Here, we demonstrate that S-nitrosylation of vasodilator-stimulated phosphoprotein (VASP) on C64 is a mechanism for the onset of platelet-activating factor-induced hyperpermeability. Our results reveal a dual role of VASP in endothelial permeability. In addition to its well-documented function in barrier integrity, we show that S-nitrosylation of VASP contributes to the onset of endothelial permeability.
Keywords: nitric oxide, inflammation, endothelial function, vasodilator-activated phosphoprotein
Abstract
We tested the hypothesis that platelet-activating factor (PAF) induces S-nitrosylation of vasodilator-stimulated phosphoprotein (VASP) as a mechanism to reduce microvascular endothelial barrier integrity and stimulate hyperpermeability. PAF elevated S-nitrosylation of VASP above baseline levels in different endothelial cells and caused hyperpermeability. To ascertain the importance of endothelial nitric oxide synthase (eNOS) subcellular location in this process, we used ECV-304 cells transfected with cytosolic eNOS (GFPeNOSG2A) and plasma membrane eNOS (GFPeNOSCAAX). PAF induced S-nitrosylation of VASP in cells with cytosolic eNOS but not in cells wherein eNOS is anchored to the cell membrane. Reconstitution of VASP knockout myocardial endothelial cells with cysteine mutants of VASP demonstrated that S-nitrosylation of cysteine 64 is associated with PAF-induced hyperpermeability. We propose that regulation of VASP contributes to endothelial cell barrier integrity and to the onset of hyperpermeability. S-nitrosylation of VASP inhibits its function in barrier integrity and leads to endothelial monolayer hyperpermeability in response to PAF, a representative proinflammatory agonist.
NEW & NOTEWORTHY Here, we demonstrate that S-nitrosylation of vasodilator-stimulated phosphoprotein (VASP) on C64 is a mechanism for the onset of platelet-activating factor-induced hyperpermeability. Our results reveal a dual role of VASP in endothelial permeability. In addition to its well-documented function in barrier integrity, we show that S-nitrosylation of VASP contributes to the onset of endothelial permeability.
focal adhesions are important cellular structures that modulate endothelial barrier function. These dynamic structures mediate the attachment of cells to the extracellular matrix and serve to transmit mechanical force and regulatory signals. One of the main constituents of focal adhesions is vasodilator-stimulated phosphoprotein (VASP) (23), which belongs to the Ena/VASP family of actin regulatory proteins. In the endothelium, VASP associates with actin stress fibers, adherens junctions (28), tight junctions (3), and focal adhesions (4, 25). The key role of VASP in maintaining barrier function is supported by evidence showing that mice lacking proteins of the VASP family die from edema formation due to defective vascular barrier function (8) and that VASP-deficient endothelial cells show increased permeability under basal conditions (22–24).
Nitric oxide (NO), produced by endothelial NO synthase (eNOS), is a key factor in the regulation of endothelial barrier. We have demonstrated that in the absence of eNOS, there is minimal or no increase in permeability in response to proinflammatory agents and that the subcellular location of eNOS regulates the development of hyperpermeability (11, 20, 21). NO acts through two independent pathways: 1) soluble guanylate cyclase (sGC)-PKG and 2) S-nitrosylation, which consists of the posttranslational modification of free-thiol cysteines. We demonstrated that platelet-activating factor (PAF) via S-nitrosylation can increase endothelial permeability independent of PKG activation. Importantly, PAF-induced hyperpermeability correlated with S-nitrosylation of vascular endothelial (VE)-cadherin, p120, and β-catenin, which are components of endothelial adherens junctions (10, 14).
Attention has focused so far on NO regulation of VASP function in barrier integrity by phosphorylation through PKG activation (13, 18). We explored whether VASP can be regulated by S-nitrosylation as a mechanism to reduce endothelial barrier and increase microvascular permeability. To accomplish these goals, we used different endothelial cell types and applied PAF as a representative proinflammatory agonist (16, 17). Our work shows that S-nitrosylation of VASP reduces endothelial barrier and increases permeability. Importantly, we identified C64 as the VASP cysteine that becomes S-nitrosylated and leads to hyperpermeability.
MATERIALS AND METHODS
Reagents and antibodies.
PAF was purchased from Calbiochem (Billerica, MA). TNF-α was from Roche (New York, NY). FITC-labeled dextran 70 (FITC-Dx-70; molecular weight: 70,000 Da) and N-methyl-l-arginine were from Sigma-Aldrich Chemicals (St. Louis, MO). All reagents for cell culture were obtained from Life Technologies (Carlsbad, CA). Mouse anti-VASP antibody was from BD Transduction Laboratories (San Jose, CA), and mouse anti-β-actin antibody was from Sigma.
Cell culture.
Immortalized human venous endothelial cells [EAhy926 cells (derived from the human umbilical vein, kindly donated by Dr C. J. S. Edgell, University of North Carolina, Chapel Hill, NC)] (6) were grown in DMEM supplemented with FBS [10% (vol/vol)], l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), fungizone (2.5 μg/ml), and hypoxanthine (100 µM)-aminopterin (0.4 µM)-thymidine (16 µM) (HAT). Bovine coronary postcapillary venular endothelial cells were grown in the same medium as EAhy926 cells with no HAT and supplemented with 20 U/ml heparin. ECV-304 cells were originally reported as derived from human umbilical vein endothelial cells. Later, DNA fingerprinting revealed that these cells are derived genetically from a human urinary bladder carcinoma T24 cell line but exhibit both endothelial and epithelial markers (27). An advantage of our batch of ECV-304 cells is that they resemble endothelial cells functionally but do not express endogenous eNOS (19). ECV-304 cells transfected with green fluorescent protein (GFP)-conjugated eNOS or the constructs GFPeNOS-G2A (which targets eNOS to the cytoplasm, ECV-GFPeNOS-G2A) and GFPeNOS-CAAX (which targets eNOS to the plasma membrane, ECV-GFPeNOS-CAAX) were grown in DMEM supplemented with 10% (vol/vol) FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone and supplemented with 400 μg/ml geneticin (G418). Immortalized mouse microvascular endothelial cell lines from the myocardium (MyEnd and MyEnd-VASP-knockout (KO) (22)] were grown in EGM2-MV media from Lonza (Walkersville, MD).
Plasmids, site-directed mutagenesis, and cell transfections.
Wild-type human VASP cDNA in pEGFP-C1 was kindly donated by Dr. Otgonchimeg Rentsendorj (Johns Hopkins University, Baltimore, MD). Using site-directed mutagenesis, we substituted C7, C64 and C334 in VASP with serine. We removed all cysteines to generate a cysteine-less VASP construct (triple mutant). The mutations were produced using the QuikChange II Site-Directed Mutagenesis Kit from Agilent Technologies (Santa Clara, CA). The mutations were verified by DNA sequencing. Wild-type VASP and the mutants were transiently transfected in EAhy926 cells and in MyEnd-VASP-KO cells using Lipofectin (Invitrogen) according to the manufacturer’s instructions. Experiments were run 3 days after transfection.
Western blot analysis and biotin switch assay.
These methods are standard in our laboratory and were applied according to previously published protocols (14, 21, 22). Cells were grown to confluence in 60-mm plates. Protein was extracted on ice with lysis buffer containing 1% Triton X-100, 50 mM Tris (pH 7.4), 150 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM PMSF, 10 µg/ml aprotinin, and 10 µg/ml leupeptin. After 15 min of incubation at 4°C, the lysate was centrifuged at 14,000 g for 15 min at 4°C, and the pellet was discarded. For the biotin switch assay, 100 μg of proteins were denatured with SDS in the presence of methyl methanethiosulfonate. After acetone precipitation, 1 mmol/l ascorbate and biotin-HPDP were added to reduce the SNO bond and label the reduced thiol with biotin, respectively. Biotinylated proteins were captured with streptavidin-agarose beads, separated by SDS-PAGE, and then detected with specific antibodies. An equal amount of protein from the same sample (100 μg) was loaded in separate gels to detect input (VASP total). In the series of experiments involving site-directed mutagenesis, we used β-actin, after total VASP-GFP membranes had been stripped, as a second loading control for every lane. To permit quantitative comparisons, we ran S-nitrosylated VASP and total VASP in separate gels simultaneously and under the same conditions. Band intensities were determined using the gel imaging and analysis system G:BOX Chemi XX6 (Syngene USA, Frederick, MD). In accordance with American Journal of Physiology-Heart and Circulatory Physiology's instructions regarding Western blot images and analysis, we 1) placed a molecular weight marker on every Western blot and 2) identified samples from different blots by placing dividing lines.
Endothelial permeability assay.
Monolayer permeability was determined as previously described (21, 22).
Statistical analysis.
Experiments were conducted in groups with a minimum of n = 5 independent experiments. Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using Sigmaplot 11 and GraphPad Prism software. Significance was accepted at P < 0.05. Specific methods are indicated in each figure.
RESULTS
PAF induces S-nitrosylation of VASP.
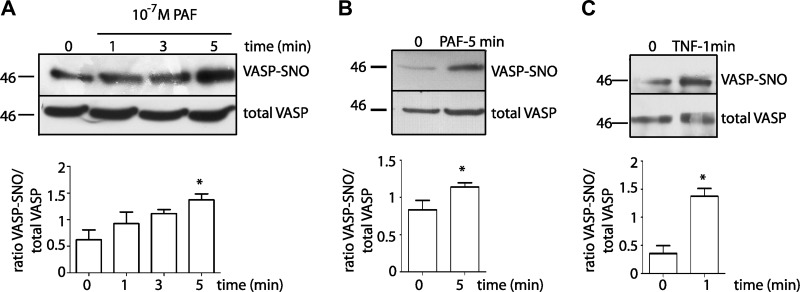
NO derived from eNOS is an important regulatory signal for endothelial permeability through posttranslational modifications of adherens junction proteins (10, 14, 29); however, whether or not NO modifies focal adhesions proteins has not been addressed in regard to the regulation of endothelial permeability. To test whether or not PAF induces S-nitrosylation of VASP in vitro, we applied 10−7 M PAF to confluent monolayers of EAhy926 cells for different times. Figure 1A shows that PAF significantly increased VASP S-nitrosylation at 5 min of stimulation. We observed similar results in primary cultures of coronary postcapillary venular endothelial cells (Fig. 1B). We applied TNF-α, a well-known proinflammatory agonist, to EAhy926 monolayers to test whether induction of S-nitrosylation of VASP is a common property of proinflammatory agents. TNF-α recapitulated PAF-induced S-nitrosylation (Fig. 1C). Interestingly, TNF-α induced significant S-nitrosylation of VASP within 1 min of application. These results indicate that S-nitrosylation of VASP is a mechanism shared by agents that increase microvascular permeability.
Fig. 1.
Platelet-activating factor (PAF) causes S-nitrosylation of vasodilator-stimulated phosphoprotein (VASP). A: EAhy926 monolayers were treated with 10−7 M PAF for 1, 3, and 5 min and then processed for the biotin switch assay with anti-VASP antibodies. PAF-induced S-nitrosylation, while increasing at every exposure time, was significant at 5 min. n = 5. *P < 0.05. One-way ANOVA, Dunnett’s multiple-comparison test. B: coronary postcapillary venular endothelial cell monolayers were treated with 10−7 M PAF for 5 min, processed for the biotin switch assay, and probed with anti-VASP antibodies. n = 5. *P < 0.05, unpaired t-test. C: EAhy926 monolayers were treated with 3 nM TNF-α for 1 min and then processed for the biotin switch assay with anti-VASP antibodies. n = 5. *P < 0.05, unpaired t-test. 0 represents control untreated cells in all graphs; SNO, S-nitrosylation. The bar graphs show the quantification of S-nitrosylation of VASP by image analysis of Western blots. Western blots for S-nitrosylated (VASP-SNO) and input (VASP total) proteins were run simultaneously and under the same conditions in separate gels. Images were cropped subsequently to construct the final figure.
S-nitrosylation of VASP depends on eNOS location.
Even though NO is a diffusible molecule, its half-life is reduced by surrounding scavengers; therefore, the location of its synthesis with respect to time and space is crucial in determining the biological functions of NO. Previously, we demonstrated that eNOS produces NO regardless of its subcellular location (cytosolic or plasma membrane) in response to PAF, but only cytosolic eNOS-derived NO increased permeability in response to PAF (22). Furthermore, S-nitrosylation of p120 and β-catenin in response to PAF occurs only in cells wherein eNOS is targeted to the cytosol (ECV-GFPeNOS-G2A) but not in cells with eNOS targeted to the plasma membrane (ECV-GFPeNOS-CAAX) (14). Based on those observations, we tested whether or not S-nitrosylation of VASP depends on eNOS location. Figure 2A shows that PAF stimulated S-nitrosylation of VASP in cells transfected with wild-type eNOSGFP (ECV-eNOSGFP). In these cells, we demonstrated that eNOS moves from the plasma membrane to the cytosol (20, 21). PAF induced significant S-nitrosylation of VASP in cells transfected with eNOS targeted to the cytosol (ECV-GFPeNOS-G2A; Fig. 2B). However, PAF failed to induce VASP S-nitrosylation in cells with eNOS targeted to the plasma membrane (ECV-GFPeNOS-CAAX; Fig. 2C). These results demonstrate that S-nitrosylation of VASP depends on eNOS location.
Fig. 2.
Subcellular location of endothelial nitric oxide synthase (eNOS) determines S-nitrosylation of VASP. Protein extracts from ECV-eNOSGFP, ECV-GFPeNOS-CAAX, and ECV-GFPeNOS-G2A cells, control or treated with 10−7 M PAF, were processed for the biotin switch assay and probed with anti-VASP antibodies. Time 0 indicates baseline control; time = 5 min indicates treatment with PAF. Top: Western blots showing S-nitrosylation of VASP. Bottom: bar graphs showing the quantification of S-nitrosylation of VASP by image analysis of the Western blots. n = 5. *P < 0.05 compared with baseline (time 0) control, one-way ANOVA and Bonferroni t-test as the post hoc test. Western blots for S-nitrosylated (VASP-SNO) and input (VASP total) proteins were run simultaneously and under the same conditions in separate gels. Images were cropped subsequently to construct the final figure. GFP, green fluorescent protein.
PAF induces S-nitrosylation of VASP on C64.
Human VASP has three cysteines, C7, C64, and C334, which are potential candidates for S-nitrosylation. We performed site-directed mutagenesis and changed each cysteine at a time to serine to determine whether all or only specific cysteines are candidates for S-nitrosylation. We also generated a cysteine-less VASP construct by simultaneously mutating the three cysteines to serine (C7,64,334S). Subsequently, we transfected mouse MyEnd-VASP-KO cells with wild-type VASP-GFP and cysteine mutants and performed a biotin switch assay to detect S-nitrosylation of VASP. Figure 3 shows that PAF induced S-nitrosylation of VASP in cells transfected with VASP-GFP and in VASP KO cells transfected with C7S and C334S mutants. In contrast, PAF failed to cause S-nitrosylation in VASP KO cells transfected with the C64S and the triple mutant. These results demonstrate that C64 is the cysteine responsible for S-nitrosylation of VASP.
Fig. 3.
Identification of VASP S-nitrosylated cysteines by site-directed mutagenesis. MyEnd-VASP-KO cells were transfected transiently with VASP-GFP and cysteine mutants and processed by a biotin switch assay to determine VASP cysteine S-nitrosylated by PAF. Top: Western blots showing that PAF promoted S-nitrosylation in VASP-GFP, C7S, and C334S. PAF failed to induce S-nitrosylation in C64S and in the cysteine-less mutant C7,64,334S. Bottom: bar graphs showing the quantification of S-nitrosylation of VASP by image analysis of Western blots, confirming the relevance of C64. n = 5. *P < 0.05, unpaired t-test. Western blots for S-nitrosylated (VASP-SNO) and input (VASP-GFP total) proteins were run simultaneously and under the same conditions in separate gels. β-Actin was determined after membranes were stripped of VASP-GFP total. Images were cropped subsequently to constructing the final figure. KO, knockout.
VASP functions in barrier integrity and hyperpermeability: role of S-nitrosylation.
To assess the role of S-nitrosylation of VASP in endothelial permeability, we reconstituted MyEnd-VASP-KO cells with VASP wild-type and cysteine mutants by transient plasmid transfection and measured permeability to FITC-Dx-70. As a control, we used MyEnd wild-type cells with a basal permeability of 4.00 ± 1.23 × 10−6 cm/s. MyEnd-VASP-KO cells, in contrast, showed a basal permeability of 11.5 ± 1.37 × 10−6 cm/s, which was about three times the permeability coefficient measured for MyEnd wild-type cells (Fig. 4A). MyEnd-VASP-KO cells reconstituted with wild-type VASP-GFP and the cysteine mutants displayed permeability coefficients similar to MyEnd wild-type cells and significantly lower than VASP KO cells (Fig. 4A). These results corroborate previous results showing that VASP is an important protein that regulates barrier integrity (23, 24). Importantly, we showed that S-nitrosylation of VASP is not necessary for the regulation of basal permeability of endothelial cells. On the contrary, when we measured PAF-stimulated permeability, we found that PAF failed to increase permeability to FITC-Dx-70 in MyEnd-VASP-KO cells. PAF significantly increased permeability to macromolecules in cells reconstituted with VASP-GFP, C7S-GFP, and C334S-GFP (Fig. 4B). Importantly, PAF did not increase permeability in cells transfected with the triple mutant C7,64,334S and C64S, indicating that C64 is the target cysteine for S-nitrosylation of VASP and PAF-stimulated hyperpermeability.
Fig. 4.
Role of S-nitrosylation of VASP in endothelial permeability. Loss and rescue of function experiments are shown. MyEnd-VASP-KO cells were transfected with VASP-GFP and different cysteine VASP mutants labeled with GFP. A: baseline permeability to FITC-labeld 70-kDa dextran (FITC-Dx-70). Loss of VASP in MyEnd-VASP-KO cells caused a large increase in permeability to FITC-Dx-70. Transfection with VASP or with cysteine VASP mutants restored barrier integrity and baseline permeability values. n = 5. #P < 0.05 compared with MyEnd wild-type (WT) cells and *P < 0.05 compared with VASP KO cells, one-way ANOVA and Bonferroni t-test as the post hoc test. B: PAF-stimulated permeability to FITC-Dx-70. PAF (10−7 M) failed to increase the already elevated permeability to FITC-Dx-70 in MyEnd-VASP-KO cells. PAF stimulated hyperpermeability in MyEnd WT cells and cells transfected with VASP-GFP, C7S, and C334S mutants. Importantly, PAF failed to stimulate hyperpermeability in MyEnd cells transfected with C64S or C7,64,334S, demonstrating that S-nitrosylation of VASP at C64 is required for PAF-induced hyperpermeability. n = 8. *P < 0.05, paired t-test.
DISCUSSION
Here, we demonstrate that VASP 1) is subject to S-nitrosylation on C64, 2) S-nitrosylation of VASP depends on eNOS localization, and 3) S-nitrosylation of VASP leads to increased endothelial permeability to macromolecules. This novel functional process adds to the well-known role of VASP in baseline permeability. Our findings lead us to propose that VASP has a dual role in the regulation of endothelial/microvascular permeability, i.e., maintenance of barrier integrity and onset of hyperpermeability.
We and others have previously demonstrated that S-nitrosylation of adherens junction proteins (β-catenin, p120, and VE-cadherin) (10, 14, 29) contributes to endothelial hyperpermeability. Here, we demonstrate that S-nitrosylation of VASP, a focal adhesion protein, is required for the onset of permeability. The causal link between S-nitrosylation of VASP and hyperpermeability was demonstrated by our experiments showing that endothelial cells transfected with C64S mutant have normal baseline permeability but are unable to increase permeability in response to PAF (Fig. 4). Several studies have indicated that focal adhesions play an important role in the control of microvascular permeability (32). Vinculin (1, 2) and paxillin (33) are fundamental to maintain the integrity of the microvascular/endothelial barrier. Depletion of vinculin or paxillin inhibited thrombin- and LPS-induced hyperpermeability due to a lack of vinculin as a scaffold to support cell contraction (1) and lack of phosphorylation of paxillin and internalization of VE-cadherin (7).
VASP, a protein located in the cytosolic side of focal adhesions, is necessary for barrier integrity inasmuch as mice lacking VASP exhibit significant extravasation of water and macromolecules and die as a consequence of edema (8). Similarly, stimulation of signaling pathways that phosphorylate VASP contributes to protect endothelial barrier function (3). Our results confirm that the presence of VASP is required for normal baseline permeability because MyEnd-VASP-KO endothelial cells displayed highly increased baseline permeability while transfection with VASP restored normal permeability to macromolecules (Fig. 4A). It is worth noting that cysteine-to-serine mutations on VASP by site-directed mutagenesis did not alter baseline permeability (Fig. 4A), an observation that suggests that other mechanisms not involving cysteine modifications contribute to VASP function in the maintenance of baseline barrier integrity.
Our results advance the novel concept that VASP is a component of mechanisms that trigger the onset of hyperpermeability. We identified S-nitrosylation as an important posttranslational modification that signals disruption of barrier integrity and enhances permeability to macromolecules upon stimulation with PAF (Fig. 4). Here, we provide evidence that PAF and TNF-α, two recognized proinflammatory agents, cause significant S-nitrosylation of VASP (Fig. 1). This novel concept in the regulation of microvascular/endothelial permeability is important because PAF and TNF-α are among the early mediators involved as causative factors in the hyperpermeability response associated with inflammation and ischemia-reperfusion (17, 28).
Unlike vasodilation, where NO diffuses from the endothelium to smooth muscle, S-nitrosylation requires proximity between eNOS and target proteins for appropriate NO delivery (12, 14). We observed that S-nitrosylation of VASP in response to PAF depends on eNOS cytosolic localization (Fig. 2), confirming the requirement of proximity to induce the posttranslational modification. VASP as a constituent of focal adhesions locates in the cytosolic side of the plasma membrane. The fact that plasma membrane eNOS fails to induce S-nitrosylation of VASP despite that it efficiently produces NO (22) strongly supports the concept that S-nitrosylation is tightly regulated by time and space and demonstrates that cytosolic eNOS has direct access to VASP.
We identified C64 in VASP as the cysteine that becomes S-nitrosylated by PAF and showed that C64 is a key posttranslational modification for enabling the onset of hyperpermeability. This conclusion is supported by the experiments showing that endothelial cells transfected with the C64S mutant failed to become S-nitrosylated and did not develop robust hyperpermeability in response to PAF (Fig. 4). Since S-nitrosylation affects interactions between proteins, protein phosphorylation, and localization (14), we speculate that S-nitrosylation regulates the localization of VASP to focal adhesions. It is important to note that C64 is located in the EVH1 domain of VASP, which mediates the subcellular targeting of VASP to focal adhesions through interactions with zyxin and vinculin (9, 15). Reorganization of focal adhesions represents an important step in the process of increased vascular permeability (1, 7). VASP function is also regulated by phosphorylation through PKG (4). Even though S-nitrosylation is required for VE-cadherin phosphorylation on tyrosine (10), we cannot discount the possibility that S-nitrosylation of VASP may influence the activity of serine/threonine kinases. Finally, S-nitrosylation of VASP is likely to modify its interaction with Rac-GTP (23) and the function of Rac-1 activity, which is key to maintain endothelial barrier functions (31) inasmuch as VASP KO cells showed diminished Rac-GTP activity (23, 24).
Overall, our present results support the concept that S-nitrosylation of VASP is necessary for the regulation of hyperpermeability in endothelial monolayers. In addition, our results highlight the novel dual role of VASP in the regulation of the microvascular barrier, i.e., barrier integrity and transport functions.
GRANTS
This work was supported by Fondecyt 1130769 and National Heart, Lung, and Blood Institute Grants 6-RO1-HL-070634 and 6-RO1-HL-088479.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.Z., N.M., F.C., A.A., J.C., and F.A.S. performed experiments; P.Z., N.M., M.P.B., J.S., and F.A.S. analyzed data; P.Z., N.M., J.S., and F.A.S. interpreted results of experiments; P.Z., N.M., C.J.M., M.P.B., N.G., J.S., W.N.D., and F.A.S. edited and revised manuscript; P.Z., F.C., A.A., C.J.M., M.P.B., N.G., J.C., W.N.D., and F.A.S. approved final version of manuscript; N.M. and F.A.S. conceived and designed research; N.M. and F.A.S. prepared figures; C.J.M., M.P.B., J.S., W.N.D., and F.A.S. drafted manuscript.
REFERENCES
- 1.Birukova AA, Shah AS, Tian Y, Gawlak G, Sarich N, Birukov KG. Selective role of vinculin in contractile mechanisms of endothelial permeability. Am J Respir Cell Mol Biol 55: 476–486, 2016. doi: 10.1165/rcmb.2015-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birukova AA, Shah AS, Tian Y, Moldobaeva N, Birukov KG. Dual role of vinculin in barrier-disruptive and barrier-enhancing endothelial cell responses. Cell Signal 28: 541–551, 2016. doi: 10.1016/j.cellsig.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16: 583–585, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Draijer R, Vaandrager AB, Nolte C, de Jonge HR, Walter U, van Hinsbergh VW. Expression of cGMP-dependent protein kinase I and phosphorylation of its substrate, vasodilator-stimulated phosphoprotein, in human endothelial cells of different origin. Circ Res 77: 897–905, 1995. doi: 10.1161/01.RES.77.5.897. [DOI] [PubMed] [Google Scholar]
- 5.Durán WN, Beuve AV, Sánchez FA. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 65: 819–826, 2013. doi: 10.1002/iub.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80: 3734–3737, 1983. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu P, Usatyuk PV, Lele A, Harijith A, Gregorio CC, Garcia JG, Salgia R, Natarajan V. c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury. Am J Physiol Lung Cell Mol Physiol 308: L1025–L1038, 2015. doi: 10.1152/ajplung.00306.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fässler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol 179: 761–775, 2007. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87: 227–239, 1996. doi: 10.1016/S0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 10.Guequén A, Carrasco R, Zamorano P, Rebolledo L, Burboa P, Sarmiento J, Boric MP, Korayem A, Durán WN, Sánchez FA. S-nitrosylation regulates VE-cadherin phosphorylation and internalization in microvascular permeability. Am J Physiol Heart Circ Physiol 310: H1039–H1044, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatakeyama T, Pappas PJ, Hobson RW II, Boric MP, Sessa WC, Durán WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA 103: 19777–19782, 2006. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsay SL, Ramsey S, Aitchison M, Renné T, Evans TJ. Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J Cell Sci 120: 3011–3021, 2007. doi: 10.1242/jcs.003061. [DOI] [PubMed] [Google Scholar]
- 14.Marín N, Zamorano P, Carrasco R, Mujica P, González FG, Quezada C, Meininger CJ, Boric MP, Durán WN, Sánchez FA. S-nitrosation of β-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability. Circ Res 111: 553–563, 2012. doi: 10.1161/CIRCRESAHA.112.274548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J 16: 5433–5444, 1997. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel AA, Fallek SR, Hobson RW II, Durán WN. Inhibition of nitric oxide synthase attenuates primed microvascular permeability in the in vivo microcirculation. J Vasc Surg 22: 661–669, 1995. doi: 10.1016/S0741-5214(95)70056-0. [DOI] [PubMed] [Google Scholar]
- 17.Noel AA, Hobson RW II, Durán WN. Platelet-activating factor and nitric oxide mediate microvascular permeability in ischemia-reperfusion injury. Microvasc Res 52: 210–220, 1996. doi: 10.1006/mvre.1996.0059. [DOI] [PubMed] [Google Scholar]
- 18.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Münzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 87: 999–1005, 2000. doi: 10.1161/01.RES.87.11.999. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez FA, Savalia NB, Durán RG, Lal BK, Boric MP, Durán WN. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol 291: H1058–H1064, 2006. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez FA, Kim DD, Durán RG, Meininger CJ, Durán WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am J Physiol Heart Circ Physiol 295: H1642–H1648, 2008. doi: 10.1152/ajpheart.00629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Durán WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA 106: 6849–6853, 2009. doi: 10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez FA, Rana R, González FG, Iwahashi T, Durán RG, Fulton DJ, Beuve AV, Kim DD, Durán WN. Functional significance of cytosolic endothelial nitric-oxide synthase (eNOS): regulation of hyperpermeability. J Biol Chem 286: 30409–30414, 2011. doi: 10.1074/jbc.M111.234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlegel N, Burger S, Golenhofen N, Walter U, Drenckhahn D, Waschke J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am J Physiol Cell Physiol 294: C178–C188, 2008. doi: 10.1152/ajpcell.00273.2007. [DOI] [PubMed] [Google Scholar]
- 24.Schlegel N, Waschke J. Impaired integrin-mediated adhesion contributes to reduced barrier properties in VASP-deficient microvascular endothelium. J Cell Physiol 220: 357–366, 2009. doi: 10.1002/jcp.21772. [DOI] [PubMed] [Google Scholar]
- 25.Schlegel N, Waschke J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am J Physiol Cell Physiol 296: C453–C462, 2009. doi: 10.1152/ajpcell.00360.2008. [DOI] [PubMed] [Google Scholar]
- 26.Smolenski A, Poller W, Walter U, Lohmann SM. Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase I. J Biol Chem 275: 25723–25732, 2000. doi: 10.1074/jbc.M909632199. [DOI] [PubMed] [Google Scholar]
- 27.Suda K, Rothen-Rutishauser B, Günthert M, Wunderli-Allenspach H. Phenotypic characterization of human umbilical vein endothelial (ECV304) and urinary carcinoma (T24) cells: endothelial versus epithelial features. In Vitro Cell Dev Biol Anim 37: 505–514, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka H, Oshiro H, Kim DD, Thompson PN, Seyama A, Hobson RW II, Durán WN. Microvascular transport is associated with TNF plasma levels and protein synthesis in postischemic muscle. Am J Physiol 274: H1914–H1919, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, Gratton JP. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell 39: 468–476, 2010. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol 13: 76–84, 2001. doi: 10.1016/S0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 31.Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114: 1343–1355, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wu MH. Endothelial focal adhesions and barrier function. J Physiol 569: 359–366, 2005. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y, Meng FY, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin/pp125FAK and microvascular endothelial barrier function. Am J Physiol 275: H84–H93, 1998. [DOI] [PubMed] [Google Scholar]