Abstract
Endothelin-1 (ET-1) contributes to age-related endothelial dysfunction in men via the ETA receptor. However, there are sex differences in the ET-1 system, and ETB receptors are modulated by sex hormones. The purpose of this study was to test the hypothesis that ETB receptors contribute to impaired vasodilatory function in postmenopausal women (PMW). We measured flow-mediated dilation (FMD) using ultrasound, and cutaneous nitric oxide-mediated vasodilation during local heating (42°C) via laser Doppler flowmetry in 18 young women (YW; 22 ± 1 yr) and 16 PMW (56 ± 1 yr). Cutaneous microdialysis perfusions of lactated Ringer (control), an ETB receptor antagonist (BQ-788, 300 nM), and an ETA receptor antagonist (BQ-123, 500 nM), were done through separate fibers, followed by perfusions of sodium nitroprusside (28 mM) and local heating to 43°C (max). Cutaneous vascular conductance (CVC) was calculated as cutaneous blood flow/mean arterial pressure and expressed as a percent of maximal dilation. FMD (YW: 7.5 ± 0.5 vs. PMW: 5.6 ± 0.6%) and cutaneous vasodilation (YW: 93 ± 2 vs. PMW: 83 ± 4%CVCmax) were lower in PMW (both P < 0.05). Blockade of ETB receptors decreased cutaneous vasodilation in YW (87 ± 2%CVCmax; P < 0.05 vs. control) but increased vasodilation in PMW (93 ± 1%CVCmax; P < 0.05 vs. control). ETA receptor blockade had minimal effect in YW (92 ± 1%CVCmax) but increased cutaneous vasodilation in PMW (91 ± 2%CVCmax; P < 0.05 vs. control). In conclusion, ETB receptors mediate vasodilation in YW, but this effect is lost after menopause. Impaired vasodilatory function in PMW is due in part to a loss of ETB-mediated dilation.
Keywords: aging, endothelin-1, endothelial function, cutaneous circulation
primary aging is a major risk factor for cardiovascular disease (CVD) (47a). One mechanism linking the age-related increase in CVD may be impaired endothelial function or impaired vasodilatory function of the vasculature (32, 59, 66). Although it has been well established that declines in endothelial function occur with healthy aging (6, 15, 45, 52, 56, 65), the age at which endothelial function becomes impaired differs between men and women; endothelial function declines at a faster rate in women after menopause (6). With the use of flow-mediated dilation (FMD), an established measure of conduit artery endothelial function, several studies have demonstrated impaired endothelial function in postmenopausal women (PMW) (5, 14, 44, 45). However, the underlying mechanisms remain unclear. Because cardiovascular disease rates are higher in PMW (33, 47a, 69), understanding the mechanisms contributing to vascular dysfunction with age in women are important.
The vascular endothelium releases several vasoactive substances to mediate dilation and constriction. It is well known that impaired nitric oxide (NO) production or availability contributes to endothelial dysfunction. Recently, age-related impairments in endothelial function have also been associated with endothelin-1 (ET-1) in men (10, 65). ET-1 is a potent vasoconstrictor produced and released from endothelial cells that acts on two receptor subtypes, ETA and ETB. Both receptors are located on the vascular smooth muscle and mediate vasoconstriction (3, 17, 34); however, ETB receptors are also located on the endothelium and mediate vasodilation (17, 23). In a recent review article, Kitada et al. suggest that the efficacy of ET-1 receptor antagonists differs between men and women, and sex differences in CVD may be related to ETB receptors (28). ET-1 preferentially binds to ETB receptors in women (12), and prior studies demonstrate sex differences in ET-1 receptor responses (24, 53). Moreover, it has recently been demonstrated that ET-1 contributes to the age-associated decline in endothelial function in men via the ETA receptor (10, 65). To our knowledge, there have been no prior studies examining ET-1 receptor responses between young women (YW) and PMW. Because of sex differences in the ET-1 system, it is important to understand the impact of aging on ET-1-mediated vascular dysfunction in women. Because changes in sex hormones may alter ET-1 receptor function, particularly ETB receptors (49, 62, 63), it is possible that the impaired vascular function that occurs after menopause may be related to the ET-1 system.
The cutaneous circulation is an excellent model to examine mechanisms regulating vasodilatory function (20, 31). The same vasoactive substances that are active in other circulatory beds also operate in the cutaneous vasculature (20). Furthermore, both ETA and ETB receptors are present in the cutaneous circulation (19, 20, 25, 26, 41). Measuring vasodilatory responses in the cutaneous circulation during microdialysis perfusions of pharmacological antagonists is a minimally invasive approach to examine in vivo mechanisms regulating vascular function in humans (20). Cutaneous vasodilatory responses to local heating are largely mediated by NO (41) and have been shown to be eNOS dependent (4, 26). Importantly, changes in vasodilatory responses in the cutaneous circulation mirror changes in microvascular function of other beds, such as the heart and kidneys, and impairments of microvascular vasodilatory function are associated with CVD risk (1, 8, 21, 27).
The purpose of this study was to examine the role of ET-1 in contributing to vascular dysfunction in PMW. We assessed endothelial function using FMD along with measuring cutaneous vasodilatory responses to local heating. We further examined cutaneous vasodilatory responses during pharmacological blockade of ETA and ETB receptors. We hypothesized that ETB receptors would mediate vasodilation in YW but that this vasodilatory response would be lost in PMW.
METHODS
Subjects
All experimental procedures and protocols were approved by the University of Delaware Institutional Review Board, and the study conformed to the standards outlined in the Declaration of Helsinki. Verbal and written consent were obtained voluntarily from all subjects before participation. Eighteen YW (22 ± 1 yr) and 16 PMW (56 ± 1 yr) participated in this study. All women completed a standard medical screening at the University of Delaware Nurse Managed Primary Care Center. A fasting blood sample was obtained and analyzed for plasma glucose, lipid profile, and kidney and liver function (Quest Laboratories). Inclusion criteria included a resting blood pressure <140/90 mmHg, nondiabetic, and not taking over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g., statins, antihypertensives, anticoagulants). Women were nonobese (body mass index <30 kg/m2), did not use tobacco products, and were free from known cardiovascular disease. Women were recreationally active, participating in physical activity 3–5 days/wk, as indicated on a medical history questionnaire. Two PMW and three YW reported not engaging in regular exercise. Young women were tested either during the early follicular phase of their menstrual cycle or during the placebo phase if using oral contraceptives (n = 6) to minimize the impact of fluctuating ovarian hormones on vascular function (7, 14, 16, 38). Postmenopausal women were at least 1 yr since their last menses and not taking hormone therapy. The average length since last menses for PMW was 8 ± 1 yr. All women were familiarized with the equipment and experimental protocol before the testing visit.
Experimental Protocol
Women reported to the laboratory (22°C) either in the morning or afternoon after refraining from exercise and alcohol for 24 h, caffeine for 12 h, and food for at least 4 h. An over-the-counter urine pregnancy test was performed in young women. Women lay supine for ~20–30 min, and a venous blood sample was obtained from an antecubital vein to assess sex steroids and plasma ET-1. Conduit artery endothelial function was assessed in the brachial artery using FMD as previously described (11, 37). Briefly, a longitudinal image of the brachial artery was obtained via ultrasound (GE P5; Healthcare, Waukesha, WI). Baseline artery diameters were assessed on a continuous basis for 1 min, at which time a cuff placed below the antecubital fossa was inflated to suprasystolic pressure (Hokanson Rapid Cuff Inflator, Bellevue, WA) and remained inflated for 5 min. Upon cuff deflation, brachial artery diameters were assessed continuously for an additional 2 min. Brachial artery diameters were assessed offline on a continuous basis using a custom LabVIEW program; FMD was calculated as a percent change from baseline to peak diameter.
Cutaneous microdialysis.
Under sterile conditions, three microdialysis fibers (CMA 31 Linear Microdialysis Probe; Harvard Apparatus, Kista, Sweden) were placed in the intradermal space on the dorsal side of the right forearm as previously described (62, 64). Briefly stated, a 23-gauge needle was placed in the intradermal space, with an entrance and exit site of 2 cm. Each microdialysis site was separated by at least 1 in. The microdialysis fibers were then threaded through the lumen of the needle, and the needle was removed, leaving the microdialysis fiber situated in the intradermal space of the skin. The microdialysis fibers were taped in place, and laser Doppler flow probes (MoorLAB, Temperature Monitor SH02; Moor Instruments, Devon, UK) were secured to the surface of the skin over the site of each microdialysis fiber. Laser Doppler probes measure red blood cell flux, which is used as an index of blood flow, and also control local skin temperature. Each site was perfused with lactated Ringer (2 μl/min) for 60–90 min after microdialysis fiber insertion to allow local blood flow recovery (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytic Systems).
We measured baseline cutaneous blood flow for 5 min with skin temperature clamped at 33°C. Microdialysis fibers were then perfused with one of the following agents at 5 μl/min for 45 min: 1) lactated Ringer; 2) ETB receptor antagonist BQ-788 (300 nM; Sigma); or 3) ETA receptor antagonist BQ-123 (500 nM; Sigma). Doses of ET-1 receptor antagonists were based off previously published data (63). The heating device in the laser Doppler probes was then increased to 42°C (at a rate of 0.1°C/s) to induce vasodilation (41, 62). The vasodilatory response to local heating is biphasic, with the initial peak attributed to an axon reflex followed by a secondary prolonged plateau phase (~25–35 min after heating begins) that is predominantly mediated by NO (4, 26, 41). As such, we used this prolonged plateau phase as our primary indicator of cutaneous vasodilatory responsiveness. After the heating-induced plateau was established and maintained for 5 min, 28 mM of sodium nitroprusside was perfused (5 μl/min) through all microdialysis fibers, and the temperature in the local heating units was increased to 43°C to elicit maximal vasodilation as previously described (62). Blood pressure was measured throughout the protocol using an automated brachial blood pressure machine on the contralateral arm (Dinamap Dash 2000; GE Medical Systems).
Blood Analysis
Blood samples for the analysis of serum estradiol (s[E2]) and progesterone (s[P4]) concentrations were collected in separate tubes without an anticoagulant. A separate blood sample was collected in an EDTA tube for the analysis of plasma ET-1 concentration (p[ET-1]). All tubes were centrifuged, to separate the serum or plasma, which was then pipetted off and frozen at −80°C until time of analysis. Serum [E2] and [P4] were determined using competitive enzyme-linked immunosorbent assays (ELISA; Alpco, Salem, NH). The range for the E2 assay was 0–200 pg/ml with a sensitivity of 1.399 pg/ml. Intra-assay and interassay coefficients of variation for the E2 assay were 2.72 and 0.17%, respectively. The range for the P4 assay was 0.3–60 ng/ml with a sensitivity of 0.1 ng/ml. Intra-assay and interassay coefficients of variation for the P4 assay were 3.52 and 1.42%, respectively. Plasma ET-1 was analyzed using an ELISA (R&D Systems, Minneapolis, MN). Intra- and interassay coefficients of variation were <2.5%. All samples were measured at a wavelength of 450 nm on an Infinite F200 Pro microplate reader, and data were analyzed with Magellan IQ software (Tecan Group, Männedorf, CH).
Data Analysis and Statistics
Cutaneous blood flow was recorded at 1,000 Hz using Powerlab and LabChart (ADInstruments, Bella Vista, NSW, Australia). After a stable plateau was achieved in response to local heating, 2 min of cutaneous blood flow at each microdialysis site were analyzed. Subsequently, maximal vasodilatory capacity was also measured at each site (2 min) after a stable plateau was achieved from the SNP + 43°C. Data are presented as cutaneous vascular conductance (CVC; cutaneous blood flow/mean arterial pressure) and normalized to the maximal vasodilatory capacity reached from SNP + 43°C (%CVCmax) to account for site-to-site variations in blood flow (62, 64).
Subject demographics, serum hormones, and plasma ET-1 were compared between groups using unpaired t-tests. The effect of ET-1 receptor blockade (drug) on vasodilatory responses in YW and PMW (group) was conducted using 3 × 2 Mixed Design ANOVA. Additionally, MAP and CVCmax were compared across protocol periods/microdialysis sites between groups using a 4 × 2 Mixed Design ANOVA. Model assumptions were tested using the Shapiro-Wilk test for normality and Box’s M and Mauchly’s test for sphericity. If sphericity was violated, the Greenhouse-Geisser correction was used. Last, post hoc tests using Fisher’s least-significant difference procedure for significant main and interaction effects were completed through pairwise comparisons of marginal means and simple main effects, respectively. Results are reported as means ± SE, and the alpha level was set at P < 0.05.
RESULTS
Subject characteristics are presented in Table 1. By design, PMW were older compared with YW. All women were nonobese. Although systolic and diastolic blood pressure was greater in PMW, all women were normotensive. Cholesterol levels were higher in PMW; however, the ratio of cholesterol to high-density lipoprotein-cholesterol was similar between YW and PMW. Fasting plasma glucose was within normal clinical limits. Sex steroids are also presented in Table 1. Serum [E2] and [P4] were similar in both groups. Plasma ET-1 was higher in PMW (Table 1).
Table 1.
Participant characteristics
| YW (n = 18) | PMW (n = 16) | |
|---|---|---|
| Age, yr | 22 ± 1 | 56 ± 1* |
| Height, cm (range) | 163 ± 2 (142–182) | 162 ± 2 (140–172) |
| Mass, kg (range) | 60 ± 2 (46–72) | 63 ± 2 (45–78) |
| BMI, kg/m2 (range) | 23 ± 1 (17–28) | 24 ± 1 (19–29) |
| Systolic BP, mmHg | 109 ± 3 | 123 ± 3* |
| Diastolic BP, mmHg | 67 ± 2 | 80 ± 2* |
| Mean arterial BP, mmHg | 81 ± 2 | 94 ± 2* |
| Heart rate, beats/min | 60 ± 2 | 57 ± 2 |
| Total cholesterol, mg/dl | 163 ± 6 | 216 ± 7* |
| HDL cholesterol, mg/dl | 67 ± 3 | 85 ± 4* |
| LDL cholesterol, mg/dl | 82 ± 6 | 117 ± 6* |
| Triglycerides, mg/dl | 79 ± 9 | 73 ± 7 |
| Chol-to-HDL-C ratio | 2.6 ± 0.2 | 2.6 ± 0.1 |
| Fasting plasma glucose, mg/dl | 80 ± 2 | 89 ± 3* |
| Serum [E2], pg/ml | 31.8 ± 19.8 | 22.0 ± 14.0 |
| Serum [P4], ng/ml | 0.76 ± 0.59 | 0.67 ± 0.57 |
| Plasma [ET-1], pg/ml | 1.39 ± 0.41 | 1.74 ± 0.42* |
Values are means ± SE; n = 18 young women (YW) and 16 postmenopausal women (PMW) except for estradiol (E2), progesterone (P4), and endothelin-1 (ET-1) where n = 17 YW and 15 PMW. BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Chol, cholesterol; HDL-C, HDL-cholesterol; [E2], [P4], and [ET-1], E2, P4, and ET-1 concentration, respectively.
P < 0.05 vs. YW for serum [E2], [P4], and plasma [ET-1], YW n = 17, PMW n = 15.
Vascular Function
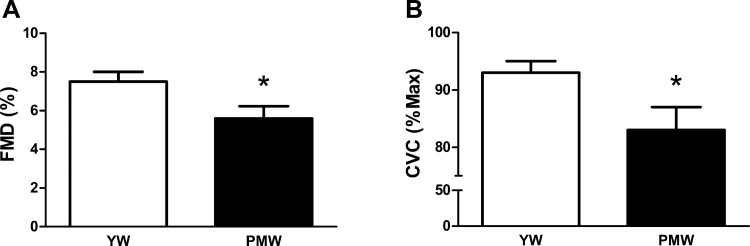
As expected, endothelial function as a measure by brachial artery FMD was lower in PMW (7.5 ± 0.5 vs. 5.6 ± 0.6%, P < 0.05; Fig. 1A). Consistent with these findings, the plateau phase of cutaneous vasodilation in response to local heating was also blunted in PMW (92 ± 2 vs. 83 ± 4%, P < 0.05; Fig. 1B).
Fig. 1.
Measures of vasodilatory function using flow-mediated dilation (FMD, A) and local heating-induced plateau (B) expressed as cutaneous vascular conductance (CVC) in young women (YW) and postmenopausal women (PMW). *P < 0.05 vs. YW.
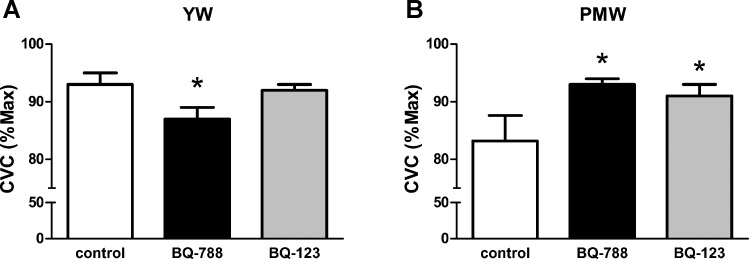
There was a significant drug × group interaction detected for the ET-1 receptor antagonists (P < 0.05; Fig. 2, A and B). In YW, blockade of ETB receptors reduced cutaneous vasodilation (P < 0.05; Fig. 2A). However, ETA receptor blockade had no effect on vasodilatory responses in YW (Fig. 2A). In contrast to YW, ETB receptor blockade increased vasodilatory responses to local heating in PMW (P < 0.05; Fig. 2B); a similar response in PMW was also observed during ETA receptor blockade (P < 0.05; Fig. 2B). Blood pressure was higher in PMW than YW throughout the microdialysis protocol. There was a small but significant reduction in BP in both groups during the heating at 43°C + SNP compared with the plateau phase achieved with heating at 42°C (Table 2); this is taken into account when CVC is calculated. Maximal vasodilatory capacity (CVC) was similar across microdialysis sites and between groups (YW: control = 0.71 ± 0.05, ETB = 0.70 ± 0.06, ETA = 0.58 ± 0.06; PMW: control = 0.46 ± 0.05, ETB = 0.57 ± 0.05, ETA = 0.54 ± 0.05; P > 0.07).
Fig. 2.
Cutaneous vasodilatory responses to local heating during cutaneous microdialysis perfusions of ETB (BQ-788) and ETA (BQ-123) receptor antagonists in YW (A) and PMW (B). *P < 0.05 vs. control.
Table 2.
Mean arterial blood pressure during microdialysis protocol
| YW | PMW | |
|---|---|---|
| Baseline | 78 ± 2 | 90 ± 2* |
| ET-1 blockade | 77 ± 2 | 90 ± 2* |
| Heat 42°C | 78 ± 2 | 91 ± 2* |
| Heat 43°C + SNP | 76 ± 2† | 88 ± 3*† |
Values are means ± SE in mmHg. ET-1, endothelin-1; SNP, sodium nitroprusside.
P < 0.05 vs. YW.
P < 0.05 vs. heat 42°C.
DISCUSSION
The main novel finding of the current study is that impaired vasodilation in PMW is due in part to a loss of ETB receptor-mediated vasodilation. In YW, blockade of ETB receptors resulted in a reduction in vasodilation, demonstrating that ETB receptors mediate vasodilation. In contrast, blockade of ETB receptors increased vasodilatory responses in PMW, demonstrating a loss of ETB receptor-mediated dilation. These data suggest a shift in ETB receptor function with aging/menopause. We also show that blockade of ETA receptors increased vasodilatory responses in PMW, indicating a removal of ETA-mediated constriction. Collectively, our study adds to the understanding of the mechanisms involved in vascular dysfunction in PMW by demonstrating the ET-1 system is involved in the impaired vasodilatory function that occurs after menopause.
It is well recognized that PMW have impaired vascular function. Consistent with prior investigations (45, 46, 55), our data show that PMW have impaired endothelial function, as evident by a lower flow-mediated dilation. Our data also show that this blunted vasodilatory capacity is evident in microcirculation. Although prior studies have demonstrated impaired cutaneous vasodilation with aging (42), to our knowledge, this study is the first to report these findings in the cutaneous circulation of PMW.
The mechanisms contributing to vascular dysfunction in PMW are complex because of the dramatic changes in ovarian hormones concomitant with an aging vascular system. Although there have been significant advances in our understanding of impaired vasodilatory function in women during and after menopause, the underlying mechanisms are not completely understood (18, 43, 44, 46). It has previously been established that aging and estrogen deprivation are associated with a reduction in NO bioavailability (6, 14, 58). Furthermore, this reduction in NO bioavailability is associated with eNOS uncoupling and reactive oxygen species in PMW, and estrogen deficiency plays an obligatory role in this response (46, 47). Thus, it has become increasingly apparent that dysfunction in the production and/or release of NO is a primary mediator of impaired vasodilatory function in PMW. However, impaired NO production and bioavailability may also be related to ET-1 (54, 60), and ET-1 signaling has recently been implicated in the development of endothelial dysfunction in men (10, 65).
To our knowledge, our study is the first to examine a role for ET-1 in contributing to impaired vasodilatory function in PMW. Plasma levels of ET-1 generally increase in PMW (36), and our data are consistent with these previous findings. These increases in p[ET-1] may be related to changes in sex hormones, since estrogen administration decreases p[ET-1] in YW and PMW (39, 70). Because ET-1 is produced by endothelial cells and is secreted abluminally, it is unclear whether this increase in p[ET-1] is related to an increase in production or a reduction in clearance of ET-1. Donato et al. recently reported that age-related endothelial dysfunction in men was related to greater ET-1 expression and bioactivity in endothelial cells (10), suggesting that aging may be associated with greater ET-1 production. Alternatively, because the endothelial ETB receptors also function as a clearance receptor for ET-1 (13), it is also possible that a downregulation of endothelial ETB receptors after menopause contributes to a greater p[ET-1]. Further investigation is needed to understand the mechanisms by which p[ET-1] increases with age.
As previously mentioned, ET-1 acts on two primary receptors: ETA and ETB. Healthy aging has been associated with an increase in ET-1 receptor expression in experimental animals (22). Furthermore, fluctuations in ovarian hormones modulate the expression of ET-1 receptors, particularly ETB receptors, in cell and animal models (9, 48, 49). In humans, the proportion of ETA and ETB receptors differs between men and women (12), and alterations in sex steroids may influence ETB receptor control of vascular function (62, 63). As such, we hypothesized that ETB receptor control of vascular function would be altered after menopause. In the current study, we show a differential regulation of cutaneous vasodilatory responses by the ETB receptor in YW and PMW: in YW, ETB receptors mediate vasodilation; however, this vasodilatory effect is lost after menopause. We speculate that either endothelial ETB receptors are downregulated or vascular smooth muscle ETB receptors are upregulated after menopause. Because ETB receptors are also within peripheral nerves and mediate vasoconstriction (35), it is possible that greater sympathetic tone in PMW is contributing to our findings of reduced dilation. Although we are not able to discern the precise mechanism or changes in receptor expression from the current study, our data are an important first step in showing functional differences in the regulation of vascular function in humans with age/menopause via the ETB receptor. Importantly, our findings are in contrast to previous findings in men showing that impaired endothelial function with aging in men occurs in part because of greater ETA receptor-mediated vasoconstriction (65). Therefore, sex differences in the ET-1 system may be a primary contributing factor to the known sex differences in endothelial dysfunction and CVD (6, 47a, 55).
It is not clear if the loss of ET-B-mediated dilation in PMW is primarily an aging effect or a result of changes in reproductive hormone status. It is challenging to completely separate the effect of aging from the hormonal changes that occur with menopause. We tested young women during the early follicular phase of menstruation or during the placebo week of oral contraceptive use to minimize the impact of circulating reproductive hormones, consistent with prior studies examining vascular function in women (7, 14, 16, 38). However, the composition of synthetic hormones can cause differential action on endothelial function (38, 40, 57). We were not powered to examine these differences in the current study, but it is an interesting area for future investigation given the use of synthetic hormones not only in young women for contraceptive use but also in postmenopausal women for the treatment of menopausal symptoms.
Limitations.
We recognize that there are other vasoactive substances regulating vascular endothelial function, such as NO and prostaglandins. Our current analysis was focused on the role of ET-1, and we were not able to explore additional mechanisms or interactions with ET-1. However, the findings of our study provide a foundation for future studies to explore additional mechanisms and interactions with ET-1. Second, ET-1 exerts other effects that can contribute to impaired vasodilatory function. For example, ET-1 can stimulate reactive oxygen species (50, 61), and oxidative stress has been shown to contribute to impaired endothelial function in PMW (47). Increased arterial stiffness is also evident in PMW (18, 67); this increased stiffness in PMW was attenuated after infusion of ascorbic acid, indicating a role for oxidative stress (18). In addition, ET-1 can also activate matrix metalloproteinases and increase collagen production, cause smooth muscle proliferation, induce fibrosis, and lead to inflammation (2, 30, 51). Therefore, ET-1 may be involved in the greater arterial stiffness observed in PMW. Unfortunately, we did not measure arterial stiffness in the current investigation and cannot determine whether there was a relationship between ET-1 receptor function and arterial stiffness. Taken together, the interactions of multiple regulatory pathways are likely involved in the impaired vascular function that occurs after menopause.
In conclusion, we demonstrate that the ET-1 system plays an important role in the impaired vascular function observed in PMW. Specifically, we show that ETB receptor-mediated dilation in young women is lost after menopause. These data add to our understanding of the mechanisms that contribute to impaired vasodilatory function that occurs after menopause.
Perspectives and Significance
It is now well recognized that sex difference are apparent in the prevalence of CVD with aging, such that CVD is more prevalent in women after menopause. Furthermore, age-related declines in endothelial function are also different between men and women. Dysregulation of ETB receptors has received increasing attention for being a proposed link for the known sex differences in CVD pathologies (28, 29), and our findings support this notion by showing important functional differences in vasodilatory function via the ETB receptor with menopause.
GRANTS
This work was supported in part by National Institute of General Medical Sciences Grants U54-GM-104941 (principal investigator S. A. Binder-Macleod), 5 P20-GM-103446–13 (DE-INBRE Program), and P20-GM-113125 (Center of Biomedical Research Excellence) and by the University of Delaware Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.W. conceived and designed research; M.M.W., K.N.S., and A.V.K. performed experiments; M.M.W., K.N.S., A.V.K., and R.T.P. analyzed data; M.M.W., K.N.S., A.V.K., R.T.P., and D.G.E. interpreted results of experiments; M.M.W. prepared figures; M.M.W., K.N.S., A.V.K., R.T.P., and D.G.E. drafted manuscript; M.M.W., K.N.S., A.V.K., R.T.P., and D.G.E. edited and revised manuscript; M.M.W., K.N.S., A.V.K., R.T.P., and D.G.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stephanie Mraz for assistance with data collection/analysis, Ken Kirshner, Allen Prettyman, Carolyn Haines and the University of Delaware Nurse Managed Primary Care Center, Kathy Masso, Liza Walker, and the participants for their time.
REFERENCES
- 1.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res 84: 55–59, 2012. doi: 10.1016/j.mvr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Agui T, Xin X, Cai Y, Sakai T, Matsumoto K. Stimulation of interleukin-6 production by endothelin in rat bone marrow-derived stromal cells. Blood 84: 2531–2538, 1994. [PubMed] [Google Scholar]
- 3.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348: 730–732, 1990. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 4.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush DE, Jones CE, Bass KM, Walters GK, Bruza JM, Ouyang P. Estrogen replacement reverses endothelial dysfunction in postmenopausal women. Am J Med 104: 552–558, 1998. doi: 10.1016/S0002-9343(98)00117-X. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol (1985) 87: 1719–1723, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 9.David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Rebouças NA, Tostes RC. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension 38: 692–696, 2001. doi: 10.1161/01.HYP.38.3.692. [DOI] [PubMed] [Google Scholar]
- 10.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther 285: 511–517, 1998. [PubMed] [Google Scholar]
- 13.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun 199: 1461–1465, 1994. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 14.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996. doi: 10.1161/01.HYP.27.4.849. [DOI] [PubMed] [Google Scholar]
- 16.Harris RA, Tedjasaputra V, Zhao J, Richardson RS. Premenopausal women exhibit an inherent protection of endothelial function following a high-fat meal. Reprod Sci 19: 221–228, 2012. doi: 10.1177/1933719111418125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes WG. Endothelins as regulators of vascular tone in man. Clin Sci (Lond) 88: 509–517, 1995. doi: 10.1042/cs0880509. [DOI] [PubMed] [Google Scholar]
- 18.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. doi: 10.1097/GME.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 20.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 21.I Jzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serné EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin . Eur J Clin Invest 33: 536–542, 2003. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishihata A, Katano Y. Role of angiotensin II and endothelin-1 receptors in aging-related functional changes in rat cardiovascular system. Ann NY Acad Sci 1067: 173–181, 2006. doi: 10.1196/annals.1354.021. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA 91: 4892–4896, 1994. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellogg DL Jr, Liu Y, Pérgola PE. Selected contribution: gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol (1985) 91: 2407–2411, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol (1985) 98: 629–632, 2005. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 28.Kitada K, Ohkita M, Matsumura Y. Pathological importance of the endothelin-1/ET(B) receptor system on vascular diseases. Cardiol Res Pract 2012: 731970, 2012. doi: 10.1155/2012/731970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 40: 362–370, 2013. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett 238: 249–252, 1988. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 31.Kuliga KZ, McDonald EF, Gush R, Michel C, Chipperfield AJ, Clough GF. Dynamics of microvascular blood flow and oxygenation measured simultaneously in human skin. Microcirculation 21: 562–573, 2014. doi: 10.1111/micc.12136. [DOI] [PubMed] [Google Scholar]
- 32.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 33.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 14: 254–260, 2012. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci USA 88: 3185–3189, 1991. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol (Oxf) 203: 37–45, 2011. doi: 10.1111/j.1748-1716.2010.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol (1985) 95: 336–341, 2003. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 37.Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J Appl Physiol (1985) 118: 1510–1515, 2015. doi: 10.1152/japplphysiol.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. A combined oral contraceptive containing 30 mcg ethinyl estradiol and 3.0 mg drospirenone does not impair endothelium-dependent vasodilation. Contraception 82: 366–372, 2010. doi: 10.1016/j.contraception.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol 294: H1630–H1637, 2008. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Ethinyl estradiol-to-desogestrel ratio impacts endothelial function in young women. Contraception 79: 41–49, 2009. doi: 10.1016/j.contraception.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 93: 1644–1649, 2002. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 43.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. doi: 10.1155/2014/204390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 48.Nuedling S, van Eickels M, Alléra A, Doevendans P, Meyer R, Vetter H, Grohé C. 17 Beta-estradiol regulates the expression of endothelin receptor type B in the heart. Br J Pharmacol 140: 195–201, 2003. doi: 10.1038/sj.bjp.0705409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedersen SH, Nielsen LB, Mortensen A, Nilas L, Ottesen B. Progestins oppose the effects of estradiol on the endothelin-1 receptor type B in coronary arteries from ovariectomized hyperlipidemic rabbits. Menopause 15: 503–510, 2008. doi: 10.1097/gme.0b013e318156f803. [DOI] [PubMed] [Google Scholar]
- 50.Pollock DM, Pollock JS. Endothelin and oxidative stress in the vascular system. Curr Vasc Pharmacol 3: 365–367, 2005. doi: 10.2174/157016105774329408. [DOI] [PubMed] [Google Scholar]
- 51.Rizvi MA, Katwa L, Spadone DP, Myers PR. The effects of endothelin-1 on collagen type I and type III synthesis in cultured porcine coronary artery vascular smooth muscle cells. J Mol Cell Cardiol 28: 243–252, 1996. doi: 10.1006/jmcc.1996.0023. [DOI] [PubMed] [Google Scholar]
- 52.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261–R265, 2010. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sud N, Black SM. Endothelin-1 impairs nitric oxide signaling in endothelial cells through a protein kinase Cdelta-dependent activation of STAT3 and decreased endothelial nitric oxide synthase expression. DNA Cell Biol 28: 543–553, 2009. doi: 10.1089/dna.2009.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 56.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. doi: 10.1161/01.CIR.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 57.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol 292: H2874–H2880, 2007. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]
- 58.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 59.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 60.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 61.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res 89: 357–364, 2001. doi: 10.1161/hh1601.094983. [DOI] [PubMed] [Google Scholar]
- 62.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol 589: 4671–4679, 2011. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenner MM, Taylor HS, Stachenfeld NS. Peripheral microvascular vasodilatory response to estradiol and genistein in women with insulin resistance. Microcirculation 22: 391–399, 2015. doi: 10.1111/micc.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westby CM, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin Sci (Lond) 120: 485–491, 2011. doi: 10.1042/CS20100475. [DOI] [PubMed] [Google Scholar]
- 66.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 67.Woodard GA, Mehta VG, Mackey RH, Tepper P, Kelsey SF, Newman AB, Sutton-Tyrrell K. C-reactive protein is associated with aortic stiffness in a cohort of African American and white women transitioning through menopause. Menopause 18: 1291–1297, 2011. doi: 10.1097/gme.0b013e31821f81c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens 24: 740–749, 2011. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ylikorkala O, Orpana A, Puolakka J, Pyörälä T, Viinikka L. Postmenopausal hormonal replacement decreases plasma levels of endothelin-1. J Clin Endocrinol Metab 80: 3384–3387, 1995. [DOI] [PubMed] [Google Scholar]