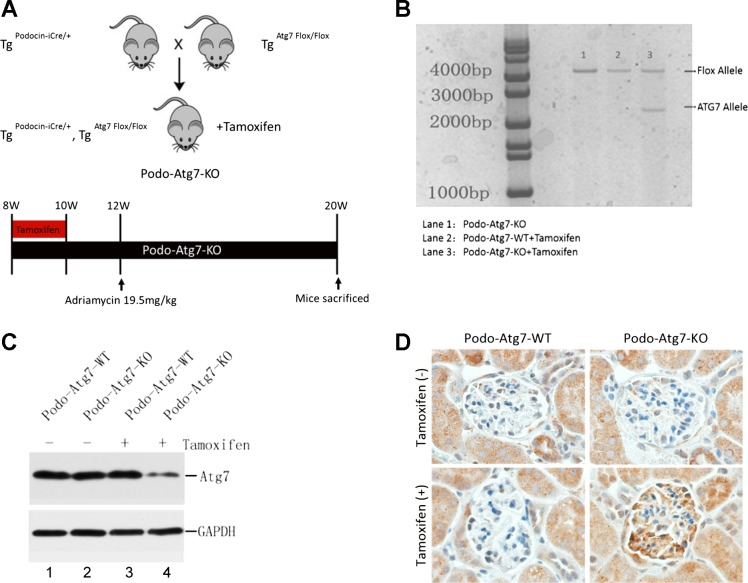
Tamoxifen-inducible podocyte-specific Cre recombinase transgenic mice were kindly provided by Dr. Farhad Danesh (MD Anderson Cancer Center, Houston, TX), whereas Atg7–Floxed mice (Atg7Flox/Flox) were obtained from Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). These two lines of mouse were crossed to generate tamoxifen-inducible podocyte-specific Atg7 knockout mice (Podo-Atg7-KO), and Atg7–Floxed Cre- littermate mice were used as wild-type control (Podo-Atg7-WT). Upon induction, Cre recombinase was driven to express only in podocytes by podocin promoter in Podo-Atg7-KO mice (22), resulting in podocyte specific Atg7 ablation. To induce Cre expression in podocytes for Atg7 ablation, tamoxifen was administered via intraperitoneal injection at a dose of 2 mg/day for 10 days. Genotyping was conducted to verify Atg7 ablation by detecting the presence of a Atg7 deletion band in renal cortex samples. We further analyzed Atg7 expression in isolated glomeruli by immunoblotting. Functionally, we showed the accumulation of P62 in glomeruli in Podo-Atg7-KO mice after tamoxifen induction.
Genotyping of Podo-Atg7-KO Mice
The genotypes of transgenic mice were identified by PCR using DNA extracted from tail and ear biopsies. The presence of podocin-iCre was detected by PCR using the following primers: iCre-Forward: 5′-TCAACATGCTGCACAGGAGAT-3′ and iCre-Reverse: 5′- ACCATAGATCAGGCGGTGGGT-3′. Atg7-floxed allele was detected by PCR-based genotyping using two sets of primers: the first set (Atg7s: 5′- TGGCTGCTACTTCTGCAATGATGT-3′ and 5′-GAATATTCTAATTCAACCAGACCTAGGT-3′) and the second set (5′- GCTGGTTAAAGACTGTCTAATAAAGAGCA-3′ and 5′- CTGCCGCTGAGCCCTGAGAGAGGCCT-3′). Atg7–Floxed allele was detected by the presence of a 500-bp fragment in PCR with the first set of primers, and wild-type allele by the presence of a 600-bp product in PCR with the second set of primers.
Adriamycin Treatment of Mice
The Podo-Atg7-KO model was mainly generated on the background of C57BL/c mice, which are relatively resistant to adriamycin-induced nephropathy. In addition, female mice are more resistant than male mice (10). In pilot studies, we tested different dosages of adriamycin in iPodo-Atg7-WT male mice following tamoxifen induction and observed significant nephropathy in dosages above 19 mg/kg and animal death at dosages exceeding 20 mg/kg in 2–4 wk. Thus the reported results were from the experiments using 19.5 mg/kg adriamycin. Briefly, at 2 wk after tamoxifen induction iPodo-Atg7-WT mice were administered 19.5 mg/kg adriamycin intravenously by Podo-Atg7-KO tail vein injection. Blood and urine sample were collected at 0, 2, 4, and 8 wk later. Mice were euthanized at 8 wk after adriamycin treatment.
Visualizing Autophagy in Autophagy Reporter Mice
Autophagy reporter mice with CAG-red fluorescent protein (RFP)-enhanced GFP (EGFP)-LC3 transgene were injected with 19.5 mg/kg adriamycin and euthanized 2 wk later for perfusion fixation with 4% paraformaldehyde in PBS. Kidney tissues were further fixed with 4% paraformaldehyde overnight and incubated in PBS with 30% sucrose for 24 h. Finally, fixed tissues were embedded in OCT compound and sectioned at 5 μm at −20°C. Following PBS washes, the tissue sections were mounted with ProLong Diamond antifade reagent for examination by Zeiss 780 laser confocal fluorescence microscopy.
Isolation of Glomeruli from Mice for Immunoblotting
Glomeruli were isolated by Iron (II, III) oxide (Fe3O4) perfusion. Mice were anesthetized and perfused with Hanks’ buffer containing 2.5 mg/ml Fe3O4 and 1% BSA. Then kidney cortical tissues were sliced and digested with 150 μg/ml collagenase I for 30 min at 37°C. Digested tissue suspension was passed through 100 μm cell strainer and centrifuged. The precipitate was resuspended in Hanks’ buffer and glomeruli were collected using a magnetic Magna GrIP Rack (EMD Millipore). The purity of glomeruli was confirmed under microscopy.
Immunoblotting Assay
Cell and isolated glomeruli were lysed by 2% SDS buffer containing protease inhibitor cocktail (1:1,000) and nuclease Benzonase (1:500). Protein concentration was measured with the Pierce BCA protein assay kit. Equal amounts of denatured protein were loaded in each lane and separated by 12% SDS-PAGE gel, then transferred to PVDF membrane. After blocking with 5% nonfat milk, blots were incubated with primary antibody diluted in 5% nonfat milk overnight in 4°C and secondary antibody for 1 h in room temperature sequentially. Finally, antigens were detected with chemiluminescent substrate (Thermo Scientific). Primary antibodies used included: anti-LC3B (Novus Biologicals, NBP-30054), anti-p62/SQSTM1 (Abcam, ab56416), anti-Atg7 (Abcam, ab53255), anti-caspase-3 (Cell Signaling, 8G10), anti-phospho-P70S6K (Cell Signaling, 108D2), anti-GAPDH (Sigma-Aldrich, G9545) and anti-β-actin (Sigma-Aldrich, G5441).
Analysis of Blood Urea Nitrogen and Serum Creatinine
Blood urea nitrogen (BUN) and serum creatinine were measured to monitor general renal function with commercial kits from Stanbio Laboratory (Boerne, TX). Briefly, serum was collected from blood samples by coagulation and centrifugation. For BUN, the serum sample was added to the reaction and heated to 100°C for 12 min and then cooled on ice for 3 min to record the absorbance at 520 nm. For serum creatinine, the serum sample was added to prewarmed (37°C) reaction mixture, and the absorbance of 510 nm was recorded, respectively, at 20 and 80 s of reaction. The calculation of BUN and serum creatinine was based on the standard curves.
Urine Analysis
Urinary albumin and creatinine were determined using mouse albumin ELISA kit and creatinine kit from Exocell (Philadelphia, PA). Urinary albumin excretion was expressed as the ratio of urinary albumin over creatinine (UACR). To reveal urinary proteins, 5 μl urine was heated in loading buffer (Bio-Rad, Hercules, CA) and resolved on SDS-PAGE gel, followed by Coomassie blue staining.
Morphological Analysis of Kidney Tissues
Periodic acid-Schiff staining.
Kidney tissues were fixed with 4% paraformaldehyde and then embedded in paraffin and sectioned at 4 μm. Periodic acid-Schiff (PAS) Kit (Sigma-Aldrich, St. Louis, MO) was used for assessment of kidney histology. Images of 20 glomeruli per mouse were taken and PAS-positive (purple) area per glomeruli was quantified by NIH ImageJ software.
Electron microscopy.
Kidneys were perfused and fixed with glutaraldehyde and then cut into ~1-mm3 tissue blocks for epoxy embedding. Transmission electron microscopy was performed by standard procedures.
Immunostaining of kidney tissues.
For immunofluorescence, the paraffin-embedded tissue sections were rehydrated sequentially and antigen retrieved for 1 h with 10 mM EDTA (pH 8.0) buffer, then sections were incubated for 30 min in PBS containing 0.25% Triton X-100. To remove and decrease nonspecific signals, sections were incubated with blocking buffer (2% BSA, 0.2% nonfat milk, 0.8% Triton X-100, and 2% normal goat/donkey serum in PBS) for 1 h. Then the sections were incubated with a primary antibody [anti-synaptopodin (Acris Antibodies, BM5086P) and anti-nephrin (R & D Systems, AF3159)], followed by second antibody. After mounting with mount media (Fisher), slides were examined by laser scanning confocal fluorescence microscopy. The fluorescence signal of 20 glomeruli per mice was quantified by NIH ImageJ software. For immunohistochemistry, the paraffin sections were stained with p62/SQSTM1 (Abcam, ab56416) or WT-1 (Santa Cruz Biotechnology, sc-192). Images were taken by using a Zeiss microscope. The density of podocytes was measured as previously described (21).
Statistics
All data are expressed as means ± SD. Statistical analysis was conducted by ANOVA using GraphPad Prism 6 software. P < 0.05 was considered to reflect significant differences between the groups of comparisons.
RESULTS
Adriamycin Induces Apoptosis in Cultured Podocytes Dose and Time Dependently
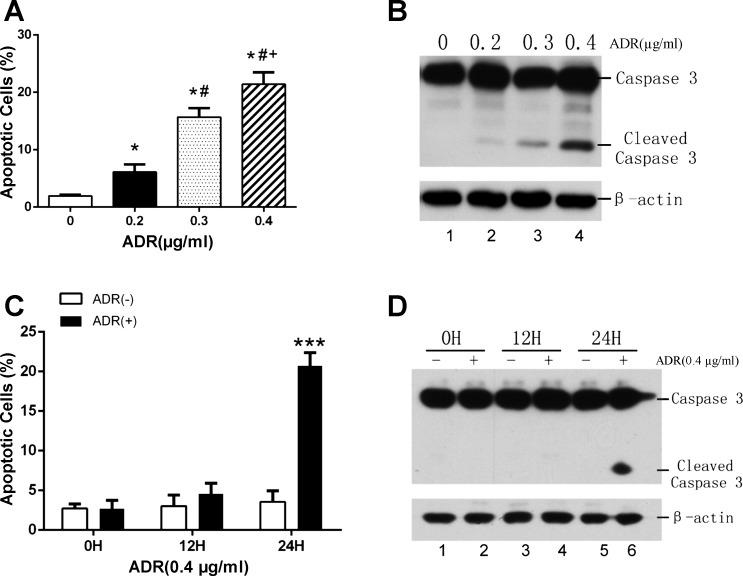
Our initial work characterized podocyte injury induced by adriamycin in podocyte cultures. To this end, podocytes were cultured and differentiated as described previously (15) and then treated with adriamycin (abbreviated as ADR in all figures). Following treatment, the cells were subjected to FITC-Annexin V and PI staining followed by flow cytometry analysis of apoptotic cells that were FITC-Annexin V-positive and PI-negative cells. The analysis showed that adriamycin induced apoptosis in podocytes within 24 h in a dose-dependent manner (Fig. 1A). The control group had 1.94% of apoptosis, which was increased to 6.13%, 15.69%, and 21.41%, respectively, by 0.2, 0.3, and 0.4 μg/ml adriamycin in 24 h (Fig. 1A). Biochemically, we analyzed the proteolytic processing of caspase-3 into active fragments, a hallmark of apoptosis (4). As shown in Fig. 1B, no active or cleaved caspase 3 was detected in control cells, but following 24 h of adriamycin treatment there was a dose-dependent increase in cleaved caspase-3. Apoptosis induction by adriamycin in podocytes was also treatment time dependent. As shown in Fig. 1C, flow cytometry of FITC-Annexin V-positive and PI-negative cells showed that significant apoptosis was induced by 0.4 μg/ml of adriamycin at 24 h, but not at 12 h. Consistently, the treatment of 0.4 μg/ml of adriamycin for 24 h induced a significant increase in active/cleaved caspase-3 in podocytes, whereas treatment for 12 h did not (Fig. 1D). Together, these results indicate that adriamycin can induce apoptosis in cultured podocytes in a dose- and treatment time-dependent manner.
Fig. 1.
Adriamycin induces apoptosis in cultured podocytes. A: podocytes in culture were treated with different concentrations of adriamycin for 24 h. The cells were then labeled with FITC-Annexin V and PI, followed by analysis using a flow cytometer. Compared with control group (0 µg/ml adriamycin), adriamycin induced a dose-dependent increase of apoptosis in podocytes. n = 3 independent experiments. *P < 0.05 vs. 0 µg/ml; #P < 0.005 vs. 0.2 µg/ml; +P < 0.01 vs. 0.3 µg/ml. B: podocytes were treated with adriamycin (ADR) for 24 h at indicated concentrations. Whole cell lysate extracted with 2% SDS buffer was subjected to SDS-PAGE and immunoblotting of caspase 3 and its cleaved active form with β-actin as loading control. n = 3 independent experiments. C: podocytes were treated with 0.4 μg/ml adriamycin for 0, 12, and 24 h. The cells were then labeled with FITC-Annexin V and PI, followed by analysis using a flow cytometer. Adriamycin induced a time-dependent increase of apoptosis in podocytes. n = 3 independent experiments; ***P < 0.005 vs. adriamycin (−) group. D: immunoblot analysis of time-dependent caspase 3 cleavage during adriamycin treatment of cultured podocytes. n = 3 independent experiments.
Autophagy Induction During Adriamycin Treatment of Podocytes
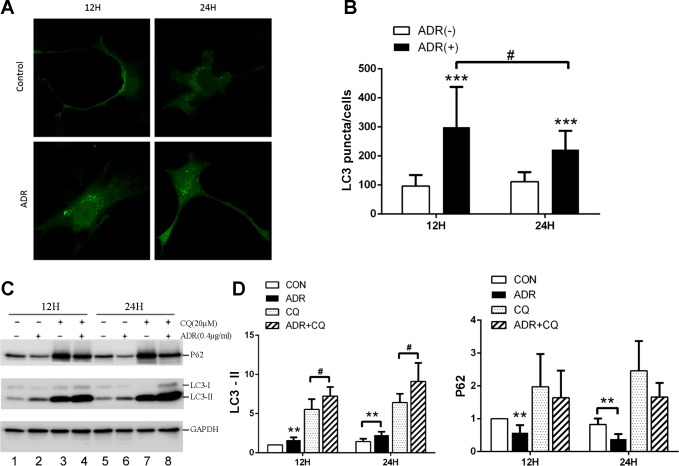
Along with apoptosis, we observed autophagy during adriamycin treatment of cultured podocytes. It is known that a critical event and hallmark of autophagy is the conversion of LC3-I to LC3-II via phosphatidylethanolamine conjugation (8). After the conversion, LC3 becomes associated with both the inner and outer membranes of autophagosome (14). Thus we first analyzed the formation of LC3 puncta to indicate autophagy in GFP-LC3-transfected podocytes. As shown in Fig. 2, compared with control groups, more GFP-LC-3 puncta appeared in the cells treated with adriamycin for both 12 and 24 h. To quantify autophagy, we determined the number of GFP-LC-3 puncta in these cells. About 100 puncta were shown in control cells, whereas 300 and 222 puncta per cell were detected after 12 and 24 h of adriamycin treatment respectively, suggesting autophagy activation (Fig. 2B). To verify the morphological observation, we analyzed LC3-I to LC3-II conversion and the degradation of p62 (an autophagy substrate) by immunoblot analysis. In this experiment, we also included the groups with chloroquine (CQ), an inhibitor of autolysosomal degradation. Representative blots are shown in Fig. 2C. In the absence of CQ, adriamycin treatment increased LC3-II and decreased p62 (Fig. 2C: lanes 2 and 6 vs. lanes 1 and 5), suggesting autophagy activation. The presence of CQ led to the accumulation of LC3-II and p62 in both control (lanes 3 and 7 vs. lanes 1 and 5) and adriamycin-treated cells (lanes 4 and 8 vs. lanes 2 and 6). Notably, adriamycin + CQ groups showed higher LC3-II accumulation than CQ-only groups (lanes 4 and 8 vs. lanes 3 and 7), further verifying the induction of autophagic flux by adriamycin. We further quantified LC3-II and P62 signals by densitometry to verify adriamycin-induced activation of autophagy in this podocyte culture model (Fig. 2D).
Fig. 2.
Autophagy induction during adriamycin treatment of podocytes. A and B: podocytes were transfected with GFP-LC3, and then treated with adriamycin (0.4 μg/ml) for 12 and 24 h for confocal microscopy. Representative images are shown in A. Control cells had a few GFP-LC3 puncta per cell, whereas adriamycin-treated cells showed significantly more GFP-LC3 puncta. GFP-LC3 puncta per cell were counted to have quantitative data (B). ***P < 0.005 vs. respective control group, #P < 0.01 between two adriamycin treatment time points. n = 3 independent experiments, 50 cells evaluated for each group. C, D, and E: podocytes were treated with 0.4 μg/ml adriamycin with and without 20 μM chloroquine (CQ) for 12 and 24 h to collect whole cell lysate for immunoblot analysis of indicated proteins. Representative immunoblots are shown in C along with quantitative data of densitometry in D. LC3-II expression was increased and p62 accumulation was decreased in adriamycin-treated group compared with control group. The addition of chloroquine resulted in further LC3-II accumulation and p62 decrease during adriamycin treatment. n = 6 independent experiments; **P < 0.01 vs. control group, #P < 0.05 vs. adriamycin only group.
Effects of Pharmacological Modulators of Autophagy on Adriamycin-Induced Apoptosis in Podocytes
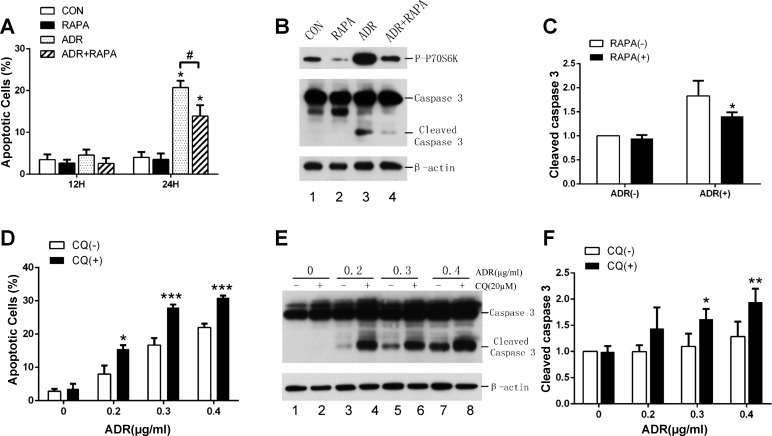
Under cell stress, autophagy may be protective; however, excessive autophagy may induce cell death (11). To determine the role of autophagy during adriamycin treatment of podocytes, we examined the effects of pharmacological modulators of autophagy. We first tested rapamycin, which is known to induce autophagy by inhibiting mammalian target of rapamycin (mTOR) in various cell types. Podocytes in culture were pretreated with rapamycin at 1 nM for 2 h before the addition of 0.4 μg/ml adriamycin for 12 and 24 h. Apoptosis was quantified by flow cytometry after FITC-Annexin V and PI staining. Consisted with the results above, significant apoptosis was not observed until at 24 h of adriamycin treatment. Notably, rapamycin significantly reduced adriamycin-induced apoptosis at 24 h from 20.74% to 13.9% (Fig. 3A). We further analyzed the cleavage or processing of caspase-3 into active forms by immunoblotting. As shown in Fig. 3B, adriamycin treatment for 24 h resulted in the appearance of significant amount of active/cleaved caspase-3, which was largely attenuated by rapamycin (Fig. 3B, lane 3 vs. lane 4). In the same samples, we verified the inhibitory effect of rapamycin on mTOR by analyzing the phosphorylation of P70S6 kinase (P70S6K), a well-documented substrate of mTOR (Fig. 3B). Interestingly, adriamycin induced marked phosphorylation of P70S6K (Fig. 3B, lane 3 vs. lane 1), suggesting mTOR activation during adriamycin treatment of podocytes. Quantification via densitometry of the blots verified that rapamycin could suppress caspase 3 cleavage or activation during adriamycin treatment (Fig. 3B), supporting the cytoprotective effect of rapamycin. We further evaluated the effect of chloroquine (autophagy inhibitor) on adriamycin-induced apoptosis in podocytes. By FITC-Annexin V and PI staining, we showed that apoptosis was induced by adriamycin in a dose-dependent manner and, notably, chloroquine increased apoptosis at each of the adriamycin concentrations tested (Fig. 3D). Consistently, chloroquine increased caspase 3 activation as shown by its cleavage during adriamycin treatment (Fig. 3, E and F). Thus inhibition of autophagy by chloroquine enhanced adriamycin-induced apoptosis in podocytes. Together with the effect of rapamycin, these results support a cytoprotective role of autophagy in podocytes.
Fig. 3.
Effects of pharmacological modulators of autophagy on adriamycin-induced apoptosis in podocytes. A: podocytes were pretreated with 1 nM rapamycin for 2 h and then coincubated with or without 0.4 μg/ml adriamycin for 12 and 24 h. The cells were labeled by FITC-Annexin V and PI for flow cytometry; n = 3 independent experiments, *P < 0.05 vs. control, #P < 0.05 vs. adriamycin only group. B and C: podocytes were treated with 0.4 μg/ml adriamycin at 1 nM with or without rapamycin for 24 h to collect whole cell lysate for immunoblot analysis of indicated proteins. Representative blots are shown in B along with densitometry quantification of cleaved caspase 3 (C). n = 4 independent experiments. *P < 0.05 vs. adriamycin (+) only group. D, E, and F: podocytes were treated with indicated concentrations of adriamycin in the presence or absence of 20 nM chloroquine for 24 h. Apoptotic cells were quantified by FITC-Annexin V and PI labeling followed by flow cytometry (A). n = 3 independent experiments; *P < 0.05, ***P < 0.005 compare with CQ (−) group. Whole cell lysate was analyzed by immunoblot analysis of caspase 3 and the loading control β-actin. Representative blots are shown in E and densitometry quantification data of cleaved caspase 3 in F. *P < 0.05 and **P < 0.01 vs. CQ (−) group.
Autophagy Is Induced in Podocytes During Adriamycin Treatment In Vivo
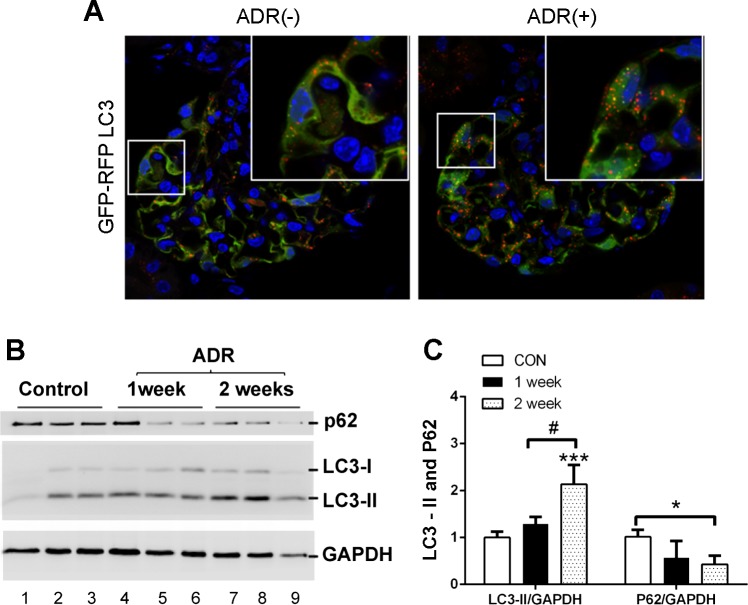
To monitor autophagy in vivo, we used an autophagy reporter mouse model that is transgenic of CAG-RFP-EGFP-LC3 (12, 25). In this model, the RFP-EGFP-LC3 transgene is driven by the CAG promoter to express ubiquitously in various cell types including podocytes. The green fluorescence of EGFP (pKa 5.9) is acid sensitive and thus quenched in acidic environment, whereas the red fluorescence of RFP (pKa 4.5) is stable at low pH. As a result, both red RFP and green EGFP signals show up in autophagosomes with neutral pH, whereas only red RFP signal is detected in autolysosomes with acidic pH. We treated CAG-RFP-EGFP-LC3 mice with adriamycin, or saline as control. As shown in Fig. 4A, RFP-GFP-LC3 puncta were detected in podocytes of control kidney tissues, indicative of a relatively high level of autophagy that was considered critical for the maintenance of podocyte homeostasis and integrity (6). Following adriamycin treatment, there appeared to be more RFP-EGFP-LC3 puncta in podocytes in glomeruli. Consistently, adriamycin induced a marginal increase of LC3-II at day 7, which became more noticeable (2-fold over control) at day 14 (Fig. 4, B and C). Adriamycin also induced the degradation of p62 (Fig. 4, B and C). Collectively, these results indicate that autophagy is induced in podocytes by adriamycin in kidneys.
Fig. 4.
Autophagy is induced in podocyte during adriamycin treatment in vivo. A: CAG-RFP-EGFP-LC3 transgenic mice were injected with 19.5 mg/kg adriamycin (or saline as control) to collect kidney tissues 2 wk later for fluorescence microscopy. The representative images show a significant increase in RFP-EGFP-LC3 green and red puncta in podocytes after adriamycin treatment. n = 3 independent experiments. B and C: glomeruli were isolated from control and adriamycin-treated mice for immunoblot analysis of LC3, p62, and GAPDH as loading control. Representative blots (B) are shown along with densitometry quantification of p62 and LC3-II. n = 3 independent experiments; *P < 0.05; ***P < 0.005 vs. control group, #P < 0.05 vs. 1 wk adriamycin group. The results show LC3-II accumulation and p62 decrease in glomeruli during adriamycin treatment of mice.
Generation of an Inducible Podocyte-Specific Atg7 Knockout Mouse Model
To determine the role of podocyte autophagy in adriamycin-induced nephropathy in vivo, we decided to examine an autophagy-deficient mouse model. Global ablation of autophagy genes leads to embryonic lethality, and constitutive autophagy deficiency in podocytes per se results in nephropathy (6). Therefore, we established a conditional model where autophagy-related gene 7 (Atg7) was specifically ablated in podocytes upon induction. As depicted in Fig. 5A, tamoxifen-inducible podocin-Cre mice (22) were bred with Atg7–Floxed mice to generate inducible podocyte-specific iCre recombinase transgenic mice (Podo-Atg7-KO). We then conducted pilot tests in these mice to titrate the condition of tamoxifen induction and timing of adriamycin treatment (Fig. 5A, bottom panel). To evaluate the efficiency and specificity of this model, kidney samples were obtained 2 wk after tamoxifen injection for analysis. In genotyping, Atg7 deletion allele only appeared in the kidney cortical tissue of tamoxifen-treated Podo-Atg7-KO mice (Fig. 5B). In immunoblots, Atg7 expression was decreased in the glomeruli isolated from tamoxifen-treated Podo-Atg7-KO mice (Fig. 5C, lane 4). Functionally, tamoxifen treatment led to the accumulation of the autophagic substrate p62 in glomeruli (likely podocytes) of Podo-Atg7-KO mice, but not in Podo-Atg7-WT mice (Fig. 5D), further validating this conditional autophagy-deficient mouse model.
Fig. 5.
Generation of an inducible, podocyte-specific Atg7 knockout mouse model. A: breeding scheme and treatment protocol of inducible Podo-Atg7-KO mice. B: representative genotyping results were induced with tamoxifen for 2 wk to collect kidney cortical tissues for genotyping. C: representative immunoblot analysis of Atg7 expression in glomeruli isolated from Podo-Atg7-KO and WT mice with or without tamoxifen induction. D: immunohistochemical staining of p62 in Podo-Atg7-KO and WT mice with or without tamoxifen induction. The results show the ablation of Atg7 from glomeruli (including podocytes) associated with p62 accumulation, indicative of autophagy deficiency.
Adriamycin-Induced Proteinuria Is Aggravated in Podo-Atg7-KO Mice
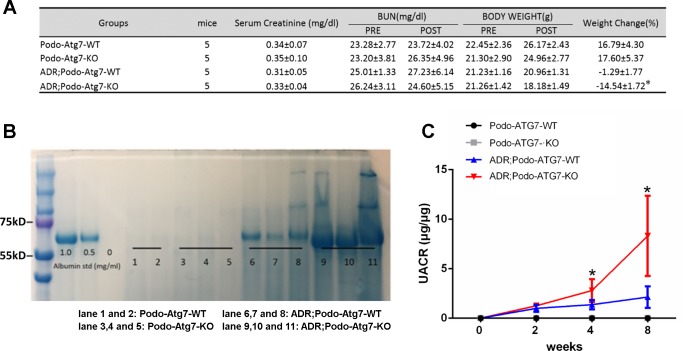
We next used the Podo-Atg7-KO mouse model to determine the role of podocyte autophagy in adriamycin-induced nephropathy. To this end, Podo-Atg7-KO and -WT mice were treated with tamoxifen and then given one injection of adriamycin. As shown in Fig. 6A, Podo-Atg7 WT mice and Podo-Atg7 KO mice showed similarly low levels of serum creatinine and BUN before and after adriamycin treatment. However, Podo-Atg7-KO mice had significantly more severe loss of body weight following adriamycin injection (Fig. 6A), suggesting a decline of general health. To monitor adriamycin-induced podocyte or glomerular dysfunction, we analyzed the leakage of proteins in urine, i.e., proteinuria. As shown in Fig. 6B, adriamycin induced moderate proteinuria in Podo-Atg7-WT mice (lanes 6–8) and the proteinuria in Podo-Atg7-KO mice was much more severe (lanes 9–11). According to molecular weight, most of the proteins leaked into urine were albumin (Fig. 6B). To further confirm the results, urinary albumin-to-creatinine ratio (UACR) was measured by ELISA to indicate urinary albumin excretion. As shown in Fig. 6C, adriamycin induced a time-dependent increase of UACR in Podo-Atg7-WT mice (blue line) and notably, the UACR increase in Podo-Atg7-KO mice was significantly higher (red line). Thus adriamycin induced more severe podocyte dysfunction in autophagy-deficient mice. Together, these data suggest a protective role of autophagy in podocytes during adriamycin nephropathy.
Fig. 6.
Exaggerated albuminuria in Podo-Atg7-KO mice during adriamycin treatment. A: body weight, serum urea nitrogen, and creatinine. n = 5; *P < 0.05 compared with the adriamycin; Podo-Atg7-WT group. B: urine samples were collected for SDS-PAGE followed by Coomassie blue staining. Albumin was loaded to show the main protein in urine samples was albumin. The representative gel staining shows proteinuria is mainly albuminuria. The representative gel shows that adriamycin induced significantly higher albuminuria in Podo-Atg7-KO mice. C: urinary albumin excretion ratio (UACR). The UACR was determined in Podo-Atg7-WT and Podo-Atg7-KO mice treated with adriamycin (19.5 mg/kg) or saline as control. n = 5; *P < 0.05 vs. adriamycin; Podo-Atg7-WT group. The results indicate that adriamycin induced significantly higher urinary albumin excretion in Podo-Atg7-KO mice than Podo-Atg7-WT mice.
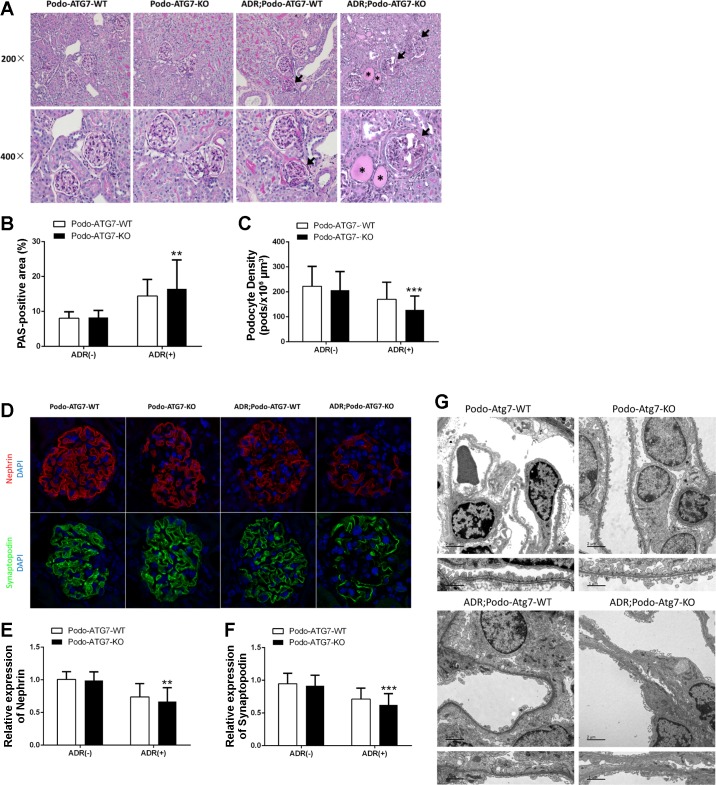
Adriamycin-Induced Podocyte Injury Is Aggravated in Podo-Atg7-KO Mice
The observed proteinuria during adriamycin treatment of Podo-Atg7-KO mice was consistent with the histopathological analysis. In comparison to Podo-Atg7-WT mice, Podo-Atg7-KO mice showed a higher degree of glomerulosclerosis after adriamycin treatment, which was associated with a decreased podocyte density (Fig. 7, A–C). In addition, whereas adriamycin decreased the expression of podocyte marker proteins (e.g., nephrin and synaptopodin) in both Podo-Atg7-WT and -KO mice, the decrease was more dramatic in Podo-Atg7-KO mice (Fig. 7, D–F). Finally, we examined ultrastructural changes of podocytes by electron microscopy. Without treatment, podocytes appeared healthy with well-organized foot processes in both Podo-Atg7-WT and -KO mice. Following adriamycin treatment, podocytes were disorganized with obvious loss and effacement of foot processes in both Podo-Atg7-WT and -KO mice, but the pathological changes appeared more severe in Podo-Atg7-KO mice.
Fig. 7.
Adriamycin-induced podocyte injury is aggravated in Podo-Atg7-KO mice. Following tamoxifen induction, Podo-Atg7-WT and Podo-Atg7-KO mice were injected with 19.5 mg/kg adriamycin (or saline as control) to collect kidney tissues for analysis 8 wk later. A: representative images of periodic acid-Schiff (PAS) staining showing glomerulosclerosis (arrows) and tubular protein cast (asterisks). Original magnification, ×200 in top row, ×400 in bottom row. B: quantification of PAS-positive area. n = 5 mice in each condition; 20 glomeruli were evaluated in each mouse; **P < 0.01 vs. adriamycin; Podo-Atg7-WT group. C: quantification of the density of podocytes. n = 5 mice in each condition; 20 glomeruli were evaluated in each mouse; ***P < 0.005 vs. adriamycin; Podo-Atg7-WT group. D: representative images of immunofluorescence staining of nephrin (red) and synaptopodin (green) showing the loss of these podocyte marker proteins during adriamycin treatment, especially in Podo-Atg7-KO mice. Nuclei were labeled by DAPI (blue). E and F: quantification of nephrin and synaptopodin expression. The immunofluorescence signal of adriamycin-treated mice glomeruli was normalized with that of control [adriamycin (−)] mice, which was arbitrarily set as 1. n = 5 mice in each condition; 20 glomeruli were evaluated in each mouse; **P < 0.01, ***P < 0.005 vs. adriamycin; Podo-Atg7-WT group. G: representative EM micrographs of kidney tissues from Podo-Atg7-WT and Podo-Atg7-KO mice showing adriamycin-induced podocyte disruption including foot process effacement, especially in Podo-Atg7-KO tissues. Scale bars: 2 μm (top), 1 μm (bottom).
DISCUSSION
Autophagy plays a critical role in supporting podocytes, which are known to be highly differentiated and long-lived in kidneys. In this regard, ablation of autophagy gene Atg5 in podocytes leads to podocyte injury, glomerular pathologies, and albuminuria at 8–12 mo of age in mice (6). In the present study, we have determined the effect of autophagy deficiency in podocytes by timed ablation of Atg7 in adult mice. Our results show that, without insults, these mice did not have noticeable pathologies in podocytes or glomeruli and defects in renal function for at least 2 mo. Our observation is consistent with previous work (6) that the effect of an autophagy defect is gradual and accumulating, and requires time to reveal under unchallenged, physiological conditions.
Although the ablation of Atg7 from podocytes did not induce significant phenotypes under physiological conditions, it exaggerated adriamycin-induced podocyte injury that was associated with glomerular pathologies and proteinuria in our study (Figs. 6 and 7). Indeed, both biochemical analysis and autophagy reporter mice showed the activation of autophagy in podocytes by adriamycin in mice (Fig. 4). In our study, significant autophagy activation was detected after 2 wk of adriamycin treatment (Fig. 4), whereas nephropathy occurred much later (Figs. 6 and 7), suggesting that autophagy activation in this model is not secondary to nephropathy. Autophagy was also induced in the in vitro model of cultured podocytes (Fig. 2). The temporal relationship between autophagy and apoptosis during adriamycin treatment of cultured podocytes is noteworthy. We detected the increase of GFP-LC3 puncta or autophagic vesicles starting from 6 h of 0.4 μg/ml adriamycin treatment (data not shown) and peaking at 12 h (Fig. 2). In addition, the accumulation of LC3-II was observed by immunoblot analysis at 12 h of adriamycin treatment (Fig. 2). However, significant apoptosis shown by the appearance of apoptotic morphology and cleaved/active caspase-3 expression was detected only after 24 h of adriamycin treatment (Fig. 1). Thus autophagy is induced before apoptosis in this model, an observation that is consistent with the idea that autophagy is induced at the early stage of cell stress for protection and, when the injury or stress is prolonged, the protective effect of autophagy is overwhelmed with ensuing cell death. Such a notion is further supported by the effects of autophagy modulators. We showed that rapamycin, a known inducer of autophagy, reduced podocyte apoptosis during adriamycin treatment, whereas the autophagy inhibitor chloroquine significantly enhanced adriamycin-induced apoptosis (Fig. 3). Of note, in addition to the effect on autophagy, chloroquine may have other effects. Nonetheless, we further examined the effect of autophagy blockade in a conditional Atg7 gene knockout model. We showed that autophagy deficiency in podocytes by ablating Atg7 during adriamycin treatment significantly sensitized mice to podocyte injury, glomerulosclerosis, and proteinuria or albuminuria (Figs. 6 and 7). These effects were initiated in podocytes as shown by podocyte-specific autophagy deficiency in this inducible, conditional knockout model (Fig. 5). Moreover, in this model we detected the decrease of podocyte proteins, reduction of podocyte number, and structural damage of existing podocytes, such as effacement of foot processes (Fig. 7). Together, these results support a crucial role of autophagy in protecting podocytes under pathological conditions.
In a rat model of puromycin aminonucleoside-induced glomerular disease, Liu and colleagues (28) suggested a protective role of autophagy by showing the effects of pharmacological modulators of autophagy. In the present study, we examined the mouse model of adriamycin-induced nephropathy. Moreover, in addition to pharmacological modulators of autophagy, we have established the inducible, conditional Atg7 knockout model that allowed us to block autophagy specifically in podocytes in adult mice. The use of both pharmacological inhibitors and genetic models demonstrates convincing evidence for the involvement of autophagy in the pathogenesis of podocytopathy or glomerular diseases.
Despite the demonstration of the protective role of autophagy in podocytes, it remains unclear how autophagy protects. Nonetheless, several possibilities exist. First, autophagy removes obsolete or dysfunctional organelles (e.g., damaged mitochondria) that are expected to accumulate in podocytes under pathological conditions of kidneys (20). By clearing these potentially toxic organelles, autophagy may help podocytes cope with the stress. Second, autophagy degrades protein aggregates and misfolded proteins, which would reduce endoplasmic reticulum stress and associated apoptosis in podocytes. In this regard, it has been reported that inhibition of autophagy may induce podocyte apoptosis by activating the proapoptotic pathway of endoplasmic reticulum stress (1, 2). In addition, autophagy and apoptosis share several critical proteins and as a result, the activation of autophagy may deploy these proteins for autophagy and deprive them from apoptosis. A good example in this regard is Beclin 1, which is crucial in the assembly of autophagic protein complex on the one hand but an apoptosis regulator by associating and antagonizing Bcl-2 on the other hand. The activation of autophagy would therefore lead to the dissociation of Beclin 1 from Bcl-2 to release this powerful anti-apoptotic protein for cytoprotection (23). Future investigation needs to delineate these mechanisms to understand how autophagy protects podocytes under physiological and pathological conditions.
In cultured podocytes, adriamycin induced a notable phosphorylation of P70S6K, a well-documented substrate and hallmark of mTOR activation (Fig. 3). mTOR is a major negative regulatory of autophagy (26). It is known that there are two distinctive mTOR complexes, mTORC1 and mTORC2, and mTORC1 is involved in autophagy regulation (9). Upon activation, mTORC1 phosphorylates ULK1 (and probably other key components) to prevent the formation of the autophagy-initiating complex (7). Thus it is perplexing how autophagy is activated during adriamycin treatment of podocytes in the presence of mTOR activation. One possible explanation is the presence of mTOR-autophagy spatial coupling compartment (TASCC), which involves a spatiotemporal arrangement of mTOR and autophagy in distinct cellular compartments (16, 27). The sequestration of mTOR from autophagy-initiating complex and autophagosomes would facilitate autophagy activation even in the presence of mTOR activation.
In cultured podocytes, rapamycin could significantly reduce adriamycin-induced apoptosis (Fig. 3). However, Guzman et al. (5) showed that rapamycin worsened adriamycin-induced nephropathy in mice. The discrepancy between these studies is likely related to the model differences and the multiple effects of rapamycin. Obviously, the in vivo situation is much more complex and rapamycin may affect not only autophagy in podocytes, but also inflammation, vasculature, and the regeneration and repair in various kidney cell types (3). As such, it is critically important to develop and test specific autophagy inducers for the protection of podocytes and other kidney cells for the therapy of chronic kidney diseases including glomerulosclerosis.
GRANTS
The study was supported in part by grants from National Natural Science Foundation of China (81528004 and 81570622), the Mason Trust Foundation, the National Institute of Diabetes and Digestive and Kidney Diseases (DK-058831 and DK-087843), and the Department of Veterans Affairs (5I01BX000319).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Y., J.-K.C., and Z.D. conceived and designed research; M.Y., L.Z., Y.L., M.J.L., and Z.D. performed experiments; M.Y. and L.Z. analyzed data; M.Y., L.Z., Y.L., M.J.L., J.-K.C., N.S.N., F.L., and Z.D. interpreted results of experiments; M.Y. and Y.L. prepared figures; M.Y. drafted manuscript; M.Y., L.Z., Y.L., J.-K.C., N.S.N., F.L., and Z.D. approved final version of manuscript; F.L. and Z.D. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Farhad Danesh at The University of Texas MD Anderson Cancer Center (Houston, TX) for providing the tamoxifen-inducible podocyte-specific Cre recombinase transgenic mouse line, Dr. Masaaki Komatsu at Tokyo Metropolitan Institute of Medical Science (Tokyo, Japan) for providing the Atg7-floxed mouse line, and Dr. Joseph Hill at The University of Texas Southwest Medical Center for providing the CAG-RFP-GFP-LC3 autophagy reporter mouse line.
REFERENCES
- 1.Cheng YC, Chang JM, Chen CA, Chen HC. Autophagy modulates endoplasmic reticulum stress-induced cell death in podocytes: a protective role. Exp Biol Med (Maywood) 240: 467–476, 2015. doi: 10.1177/1535370214553772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang L, Li X, Luo Y, He W, Dai C, Yang J. Autophagy inhibition induces podocyte apoptosis by activating the pro-apoptotic pathway of endoplasmic reticulum stress. Exp Cell Res 322: 290–301, 2014. doi: 10.1016/j.yexcr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol 11: 34–45, 2015. doi: 10.1038/nrneph.2014.201. [DOI] [PubMed] [Google Scholar]
- 4.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: mutations within caspase genes. J Med Genet 46: 497–510, 2009. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 5.Guzman J, Jauregui AN, Merscher-Gomez S, Maiguel D, Muresan C, Mitrofanova A, Diez-Sampedro A, Szust J, Yoo TH, Villarreal R, Pedigo C, Molano RD, Johnson K, Kahn B, Hartleben B, Huber TB, Saha J, Burke GW III, Abel ED, Brosius FC, Fornoni A. Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes 63: 701–714, 2014. doi: 10.2337/db13-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoki K. mTOR signaling in autophagy regulation in the kidney. Semin Nephrol 34: 2–8, 2014. doi: 10.1016/j.semnephrol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky DJ. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 11.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12: 976–998, 2016. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 16.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, Tavaré S, Inoki K, Shimizu S, Narita M. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332: 966–970, 2011. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsumi Y. Historical landmarks of autophagy research. Cell Res 24: 9–23, 2014. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 19.Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwara N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes 65: 755–767, 2016. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 20.Tang C, He L, Liu J, Dong Z. Mitophagy: basic mechanism and potential role in kidney diseases. Kidney Dis (Basel) 1: 71–79, 2015. doi: 10.1159/000381510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014. doi: 10.1681/ASN.2013080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Wang Y, Long J, Chang BH, Wilson MH, Overbeek P, Danesh FR. Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis 48: 446–451, 2010. doi: 10.1002/dvg.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 4: 949–951, 2008. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 25.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 129: 1139–1151, 2014. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822, 2010. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young AR, Narita M, Narita M. Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy 7: 1387–1388, 2011. doi: 10.4161/auto.7.11.17348. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong Y, Zhang C, Zen K, Liu Z. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J Pathol 234: 203–213, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Livingston MJ, Chen JK, Dong Z. Autophagy in podocytes. Contrib Nephrol 183: 83–100, 2014. doi: 10.1159/000360015. [DOI] [Google Scholar]