Abstract
Adult rats exposed to maternal separation (MatSep) are normotensive but display lower glomerular filtration rate and increased renal neuroadrenergic drive. The aim of this study was to determine the renal α-adrenergic receptor density and the renal vascular responsiveness to adrenergic stimulation in male rats exposed to MatSep. In addition, baroreflex sensitivity was assessed to determine a component of neural control of the vasculature. Using tissue collected from 4-mo-old MatSep and control rats, α1-adrenergic receptors (α1-ARs) were measured in renal cortex and isolated renal vasculature using receptor binding assay, and the α-AR subtype gene expression was determined by RT-PCR. Renal cortical α1-AR density was similar between MatSep and control tissues (Bmax = 44 ± 1 vs. 42 ± 2 fmol/mg protein, respectively); however, MatSep reduced α1-AR density in renal vasculature (Bmax = 47 ± 4 vs. 62 ± 4 fmol/mg protein, P < 0.05, respectively). In a separate group of rats, the pressor, bradycardic, and renal vascular constrictor responses to acute norepinephrine injection (NE, 0.03–0.25 μg/μl) were determined under anesthesia. Attenuated NE-induced renal vasoconstriction was observed in rats exposed to MatSep compared with control (P < 0.05). A third group of rats was infused at steady state with the α1 agonist phenylephrine (10 μg/min iv) and vasodilator sodium nitroprusside (5 μg/min iv). The difference between the change in heart rate/mean arterial pressure slopes was indicative of reduced baroreflex sensitivity in MatSep vs. control rats (−0.45 ± 0.04 vs. −0.95 ± 0.07 beats·min−1·mmHg−1, P < 0.05). These data support the notion that reduced α-adrenergic receptor expression and function in the renal vasculature could develop secondary to MatSep-induced overactivation of the renal neuroadrenergic tone.
Keywords: maternal separation, α-adrenergic receptors, binding receptor assay, arterial baroreceptor-heart rate reflex
cardiovascular disease (CVD) affects over 70 million people in the United States (26, 41). Elucidating the developmental origins of adult disease has become a strategy to prevent and/or delay the development of CVD (12, 18, 39, 55). As such, exposure to early life stress (ELS) has been identified as an independent risk factor for CVD later in life (8, 9, 44, 57). It has been consistently reported that children raised under stressful conditions show elevated blood pressure over time and enhanced vascular reactivity to stress (4, 8, 9, 29, 60). Recent findings from the Georgia Stress and Heart Study showed that individuals exposed to ELS display greater blood pressure in early adulthood compared with control subjects (58). However, in what fashion ELS “primes” the cardiovascular risk remains unclear.
In rodents, maternal separation (MatSep) is a chronic behavioral stress model that has been shown to mimic the behavioral, neuroendocrine, and cardiovascular effects of ELS in humans (32, 35, 37, 47, 49, 61). While numerous experimental studies conducted in normal adult rats (not exposed to MatSep) have provided insights on the mechanisms by which chronic stress exacerbates blood pressure responses (7, 28, 46), a handful of reports have investigated the long-term effects of chronic stress during postnatal life on blood pressure control (34, 49, 61). Particularly, postnatal MatSep increased heart rate (HR) in adult borderline hypertensive rats (49) and reduced glomerular filtration rate (GFR) in adult normotensive Wistar-Kyoto rats by exaggerating the renal neuroadrenergic drive (37). Overall, MatSep induced impaired cardiac function, exacerbated locomotor activity, brain neuronal activation and HR responses to restraint stress, and resulted in a greater blood pressure reduction in response to ganglionic blockade (34, 49, 61, 62). These data support the notion that alterations in autonomic function at various levels are connected to mechanisms by which MatSep causes dysfunctional control of acute and chronic changes on blood pressure.
The neural control of the cardiovascular system is accomplished through the autonomic nervous system. Particularly, the sympathetic nervous system (SNS) branch plays an important role in the acute regulation of blood pressure (17, 23, 38, 66). However, when chronically stimulated, it compromises the cardiovascular function by eliciting end-organ damage (50, 53). The kidney is an essential organ associated with blood pressure regulation and subjected to the influence of the SNS. The efferent sympathetic fibers innervate all essential parts of the kidney, including arterioles and renal tubules (22). Thus increased neuroadrenergic activity is associated with high blood pressure and renal dysfunction in patients with essential hypertension (11, 52).
It is well known that short-term regulation of blood pressure through baroreflex mechanisms is especially relevant in response to transient changes in arterial pressure (15, 42). A critical controller of sympathetic outflow is localized around high pressure arterial baroreceptor sensors (5, 27, 42). Several studies document that baroreflex control of HR is diminished in most forms of experimental hypertension (15, 33, 67). This impairment usually occurs at different levels: secondary to dysfunction in the afferent sensing of arterial pressure, altered central processing, and/or changes in efferent effector mechanisms (27, 31, 40).
The present study tested the hypothesis that MatSep during postnatal life induces a long-term dysfunction of the neural mechanisms implicated in blood pressure regulation. Therefore, we determined the α1-adrenergic receptor (ARs) density and the α1-AR subtype mRNA expression in the renal cortex and vasculature in vitro. We also measured systemic resistance and renovascular blood flow responses to acute α-AR stimulation [norepinephrine (NE)] in vivo. Finally, to assess the effects of MatSep on regulation of sympathetic outflow, baroreceptor-HR reflex sensitivity was determined as a potential factor underlying exaggerated SNS activation.
METHODS
MatSep protocol.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the University of Kentucky Institutional Animal Care and Use Committee. In a Wistar-Kyoto in-house breeding colony, approximately one-half of the male pups (MatSep group) were removed from their mother’s cage from postnatal days 2 to 14 of life at the same time of day by transferring the pups to a clean cage in an incubator (30 ± 1°C) for 3 h (36). Nonhandled littermates that remained with their mother at all times served as the control group. Each dataset was composed of rats from at least three different litters. Weaning was performed at postnatal day 28, and experiments were conducted in 16-wk-old control or MatSep male rats.
Membrane preparations.
Renal tissue was collected under anesthesia (80 mg/kg ketamine + 10 mg/kg xylazine) from rats that were not exposed to prior surgical procedures. After removal of the renal capsule, the renal cortex was dissected from the renal medulla (n = 6 control and n = 8 MatSep). For the renal vasculature studies, vessels from a different set of kidneys were pooled using eight rats per curve (n = 4/group) using the sieving method. Briefly, kidneys were removed under anesthesia and immediately placed in a petri dish with cold physiological saline solution. The renal capsule was removed, and a 4.7-cm-diameter 100-μM pore mesh screen was folded around the kidney (Biodesign, Carmel, NY). Next, a gentle grating of renal tissue in cold physiological saline solution was performed under the microscope. The kidney vessels were subsequently frozen in liquid nitrogen and stored at −80°C. Cortex and renal vasculature were snap-frozen and stored at −80°C. Frozen tissues were homogenized in buffer (50 mM Tris, 5 mM EDTA, 250 mM sucrose, and 15 μM PMSF, pH = 7.4) and centrifuged for 30 min, 4°C, 1,000 g (16). Next, the supernatant obtained was ultracentrifuged for 45 min, 4°C, 45,000 revolutions/min using a TLA 120.2 rotor (Beckman Coulter, Brea, CA). Protein concentrations were determined by Coomassie blue reaction. Renal cortical tissue saturation curves were generated from each membrane preparation (80 μg protein). Renal vasculature was pooled from eight rats to achieve 50 μg of protein/curve.
Radioligand binding studies for α-AR.
Under standard assay conditions, each sample was brought to a final volume of 1 ml, consisting of 100 μl of membrane preparation in 900 μl of binding buffer (20 mM Tris, 100 mM NaCl, 20 mM MgCl2, 3 mM EDTA, and 100 μM PMSF, pH = 7.4), or 800 μl of binding buffer plus 100 μl phentolamine (500 μM), and 100 μl of radioligand [3H]prazosin 0.125, 1.25, 2.5, 5, 10, and 20 nM solutions (6, 16, 30). The mixture was incubated at room temperature for 90 min in an orbital shaker. The reaction was stopped using a harvester apparatus (Brandel, Gaithersburg, MD). Under low negative pressure, the bound and free radioligands were separated with a Whatman GF/C glass-fiber filter (Brandel) previously treated with 0.15% polyethylenimine (Sigma-Aldrich). The incubation tubes were immediately rinsed three times with 3 ml of ice-cold binding buffer and poured over the same GF/C filters to wash away any unbound radioactivity. The filters were subsequently collected in polypropylene scintillation vials, and radioactive decay was measured in a Tri-Carb 2300 TR liquid scintillation counter (Packard Instrument, Meriden, CT) standard scintillation fluid (Optiphase, Highphase 2; PerkinElmer, Waltham, MA). Specific binding was defined as the difference between the amounts of radioligand bound in the absence (total binding) and presence (nonspecific binding) of phentolamine. The Bmax and Kd were calculated from Scatchard data plots and analyzed by nonlinear regression (Graph Pad Prism 6 for Mac, version 6.0; Graph Pad Software, La Jolla, CA).
Real-time quantitative PCR.
Total RNA was extracted from homogenized rat renal cortical (n = 6) and vascular (n = 8) tissue using Purezol in conjunction with the Aurum Total RNA Mini kit (Bio-Rad, Berkeley, CA). The Script cDNA Synthesis Kit (Bio-Rad) was used for reverse transcription. Primers were designed for each gene of interest to produce an amplicon of <100 bp (IDT, Coralville, IA) as follows: α1A forward = 5′-TCTCGAGAGAAGAAAGCTGCC-3′, reverse = 5′-AAAGACCCAATGGGCATCAC-3′; α1B forward = 5′-TTGGCTCCCTGTTCTCCAC-3′, reverse = 5′-AAGTAGCCCAGCCAGAACAC-3′; α1D forward = 5′-TGCCTCTGGGCTCTCTGTT-3′, reverse = 5′-TGAAGTAGCCCAGCCAGAAG-3′; and GAPDH forward = 5′-TCTCTGCTCCTCCCTGTTCT-3′, reverse = 5′-TACGGCCAAATCCGTTCACA-3′. Primer quality control was run on each primer set with inclusion criteria, including a minimum of 90% efficiency, production of a single melting peak, and the determination of optimum primer concentration. The mRNA levels were measured by real-time quantitative PCR using I-Taq Universal Sybr Green (Bio-Rad). All samples were analyzed using the ΔΔCT method and standardized to Gapdh expression.
Acute dose-response to NE.
Rats (320–335 g, n = 4 control, n = 5 MatSep) were anesthetized with isoflurane (1–4% in O2, inhaled-nose cone). Briefly, a femoral vein and femoral artery catheter were placed for infusion administration and hemodynamic monitoring, respectively. Body temperature was monitored continuously throughout the experiment using a rectal probe and controlled using a thermoregulated heating pad. In addition, the left kidney was exposed, and the renal artery was isolated from the vein by dissection. A flow probe was placed around the artery, using a micromanipulator to stabilize the flow probe position on the artery (Transonic, Ithaca, NY) for renal blood flow (RBF) measurement. Acoustic gel was placed around the probe to maximize the ultrasonic signal. After surgical preparation, rats were infused with saline (0.9% NaCl, 3 ml/h) and allowed to equilibrate (40 min) to obtain baseline measurements. Changes in mean arterial pressure (MAP), HR, and RBF were measured in response to acute doses of NE delivered via the femoral vein. A randomized dose-response to NE (0.03–0.25 μg/μl; Sigma Aldrich, St. Louis, MO) was performed allowing measured parameters to return to the baseline MAP and HR levels before administering the subsequent drug bolus. Data were calculated before and after the NE administration for every 10 mmHg MAP change using an averaging window of ~30 data points every 0.1 s. Renal vascular resistance (RVR) was calculated as the MAP-to-RBF ratio.
Blood pressure, heart rate measurement in steady state.
In a separate group (n = 5 each group), rats were anesthetized with isoflurane and instrumented with femoral vein and artery catheters as described above. Following an equilibration period (40 min), rats were infused to achieve steady state with phenylephrine (PE, 10 μg/min; Sigma-Aldrich) at a rate of 150 μl/min for 2 min. After a 30-min recovery period, a 5-min average baseline measurement was performed followed by a blood pressure and HR response to sodium nitroprusside (SNP) infusion by a continuous infusion at 5 μg/min (Sigma-Aldrich). Changes in HR from baseline were measured at each progressive 10-mmHg increment of blood pressure (Power Laboratory Instruments; ADI, Colorado Springs, CO). The sensitivity of the baroreflex control of HR was determined by linear regression analysis between ΔMAP/ΔHR (Graph Pad Prism 6 for Mac).
Statistical analysis.
Data are reported as means ± SE. For the α1-AR binding assays, Bmax and Kd were calculated by nonlinear regression, assuming one binding site and the least-square fitting method (Prism, version 6.0e, La Jolla, CA). RT-PCR data for adrenergic subtypes were analyzed by a Student’s t-test. To determine the effect of MatSep on MAP, HR, RBF, and RVR data were analyzed using a repeated-measures ANOVA test, with post hoc test corrected for multiple comparisons. The criterion for significance was P < 0.05.
RESULTS
Renal cortex and vasculature α1-AR density.
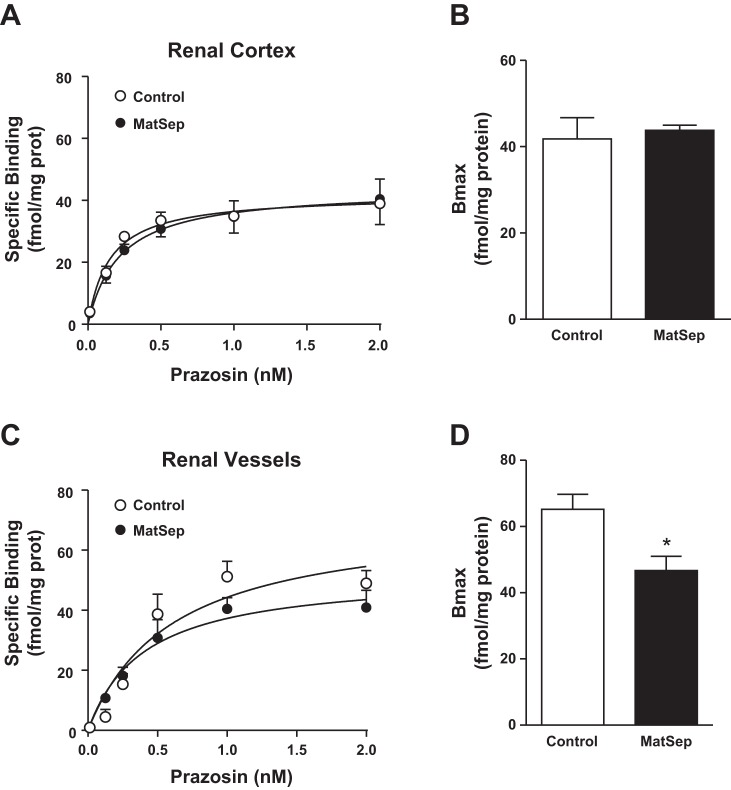
The α1-AR saturation curves in cortical tissue were not different between control and MatSep rats (Fig. 1A), showing similar Bmax and Kd in both groups. However, the α1-AR saturation curves in pooled renal vascular tissue showed lower α1-AR density in tissue from MatSep rats (P < 0.05 vs. control; Fig. 1B). The Kd values were not different between MatSep and control rats in renal cortex (0.15 ± 0.03 and 0.21 ± 0.02 nM, respectively) and renal vasculature (0.57 ± 0.12 and 0.35 ± 0.08 nM, respectively).
Fig. 1.
Saturation curves to α1-adrenergic receptors (α1-ARs) in renal tissue were similar between control and rats exposed to maternal separation (MatSep). A: renal cortical tissue specific binding. B: renal cortical tissue Bmax. C: isolated renal vascular tissue specific binding. D: isolated renal vascular tissue Bmax. Renal cortex: n = 6 control, n = 8 MatSep. Renal vasculature: n = 4/group (pooled samples from 8 rats each).
Renal mRNA expression of α1-AR subtypes.
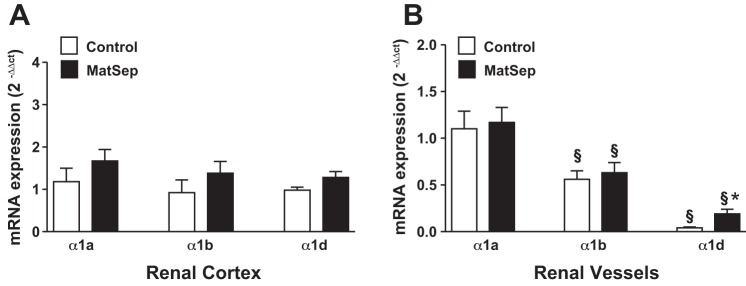
The AR subtype α1A, α1B, and α1D mRNA levels showed no significant effect of MatSep in cortical tissue (Fig. 2A). In isolated renal vasculature, α1A-B subtypes also were similar between groups (P dose <0.05; Fig. 2B); however, the α1D receptor subtype was significantly increased in tissue from MatSep rats (P < 0.05 vs. control).
Fig. 2.
Renal tissue α1-AR subtype mRNA expression. A: renal cortical tissue. B: isolated renal vascular tissue. P < 0.05 vs. other subtypes (§) and control (*).
Renal vascular responsiveness to NE.
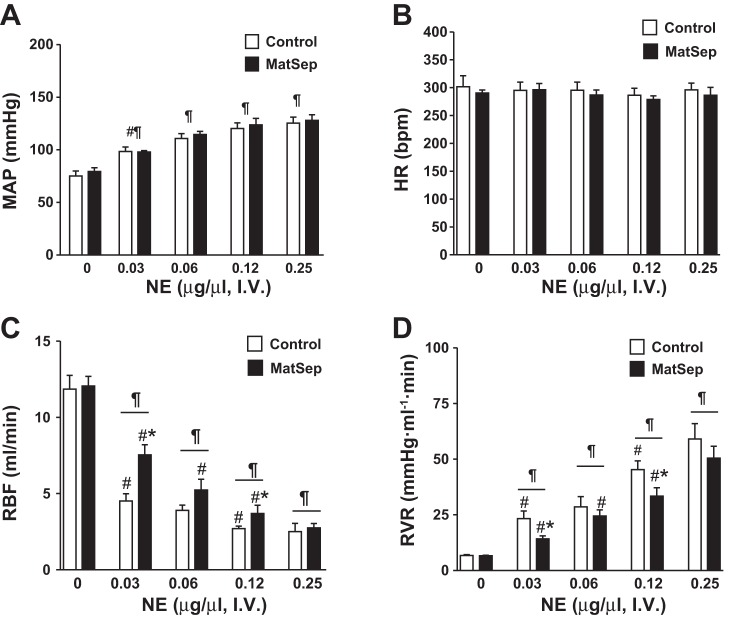
In a separate group of rats, surgical preparation was performed to measure hemodynamic parameters. No significant differences in baseline MAP, HR, RBF, or RVR were observed during this experiment (see dose = 0 µg/µl in Fig. 3). Bolus of NE increased MAP in response to 0.03–0.25 µg/µl NE doses from baseline (Fig. 3A). No changes in HR were significant among the groups (Fig. 3B). The NE-induced reduction in RBF was significantly greater in both groups in response to 0.03 and 0.12 µg/µl NE doses; however, this response was significantly attenuated in MatSep rats compared with control rats (Fig. 3C). Consequently, the NE-induced increase in RVR was reduced in MatSep rats compared with control rats (Fig. 3D). Figure 4 shows the changes in RBF from baseline after NE-induced constriction, exhibiting a significant MatSep effect at 0.03 and 0.12 µg/µl NE doses. A similar response was observed using a 0.06 µg/µl NE dose; however, this response did not reach statistical significance.
Fig. 3.
Acute dose response to norepinephrine (NE) in anesthetized control and MatSep rats. A: MatSep did not affect NE-induced increases in mean arterial pressure (MAP). B: no dose-dependent changes in HR were found between groups. C: renal blood flow (RBF) response was significantly attenuated in MatSep rats. D: calculated renal vascular resistance (RVR) was reduced in MatSep rats. n = 4 Control and 5 MatSep. P < 0.05 vs. baseline (¶), previous dose (#), and control same dose (*).
Fig. 4.
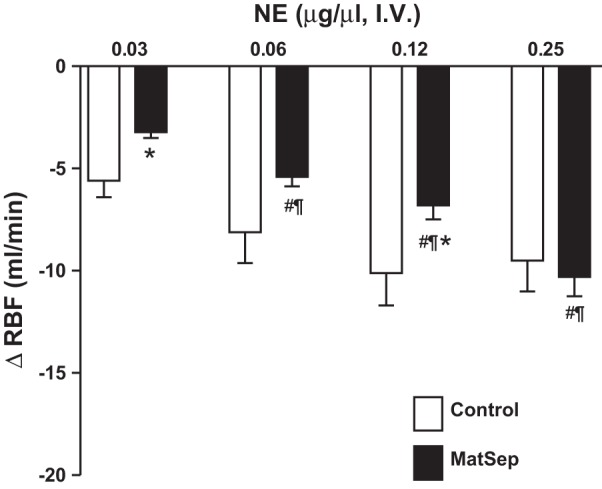
Changes from baseline RBF in response to an acute dose-response to NE in anesthetized control and MatSep rats; n = 5. P < 0.05 vs. baseline (¶), previous dose (#), and control same dose (*).
Baroreflex sensitivity assessment.
When each dose was compared between groups, PE- and SNP-induced reflex changes in HR were not significant between MatSep and control rats (Fig. 5). However, rats exposed to MatSep displayed impaired baroreflex sensitivity, since the PE- and SNP-induced reflex changes in the ΔHR/ΔMAP slope were decreased compared with control rats (−0.45 ± 0.04 vs. −0.95 ± 0.07 beats·min−1·mmHg−1, respectively, F = 33.11, degrees of freedom in the numerator = 1, degrees of freedom in the denominator = 18, P < 0.05).
Fig. 5.
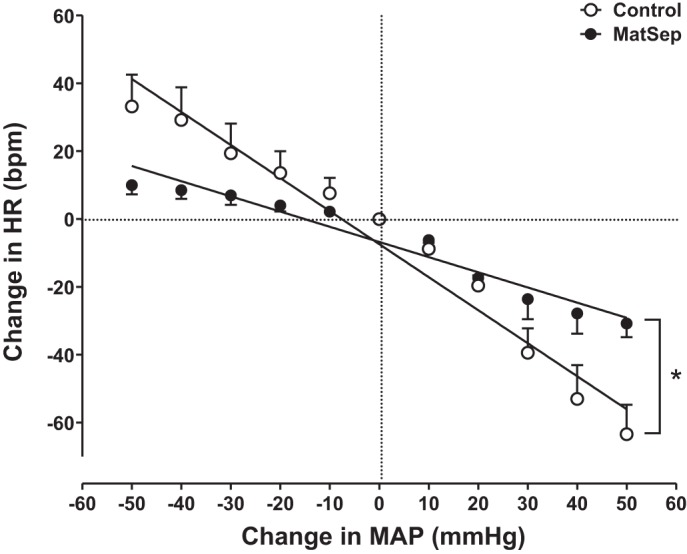
MatSep reduces the baroreflex sensitivity in response to a steady-state infusion (phenylephrine and sodium nitroprusside) in male rats under anesthesia; n = 5, F = 33.11, degrees of freedom in the numerator = 1, degrees of freedom in the denominator = 18, P < 0.05.
DISCUSSION
This study demonstrates that adult normotensive male rats exposed to chronic stress during early postnatal life display 1) lower density of α1-ARs in the renal vasculature, 2) attenuated renal vasoconstrictor responses to acute α-adrenergic stimulation, and 3) diminished baroreceptor control of HR. Taken together, these data support the existence of a potential adrenergic receptor desensitization in renal vasculature of MatSep rats, most likely due to chronically increased renal sympathetic outflow to the kidneys. Moreover, MatSep induces a long-term autonomic dysfunction that could be associated with the impaired blood pressure regulation in response to significant hypertensive stimuli.
Numerous clinical studies have shown a direct relationship between exposure to ELS and increased risk for hypertension, obesity, depression, and anxiety (1, 56, 57, 60). All of these are important risk factors for CVD. It is consistently reported that children raised in low socioeconomic status households and/or under stressful conditions have an increased risk to elevations in blood pressure over time and enhanced vascular reactivity to stress (4, 8, 9, 29, 60). Furthermore, it has been shown that individuals exposed to ELS display greater blood pressure in early adulthood compared with control subjects (58). MatSep is a well-established animal model of ELS that has been widely used in psychoneuroendocrinology to assess its long-lasting behavioral effects (3, 10, 45). This experimental stress model mimics the behavioral outcomes of ELS in humans such as anxiety and depression (14, 54, 64). In the last decade, MatSep served as a suitable tool to study the mechanisms underlying the risk for CVD in rodents. A hallmark of this model is that MatSep increases the sensitivity of the mechanisms activated in response to secondary stressors, including neuroadrenergic (49, 61) and angiotensinergic (36) stimulation. In addition, similar postnatal models of chronic behavioral stress, such as neonatal handling, have shown the influence of both early environmental conditions and genetic factors on altered physiological blood pressure control (62).
Adult rats exposed to MatSep during postnatal life are normotensive but display lower GFR (34), suggesting the increased renal sympathetic outflow as a potential underlying mechanism. This notion was supported by previous findings in adult rats exposed to MatSep such as: 1) greater reduction of blood pressure after ganglionic blockade (2 mg/kg ip mecamylamine), 2) increased renal NE content, and 3) normalization of GFR following renal denervation (34). The present findings showing reduction in the acute reactivity of the renal vascular response to adrenergic stimulation and reduced α1-AR density in isolated renal vasculature of MatSep support this idea further. Thus, a suitable explanation for the reduced α1-AR density could be that adrenergic receptors undergo desensitization secondary to chronic overstimulation of renal sympathetic outflow (13, 25).
Despite the difficulties in studying the α1D-AR biological characteristics, they are functionally expressed in many arteries (21) and influence arterial blood pressure regulation via vasoconstriction, particularly in the renal artery (65). We found a greater α1D-AR mRNA expression in renal vasculature, which could be linked to increased receptor phosphorylation in response to the greater NE content in renal tissue (13). The α1D-AR mRNA abundance is less compared with the other α-AR subtypes in isolated renal vasculature. Thus, the attenuated functional response to adrenergic stimulation most likely involves a potential adrenergic desensitization of different α-AR subtypes.
The inhibition of arterial baroreceptor function is among the array of causes that can trigger excess SNS activity (5, 27, 42, 51). Several studies support that reflex control of HR is diminished in most forms of hypertension (15, 33, 67). This impairment usually occurs at different levels as follows: secondary to dysfunction in the afferent sensing of arterial pressure, altered central processing, and/or changes in efferent effector mechanisms (27, 31, 40). The role of the SNS in the developmental origins of hypertension has been described in several experimental models, including intrauterine growth restriction and early exposure to energy-dense nutrients (20, 24, 48). Specifically in the MatSep paradigm, behavioral stress seems to exert a permanent disruption of the autonomic function. During postnatal week 3 in rodents, a surge in activity of postganglionic neurons is associated with an overall sympathetic hyperactivity and development of the baroreceptor regulation of sympathetic tone (2). However, exposure to behavioral stressors may alter the signaling cascades that control stress system activity in the neonate, leading to long-term derangements in autonomic control implicated in blood pressure regulation such as the HR reflex in response to changes in MAP. This finding is supported by other reports showing that MatSep alters the cardiovascular stress response induced by 30 min of restraint stress. These animals had exaggerated tachycardia compared with controls, despite no differences in MAP (50) and normal cardiac pacemaker intrinsic activity (61). In our hands, rats exposed to MatSep do not display changes in baseline MAP or HR (36). In addition, the heart weight-to-left ventricle weight ratio is similar between MatSep and control rats (0.78 ± 0.01 vs. 0.79 ± 0.01, respectively, unpublished observations). However, we found HR impairments to achieve the adaptive response to hypertensive stimuli such as acute adrenergic stimulation due to ELS. In light that arterial baroreflex sensitivity is blunted with the initiation of hypertension (19, 43), our data indicate that exposure to MatSep may lead to altered baroreflex sensitivity and predispose rats to impaired blood pressure control during adult life. Two important considerations need special attention: 1) the HR control is only one arm of the efferent regulation of the baroreceptor reflex, and 2) changes in RBF are just a last resort of baroreflex vasoconstriction, in which case the mesenteric vascular bed or skeletal muscle reactivity would provide a better explanatory approach.
A special acknowledgment should be prompted to other factors that could be influenced by MatSep regarding α-AR stimulation, i.e., α2-AR NE reuptake mechanisms and changes in the smooth muscle contraction such as intracellular pathways following receptor activation.
In summary, we have shown that rats exposed to MatSep display long-term alterations in the renal vasculature adrenergic receptor density and the HR reflex of the MAP. This study endorses the impact of ELS overactivating the neuroadrenergic drive thereby increasing CVD risk in response to secondary stressors such as hypertensive stimuli.
Perspectives and Significance
The prevalence of chronic kidney disease and end-stage renal disease continues to increase worldwide, and hypertension is recognized as a major contributing factor to chronic kidney disease. Importantly, progressive renal impairment increases cardiovascular morbidity and mortality. The relevance of sympathetic activation for the development and progression of chronic kidney disease is well established. In light of our findings, the translational studies designed to investigate the autonomic function in patients exposed to ELS might provide novel therapeutic targets to address better the origin of these health disparities.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R00-HL-111354 to A. S. Loria.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.L. conceived and designed research; A.S.L. performed experiments; A.S.L. and J.L.O. analyzed data; A.S.L. and J.L.O. interpreted results of experiments; A.S.L. prepared figures; A.S.L. drafted manuscript; A.S.L. and J.L.O. edited and revised manuscript; A.S.L. and J.L.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dianne Cohn for technical support, Dr. Michael Piascik for expertise on the binding assay optimization, Dr. Richard Charnigo for statistical analysis interpretation, and Dr. David Randall for scientific feedback.
REFERENCES
- 1.Alastalo H, Räikkönen K, Pesonen A-K, Osmond C, Barker DJP, Heinonen K, Kajantie E, Eriksson JG. Cardiovascular morbidity and mortality in Finnish men and women separated temporarily from their parents in childhood--a life course study. Psychosom Med 74: 583–587, 2012. doi: 10.1097/PSY.0b013e31825b3d76. [DOI] [PubMed] [Google Scholar]
- 2.Bartolome J, Mills E, Lau C, Slotkin TA. Maturation of sympathetic neurotransmission in the rat heart. V. Development of baroreceptor control of sympathetic tone. J Pharmacol Exp Ther 215: 596–600, 1980. [PubMed] [Google Scholar]
- 3.Beutel ME, Wiltink J, Kirschner Y, Sinning C, Espinola-Klein C, Wild PS, Münzel T, Blettner M, Zwiener I, Lackner K, Michal M. History of depression but not current depression is associated with signs of atherosclerosis: data from the Gutenberg Health Study. Psychol Med 44: 919–925, 2014. doi: 10.1017/S0033291713001542. [DOI] [PubMed] [Google Scholar]
- 4.Bosma H, van de Mheen HD, Borsboom GJJM, Mackenbach JP. Neighborhood socioeconomic status and all-cause mortality. Am J Epidemiol 153: 363–371, 2001. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 5.Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation 39: 48–54, 1969. doi: 10.1161/01.CIR.39.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Bylund DB, Toews ML. Radioligand binding methods: practical guide and tips. Am J Physiol Lung Cell Mol Physiol 265: L421–L429, 1993. [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo G, Loria AS, Pollock DM, Pollock JS. Endothelin activation of reactive oxygen species mediates stress-induced pressor response in Dahl salt-sensitive prehypertensive rats. Hypertension 56: 282–289, 2010. doi: 10.1161/HYPERTENSIONAHA.110.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 163: 1135–1143, 2009. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110: 1761–1766, 2004. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 10.Filipović D, Gavrilović L, Dronjak S, Radojcić MB. Brain glucocorticoid receptor and heat shock protein 70 levels in rats exposed to acute, chronic or combined stress. Neuropsychobiology 51: 107–114, 2005. doi: 10.1159/000084168. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JP, Paton JFR. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26: 463–475, 2012. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- 12.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol 16: 91–104, 2006. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 13.García-Sáinz JA, Rodríguez-Pérez CE, Romero-Avila MT. Human alpha1D-adrenoceptor phosphorylation and desensitization. Biochem Pharmacol 67: 1853–1858, 2004. doi: 10.1016/j.bcp.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 233: 102–111, 2012. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FCGJ, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55: 619–626, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda C, Koshimizu T-A, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, Fukuda S, Shinoura H, Oshikawa S, Takeo S, Kitamura T, Cotecchia S, Tsujimoto G. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol 67: 912–922, 2005. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- 17.Hoyt RE, Speakman RO, Brown DR, Cassis LA, Silcox DL, Anigbogu CN, Randall DC. Chronic angiotensin-II treatment potentiates HR slowing in Sprague-Dawley rat during acute behavioral stress. Auton Neurosci 174: 42–46, 2013. doi: 10.1016/j.autneu.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, Oddy WH, Blake KV, Palmer LJ, Beilin LJ. Perinatal and childhood origins of cardiovascular disease. Int J Obes 31: 236–244, 2007. doi: 10.1038/sj.ijo.0803394. [DOI] [PubMed] [Google Scholar]
- 19.Iliescu R, Tudorancea I, Irwin ED, Lohmeier TE. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol 305: H1080–H1088, 2013. doi: 10.1152/ajpheart.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension 61: 828–834, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen BC, Swigart PM, Laden ME, DeMarco T, Hoopes C, Simpson PC. The alpha-1D Is the predominant alpha-1-adrenergic receptor subtype in human epicardial coronary arteries. J Am Coll Cardiol 54: 1137–1145, 2009. doi: 10.1016/j.jacc.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 23.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715–724, 2008. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4: e5870, 2009. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiuchi K, Vatner DE, Uemura N, Bigaud M, Hasebe N, Hempel DM, Graham RM, Vatner SF. Mechanisms of alpha 1-adrenergic vascular desensitization in conscious dogs. Circ Res 71: 1185–1199, 1992. doi: 10.1161/01.RES.71.5.1185. [DOI] [PubMed] [Google Scholar]
- 26.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep 60: 1–116, 2011. [PubMed] [Google Scholar]
- 27.Korner PI. Baroreceptor resetting and other determinants of baroreflex properties in hypertension. Clin Exp Pharmacol Physiol Suppl 15, s15: 45–64, 1989. doi: 10.1111/j.1440-1681.1989.tb02995.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol 287: R1394–R1398, 2004. doi: 10.1152/ajpregu.00306.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol 28: 338–346, 2009. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leppik RA, Mynett A, Lazareno S, Birdsall NJ. Allosteric interactions between the antagonist prazosin and amiloride analogs at the human alpha(1A)-adrenergic receptor. Mol Pharmacol 57: 436–445, 2000. doi: 10.1124/mol.57.3.436. [DOI] [PubMed] [Google Scholar]
- 31.Li SG, Lawler JE, Randall DC, Brown DR. Sympathetic nervous activity and arterial pressure responses during rest and acute behavioral stress in SHR versus WKY rats. J Auton Nerv Syst 62: 147–154, 1997. doi: 10.1016/S0165-1838(96)00119-1. [DOI] [PubMed] [Google Scholar]
- 32.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098, 2007. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 33.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 299: H402–H409, 2010. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol 305: F390–F395, 2013. doi: 10.1152/ajprenal.00008.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loria AS, Kang K-T, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundin S, Ricksten SE, Thorén P. Interaction between mental stress and baroreceptor control of heart rate and sympathetic activity in conscious spontaneously hypertensive (SHR) and normotensive (WKY) rats. J Hypertens Suppl 1: 68–70, 1983. [PubMed] [Google Scholar]
- 39.Martyn CN. Fetal and infant origins of cardiovascular disease. Midwifery 10: 61–66, 1994. doi: 10.1016/S0266-6138(05)80246-3. [DOI] [PubMed] [Google Scholar]
- 40.McDonald MP, Sanfilippo AJ, Savard GK. Baroreflex function and cardiac structure with moderate endurance training in normotensive men. J Appl Physiol (1985) 74: 2469–2477, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief 1–8, 2013. [PubMed] [Google Scholar]
- 42.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 288: R846–R855, 2005. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- 43.Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation 85: 497–503, 1992. doi: 10.1161/01.CIR.85.2.497. [DOI] [PubMed] [Google Scholar]
- 44.Pretty C, O’Leary DD, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. BMC Pediatr 13: 208, 2013. doi: 10.1186/1471-2431-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman I, Humphreys K, Bennet AM, Ingelsson E, Pedersen NL, Magnusson PKE. Clinical depression, antidepressant use and risk of future cardiovascular disease. Eur J Epidemiol 28: 589–595, 2013. doi: 10.1007/s10654-013-9821-z. [DOI] [PubMed] [Google Scholar]
- 46.Randall DC, Speakman RO, Silcox DL, Brown LV, Brown DR, Gong MC, Patwardhan A, Reynolds LR, Karounos DG, Burgess DE, Anigbogu CN. Longitudinal analysis of arterial blood pressure and heart rate response to acute behavioral stress in rats with type 1 diabetes mellitus and in age-matched controls. Front Physiol 2: 53, 2011. doi: 10.3389/fphys.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M. The behavioral and immunological impact of maternal separation: a matter of timing. Front Behav Neurosci 8: 192, 2014. doi: 10.3389/fnbeh.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, Pombo J, Redington K, Uppal E, Coen CW, Poston L, Taylor PD. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension 62: 627–633, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00691. [DOI] [PubMed] [Google Scholar]
- 49.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res 183: 25–30, 2007. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiller AM, Pellegrino PR, Zucker IH. The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front Physiol 6: 224, 2015. doi: 10.3389/fphys.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 43: 169–175, 2004. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 52.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 54: 1195–1201, 2009. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 53.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 20: 933–939, 2009. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt MV, Wang X-D, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 214: 131–140, 2011. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- 55.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 363: 1642–1645, 2004. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 56.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology 38: 188–200, 2013. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension 64: 201–207, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131: 1674–1681, 2015. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 109: 765–775, 2002. doi: 10.1172/JCI200214001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics 121: e1240–e1249, 2008. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- 61.Trombini M, Hulshof HJ, Graiani G, Carnevali L, Meerlo P, Quaini F, Sgoifo A. Early maternal separation has mild effects on cardiac autonomic balance and heart structure in adult male rats. Stress 15: 457–470, 2012. doi: 10.3109/10253890.2011.639414. [DOI] [PubMed] [Google Scholar]
- 62.Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and preweanling heart rate in spontaneously hypertensive and Wistar Kyoto rats. Dev Psychobiol 17: 587–600, 1984. doi: 10.1002/dev.420170603. [DOI] [PubMed] [Google Scholar]
- 64.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci 30: 15007–15018, 2010. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villalobos-Molina R, López-Guerrero JJ, Ibarra M. Alpha 1D- and alpha 1A-adrenoceptors mediate contraction in rat renal artery. Eur J Pharmacol 322: 225–227, 1997. doi: 10.1016/S0014-2999(97)00095-2. [DOI] [PubMed] [Google Scholar]
- 66.Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve 36: 595–614, 2007. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- 67.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacrétaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 54: 530–536, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 68.Zhao X, Zhang Y, Leander M, Li L, Wang G, Emmett N. Altered expression profile of renal α(1D)-adrenergic receptor in diabetes and its modulation by PPAR agonists. J Diabetes Res 2014: 725634, 2014. doi: 10.1155/2014/725634. [DOI] [PMC free article] [PubMed] [Google Scholar]