Abstract
Abrupt cessation of chronic alcohol consumption triggers signaling cascades that harm vulnerable brain regions and produce neurobehavioral deficits. We have demonstrated that a program of intermittent, normobaric hypoxia training (IHT) in rats prevents brain damage and neurobehavioral impairment resulting from abrupt ethanol withdrawal (EW). Moreover, EW induced expression of stress-activated protein kinase p38 and presenilin 1 (PS1), the catalytic subunit of γ-secretase that produces the neurotoxic amyloid-β (Aβ) peptides Aβ40 and Aβ42. We tested the hypotheses that 1) IHT limits EW-induced activation of the p38-PS1 axis, thereby attenuating γ-secretase activation and Aβ accumulation, and 2) EW disables heat shock protein 25 (HSP25), a p38 substrate, molecular chaperone, and antioxidant, and provokes protein carbonylation in a manner suppressed by IHT. Adult male rats completed two cycles of a 4-wk ethanol diet (6.5% wt/vol) and a 3-wk EW or an isocaloric, dextrin-based control diet. A 20-day IHT program (5–8 daily cycles of 5–10 min of 9.5–10% fractional inspired O2 + 4 min of 21% fractional inspired O2) was administered during the first EW phase. After the second EW phase, the brain was excised and the prefrontal cortex extracted. PS1, phosphorylated p38 (p-p38), and HSP25 were analyzed by immunoblot, PS1 messenger RNA by quantitative polymerase chain reaction, protein carbonyl content by spectrometry, and Aβ40 and Aβ42 contents by enzyme-linked immunosorbent assay. IHT attenuated the EW-associated increases in PS1, p-p38, Aβ40, Aβ42, and protein carbonyl contents, but not that of PS1 messenger RNA, while preserving functionally competent HSP25 dimers in EW rats. Collectively, these findings suggest that IHT may attenuate EW-induced γ-secretase overactivation by suppressing activation of the p38-PS1 axis and by preventing oxidative protein damage.
Keywords: amyloid-β, carbonyls, ethanol withdrawal, heat shock protein 25, intermittent hypoxia training, presenilin 1
alcohol intoxication exacts a staggering social, economic, and medical burden worldwide. Ethanol intoxication and ethanol withdrawal (EW) are associated with substantial mortality and morbidity (48). Neurological sequelae such as tremor, convulsions, and seizures occur in humans and rodents when long-term heavy drinking is abruptly stopped. These morbidities discourage many alcoholics from staying abstinent. Consequently, they repeat drinking and withdrawal, and such repeated EW sensitizes neurons to excitatory glutamate, increasing the risk of seizures (3, 5).
Intermittent hypoxia training (IHT) programs, e.g., those alternating several cycles of brief (5- to 10-min) bouts of normobaric, moderately severe hypoxia [9.5–10% fractional inspired O2 ()] with room air ventilation, bolster endogenous protective mechanisms against pathophysiological insults (36, 38, 49). The protective action of IHT could be mediated by several mechanisms. IHT can dampen the excessive formation of reactive metabolites such as superoxide and peroxynitrite (49) and prevent catastrophic opening of mitochondrial membrane pores (27). Also, IHT moderates activity of the stress-activated protein kinase p38 (26), an effect that may be linked to attenuation of reactive oxygen species, which induce p38 phosphorylation and activation (41).
We demonstrated previously that IHT prevents brain disorders associated with EW (26, 29). Specifically, single or repeated EW episodes caused behavioral hyperexcitation, cerebellar-related motoric deficit, and neurological impairment accompanied by oxidative stress and p38 activation in a manner attenuated by IHT (26, 29). A marked increase in presenilin 1 (PS1), the catalytic subunit of γ-secretase, was demonstrated in the prefrontal cortex of ethanol-withdrawn rats (28). γ-Secretase cleaves amyloid precursor protein (APP), producing amyloid-β (Aβ) peptides, aggregates of which are hallmarks and mediators of Alzheimer’s disease (25, 52). Indeed, excessive Aβ has the propensity to form misfolded aggregates of PS1 that may contribute to neurodegeneration (33). When they accumulate, the Aβ peptides Aβ40 and Aβ42 are notoriously toxic to neurons (8). Although the physiological significance of PS1 is still under investigation, the EW-induced PS1 upregulation (28) persisted for 3 wk after the termination of chronic ethanol consumption. These findings suggest that PS1 is a pivotal mediator of neurotoxic molecular signaling associated with neuronal hyperactivity and remains elevated in the brain after EW.
Manukhina et al. (37) examined IHT intervention against Aβ toxicity in rats injected with Aβ fragments in the basal magnocellular nucleus. That study demonstrated that IHT blunted oxidative stress and nitric oxide overproduction, prevented neuronal death, and preserved memory in the Aβ-injected rats. However, it is unknown whether IHT prevents the accumulation of endogenous PS1 and Aβ associated with EW. Accordingly, this study addressed the hypothesis that IHT suppresses EW-induced activation of γ-secretase and Aβ formation. Furthermore, this study examined potential IHT mechanisms involving p38, the small heat shock protein (HSP) and p38 substrate HSP25, and protein carbonylation, a footprint of oxidative stress. We show that IHT prevents increases in PS1 and the Aβ products of γ-secretase, suppresses p38 overactivation, partially preserves HSP25 dimers, and blunts protein carbonyl accumulation in the prefrontal cortex of ethanol-withdrawn rats.
METHODS
Materials.
Analytic reagents were purchased from Qiagen (Valencia, CA), Sigma-Aldrich (St. Louis, MO), Abcam (Cambridge, MA), EMD Millipore (Billerica, MA), and Cell Signaling Technology (Danvers, MA). Aβ assay kits were purchased from Sigma-Aldrich. Diet ingredients were obtained from Research Organics (Cleveland, OH) or MP Biomedicals (Irvine, CA).
Ethanol intoxication-withdrawal protocol.
Animal experimentation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council publication 85-23, Revised 2011) and was approved by the University of North Texas Health Science Center Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River, Wilmington, MA), 3-mo-old at the beginning of the ethanol diet, were housed at 22–25°C on a 12:12-h light-dark cycle and 55% humidity with ad libitum access to water.
We employed a model of repeated EW to mimic the drinking pattern of human alcoholics (4, 44, 50). Rats (6/group) were assigned to one of four groups: 1) a control group receiving a dextrin diet and nonhypoxic sham conditioning, 2) a group that received a dextrin diet and the IHT program described below, 3) an EW group that underwent the ethanol intoxication-EW protocol described below and sham conditioning, and 4) an EW + IHT group that underwent ethanol intoxication, EW, and IHT. The EW rats received a nutritionally balanced liquid diet containing 6.5% (vol/vol) ethanol for 4 wk, followed by withdrawal for 3 wk/cycle for two cycles. The ethanol concentration was gradually increased to 6.5% during the first week of each cycle to habituate the rats to ethanol consumption. The control groups consumed an identical liquid diet, with the exception that the complex carbohydrate dextrin was isocalorically substituted for ethanol. The health of the rats was monitored regularly by observing their physical appearance and measuring body weights. Animals were fed a standard chow diet during EW and were euthanized at 3 wk of the second EW to sample the prefrontal cortex, a brain region known to be vulnerable to the damaging effects of excessive glutamatergic neurotransmission, chronic ethanol, and EW (18, 40, 47). Animal requirements to obtain statistically conclusive results were estimated by power analyses (GraphPad StatMate Statistics Software, Loyola, CA) of previously reported (26) cerebellar contents of phosphorylated p38 and protein carbonyls in dextrin control, EW, and EW + IHT rats. These analyses indicated that six rats per group would be required to detect treatment effects of EW and IHT on these variables at α = 0.05 and 1-β = 0.8.
Intermittent normobaric hypoxia training program.
The IHT program described by Zong et al. (61) was applied every morning for the last 3 days of the first ethanol diet and the first 17 days of the subsequent EW. This schedule targets mainly the EW phase of the protocol. The initiation of IHT 3 days before EW helped avoid the simultaneous stress of EW and a new procedure (IHT) that would complicate the interpretation of data. Rats were hypoxia or sham trained in 267-liter acrylic chambers. These chambers accommodated multiple rat cages and allowed the chamber atmosphere to be adjusted quickly and precisely with compressed gas. The IHT program (61) consisted of brief (5–10 min) hypoxic exposures (5–8 bouts/day) with intervening 4-min reoxygenation periods. in the chamber was monitored with a precision O2 sensor (Alpha Omega Instruments model 2000). Compressed N2 was introduced into the chamber to lower O2 content to the prescribed value within 90 s. Abrupt reoxygenation was achieved by opening the top and ends of the chamber and ventilating with a handheld fan. Non-IHT groups underwent sham conditioning protocols in which compressed air instead of N2 was introduced to maintain at 21%. The rats exhibited no discomfort or distress during the IHT or sham sessions.
Immunoblotting.
The effects of EW and IHT on the contents of PS1, p38, HSP25, HSP90, and the γ-secretase product Notch intracellular domain (NICD) in the prefrontal cortex of rats were assessed by immunoblotting. Among the four p38 isoforms (α, β, γ, and δ), p38α is ubiquitously and significantly expressed in most cell types (9) and in human brain (57). We examined p38α because it is particularly responsive to and activated by stressful stimuli (22), including glutamate (51, 59) and EW (28). p38 is activated by phosphorylation; accordingly, we measured phosphorylated p38α for all p38 assessments. Prefrontal cortex biopsies were sonicated in lysis buffer. Total protein concentration was determined in an aliquot of homogenate by Bradford assay (Bio-Rad, Hercules, CA). Another aliquot was combined with an equal volume of loading buffer, electrophoresed on 10% SDS-PAGE, and electrophoretically transferred onto a polyvinylidene fluoride membrane. Nonspecific binding sites were blocked with 5% fat-free milk. The blot was washed in TBS-T and incubated overnight with mouse polyclonal antibody against PS1 (EMD Millipore) or NICD (Abcam) or rabbit monoclonal antibody against HSP25, HSP90, or phosphoryated p38α (Abcam). The blot was then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 1–2 h at room temperature. Bands were detected using the UVP (Upland, CA) luminescence system and quantified by image densitometry. β-Actin immunoblots (Santa Cruz Biotechnology, Dallas, TX) provided loading controls.
Real-time polymerase chain reaction.
The messenger RNA (mRNA) abundance of PS1 was analyzed to determine whether IHT affects PS1 at the transcriptional level. Total RNA was isolated from prefrontal cortex using TRIzol (Qiagen), following the manufacturer’s instructions. RNA was transcribed to cDNA by adding random primers and Superscript III reverse transcriptase (Thermo Fisher, Waltham, MA). cDNA was then quantified in an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). Real-time PCR was conducted to analyze mouse PS1 gene expression using an ABI Prism 7000 (Thermo Fisher) sequence detection system with TaqMan primers (Thermo Fisher). Primer sequences were as follows: PS1 forward, 5′-GCGGCGGGGAAGCGTATACC-3′; PS1 reverse, 5′-GGCCAAGCTGTCTAAGGACCGC-3′. Quantitative PCR reactions were performed as follows: 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was analyzed as an internal control. Cycle threshold (CT) values were calculated with SDS software version 2.3 (Thermo Fisher) using automatic baseline settings and a threshold of 0.2. The comparative CT method was used to calculate the relative mRNA expression. The CT value of GAPDH was also measured and subtracted from the corresponding CT value for PS1 to calculate the ΔCT value.
Protein carbonyls.
Carbonyl content was measured with 2,4-dinitrophenylhydrazine (DNPH), which forms adducts with aldehyde and ketone moieties in proteins. Brain samples were homogenized in 50 mM HEPES (pH 7.2) containing 10 mM KCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail. To 1.0 ml of homogenate, 0.2 ml of 10 mM DNPH in 2 N HCl was added. DNPH-free HCl added to 1 ml of homogenate provided a blank. The mixtures were incubated for 1 h at room temperature. The protein was precipitated with an equal volume of 20% trichloroacetic acid and washed three times with ethanol-ethyl acetate (1:1 vol/vol). The final precipitate was dissolved in 2 ml of 6 M guanidine hydrochloride (pH 2.3), and insoluble debris was removed by centrifugation. The absorbance of the DNPH derivatives was measured at 360 nm. The protein carbonyl content was calculated by applying an absorbance coefficient of 22 nM/cm and expressed as nanomoles of carbonyl per milligram protein (35).
Amyloid-β contents.
Amyloid peptides Aβ40 and Aβ42 were measured by enzyme-linked immunosorbent assay (ELISA). Approximately 100 mg of brain tissue was homogenized at 4°C in 5 M guanidine HCl and 50 mM Tris·HCl. Homogenate was then mixed for 4 h at room temperature using a rotating mixer, diluted (1:5) in BSAT-DPBS reaction buffer (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, 8.1 mM Na2HPO4, 5% BSA, and 0.03% Tween-20) supplemented with a protease inhibitor, and then centrifuged at 16,000 g for 20 min at 4°C. The supernatants were transferred to polypropylene tubes and kept on ice until use. The standards and samples were incubated in 96-well plates for 2 h at room temperature, and the wells were washed four times with wash buffer (400 μl/well). Aβ40 or Aβ42 antibody (100 μl) was added to each well. The plate was covered and incubated for 1 h at room temperature and washed four times with wash buffer. Anti-rabbit IgG horseradish peroxidase (100 μl) was added to each well except for chromogen blanks. The plate was covered, incubated at room temperature for 30 min, and washed four times with wash buffer. Stabilized chromogen (100 μl) was added to each well and incubated at room temperature in the dark. Stop solution (100 μl) was added to each well, and the plate was tapped gently to mix. The absorbance of each well, including blank wells as controls against the chromogen well, was read at 450 nm.
Data and statistical analysis.
All numerical data are expressed as means ± SE. Student’s t-test was applied for two group comparisons. Single-factor comparisons of three groups and two-factor comparisons of four groups were accomplished with single- and two-factor ANOVAs, respectively, followed by Tukey’s honestly significantly different post hoc analyses. Each immunoblot was repeated two to three times with multiple samples from each treatment group. Blots with clear band images were selected for statistical analysis and presented in the figures. P values of <0.05 were taken to indicate statistically significant differences between groups.
RESULTS
IHT attenuates PS1 expression in EW rats.
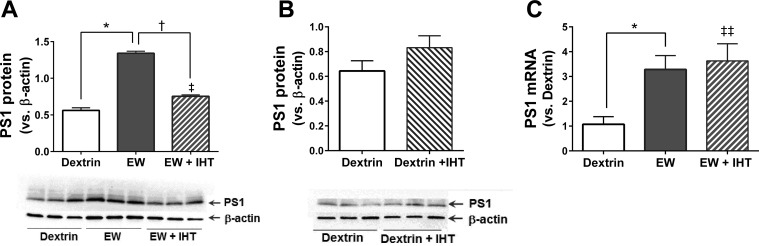
We previously demonstrated in rats that abrupt and repeated withdrawal from chronic ethanol intake provokes PS1 overexpression in cerebral cortex (28). Here, we tested whether IHT attenuates this EW effect. PS1 protein content in prefrontal cortex more than doubled following EW vs. control dextrin diet (P < 0.0001), yet IHT prevented this increase (P < 0.0001) [F (2, 15) = 214.6, P < 0.0001]. Nevertheless, PS1 protein content in EW + IHT rats was still higher than that in dextrin rats (P < 0.01) (Fig. 1A). IHT alone, in the absence of EW, produced no significant effect on PS1 content (Fig. 1B). We also assessed the effect of IHT on the abundance of PS1 mRNA to determine whether IHT affects PS1 transcription. EW sharply increased PS1 mRNA abundance (P < 0.002), but IHT (P > 0.05) did not lower PS1 mRNA in EW rats [F (2, 45) = 6.4, P = 0.0035] (Fig. 1C). PS1 mRNA abundance in EW + IHT rats was higher than that in dextrin rats (P < 0.002) (Fig. 1C). These results show that IHT prevents EW induction of PS1 protein but not PS1 mRNA.
Fig. 1.
Intermittent hypoxia training (IHT) suppresses PS1 in ethanol-withdrawn rats. Male rats aged 3 mo completed 2 cycles of consuming an ethanol (6.5% vol/vol) diet for 4 wk, followed by 3 wk of ethanol withdrawal (EW). Control diet contained dextrin in place of ethanol. IHT was applied for 20 days during the last 3 days of the first ethanol diet and the subsequent 17 days of EW. Brains were harvested after the 2nd EW cycle. PS1 protein (A) and mRNA (B) were analyzed by immunoblot and quantitative PCR, respectively. β-Actin served as loading control for PS1 protein. A: PS1 protein content was elevated in EW rats compared with dextrin control (*P < 0.0001) or EW + IHT rats (†P < 0.0001) and higher in EW + IHT rats (‡P < 0.01) than in dextrin rats. B: PS1 protein content did not significantly differ between dextrin and dextrin + IHT rats. C: PS1 mRNA abundance in EW rats was higher than in dextrin rats (*P < 0.002) but did not differ from EW + IHT rats. PS1 mRNA abundance was higher in EW + IHT rats than in dextrin rats (‡‡P < 0.002); n = 6 rats/group.
IHT prevents p38 activation and preserves HSP25 dimers in EW rats.
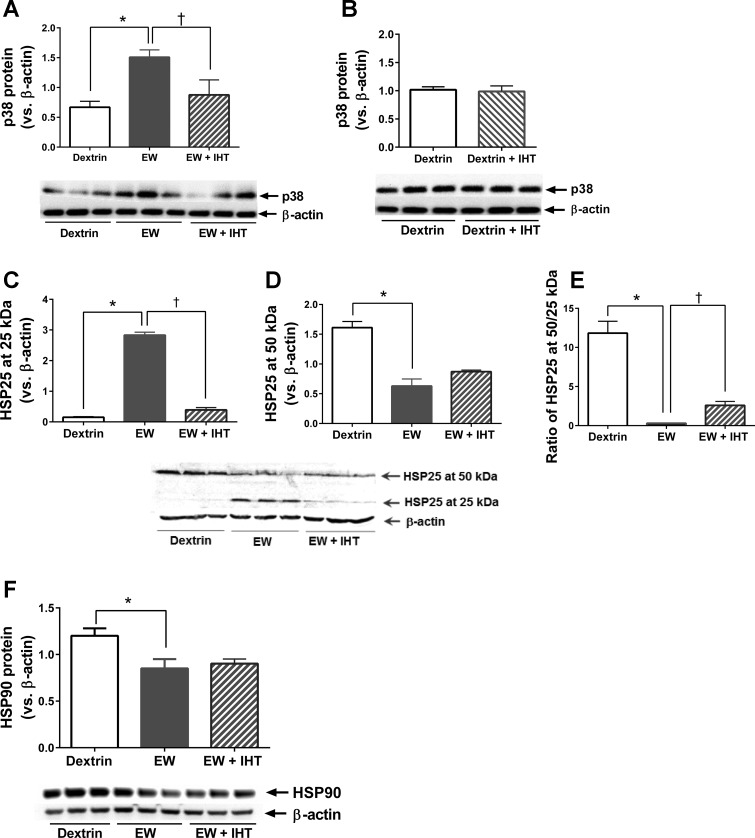
We recently demonstrated that an inhibitor of the stress-activated protein kinase p38 attenuated PS1 expression in ethanol-withdrawn mouse hippocampal HT22 cells (28), so we examined the impact of IHT on p38 activation. When compared with dextrin control rats, EW rats showed a doubling of cerebrocortical p38 phosphorylation (P < 0.0004), and IHT prevented this EW effect [F (2, 15) = 6.4, P < 0.01, P < 0.05; Fig. 2A). p38 phosphorylation did not differ between dextrin and dextrin + IHT rats (P > 0.05; Fig. 2B).
Fig. 2.
Effects of IHT on p38, heat shock protein (HSP)25, and HSP90 in EW rats. Prefrontal cortical contents of p38 (A and B), HSP25 (C–E), and HSP90 (F) were assessed by immunoblot in the same experiments as Fig. 1. β-Actin provided loading control. A: p38 phosphorylation was elevated in EW rats vs. dextrin control (*P < 0.0004), and IHT prevented this EW effect (†P < 0.05). B: p38 phosphorylation did not differ between dextrin and dextrin + IHT rats. C and D: content of HSP25 monomers (25 kDa) was higher in EW rats than in dextrin (*P < 0.0001; C) or EW + IHT (†P < 0.0001; C) rats, whereas HSP25 dimers (50 kDa) were decreased in EW vs. control rats (*P < 0.0001; D). EW + IHT rats showed a slight, not statistically significant increase (P > 0.05) in HSP25 at 50 kDa vs. EW rats (D). E: the ratio of HSP25 at 50 kDa vs. 25 kDa was lower in EW than control rats (*P < 0.0001) and higher in EW + IHT than EW rats (†P < 0.001). F: HSP90 content was lower in EW than in control rats (*P < 0.0001) and was not preserved in EW + IHT rats; n = 6 rats/group.
Because p38 phosphorylates HSP25 in response to stress, we examined the impact of EW and IHT on HSP25. EW rats showed a sharp increase in HSP25 at 25kDa, i.e., the monomeric form of HSP25 (P < 0.0001), but IHT almost completely prevented EW-induced accumulation of HSP25 monomers (P < 0.0001) [F (2, 15) = 416.9, P < 0.0001] (Fig. 2C). By contrast, EW rats had only half as much HSP25 at 50 kDa (the dimer form of HSP25) as dextrin rats (P < 0.0001) [F (2, 15) = 40.8, P < 0.0001] (Fig. 2D). Although the amount of HSP25 at 50 kDa in EW + IHT rats appeared to be higher than in EW rats, the difference did not attain statistical significance (P > 0.05). On the other hand, the ratio of HSP25 at 50 kDa (dimer) versus 25 kDa (monomer) was strikingly decreased in EW versus dextrin rats (P < 0.0001) but was partially preserved in EW + IHT rats (P < 0.001) [F (2, 15) = 50.73, P < 0.0001] (Fig. 2E). It is thus apparent that IHT at least partially prevents the structural perturbation of HSP25 induced by EW.
The effects of IHT on HSP25 raised the possibility that IHT might also protect higher molecular weight HSPs in addition to small HSPs like HSP25. Accordingly, we examined the impact of EW and IHT on a larger molecular chaperone, HSP90 (Fig. 2F). As was the case for the HSP25 dimer, EW sharply lowered HSP90 content (P < 0.0001). However, IHT did not prevent the loss of HSP90 (P > 0.05) [F (2, 15) = 61.21] (Fig. 2F). These results demonstrate that IHT protection is selective for HSP25 rather than a global effect on all HSPs.
IHT attenuates protein carbonylation in EW rats.
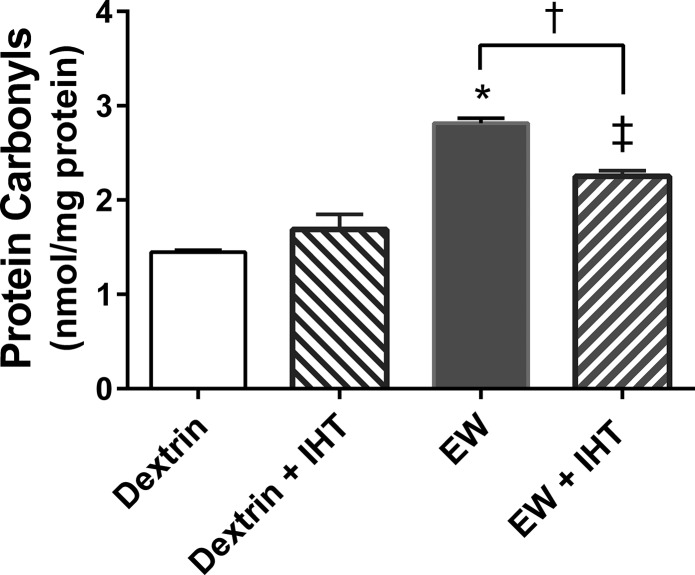
HSP25 has been found to protect proteins from oxidative damage (23). If IHT protects HSP25, it may also minimize protein oxidation. Accordingly, we assessed the impact of EW and IHT on protein carbonylation, an established marker of oxidatively modified proteins. The amount of protein carbonyls was higher in EW rats than dextrin rats (P < 0.0001), a difference that was attenuated by IHT (P < 0.001) [F (1, 20) = 116.1, P < 0.0001 by EW factor; F (1, 20) = 3.2, P < 0.05 by IHT factor] (Fig. 3). Nevertheless, protein carbonyl content in EW + IHT rats was still higher than that in dextrin rats (P < 0.001). These data show that IHT exerts antioxidant protection that attenuates EW-induced protein carbonylation.
Fig. 3.
IHT suppresses EW-induced protein carbonylation. Protein carbonyl contents were assessed by spectrophotometry in extracts of prefrontal cortex. EW rats showed greater protein carbonyl contents than dextrin, dextrin + IHT, or EW + IHT rats. *P < 0.0001; †P < 0.001; ‡P < 0.001 vs. dextrin or dextrin + IHT (n = 6 rats/group).
IHT attenuates EW-induced amyloid accumulation in prefrontal cortex.
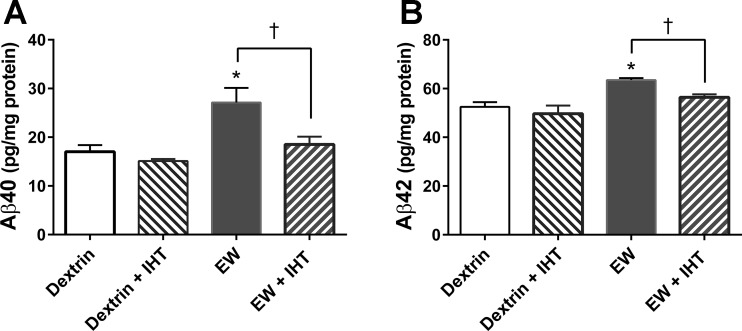
Presenilin 1 is the catalytic subunit of γ-secretase (19), a protease that cleaves APP, yielding Aβ40 and Aβ42. The accumulation and aggregation of these amyloid peptides are known to be neurotoxic (21). Our observation that PS1 was increased in EW rats and decreased in EW + IHT rats led us to propose that Aβ40 and Aβ42 would be increased by EW in a manner suppressed by IHT. Aβ40 content was indeed higher in EW rats than in dextrin (P < 0.005), dextrin + IHT (P < 0.001), and EW + IHT (P < 0.02) rats [F (1, 53) = 12.07, P < 0.001, by EW; F (1, 44) = 7.35, P < 0.01, by IHT] (Fig. 4A). Similarly, Aβ42 content was higher in EW rats than in dextrin (P < 0.0039), dextrin + IHT (P < 0.01), and EW + IHT (P < 0.005) rats [F (1, 44) = 19, P < 0.001, by EW; F (1, 53) = 6.0, P < 0.02 by IHT] (Fig. 4B). Thus EW stress provokes the accumulation of Aβ40 and Aβ42 in prefrontal cortex in a manner attenuated by IHT.
Fig. 4.
IHT prevents cerebrocortical amyloid-β (Aβ)40 and Aβ42 accumulation in EW rats. Aβ40 and Aβ42 were measured by ELISA in extracts of prefrontal cortex. A: Aβ40 contents were higher in EW rats than in dextrin (*P < 0.005), dextrin + IHT (P < 0.001), or EW + IHT (†P < 0.02) rats. B: EW rats also showed higher contents of Aβ42 than dextrin (*P < 0.0039), dextrin + IHT (P < 0.01), or EW + IHT (†P < 0.005) rats; n = 6 rats/group.
IHT does not alter transcription factor NICD in EW rats.
In addition to catalyzing Aβ production, γ-secretase cleaves the transmembrane protein Notch, producing Notch intracellular domain (NICD) (53), which then enters nuclei and regulates transcription. We have reported that EW stress increases both PS1 and NICD in rat brain (28). Here, we determined whether IHT decreases NICD as it did Aβ. As expected, NICD content was higher in EW than dextrin rats (P < 0.001; Fig. 5). Unexpectedly, the content of NICD did not differ appreciably between EW and EW + IHT rats (P > 0.05) and was even increased in dextrin + IHT versus dextrin rats (P < 0.001) [F (1, 28) = 2.050, P < 0.05 by EW; F (1, 28) = 15.30, P < 0.0005 by IHT]. These data show that IHT exerts distinct effects on the two γ-secretase pathways (Aβ production vs. NICD signaling) in EW rats.
Fig. 5.
Effects of IHT on Notch intracellular domain (NICD) content in EW rats. Cerebrocortical NICD contents were assessed by immunoblot, with β-actin as loading control. NICD contents in EW rats were higher than in dextrin rats (*P < 0.001) but were not affected appreciably by IHT (P > 0.05). NICD content was higher in dextrin + IHT than in dextrin rats (†P < 0.001); n = 6 rats/group.
DISCUSSION
This study demonstrated that IHT attenuates the overproduction of γ-secretase catalytic subunit PS1 and γ-secretase products Aβ40 and Aβ42 in prefrontal cortex of EW rats. These IHT effects were accompanied by attenuation of p38 overactivation, loss of HSP25 dimers, and protein oxidation. Importantly, IHT was applied only during the first EW cycle, yet its beneficial effects persisted throughout a second cycle of 4-wk ethanol intake and 3-wk EW. IHT’s cytoprotective actions replicate the effects of γ-secretase and p38 inhibitors that we reported recently in a mouse hippocampal cell model of EW (28). The current findings are the first evidence that IHT can protect brain against endogenous overexpression of PS1 and its Aβ products in EW rats via mechanisms involving p38 inhibition and HSP25 dimer stabilization.
Figure 6 presents a proposed integration of IHT’s protective modulation of multiple components of the EW-induced p38/PS1/Aβ40 cascade. In this scenario, EW provokes glutamate release, leading to p38 phosphorylated activation. P-p38, in turn, phosphorylates HSP25 dimers, causing their dissociation, thereby disabling the chaperone’s antioxidant function with resultant oxidative damage and carbonylation of cellular proteins. P-p38 also activates PS1 and thus γ-secretase, which in turn activates the transcription factor NICD, which in some settings has proven to be neuroprotective (56, 60), and cytotoxic overproduction of Aβ40 and Aβ42. Protein carbonylation and Aβ accumulation culminate in the death of brain cells (30, 39). IHT intervenes at several steps in the EW-initiated cascade; it suppresses p38 activation, preserves HSP25 dimers sufficiently to prevent protein carbonylation, activates NICD signaling, and dampens Aβ accumulation.
Fig. 6.
Proposed mechanisms of IHT neuroprotection against EW-induced activation of p38/PS1/Aβ cascade. See text for details. The effect indicated by the dashed arrow was not statistically significant in this study.
Mutations of the PS1 gene provoke Aβ accumulation and aggregation, hallmarks of Alzheimer’s disease, but the pathophysiological significance of overexpressing the PS1 wild type is still under investigation. We recently demonstrated increased PS1 content in ethanol-withdrawn rats (28). In that study, the PS1 increase was attenuated by a glutamate receptor antagonist, indicating that increased PS1 is pivotal to signaling activated by EW. The current study revealed that IHT blunted the increase in PS1 protein but not PS1 mRNA in EW rats. This finding suggests that IHT suppresses PS1 synthesis after its mRNA transcription. Instead, IHT may regulate posttranslational processing of PS1 through other molecules that interact with PS1, and p38 is a likely candidate for such interaction. Indeed, EW stress activates p38, which in turn upregulates PS1 in a manner suppressed by IHT. In support of this scenario, EW + IHT rats showed smaller amounts of both PS1 and p38 than non-IHT EW rats. Accordingly, we propose that IHT protects against EW induction of excessive PS1 by restraining p38 (Fig. 6).
Heat shock proteins coordinate proper folding and function of target proteins (10, 34). HSP25 belongs to the chaperone HSP family that mediates proper folding/unfolding of target proteins, including PS1 (28, 32) and confers resistance to oxidative stress (12, 14). The structural configuration of HSP25 is intimately associated with its cytoprotective and antioxidant properties (12, 14). HSP25 resides in cells as oligomers under normal conditions. Stress-activated p38 phosphorylates HSP25 oligomers, causing their dissociation into smaller units, particularly dimers, the most effective form of the chaperone (15, 34).
Overexpressed PS1 proteins aggregate; the accumulation of these PS1 “aggresomes” has been associated with Alzheimer’s disease (33). Misfolding and aggregation of PS1 may be prevented by small HSPs; indeed, HSP40 has been found to prevent aggregation of PS1 (32, 33). Wilhelmus et al. (58) demonstrated HSP22 and HSP27 binding of amyloid peptides, preventing plaque formation. Because PS1 is a component of γ-secretase that produces Aβ, these findings are indirect evidence of a small HSP:PS1 interaction. Fonte et al. (17) reported that the overexpression of another small HSP, HSP-16.2, reduced Aβ toxicity by interacting with the Aβ peptides. These findings, together with the protection of IHT against excessive p38, prompted us to examine the effect of IHT on HSP25 in EW rats. Excessive p38 induced by high-stress EW may dissociate HSP25 dimers into nonfunctioning monomers. Accordingly, the IHT-induced increase in the dimer-to-monomer ratio of HSP25 may indicate that IHT protects HSP25’s dimer configuration under EW stress, thereby preserving its chaperoning activity.
A distinct property of small HSPs is their antioxidant activity. For example, overexpression of HSP27 and HSP16.2 reduced protein carbonylation in postischemic mouse myocardium (23), and HSP27 overexpression prevented neuronal death induced by H2O2 (10). Mutant mice lacking HSP25 dimers were vulnerable to H2O2 (12), yet these mice still possessed intact HSP25 monomers (12). Thus the structural configuration of HSP25 is pivotal to its antioxidant capabilities, such that EW-induced disintegration of HSP25 dimers may leave cells vulnerable to protein carbonylation. PS1 accumulation in EW rats may be the result of HSP25 structural perturbation and impaired chaperone activity. In support of this scenario, EW rats with a decreased HSP25 dimer-to-monomer ratio showed increased PS1 expression and protein oxidation, phenomena that were prevented by IHT. To determine whether IHT’s protection is specific for small HSPs, we examined the impact of IHT on the larger HSP90. EW rats showed a decrease in HSP90 expression, but IHT was unable to reverse this effect. It is thus plausible that IHT protection may be selective for small antioxidant HSPs under EW conditions.
γ-Secretase mediates two distinct pathways (53). It cleaves APP, producing Aβ peptides, and the transmembrane protein Notch, producing NICD that enters nuclei and modulates gene transcription. γ-Secretase is minimally composed of PS1, nicastrin, and β-catenin subunits (53), and PS1 is required for Aβ production (11, 19, 42). The finding of EW-induced increase and IHT-induced decrease in PS1 prompted us to test whether EW increases γ-secretase products (Aβ40, Aβ42, and NICD) in a manner prevented by IHT (Fig. 6). All three products were indeed increased in the brains of EW versus control rats, whereas IHT blunted the increases in Aβ40 and Aβ42 but not NICD. The increases in Aβ peptides in EW rats were expected, as their accumulation is closely associated with hyperactive synaptic dysfunction (16).
A direct relationship may exist between neuronal hyperactivity or glutamate and Aβ accumulation. Hyperactivity of pyramidal neurons occurred before amyloid plaque deposition in the hippocampus of mice carrying mutated APP and PS1 genes (6). Hyperactivity of cortical and hippocampal neurons commonly occurs in transgenic mouse models of Alzheimer’s disease (6, 7, 45, 54) as well as in humans with mild cognitive impairments (2) or Alzheimer’s disease (55). Young mice carrying mutated APP/PS1 genes, a model of Alzheimer’s disease, showed increased glutamate release in hippocampal neurons (13). These studies suggest that excessive glutamate release may be one of the factors triggering accumulation of Aβ peptides. Reciprocal increases in glutamate release by Aβ also have been shown. Inhibition of Aβ-degrading enzymes augmented glutamate release from hippocampal neurons in parallel with Aβ accumulation (1), and Aβ40 overexpression augmented glutamate release (16).
In the current study, rats were healthy before undergoing the ethanol diet and EW stress. Therefore, Aβ accumulation may be a consequence rather than a cause of EW stress. Relevant to the protection of IHT against Aβ is a previous study that assessed the effect of IHT on cerebrovascular endothelial function (38). In that study, rats completed IHT, and thereafter an Aβ fragment was administered to the basal magnocellular nucleus of the forebrain (38). The Aβ fragment impaired endothelium-mediated vasodilation in a manner ameliorated by IHT. The current study extends these findings to demonstrate IHT’s protection against the overexpression of endogenous Aβ upon EW stress.
The selective effect of IHT on γ-secretase products, in which IHT attenuated Aβ40 and Aβ42 but not NICD, was unexpected. How IHT differentially affects these products of γ-secretase is unknown. EW + IHT produced an increase in NICD similar to that of EW alone; moreover, IHT alone increased NICD in dextrin control rats. Should EW or IHT alone maximally increase NICD through a common mechanism, combined EW and IHT might not produce an additive effect on NICD content. NICD enters nuclei and triggers a DNA-binding protein to activate its target genes (31), so the absence of NICD represses transcription of these genes. From a therapeutic standpoint, NICD inhibition can be beneficial for treating certain diseases, such as cancer by gene repression (43, 46). However, nonselective repression of NICD-inducible genes might not be beneficial. Notch signaling is highly conserved across species, implying that it plays a fundamental physiological role, and thus, the global suppression of Notch signaling in normal cells may be detrimental. Indeed, inhibition of Notch signaling induced apoptosis in gastric epithelial cells (20), suggesting that Notch signaling is required to maintain gastric epithelial cell homeostasis. The ability of IHT to selectively inhibit γ-secretase products may thus be a therapeutic advantage that targets a specific molecular pathway.
This study showed that IHT attenuates EW-induced upregulation of γ-secretase’s catalytic subunit PS1 and its proteolysis products Aβ40 and Aβ42, perturbation of p38 signaling (p38 and its substrate HSP25), and protein carbonylation. In HT22 cells, a mouse hippocampal cell line, EW and glutamate exposure increased PS1 and phosphorylated p38α, in parallel with loss of cell viability (28). The cytotoxic effects of EW and glutamate were blunted by the γ-secretase inhibitor DAPT. The p38α inhibitor SB-203580 lowered PS1 content in HT22 cells subjected to EW or glutamate. Collectively, this recent study and the current work demonstrate that concerted mechanisms involving p38/γ-secretase/prooxidant stress may mediate EW-induced brain disorders such as cognitive deficit. The current study shows IHT’s ability to suppress this harmful cascade in a manner that persists for several weeks after the IHT program.
The limitations of this study must be acknowledged. Technical limitations of the rat model precluded assessment of the direct interaction between HSP25 and PS1 or Aβ peptides during EW. Specifically, we did not evaluate neuronal death, and thus we do not know whether EW-induced elevation of Aβ40 and Aβ42 produces neurotoxic effects. Chronic pharmacological modulation of p38 and γ-secretase was not practical in the intact rats undergoing chronic EW and IHT, so it was not possible to determine whether p38 and γ-secretase inhibitors can duplicate the effects of IHT or that pharmacological activators of these enzymes blunt the effects of IHT on HSP25 and protein carbonylation. Also, this study was conducted in young rats that displayed a moderate elevation in Aβ after two episodes of EW.
It is premature to extrapolate the present results to human alcoholics, who many of whom are older adults and typically undergo several episodes of EW. Conceivably, multiple EW episodes might produce sufficient Aβ accumulation to cause dementia in older rats and, possibly, older adult humans. Accordingly, further studies should apply several episodes of EW to older rats to determine whether EW-induced Aβ elevation increases with age and whether IHT can dampen Aβ accumulation in the brains of older rats. Supportive of a critical role of age in Aβ-associated central nervous system disorders is the study by Hsiao et al. (24), who found that transgenic mice overexpressing human Aβ precursor genes had normal learning and memory function at 3 mo of age but marked impairment by 9–10 mo.
Despite these limitations, this study provides empirical evidence that IHT protects the brain from EW stress through the orchestrated mechanism of suppression of PS1 content and amyloid-β accumulation, blunting of p38 signaling, and preservation of antioxidant activity of HSP25 (Fig. 6). The therapeutic application of IHT to treat EW-induced damage to brain cells merits further study.
Perspectives and Significance
This study examined the cerebroprotective effects of IHT against EW stress. EW was associated with increased PS1, the catalytic component of the amyloid-β-generating protease γ-secretase (28). IHT moderates overactivation of p38 and maintains small HSPs in their physiologically active forms, e.g., dimeric HSP25. IHT suppression of EW-induced p38 phosphorylated activation is closely associated with decreased endogenous PS1 content. Therefore, IHT could be beneficial in persons who are prone to neurocognitive diseases. Based on numerous studies exploring the pathophysiology of cognitive impairment, including Alzheimer’s disease, and the current findings, it is reasonable to speculate that IHT may eventually provide an effective intervention to prevent and/or treat cognitive impairment. Additionally, activated p38 is closely associated with numerous neuropathologies. A recent search of the PubMed database identified more than 36,000 publications reporting role of p38 in pathological conditions. IHT impacts the entire organism, not the brain alone, so the effect of IHT on other organs merits investigation. Because the hypoxia imposed by the IHT regimen is moderate and transitory, IHT is minimally stressful to human subjects. Therefore, IHT may provide a noninvasive treatment that could be readily administered without harming the recipient.
GRANTS
This work was supported by research grants from the National Institute of Alcohol Abuse and Alcoholism (AA-015982), the National Institute of Aging (AG-053974), the National Institute of Neurological Disorders and Stroke (NS-076975), the University of North Texas Health Science Center (UNTHSC) Institute for Aging and Alzheimer’s Disease Research, and the UNTHSC Institute for Cardiovascular and Metabolic Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.-G.R. and M.E.J. conceived and designed research; M.-G.R., D.B.M., and M.E.J. analyzed data; M.-G.R., R.T.M., and M.E.J. interpreted results of experiments; M.-G.R., R.T.M., and M.E.J. prepared figures; M.-G.R., R.T.M., and M.E.J. drafted manuscript; M.-G.R., R.T.M., D.B.M., and M.E.J. edited and revised manuscript; M.-G.R., R.T.M., and M.E.J. approved final version of manuscript; D.B.M. performed experiments.
REFERENCES
- 1.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12: 1567–1576, 2009. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 2.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74: 467–474, 2012. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry 133: 1–14, 1978. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res 17: 94–98, 1993. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry 23: 507–514, 1988. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 6.Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 109: 8740–8745, 2012. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321: 1686–1689, 2008. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 8.Chang YJ, Chen YR. The coexistence of an equal amount of Alzheimer’s amyloid-β 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. FEBS J 281: 2674–2687, 2014. doi: 10.1111/febs.12813. [DOI] [PubMed] [Google Scholar]
- 9.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 429: 403–417, 2010. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 10.Dávila D, Jiménez-Mateos EM, Mooney CM, Velasco G, Henshall DC, Prehn JH. Hsp27 binding to the 3'UTR of bim mRNA prevents neuronal death during oxidative stress-induced injury: a novel cytoprotective mechanism. Mol Biol Cell 25: 3413–3423, 2014. doi: 10.1091/mbc.E13-08-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522, 1999. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP. Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 7: 436–445, 2005. doi: 10.1089/ars.2005.7.436. [DOI] [PubMed] [Google Scholar]
- 13.Dolev I, Fogel H, Milshtein H, Berdichevsky Y, Lipstein N, Brose N, Gazit N, Slutsky I. Spike bursts increase amyloid-β 40/42 ratio by inducing a presenilin-1 conformational change. Nat Neurosci 16: 587–595, 2013. doi: 10.1038/nn.3376. [DOI] [PubMed] [Google Scholar]
- 14.Dudich IV, Zav’yalov VP, Pfeil W, Gaestel M, Zav’yalova GA, Denesyuk AI, Korpela T. Dimer structure as a minimum cooperative subunit of small heat-shock proteins. Biochim Biophys Acta 1253: 163–168, 1995. doi: 10.1016/0167-4838(95)00135-X. [DOI] [PubMed] [Google Scholar]
- 15.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56: 385–431, 1998. doi: 10.1016/S0301-0082(98)00032-X. [DOI] [PubMed] [Google Scholar]
- 16.Fogel H, Frere S, Segev O, Bharill S, Shapira I, Gazit N, O’Malley T, Slomowitz E, Berdichevsky Y, Walsh DM, Isacoff EY, Hirsch JA, Slutsky I. APP homodimers transduce an amyloid-β-mediated increase in release probability at excitatory synapses. Cell Reports 7: 1560–1576, 2014. doi: 10.1016/j.celrep.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Fonte V, Kipp DR, Yerg J III, Merin D, Forrestal M, Wagner E, Roberts CM, Link CD. Suppression of in vivo β-amyloid peptide toxicity by overexpression of the HSP-16.2 small chaperone protein. J Biol Chem 283: 784–791, 2008. doi: 10.1074/jbc.M703339200. [DOI] [PubMed] [Google Scholar]
- 18.Fowler AK, Thompson J, Chen L, Dagda M, Dertien J, Dossou KSS, Moaddel R, Bergeson SE, Kruman II. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity. PLoS One 9: e106945, 2014. doi: 10.1371/journal.pone.0106945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3: 688–694, 2002. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gifford GB, Demitrack ES, Keeley TM, Tam A, La Cunza N, Dedhia PH, Spence JR, Simeone DM, Saotome I, Louvi A, Siebel CW, Samuelson LC. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut 66: 1001–1011, 2016. doi: 10.1136/gutjnl-2015-310811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goryacheva AV, Kruglov SV, Pshennikova MG, Smirin BV, Malyshev IY, Barskov IV, Viktorov IV, Downey HF, Manukhina EB. Adaptation to intermittent hypoxia restricts nitric oxide overproduction and prevents beta-amyloid toxicity in rat brain. Nitric Oxide 23: 289–299, 2010. doi: 10.1016/j.niox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811, 1994. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 23.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation 110: 3544–3552, 2004. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: 99–103, 1996. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 25.Jamasbi E, Wade JD, Separovic F, Hossain MA. Amyloid beta (Aβ) peptide and factors that play important roles in Alzheimer’s disease. Curr Med Chem 23: 884–892, 2016. doi: 10.2174/0929867323666160229113911. [DOI] [PubMed] [Google Scholar]
- 26.Ju X, Mallet RT, Downey HF, Metzger DB, Jung ME. Intermittent hypoxia conditioning protects mitochondrial cytochrome c oxidase of rat cerebellum from ethanol withdrawal stress. J Appl Physiol (1985) 112: 1706–1714, 2012. doi: 10.1152/japplphysiol.01428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung ME, Ju X, Simpkins JW, Metzger DB, Yan LJ, Wen Y. Ethanol withdrawal acts as an age-specific stressor to activate cerebellar p38 kinase. Neurobiol Aging 32: 2266–2278, 2011. doi: 10.1016/j.neurobiolaging.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung ME, Metzger DB, Das HK. The role of presenilin-1 in the excitotoxicity of ethanol withdrawal. J Pharmacol Exp Ther 358: 516–526, 2016. doi: 10.1124/jpet.116.233361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J Appl Physiol (1985) 105: 510–517, 2008. doi: 10.1152/japplphysiol.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamarehei M, Yazdanparast R. Modulation of notch signaling pathway to prevent H2O2/menadione-induced SK-N-MC cells death by EUK134. Cell Mol Neurobiol 34: 1037–1045, 2014. doi: 10.1007/s10571-014-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233, 2009. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koren J III, Jinwal UK, Lee DC, Jones JR, Shults CL, Johnson AG, Anderson LJ, Dickey CA. Chaperone signalling complexes in Alzheimer’s disease. J Cell Mol Med 13: 619–630, 2009. doi: 10.1111/j.1582-4934.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs I, Lentini KM, Ingano LM, Kovacs DM. Presenilin 1 forms aggresomal deposits in response to heat shock. J Mol Neurosci 29: 9–19, 2006. doi: 10.1385/JMN:29:1:29. [DOI] [PubMed] [Google Scholar]
- 34.Lelj-Garolla B, Mauk AG. Self-association and chaperone activity of Hsp27 are thermally activated. J Biol Chem 281: 8169–8174, 2006. doi: 10.1074/jbc.M512553200. [DOI] [PubMed] [Google Scholar]
- 35.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186: 464–478, 1990. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- 36.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens 29: 2265–2272, 2011. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- 37.Manukhina EB, Goryacheva AV, Barskov IV, Viktorov IV, Guseva AA, Pshennikova MG, Khomenko IP, Mashina SY, Pokidyshev DA, Malyshev IY. Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer’s disease by adaptation to hypoxia. Neurosci Behav Physiol 40: 737–743, 2010. doi: 10.1007/s11055-010-9320-6. [DOI] [PubMed] [Google Scholar]
- 38.Mashina SY, Aleksandrin VV, Goryacheva AV, Vlasova MA, Vanin AF, Malyshev IY, Manukhina EB. Adaptation to hypoxia prevents disturbances in cerebral blood flow during neurodegenerative process. Bull Exp Biol Med 142: 169–172, 2006. doi: 10.1007/s10517-006-0318-6. [DOI] [PubMed] [Google Scholar]
- 39.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by β-amyloid(1-40). Brain Res 958: 210–221, 2002. doi: 10.1016/S0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 40.Morgan PF, Nadi NS, Karanian J, Linnoila M. Mapping rat brain structures activated during ethanol withdrawal: role of glutamate and NMDA receptors. Eur J Pharmacol 225: 217–223, 1992. doi: 10.1016/0922-4106(92)90023-O. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-α, and H2O2. J Biol Chem 271: 26981–26988, 1996. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- 42.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol 228: 151–165, 2000. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 43.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood 111: 2220–2229, 2008. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 44.National Institute on Alcohol Abuse and Alcoholism The Physicians’ Guide to Helping Patients with Alcohol Problems. Bethesda, MD: National Institutes of Health, 1995. [Google Scholar]
- 45.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55: 697–711, 2007. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purow B. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol 727: 305–319, 2012. doi: 10.1007/978-1-4614-0899-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roenker NL, Gudelsky GA, Ahlbrand R, Horn PS, Richtand NM. Evidence for involvement of nitric oxide and GABA(B) receptors in MK-801- stimulated release of glutamate in rat prefrontal cortex. Neuropharmacology 63: 575–581, 2012. doi: 10.1016/j.neuropharm.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rostron B. Alcohol consumption and mortality risks in the USA. Alcohol Alcohol 47: 334–339, 2012. doi: 10.1093/alcalc/agr171. [DOI] [PubMed] [Google Scholar]
- 49.Ryou MG, Sun J, Oguayo KN, Manukhina EB, Downey HF, Mallet RT. Hypoxic conditioning suppresses nitric oxide production upon myocardial reperfusion. Exp Biol Med (Maywood) 233: 766–774, 2008. doi: 10.3181/0710-RM-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci 14: 2023–2031, 2001. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, Li M, Han J, Gu J. Sensitization of differentiated PC12 cells to apoptosis by presenilin-2 is mediated by p38. Biochem Biophys Res Commun 287: 536–541, 2001. doi: 10.1006/bbrc.2001.5598. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Chen WD, Wang YD. β-Amyloid: the key peptide in the pathogenesis of Alzheimer’s disease. Front Pharmacol 6: 221, 2015. doi: 10.3389/fphar.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tandon A, Fraser P. The presenilins. Genome Biol 3: 3014.1, 2002. doi: 10.1186/gb-2002-3-11-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149: 708–721, 2012. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70: 1158–1166, 2013. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K, Zhang L, Rao W, Su N, Hui H, Wang L, Peng C, Tu Y, Zhang S, Fei Z. Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci Lett 591: 53–58, 2015. doi: 10.1016/j.neulet.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, Delaney J, Cole CN, Chan-Hui PY, Mantlo N, Lichenstein HS, Zukowski M, Yao Z. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem 272: 23668–23674, 1997. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 58.Wilhelmus MM, Boelens WC, Otte-Höller I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-β protein aggregation and cerebrovascular amyloid-β protein toxicity. Brain Res 1089: 67–78, 2006. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 59.Xing B, Bachstetter AD, Van Eldik LJ. Inhibition of neuronal p38α, but not p38β MAPK, provides neuroprotection against three different neurotoxic insults. J Mol Neurosci 55: 509–518, 2015. doi: 10.1007/s12031-014-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang HP, Sun YY, Chen XM, Yuan LB, Su BX, Ma R, Zhao RN, Dong HL, Xiong L. The neuroprotective effects of isoflurane preconditioning in a murine transient global cerebral ischemia-reperfusion model: the role of the Notch signaling pathway. Neuromolecular Med 16: 191–204, 2014. doi: 10.1007/s12017-013-8273-7. [DOI] [PubMed] [Google Scholar]
- 61.Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood) 229: 806–812, 2004. [DOI] [PubMed] [Google Scholar]